Lightweight 3D Lithiophilic Graphene Aerogel Current Collectors for Lithium Metal Anodes
Abstract
:1. Introduction
2. Materials and Methods
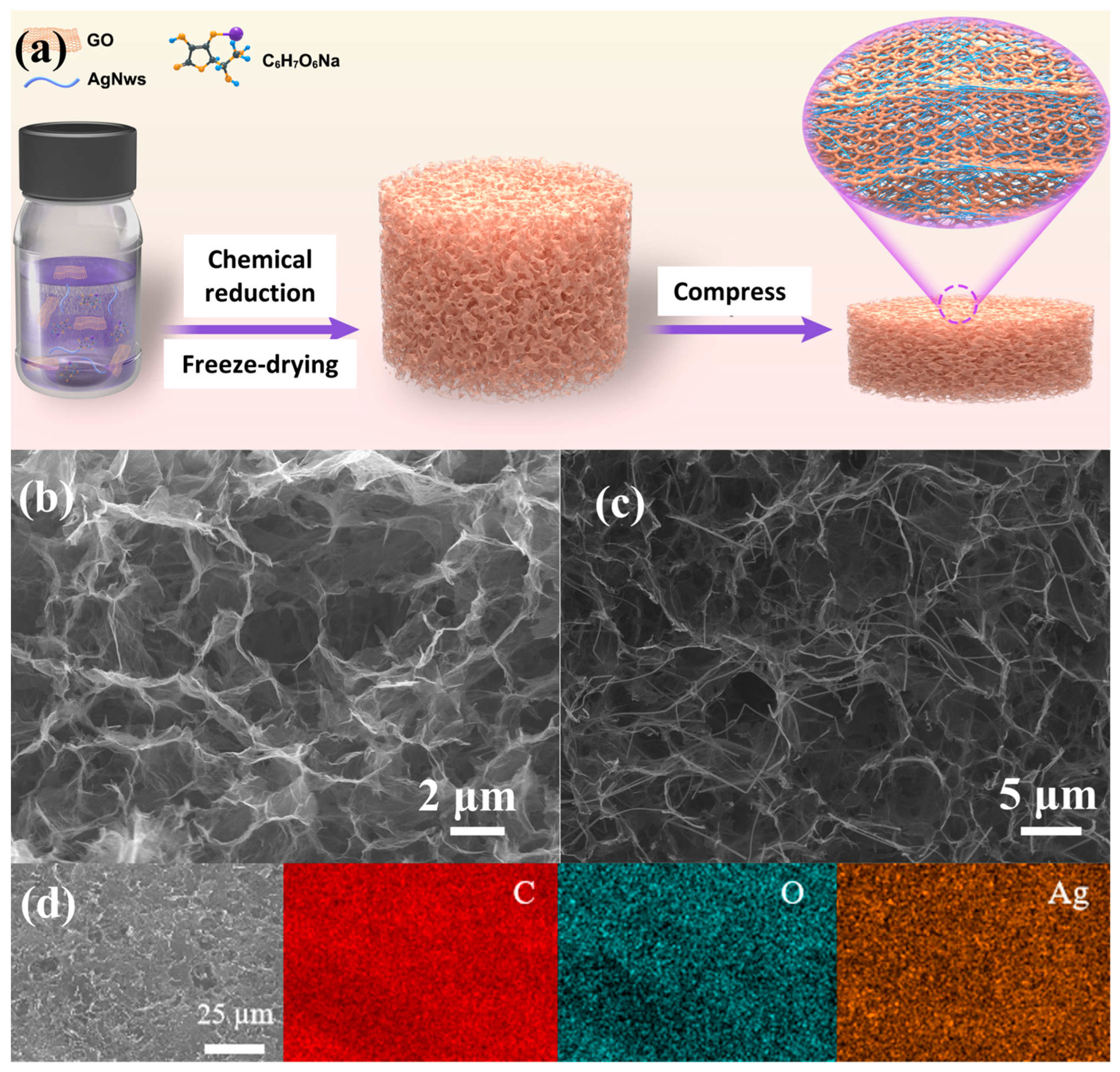
2.1. Fabrication of Reduced Graphene Oxide Aerogels with Silver Nanowires
2.2. Materials Characterization
2.3. Electrochemical Measurements
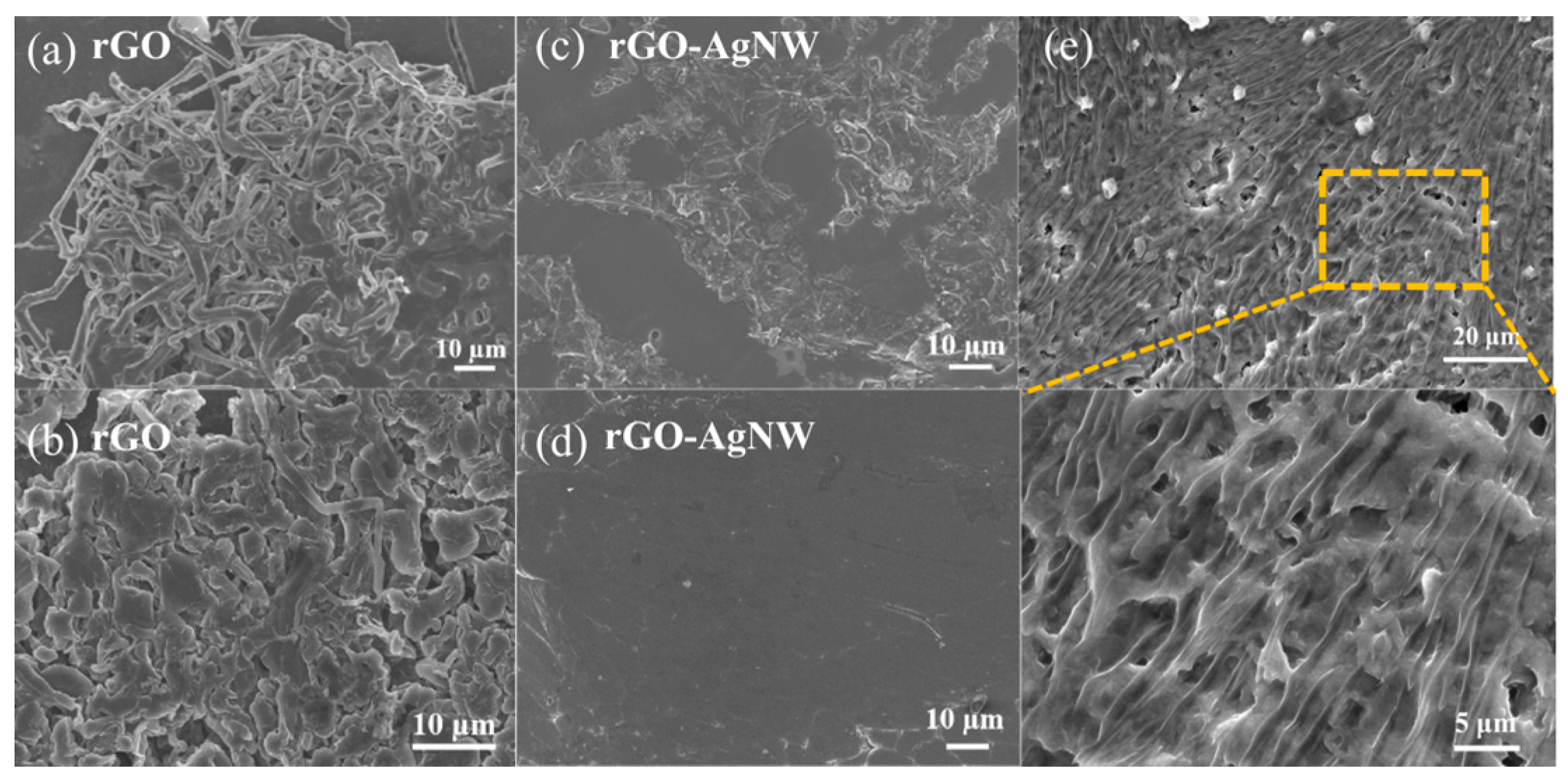
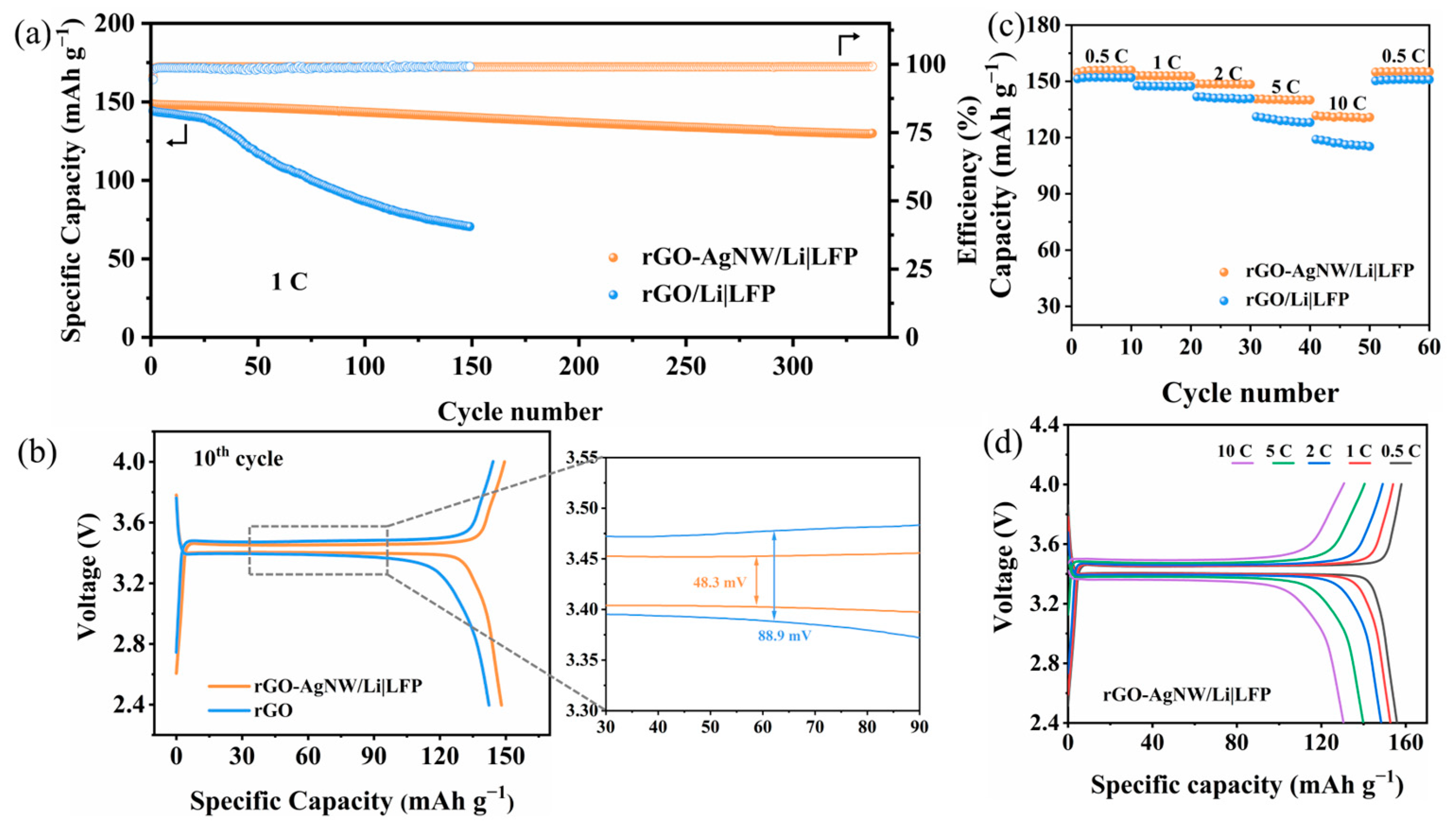
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, C.P.; Fu, K.; Zhang, Y.; Hitz, E.; Hu, L.B. Protected Lithium-Metal Anodes in Batteries: From Liquid to Solid. Adv. Mater. 2017, 29, 1701169. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.H.; Cui, W.S.; Chu, F.L.; Wu, F.X. Lithium metal anodes: Present and future. J. Energy Chem. 2020, 48, 145–159. [Google Scholar] [CrossRef]
- Albertus, P.; Babinec, S.; Litzelman, S.; Newman, A. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries. Nat. Energy 2018, 3, 16–21. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.P.; Xie, L.L.; Han, Q.; Zhu, L.M.; Chen, L.B.; Cao, X.Y. An overview of the key challenges and strategies for lithium metal anodes. J. Energy Storage 2022, 47, 103641. [Google Scholar] [CrossRef]
- Ghazi, Z.A.; Sun, Z.H.; Sun, C.G.; Qi, F.L.; An, B.G.; Li, F.; Cheng, H.M. Key Aspects of Lithium Metal Anodes for Lithium Metal Batteries. Small 2019, 15, 1900687. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.T.; Deng, Y.J.; Qu, B.H.; Huang, G.S.; Song, J.F.; Xu, C.H.; Du, A.B.; Xie, Q.S.; Wang, J.F.; Cui, G.L.; et al. Re-envisioning the Key Factors of Magnesium Metal Anodes for Rechargeable Magnesium Batteries. ACS Energy Lett. 2023, 8, 4848–4861. [Google Scholar] [CrossRef]
- Varzi, A.; Thanner, K.; Scipioni, R.; Di Lecce, D.; Hassoun, J.; Doerfler, S.; Altheus, H.; Kaskel, S.; Prehal, C.; Freunberger, S.A. Current status and future perspectives of lithium metal batteries. J. Power Sources 2020, 480, 228803. [Google Scholar] [CrossRef]
- Tikekar, M.D.; Choudhury, S.; Tu, Z.; Archer, L.A. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 2016, 1, 16114. [Google Scholar] [CrossRef]
- Zhang, Q.-K.; Zhang, X.-Q.; Wan, J.; Yao, N.; Song, T.-L.; Xie, J.; Hou, L.-P.; Zhou, M.-Y.; Chen, X.; Li, B.-Q.; et al. Homogeneous and mechanically stable solid-electrolyte interphase enabled by trioxane-modulated electrolytes for lithium metal batteries. Nat. Energy 2023, 8, 725–735. [Google Scholar] [CrossRef]
- Zhao, F.; Zhai, P.; Wei, Y.; Yang, Z.; Chen, Q.; Zuo, J.; Gu, X.; Gong, Y. Constructing Artificial SEI Layer on Lithiophilic MXene Surface for High-Performance Lithium Metal Anodes. Adv. Sci. 2022, 9, 2103930. [Google Scholar] [CrossRef] [PubMed]
- Wellmann, J.; Brinkmann, J.-P.; Wankmiller, B.; Neuhaus, K.; Rodehorst, U.; Hansen, M.R.; Winter, M.; Paillard, E. Effective Solid Electrolyte Interphase Formation on Lithium Metal Anodes by Mechanochemical Modification. ACS Appl. Mater. Interfaces 2021, 13, 34227–34237. [Google Scholar] [CrossRef] [PubMed]
- Thanner, K.; Varzi, A.; Buchholz, D.; Sedlmaier, S.J.; Passerini, S. Artificial Solid Electrolyte Interphases for Lithium Metal Electrodes by Wet Processing: The Role of Metal Salt Concentration and Solvent Choice. ACS Appl. Mater. Interfaces 2020, 12, 32851–32862. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhang, X.-Q.; Cheng, X.-B.; Peng, H.-J.; Zhao, C.-Z.; Yan, C.; Huang, J.-Q. Artificial Soft-Rigid Protective Layer for Dendrite-Free Lithium Metal Anode. Adv. Funct. Mater. 2018, 28, 1705838. [Google Scholar] [CrossRef]
- Thompson, R.S.; Schroeder, D.J.; López, C.M.; Neuhold, S.; Vaughey, J.T. Stabilization of lithium metal anodes using silane-based coatings. Electrochem. Commun. 2011, 13, 1369–1372. [Google Scholar] [CrossRef]
- Shaik, M.R.; Bissannagari, M.; Kwon, Y.M.; Cho, K.Y.; Kim, J.; Yoon, S. Soft, robust, Li-ion friendly halloysite-based hybrid protective layer for dendrite-free Li metal anode. Chem. Eng. J. 2021, 424, 130326. [Google Scholar] [CrossRef]
- Umeda, G.A.; Menke, E.; Richard, M.; Stamm, K.L.; Wudl, F.; Dunn, B. Protection of lithium metal surfaces using tetraethoxysilane. J. Mater. Chem. 2011, 21, 1593–1599. [Google Scholar] [CrossRef]
- Li, F.; He, J.; Liu, J.; Wu, M.; Hou, Y.; Wang, H.; Qi, S.; Liu, Q.; Hu, J.; Ma, J. Gradient Solid Electrolyte Interphase and Lithium-Ion Solvation Regulated by Bisfluoroacetamide for Stable Lithium Metal Batteries. Angew. Chem. Int. Ed. 2021, 60, 6600–6608. [Google Scholar] [CrossRef] [PubMed]
- Piao, N.; Liu, S.; Zhang, B.; Ji, X.; Fan, X.; Wang, L.; Wang, P.-F.; Jin, T.; Liou, S.-C.; Yang, H.; et al. Lithium Metal Batteries Enabled by Synergetic Additives in Commercial Carbonate Electrolytes. ACS Energy Lett. 2021, 6, 1839–1848. [Google Scholar] [CrossRef]
- Schedlbauer, T.; Rodehorst, U.C.; Schreiner, C.; Gores, H.J.; Winter, M. Blends of lithium bis(oxalato)borate and lithium tetrafluoroborate: Useful substitutes for lithium difluoro(oxalato)borate in electrolytes for lithium metal based secondary batteries? Electrochim. Acta 2013, 107, 26–32. [Google Scholar] [CrossRef]
- Ue, M.; Uosaki, K. Recent progress in liquid electrolytes for lithium metal batteries. Curr. Opin. Electrochem. 2019, 17, 106–113. [Google Scholar] [CrossRef]
- Yun, J.; Park, B.-K.; Won, E.-S.; Choi, S.H.; Kang, H.C.; Kim, J.H.; Park, M.-S.; Lee, J.-W. Bottom-Up Lithium Growth Triggered by Interfacial Activity Gradient on Porous Framework for Lithium-Metal Anode. ACS Energy Lett. 2020, 5, 3108–3114. [Google Scholar] [CrossRef]
- Xue, P.; Liu, S.; Shi, X.; Sun, C.; Lai, C.; Zhou, Y.; Sui, D.; Chen, Y.; Liang, J. A Hierarchical Silver-Nanowire–Graphene Host Enabling Ultrahigh Rates and Superior Long-Term Cycling of Lithium-Metal Composite Anodes. Adv. Mater. 2018, 30, 1804165. [Google Scholar] [CrossRef]
- Phattharasupakun, N.; Wutthiprom, J.; Duangdangchote, S.; Sawangphruk, M. A 3D free-standing lithiophilic silver nanowire aerogel for lithium metal batteries without lithium dendrites and volume expansion: In operando X-ray diffraction. Chem. Commun. 2019, 55, 5689–5692. [Google Scholar] [CrossRef]
- Lu, L.Q.; Pei, Y.T. Interfacial modification by lithiophilic oxide facilitating uniform and thin solid electrolyte interphase towards stable lithium metal anodes. Mater. Today Energy 2021, 21, 100748. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, X.T.; Stalin, S.; Khan, K.; Archer, L.A. Solid-state polymer electrolytes with in-built fast interfacial transport for secondary lithium batteries. Nat. Energy 2019, 4, 365–373. [Google Scholar] [CrossRef]
- Ma, Y.X.; Wan, J.Y.; Yang, Y.F.; Ye, Y.S.; Xiao, X.; Boyle, D.T.; Burke, W.; Huang, Z.J.; Chen, H.; Cui, Y.; et al. Scalable, Ultrathin, and High-Temperature-Resistant Solid Polymer Electrolytes for Energy-Dense Lithium Metal Batteries. Adv. Energy. Mater 2022, 12, 2103720. [Google Scholar] [CrossRef]
- Jones, S.D.; Nguyen, H.; Richardson, P.M.; Chen, Y.Q.; Wyckoff, K.E.; Hawker, C.J.; Clément, R.J.; Fredrickson, G.H.; Segalman, R.A. Design of Polymeric Zwitterionic Solid Electrolytes with Superionic Lithium Transport. ACS Cent. Sci. 2022, 8, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Simon, F.J.; Busche, M.R.; Nakamura, T.; Schroeder, D.; Richter, F.H.; Janek, J. From Liquid- to Solid-State Batteries: Ion Transfer Kinetics of Heteroionic Interfaces. Electrochem. Energy Rev. 2020, 3, 221–238. [Google Scholar] [CrossRef]
- Huang, H.F.; Gui, Y.N.; Sun, F.; Liu, Z.J.; Ning, H.L.; Wu, C.; Chen, L.B. In situ formed three-dimensional (3D) lithium-boron (Li-B) alloy as a potential anode for next-generation lithium batteries. Rare Met. 2021, 40, 3494–3500. [Google Scholar] [CrossRef]
- Zheng, Z.J.; Ye, H.; Guo, Z.P. Recent Progress in Designing Stable Composite Lithium Anodes with Improved Wettability. Adv. Sci. 2020, 7, 2002212. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Ahn, Y.J.; Yoon, W.Y. The surface morphology of Li metal electrode. Met. Mater. Korea 2000, 6, 345–349. [Google Scholar] [CrossRef]
- Jin, C.; Sheng, O.; Chen, M.; Ju, Z.; Lu, G.; Liu, T.; Nai, J.; Liu, Y.; Wang, Y.; Tao, X. Armed lithium metal anodes with functional skeletons. Mater. Today Nano 2021, 13, 100103. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Chen, J.B.A.; Chen, Y.M.; Ke, X.; Li, J.; Yang, Y.; Shi, Z.C. Lithium Host:Advanced architecture components for lithium metal anode. Energy Storage Mater. 2021, 38, 276–298. [Google Scholar] [CrossRef]
- Chabi, S.; Peng, C.; Hu, D.; Zhu, Y. Ideal Three-Dimensional Electrode Structures for Electrochemical Energy Storage. Adv. Mater. 2014, 26, 2440–2445. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.R.; Zhao, B.C.; Yan, C.; Zhang, Q. Review on Li Deposition in Working Batteries: From Nucleation to Early Growth. Adv. Mater. 2021, 33, 2004128. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.X.; Lu, W.Y.; Chen, Y.J.; Yang, H.; Wu, C.; Wei, W.F.; Chen, L.B.; Ouyang, X.P. Coating highly lithiophilic Zn on Cu foil for high-performance lithium metal batteries. Rare Met. 2022, 41, 1255–1264. [Google Scholar] [CrossRef]
- Yan, K.; Lu, Z.; Lee, H.-W.; Xiong, F.; Hsu, P.-C.; Li, Y.; Zhao, J.; Chu, S.; Cui, Y. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat. Energy 2016, 1, 16010. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Fujiki, S.; Jung, C.; Suzuki, N.; Yashiro, N.; Omoda, R.; Ko, D.-S.; Shiratsuchi, T.; Sugimoto, T.; Ryu, S.; et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver-carbon composite anodes. Nat. Energy 2020, 5, 299–308. [Google Scholar] [CrossRef]
- Qing, P.; Wu, Z.; Chen, Y.; Tang, F.; Yang, H.; Chen, L. Powder metallurgical 3D nickel current collectors with plasma-induced Ni3N nanocoatings enabling long-life and dendrite-free lithium metal anode. J. Energy Chem. 2022, 72, 149–157. [Google Scholar] [CrossRef]
- Jin, S.; Sun, Z.W.; Guo, Y.L.; Qi, Z.K.; Guo, C.K.; Kong, X.H.; Zhu, Y.W.; Ji, H.X. High Areal Capacity and Lithium Utilization in Anodes Made of Covalently Connected Graphite Microtubes. Adv. Mater. 2017, 29, 1700783. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Xin, S.; Yin, Y.X.; Guo, Y.G. Advanced Porous Carbon Materials for High-Efficient Lithium Metal Anodes. Adv. Energy. Mater. 2017, 7, 1700530. [Google Scholar] [CrossRef]
- Zuo, T.T.; Wu, X.W.; Yang, C.P.; Yin, Y.X.; Ye, H.; Li, N.W.; Guo, Y.G. Graphitized Carbon Fibers as Multifunctional 3D Current Collectors for High Areal Capacity Li Anodes. Adv. Mater. 2017, 29, 1700389. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, J.; Kim, P.J. A new approach to stabilize the electrochemical performance of Li metal batteries through the structure alteration of CNT scaffolds. Carbon 2023, 203, 426–435. [Google Scholar] [CrossRef]
- Yang, Y.; Ai, L.; Yu, S.; He, J.; Xu, T.; Chen, D.; Shen, L. 3D-Printed Porous GO Framework Enabling Dendrite-Free Lithium-Metal Anodes. ACS Appl. Energy Mater. 2022, 5, 15666–15672. [Google Scholar] [CrossRef]
- Singh, S.B.; Haskin, N.; Dastgheib, S.A. Coal-based graphene oxide-like materials: A comprehensive review. Carbon 2023, 215, 118447. [Google Scholar] [CrossRef]
- Raccichini, R.; Varzi, A.; Passerini, S.; Scrosati, B. The role of graphene for electrochemical energy storage. Nat. Mater. 2014, 14, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Liu, J.; Liu, X.; Hu, J.; Duan, X.; Duan, X. Rapid Electrochemical Cleaning Silver Nanowire Thin Films for High-Performance Transparent Conductors. J. Am. Chem. Soc. 2019, 141, 12251–12257. [Google Scholar] [CrossRef] [PubMed]
- Kuila, T.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Recent advances in the efficient reduction of graphene oxide and its application as energy storage electrode materials. Nanoscale 2013, 5, 52–71. [Google Scholar] [CrossRef]
- Yun, J.; Shin, H.R.; Won, E.-S.; Kang, H.C.; Lee, J.-W. Confined Li metal storage in porous carbon frameworks promoted by strong Li-substrate interaction. Chem. Eng. J. 2022, 430, 132897. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, S.; Zhang, Y.; Wang, L.; Liu, Y.; Duan, Q. One-Pot Facile Synthesis of Noble Metal Nanoparticles Supported on rGO with Enhanced Catalytic Performance for 4-Nitrophenol Reduction. Molecules 2021, 26, 7261. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, J.Q.; Chen, D.Q.; Zhao, J.B. A Facile Electrophoretic Deposition Route to the Fe3O4/CNTs/rGO Composite Electrode as a Binder-Free Anode for Lithium Ion Battery. ACS Appl. Mater. Interfaces 2016, 8, 26730–26739. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.L.; Luo, Y.X.; Li, Y.; Li, W.W.; Fang, M.H.; Shui, M.; Shu, J.; Ren, Y.L. The investigation of NaTi2(PO4)3@C/Ag as a high-performance anode material for aqueous rechargeable sodium-ion batteries. Mater. Res. Bull. 2018, 104, 194–201. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Jin, M.; Wu, L.; Guo, D.; Wu, C.; Wang, C.; Chen, X.A.; Wang, S. Rational Design of Three-Dimensional Self-Supporting Structure for Advanced Lithium Metal Anode. Batter. Supercaps 2023, 6, e202300246. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Ge, Y.; Qing, P.; Jin, Y.; Chen, L.; Mei, L. Lightweight 3D Lithiophilic Graphene Aerogel Current Collectors for Lithium Metal Anodes. Materials 2024, 17, 1693. https://doi.org/10.3390/ma17071693
Guo C, Ge Y, Qing P, Jin Y, Chen L, Mei L. Lightweight 3D Lithiophilic Graphene Aerogel Current Collectors for Lithium Metal Anodes. Materials. 2024; 17(7):1693. https://doi.org/10.3390/ma17071693
Chicago/Turabian StyleGuo, Caili, Yongjie Ge, Piao Qing, Yunke Jin, Libao Chen, and Lin Mei. 2024. "Lightweight 3D Lithiophilic Graphene Aerogel Current Collectors for Lithium Metal Anodes" Materials 17, no. 7: 1693. https://doi.org/10.3390/ma17071693




