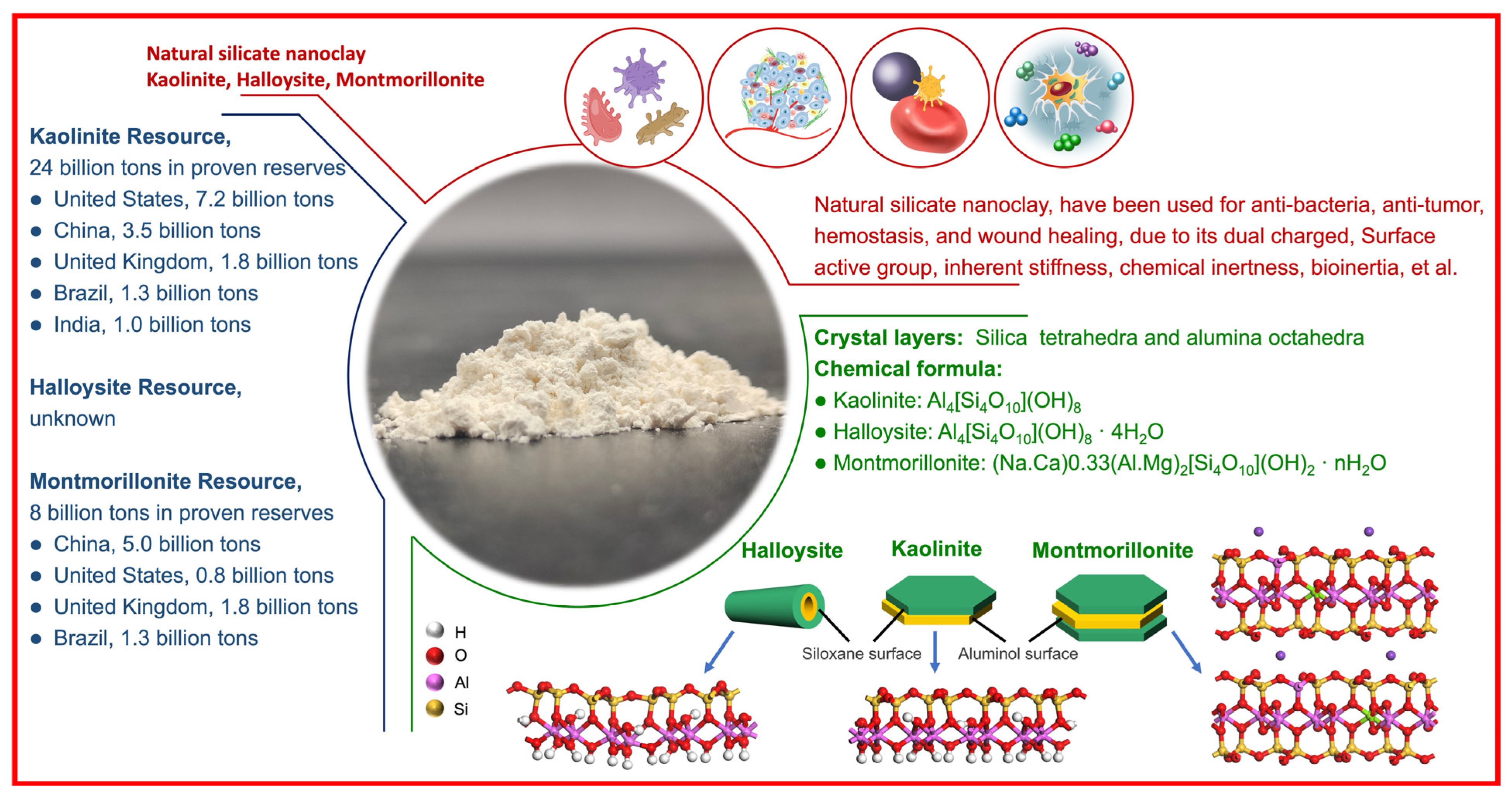
Clays and Wound Healing
Abstract
:1. Introduction
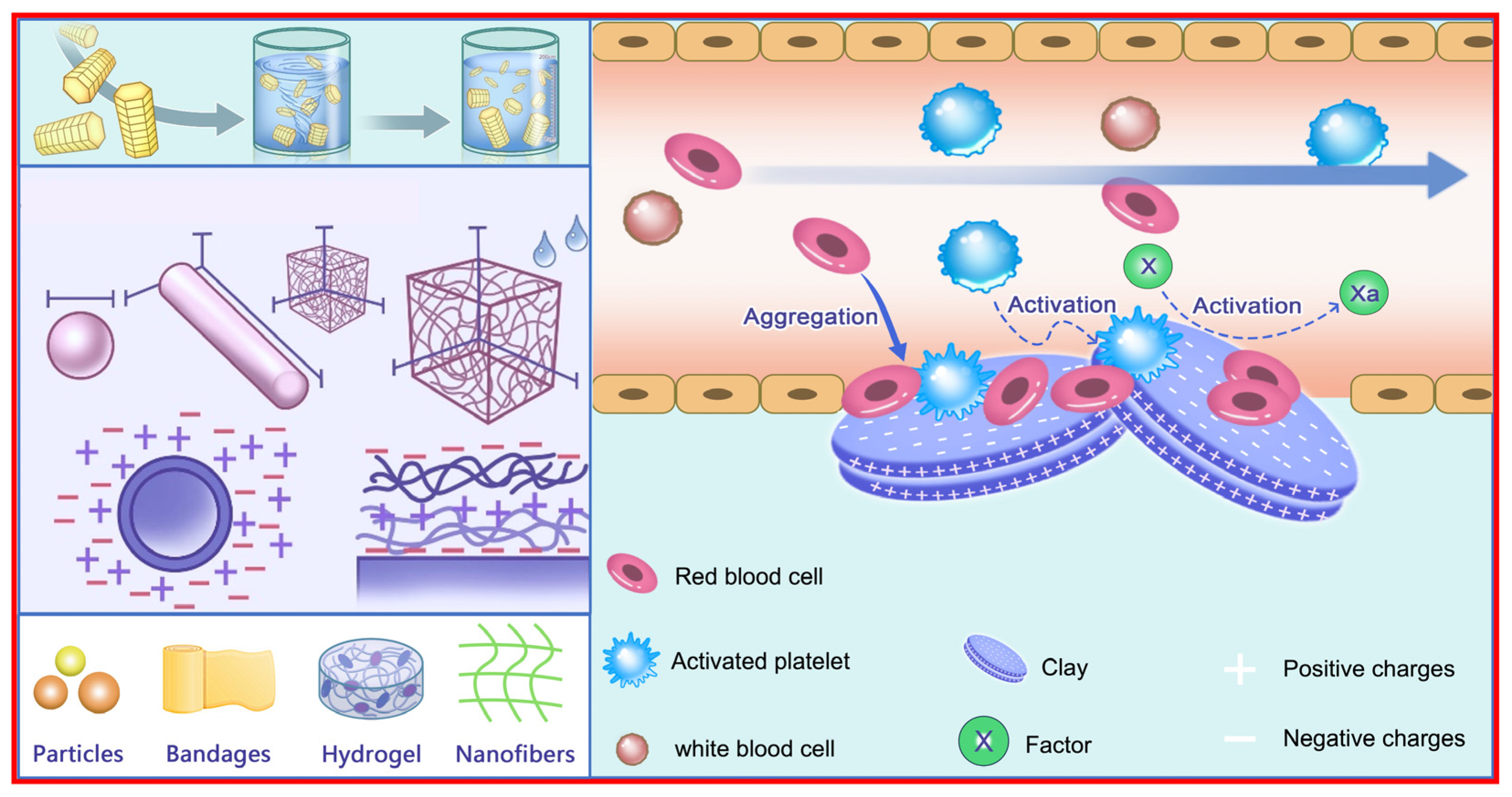
2. Hemostatic Mechanism
2.1. Hemostatic Mechanism Related to Material Characteristics
2.1.1. Surface Roughness
2.1.2. Surface Charge
2.1.3. Wettable Surface and Other Factors
2.2. Hemostatic Mechanism Related to Chemical Components
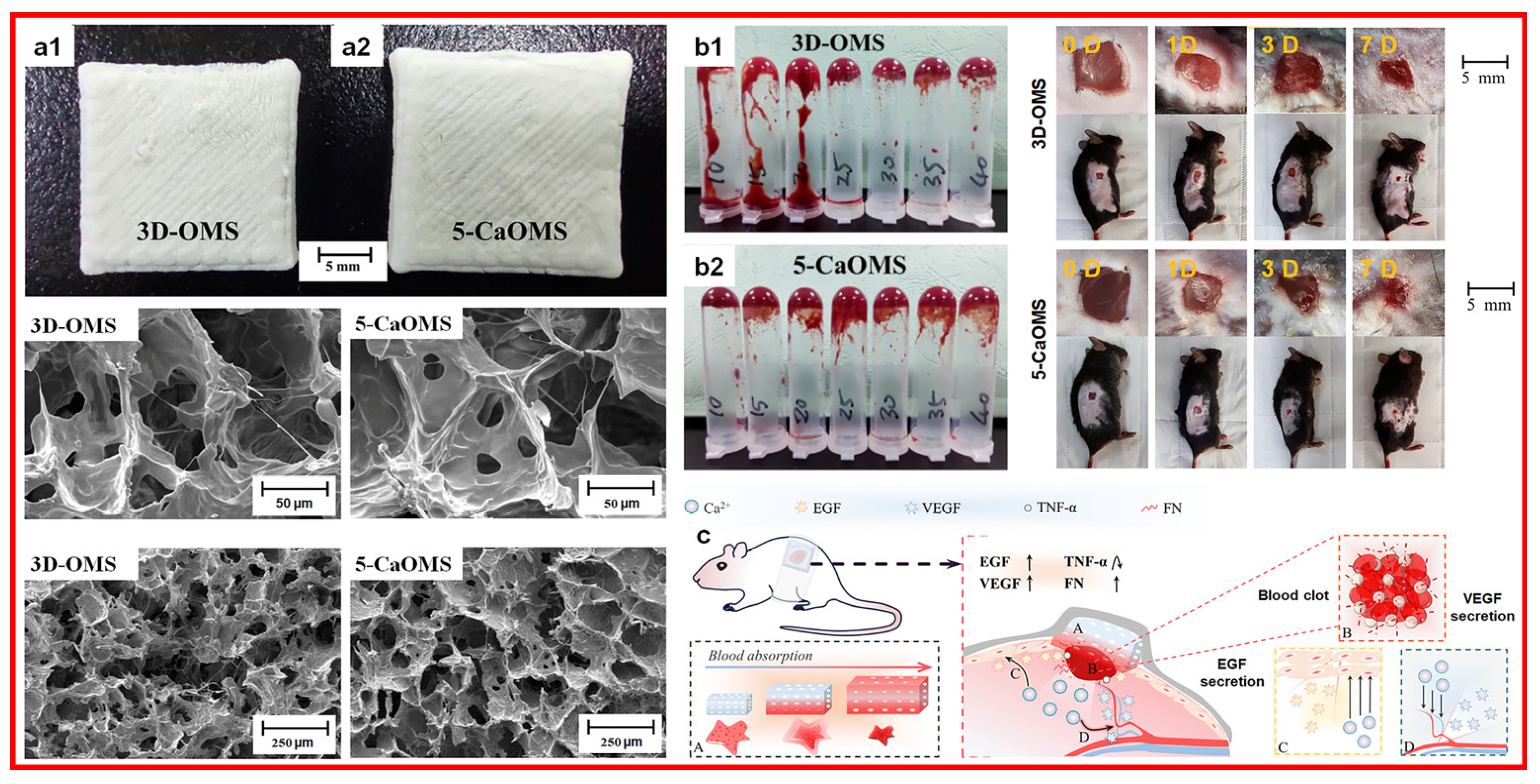
2.2.1. Calcium Ions
2.2.2. Zinc Ions
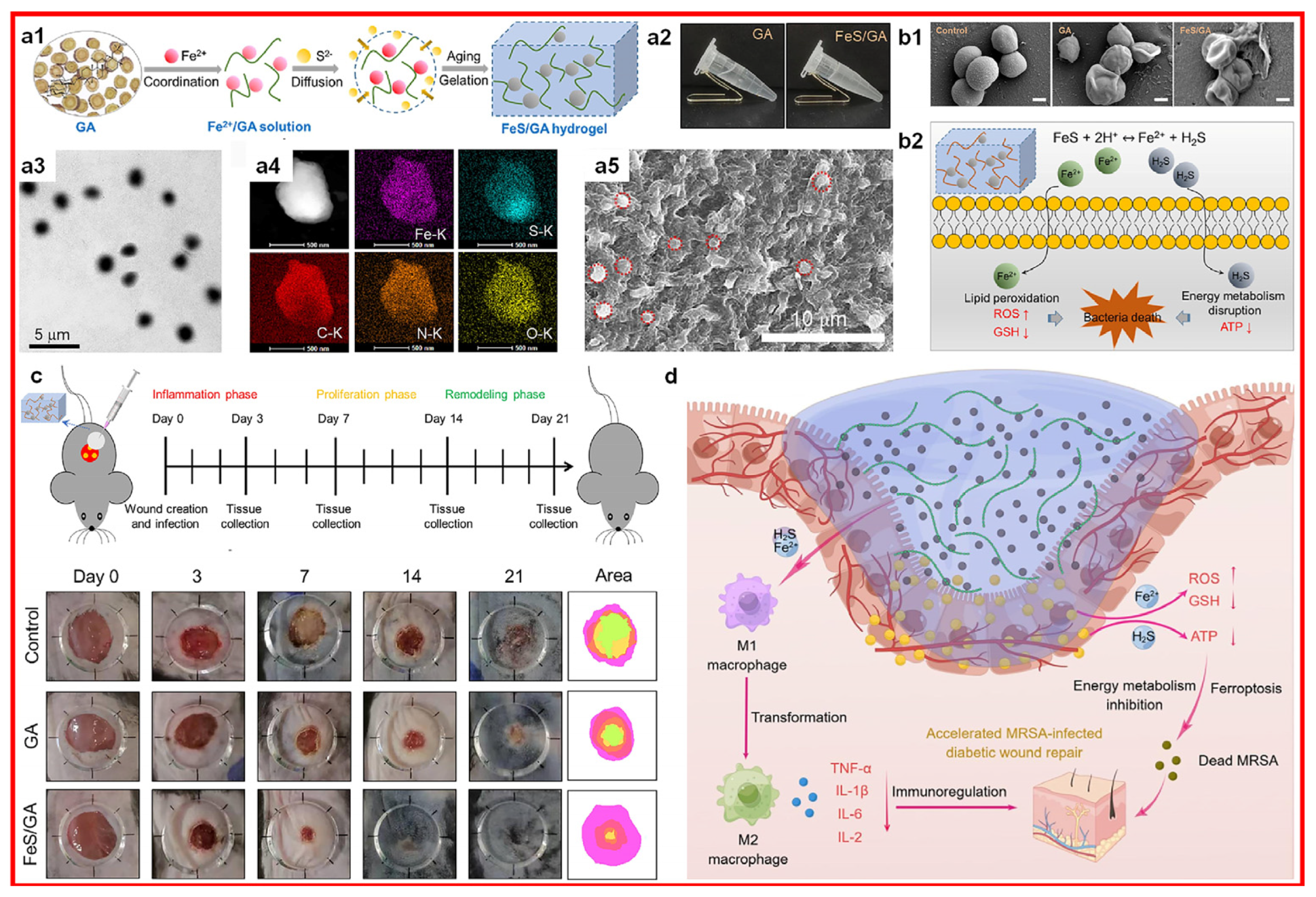
2.2.3. Iron Ions
2.2.4. Copper Ions
2.2.5. Magnesium Ions
2.2.6. Other Ions
3. Forms of Hemostatic Materials
3.1. Clay Bandages
3.2. Clay Hydrogels
3.3. Clay Electrospun Fibers
3.4. Toxicities and Limitations
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; You, X.; Dai, C.; Tong, T.; Wu, J. Hemostatic Nanotechnologies for External and Internal Hemorrhage Management. Biomater. Sci. 2020, 8, 4396–4412. [Google Scholar] [CrossRef]
- Liu, L.; Hu, E.; Yu, K.; Xie, R.; Lu, F.; Lu, B.; Bao, R.; Li, Q.; Dai, F.; Lan, G. Recent Advances in Materials for Hemostatic Management. Biomater. Sci. 2021, 9, 7343–7378. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Liu, Q.; Sui, J.X.; Ramakrishna, S.; Yu, M.; Zhou, Y.; Jiang, X.Y.; Long, Y.Z. Recent Advances in Hemostasis at the Nanoscale. Adv. Healthc. Mater. 2019, 8, 1900823. [Google Scholar] [CrossRef]
- Tian, G.; Zhang, Y.; Jiang, Y.; Hu, P.; Wang, H.; Zhang, Y. Nanoscale Zerovalent Iron-Incorporated Kaolinite for Hemostatic and Antibacterial Applications. Appl. Surf. Sci. 2023, 636, 157879. [Google Scholar] [CrossRef]
- Teng, L.; Shao, Z.; Bai, Q.; Zhang, X.; He, Y.S.; Lu, J.; Zou, D.; Feng, C.; Dong, C.M. Biomimetic Glycopolypeptide Hydrogels with Tunable Adhesion and Microporous Structure for Fast Hemostasis and Highly Efficient Wound Healing. Adv. Funct. Mater. 2021, 31, 2105628. [Google Scholar] [CrossRef]
- Dai, M.; Li, M.; Gong, J.; Meng, L.; Zhang, B.; Zhang, Y.; Yin, Y.; Wang, J. Silk Fibroin/Gelatin/Calcium Alginate Composite Materials: Preparation, Pore Characteristics, Comprehensive Hemostasis in Vitro. Mater. Des. 2022, 216, 110577. [Google Scholar] [CrossRef]
- Shi, Y.; Fang, Y.; Liang, X.; Huang, C.; Liang, Y.; Yang, Z.; Yu, J.; Wang, J.; Zhao, G. Yeast Cell Templated Porous Hollow Silica Spheres for Rapid Hemostasis Accompanied by Antibacterial Action. Biomater. Sci. 2023, 11, 3104–3113. [Google Scholar] [CrossRef]
- Pourshahrestani, S.; Zeimaran, E.; Djordjevic, I.; Kadri, N.A.; Towler, M.R. Inorganic Hemostats: The State-of-the-Art and Recent Advances. Mater. Sci. Eng. C 2016, 58, 1255–1268. [Google Scholar] [CrossRef]
- Ashfaq, M.; Wongpakham, T.; Talreja, N.; Chauhan, D.; Tharasanit, T.; Srituravanich, W. Synthesis of Polymeric Composite Grafted with Mineral Particles/Graphene Oxide-Based Biomaterial: A Promising Robust Hemostatic Bandage. Mater. Today Commun. 2022, 33, 104786. [Google Scholar] [CrossRef]
- Zhu, B.; Cheng, W.; Zhao, K.; Hu, Z.; Zhou, F.; Zhou, M.; Qian, C.; Ding, Z. Multifunctional Composite Dressings Based on Bletilla Striata Polysaccharide and Zeolite for Rapid Hemostatic and Accelerated Wound Healing. J. Mater. Sci. 2023, 58, 5427–5443. [Google Scholar] [CrossRef]
- Song, Y.; Li, S.; Chen, H.; Han, X.; Duns, G.J.; Dessie, W.; Tang, W.; Tan, Y.; Qin, Z.; Luo, X. Kaolin-Loaded Carboxymethyl Chitosan/Sodium Alginate Composite Sponges for Rapid Hemostasis. Int. J. Biol. Macromol. 2023, 233, 123532. [Google Scholar] [CrossRef]
- Li, S.; Jiang, M.; Zhang, Y.; Xie, X.; Li, W.; Ming, P.Y.; Jiang, X.; Yang, B.; He, Y.; Chen, J.; et al. Multi-Functional Carboxymethyl Chitosan/Sericin Protein/Halloysite Composite Sponge with Efficient Antibacterial and Hemostatic Properties for Accelerating Wound Healing. Int. J. Biol. Macromol. 2023, 234, 123357. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zhao, Y.; Zheng, X.; Zhang, Y.; Zhao, J.; Wang, S.; Gu, Y. Rapidly Degrading and Mussel-Inspired Multifunctional Carboxymethyl Chitosan/Montmorillonite Hydrogel for Wound Hemostasis. Int. J. Biol. Macromol. 2023, 242, 124960. [Google Scholar] [CrossRef]
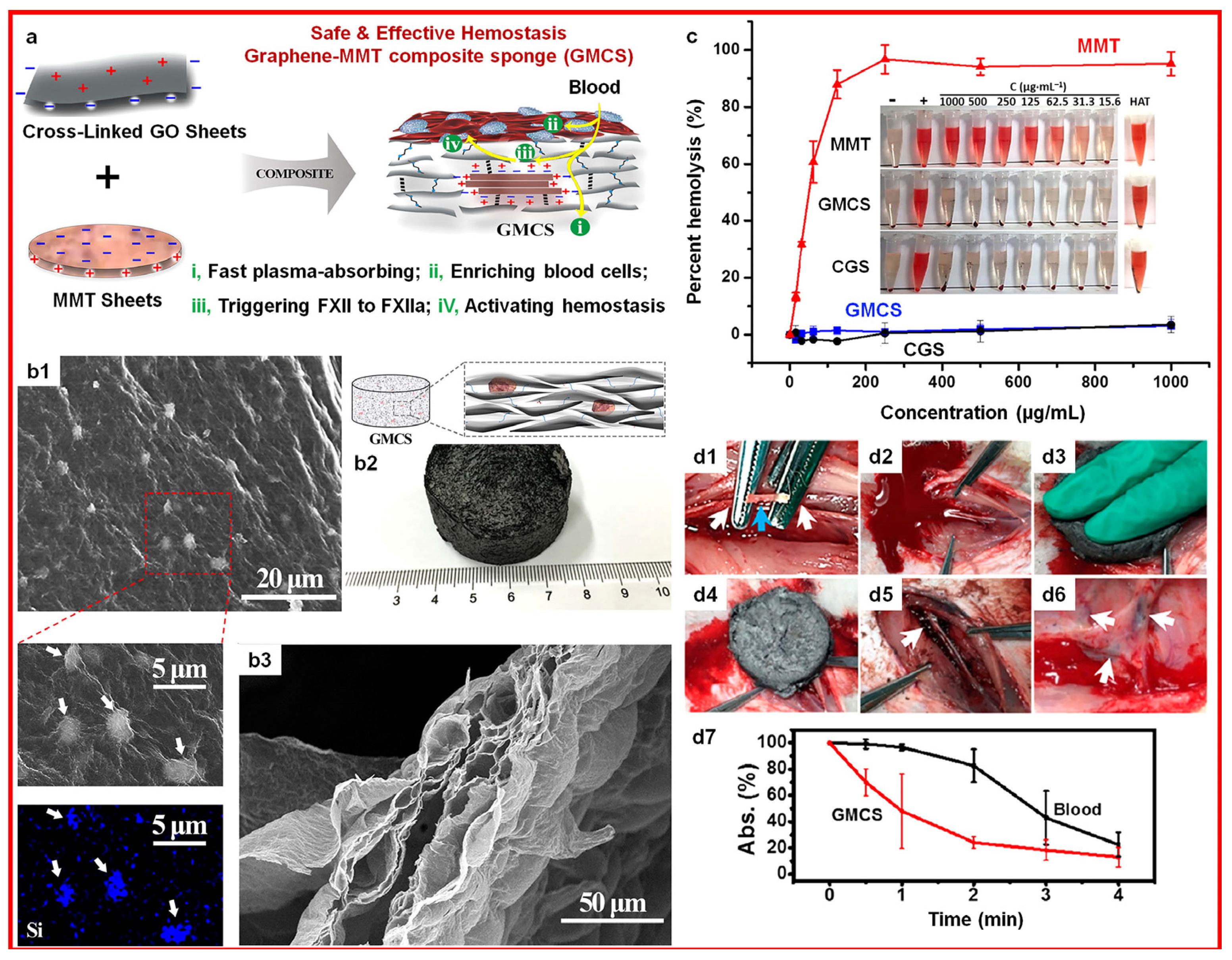
- Li, G.; Quan, K.; Liang, Y.; Li, T.; Yuan, Q.; Tao, L.; Xie, Q.; Wang, X. Graphene-Montmorillonite Composite Sponge for Safe and Effective Hemostasis. ACS Appl. Mater. Interfaces 2016, 8, 35071–35080. [Google Scholar] [CrossRef]
- Same, S.; Nakhjavani, S.A.; Samee, G.; Navidi, G.; Jahanbani, Y.; Davaran, S. Halloysite Clay Nanotube in Regenerative Medicine for Tissue and Wound Healing. Ceram. Int. 2022, 48, 31065–31079. [Google Scholar] [CrossRef]
- Awad, M.E.; López-Galindo, A.; Setti, M.; El-Rahmany, M.M.; Iborra, C.V. Kaolinite in Pharmaceutics and Biomedicine. Int. J. Pharm. 2017, 533, 34–48. [Google Scholar] [CrossRef]
- Long, M.; Zhang, B.; Peng, S.; Liao, J.; Zhang, Y.; Wang, J.; Wang, M.; Qin, B.; Huang, J.; Huang, J.; et al. Interactions between Two-Dimensional Nanoclay and Blood Cells in Hemostasis. Mater. Sci. Eng. C 2019, 105, 110081. [Google Scholar] [CrossRef]
- Liu, M.; Fakhrullin, R.; Novikov, A.; Panchal, A.; Lvov, Y. Tubule Nanoclay-Organic Heterostructures for Biomedical Applications. Macromol. Biosci. 2019, 19, 1800419. [Google Scholar] [CrossRef]
- Tarasova, E.; Naumenko, E.; Rozhina, E.; Akhatova, F.; Fakhrullin, R. Cytocompatibility and Uptake of Polycations-Modified Halloysite Clay Nanotubes. Appl. Clay Sci. 2019, 169, 21–30. [Google Scholar] [CrossRef]
- Nourizadeh, H.; Bakhshayesh, A. Nanoclay-Based Products across Global Markets: Applications and Properties; StatNano Publications: Lund, Sweden, 2020; pp. 3–33. [Google Scholar] [CrossRef]
- Yue, Q.; Zhang, Y.; Jiang, Y.; Li, J.; Zhang, H.; Yu, C.; Elzatahry, A.A.; Alghamdi, A.; Deng, Y.; Zhao, D. Nanoengineering of Core-Shell Magnetic Mesoporous Microspheres with Tunable Surface Roughness. J. Am. Chem. Soc. 2017, 139, 4954–4961. [Google Scholar] [CrossRef]
- Su, C.; Jiang, C.; Sun, X.; Cao, Z.; Mu, Y.; Cong, X.; Qiu, K.; Lin, J.; Chen, X.; Feng, C. Diatomite Hemostatic Particles with Hierarchical Porous Structure for Rapid and Effective Hemostasis. Colloids Surf. B Biointerfaces 2022, 219, 112809. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Fu, Q.; Deng, Y.; Wang, F.; Xia, B.; Chen, Z.; Chen, G. Surface Roughness of Silk Fibroin/Alginate Microspheres for Rapid Hemostasis In Vitro and In Vivo. Carbohydr. Polym. 2021, 253, 117256. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, W.; Li, N.; Wang, M.; Liang, B.; Ullah, I.; Luis Neve, A.; Feng, Y.; Chen, H.; Shi, C. Design and Development of Polysaccharide Hemostatic Materials and Their Hemostatic Mechanism. Biomater. Sci. 2017, 5, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- Björses, K.; Faxälv, L.; Montan, C.; Wildt-Persson, K.; Fyhr, P.; Holst, J.; Lindahl, T.L. In Vitro and In Vivo Evaluation of Chemically Modified Degradable Starch Microspheres for Topical Haemostasis. Acta Biomater. 2011, 7, 2558–2565. [Google Scholar] [CrossRef] [PubMed]
- Sperling, C.; Fischer, M.; Maitz, M.F.; Werner, C. Blood Coagulation on Biomaterials Requires the Combination of Distinct Activation Processes. Biomaterials 2009, 30, 4447–4456. [Google Scholar] [CrossRef] [PubMed]
- Dalisson, B.; Barralet, J. Bioinorganics and Wound Healing. Adv. Healthc. Mater. 2019, 8, 1900764. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Lei, H.; Cheng, L. Bioactive Inorganic Nanomaterials for Cancer Theranostics. Chem. Soc. Rev. 2023, 52, 2031–2081. [Google Scholar] [CrossRef]
- Guo, B.; Dong, R.; Liang, Y.; Li, M. Haemostatic Materials for Wound Healing Applications. Nat. Rev. Chem. 2021, 5, 773–791. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.S.; Lee, J.J.; Lee, A.K.X.; Ho, C.C.; Liu, Y.T.; Shie, M.Y. Calcium Silicate-Activated Gelatin Methacrylate Hydrogel for Accelerating Human Dermal Fibroblast Proliferation and Differentiation. Polymers 2021, 13, 70. [Google Scholar] [CrossRef]
- Chen, Y.W.; Shen, Y.F.; Ho, C.C.; Yu, J.; Wu, Y.H.A.; Wang, K.; Shih, C.T.; Shie, M.Y. Osteogenic and Angiogenic Potentials of the Cell-Laden Hydrogel/Mussel-Inspired Calcium Silicate Complex Hierarchical Porous Scaffold Fabricated by 3D Bioprinting. Mater. Sci. Eng. C 2018, 91, 679–687. [Google Scholar] [CrossRef]
- Chen, Y.W.; Hsu, T.T.; Wang, K.; Shie, M.Y. Preparation of the Fast Setting and Degrading Ca-Si-Mg Cement with Both Odontogenesis and Angiogenesis Differentiation of Human Periodontal Ligament Cells. Mater. Sci. Eng. C 2016, 60, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Shang, X.; Chen, H.; Xiao, L.; Zhu, Y.; Fan, J. A Tightly-Bonded and Flexible Mesoporous Zeolite-Cotton Hybrid Hemostat. Nat. Commun. 2019, 10, 1932. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sah, D.K.; Khanna, K.; Rai, Y.; Yadav, A.K.; Ansari, M.S.; Bhatt, A.N. A Calcium and Zinc Composite Alginate Hydrogel for Pre-Hospital Hemostasis and Wound Care. Carbohydr. Polym. 2023, 299, 120186. [Google Scholar] [CrossRef] [PubMed]
- Tommila, M.; Jokinen, J.; Wilson, T.; Forsback, A.P.; Saukko, P.; Penttinen, R.; Ekholm, E. Bioactive Glass-Derived Hydroxyapatite-Coating Promotes Granulation Tissue Growth in Subcutaneous Cellulose Implants in Rats. Acta Biomater. 2008, 4, 354–361. [Google Scholar] [CrossRef]
- Zheng, B.; Qiu, Z.; Xu, J.; Zeng, X.; Liu, K.; Chen, L. 3D Printing-Mediated Microporous Starch Hydrogels for Wound Hemostasis. J. Mater. Chem. B 2023, 11, 8411–8421. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in Wound Healing Modulation. Nutrients 2018, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Pires, M.E.; Ahmed, N.S.; Vara, D.; Gibbins, J.M.; Pula, G.; Pugh, N. Zinc Regulates Reactive Oxygen Species Generation in Platelets. Platelets 2021, 32, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, A.I.S.; Pitt, S.J.; Stewart, A.J. Influence of Zinc on Glycosaminoglycan Neutralisation during Coagulation. Metallomics 2018, 10, 1180–1190. [Google Scholar] [CrossRef]
- Tubek, S.; Grzanka, P.; Tubek, I. Role of Zinc in Hemostasis: A Review. Biol. Trace Elem. Res. 2008, 121, 1–8. [Google Scholar] [CrossRef]
- Pan, M.; Tang, Z.; Tu, J.; Wang, Z.; Chen, Q.; Xiao, R.; Liu, H. Porous Chitosan Microspheres Containing Zinc Ion for Enhanced Thrombosis and Hemostasis. Mater. Sci. Eng. C 2018, 85, 27–36. [Google Scholar] [CrossRef]
- Rink, L.; Gabriel, P. Extracellular and Immunological Actions of Zinc. BioMetals 2001, 14, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and Its Role in Immunity and Inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef]
- Deng, M.; Zhang, M.; Huang, R.; Li, H.; Lv, W.; Lin, X.; Huang, R.; Wang, Y. Diabetes Immunity-Modulated Multifunctional Hydrogel with Cascade Enzyme Catalytic Activity for Bacterial Wound Treatment. Biomaterials 2022, 289, 121790. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.; Rink, L. Functional Significance of Zinc-Related Signaling Pathways in Immune Cells. Annu. Rev. Nutr. 2009, 29, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Ponsen, A.C.; Proust, R.; Soave, S.; Mercier-Nomé, F.; Garcin, I.; Combettes, L.; Lataillade, J.J.; Uzan, G. A New Hemostatic Agent Composed of Zn2+-Enriched Ca2+ Alginate Activates Vascular Endothelial Cells In Vitro and Promotes Tissue Repair in Vivo. Bioact. Mater. 2022, 18, 368–382. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, R.S. The Role of Zinc in Growth and Cell Proliferation. J. Nutr. 2000, 130, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Zeng, H.; Ye, X.; Dai, M.; Fang, B.; Liu, L. Zn2+ Incorporated Composite Polysaccharide Microspheres for Sustained Growth Factor Release and Wound Healing. Mater. Today Bio 2023, 22, 100739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, W.; Ma, H.; Qin, C.; Chen, J.; Wu, C. Spindle-Like Zinc Silicate Nanoparticles Accelerating Innervated and Vascularized Skin Burn Wound Healing. Adv. Healthc. Mater. 2022, 11, 2102359. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Li, L.; Jiao, Z.; Yan, H.; Wang, Z.; Wu, Z.; Guo, M.; Wang, Y.; Zhang, P. Improved Hemostatic Effects by Fe3+ Modified Biomimetic PLLA Cotton-like Mat via Sodium Alginate Grafted with Dopamine. Bioact. Mater. 2021, 6, 2346–2359. [Google Scholar] [CrossRef]
- Ziv-Polat, O.; Topaz, M.; Brosh, T.; Margel, S. Enhancement of Incisional Wound Healing by Thrombin Conjugated Iron Oxide Nanoparticles. Biomaterials 2010, 31, 741–747. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, B.; Lu, M.; Li, S.; Guo, J.; Chen, F.; Xiong, X.; Yin, Z.; Liu, H.; Zhou, D. Ultrasmall Fe-Doped Carbon Dots Nanozymes for Photoenhanced Antibacterial Therapy and Wound Healing. Bioact. Mater. 2022, 12, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xu, Z.; Gu, J.; Zhou, J.; Sha, G.; Huang, Y.; Wang, T.; Fan, L.; Zhang, Y.; Xi, J. In Situ Formation of Ferrous Sulfide in Glycyrrhizic Acid Hydrogels to Promote Healing of Multi-Drug Resistant Staphylococcus Aureus-Infected Diabetic Wounds. J. Colloid Interface Sci. 2023, 65, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Festa, R.A.; Thiele, D.J. Copper: An Essential Metal in Biology. Curr. Biol. 2011, 21, R877–R883. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Wang, Y.; Yao, H.; Yan, L.; Yin, W.; Gu, Z. A Copper Peroxide Fenton Nanoagent-Hydrogel as an In Situ PH-Responsive Wound Dressing for Effectively Trapping and Eliminating Bacteria. ACS Appl. Bio Mater. 2022, 5, 1779–1793. [Google Scholar] [CrossRef]
- Kornblatt, A.P.; Nicoletti, V.G.; Travaglia, A. The Neglected Role of Copper Ions in Wound Healing. J. Inorg. Biochem. 2016, 161, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qiu, L.; Lin, C.; Yang, H.; Fu, H.; Li, R.; Kang, Y.J. Copper-Dependent and -Independent Hypoxia-Inducible Factor-1 Regulation of Gene Expression. Metallomics 2014, 6, 1889–1893. [Google Scholar] [CrossRef] [PubMed]
- Zimnicka, A.M.; Tang, H.; Guo, Q.; Kuhr, F.K.; Oh, M.J.; Wan, J.; Chen, J.; Smith, K.A.; Fraidenburg, D.R.; Choudhury, M.S.R.; et al. Upregulated Copper Transporters in Hypoxia-Induced Pulmonary Hypertension. PLoS ONE 2014, 9, e90544. [Google Scholar] [CrossRef] [PubMed]
- Salvo, J.; Sandoval, C. Role of Copper Nanoparticles in Wound Healing for Chronic Wounds: Literature Review. Burn. Trauma 2022, 10, tkab047. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Ping, Y.; Zhang, H.; Zhou, B.; Liu, F.; Yu, Y.; Xie, T.; Li, W.; Zhong, D.; Zhang, Y.; et al. Laser-Activatable CuS Nanodots to Treat Multidrug-Resistant Bacteria and Release Copper Ion to Accelerate Healing of Infected Chronic Nonhealing Wounds. ACS Appl. Mater. Interfaces 2019, 11, 3809–3822. [Google Scholar] [CrossRef]
- Zhou, W.; Zi, L.; Cen, Y.; You, C.; Tian, M. Copper Sulfide Nanoparticles-Incorporated Hyaluronic Acid Injectable Hydrogel with Enhanced Angiogenesis to Promote Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 417. [Google Scholar] [CrossRef]
- Qian, J.; Ji, L.; Xu, W.; Hou, G.; Wang, J.; Wang, Y.; Wang, T. Copper-Hydrazide Coordinated Multifunctional Hyaluronan Hydrogels for Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 16018–16031. [Google Scholar] [CrossRef]
- Sasaki, Y.; Sathi, G.A.; Yamamoto, O. Wound Healing Effect of Bioactive Ion Released from Mg-Smectite. Mater. Sci. Eng. C 2017, 77, 52–57. [Google Scholar] [CrossRef]
- Yin, M.; Wu, J.; Deng, M.; Wang, P.; Ji, G.; Wang, M.; Zhou, C.; Blum, N.T.; Zhang, W.; Shi, H.; et al. Multifunctional Magnesium Organic Framework-Based Microneedle Patch for Accelerating Diabetic Wound Healing. ACS Nano 2021, 15, 17842–17853. [Google Scholar] [CrossRef]
- Liu, W.; Guo, S.; Tang, Z.; Wei, X.; Gao, P.; Wang, N.; Li, X.; Guo, Z. Magnesium Promotes Bone Formation and Angiogenesis by Enhancing MC3T3-E1 Secretion of PDGF-BB. Biochem. Biophys. Res. Commun. 2020, 528, 664–670. [Google Scholar] [CrossRef]
- Yang, F.; Xue, Y.; Wang, F.; Guo, D.; He, Y.; Zhao, X.; Yan, F.; Xu, Y.; Xia, D.; Liu, Y. Sustained Release of Magnesium and Zinc Ions Synergistically Accelerates Wound Healing. Bioact. Mater. 2023, 26, 88–101. [Google Scholar] [CrossRef]
- Lin, C.Y.; Tsai, P.S.; Hung, Y.C.; Huang, C.J. L-Type Calcium Channels Are Involved in Mediating the Anti-Inflammatory Effects of Magnesium Sulphate. Br. J. Anaesth. 2010, 104, 44–51. [Google Scholar] [CrossRef]
- Li, W.; Jian, X.; Zou, Y.; Wu, L.; Huang, H.; Li, H.; Hu, D.; Yu, B. The Fabrication of a Gellan Gum-Based Hydrogel Loaded with Magnesium Ions for the Synergistic Promotion of Skin Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 709679. [Google Scholar] [CrossRef]
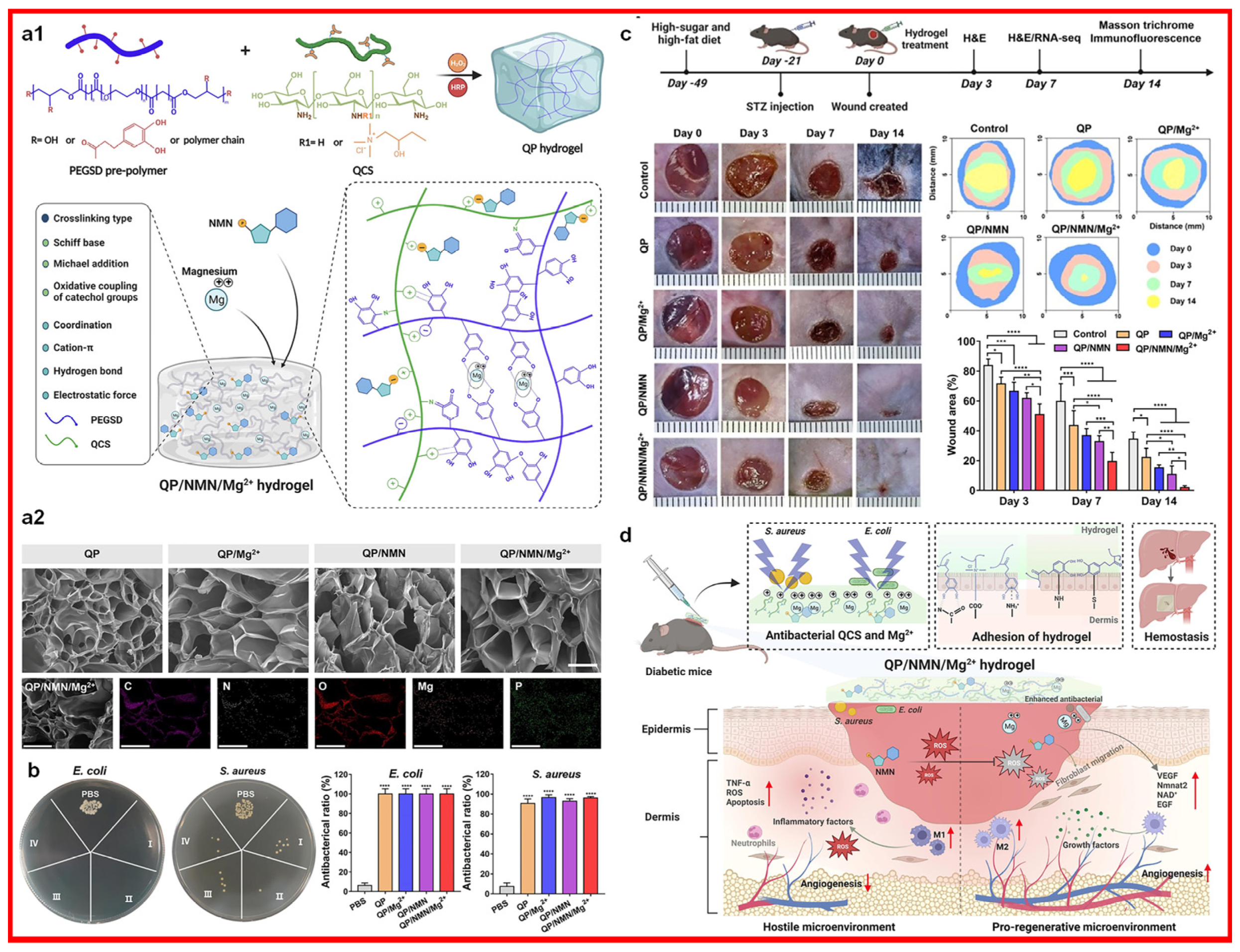
- Liang, Z.; Luo, J.; Liu, S.; Gu, Y.; Cui, Z.; Zhu, Y.; Zhao, X.; Guo, B.; Song, B. Injectable, antibacterial, ROS scavenging and pro-angiogenic hydrogel adhesives promote chronic wound healing in diabetes via synergistic release of NMN and Mg2+. Chem. Eng. J. 2023, 475, 146092. [Google Scholar] [CrossRef]
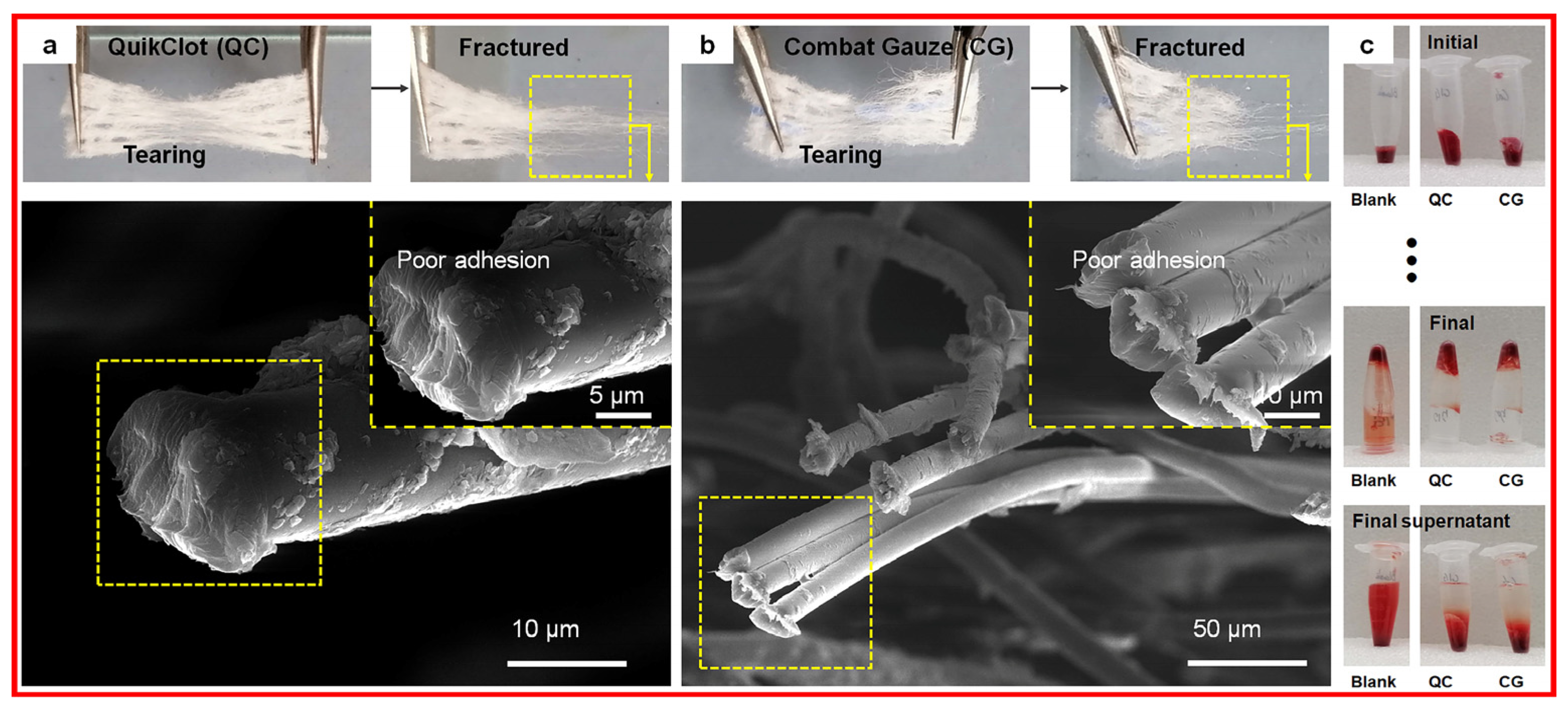
- Li, C.; Li, F.; Chen, J.; Wu, H.; Lin, Y.; Chen, C.; Zhang, P.; Wang, Q.; Liu, J.; Deng, G. Flexible Biomimetic Hollow Al2O3 Fibers for Safe and Effective Hemostasis. Mater. Des. 2022, 213, 110365. [Google Scholar] [CrossRef]
- Shie, M.Y.; Ding, S.J. Integrin Binding and MAPK Signal Pathways in Primary Cell Responses to Surface Chemistry of Calcium Silicate Cements. Biomaterials 2013, 34, 6589–6606. [Google Scholar] [CrossRef]
- Long, M.; Zhang, Y.; Huang, P.; Chang, S.; Hu, Y.; Yang, Q.; Mao, L.; Yang, H. Emerging Nanoclay Composite for Effective Hemostasis. Adv. Funct. Mater. 2018, 28, 1704452. [Google Scholar] [CrossRef]
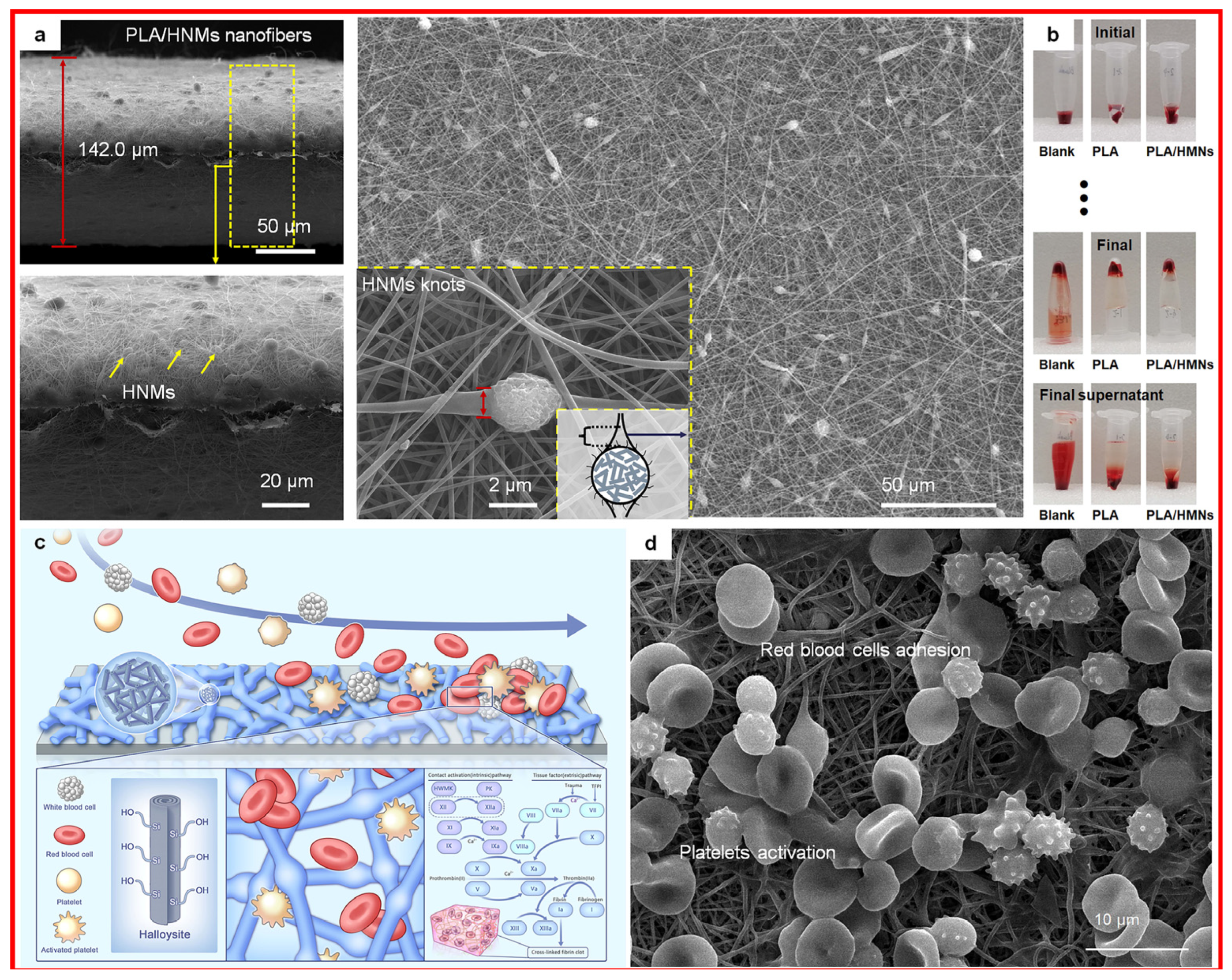
- Cui, Y.; Huang, Z.; Lei, L.; Li, Q.; Jiang, J.; Zeng, Q.; Tang, A.; Yang, H.; Zhang, Y. Robust Hemostatic Bandages Based on Nanoclay Electrospun Membranes. Nat. Commun. 2021, 12, 5922. [Google Scholar] [CrossRef]
- Huang, Z.; Qian, F.; Tian, G.; Shen, J.; Liu, S.; Yang, Z.; Zhang, Y. Halloysite Nanotube Microspheres Connected to an Electrospun Nanofiber Membrane for Effective and Riskless Hemostasis. Appl. Clay Sci. 2023, 242, 107045. [Google Scholar] [CrossRef]
- Li, X.F.; Lu, P.; Jia, H.R.; Li, G.; Zhu, B.; Wang, X.; Wu, F.G. Emerging Materials for Hemostasis. Coord. Chem. Rev. 2023, 475, 214823. [Google Scholar] [CrossRef]
- Feng, Y.; He, Y.; Lin, X.; Xie, M.; Liu, M.; Lvov, Y. Assembly of Clay Nanotubes on Cotton Fibers Mediated by Biopolymer for Robust and High-Performance Hemostatic Dressing. Adv. Healthc. Mater. 2023, 12, 2202265. [Google Scholar] [CrossRef]
- Feng, Y.; Luo, X.; Wu, F.; Liu, H.; Liang, E.; He, R.R.; Liu, M. Systematic Studies on Blood Coagulation Mechanisms of Halloysite Nanotubes-Coated PET Dressing as Superior Topical Hemostatic Agent. Chem. Eng. J. 2022, 428. [Google Scholar] [CrossRef]
- Wang, X.; Mu, B.; Zhang, H.; Du, Y.; Yang, F.; Wang, A. Incorporation of Mixed-Dimensional Palygorskite Clay into Chitosan/Polyvinylpyrrolidone Nanocomposite Films for Enhancing Hemostatic Activity. Int. J. Biol. Macromol. 2023, 237, 124213. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Bao, Z.; Gao, M.; Sun, Y.; Nian, R.; Xian, M. The Recent Progress of Tissue Adhesives in Design Strategies, Adhesive Mechanism and Applications. Mater. Sci. Eng. C 2020, 111, 110796. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Feng, Y.; Zhou, Y.; Tan, C.; Liu, M. Gold@Halloysite Nanotubes-Chitin Composite Hydrogel with Antibacterial and Hemostatic Activity for Wound Healing. Bioact. Mater. 2023, 20, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Niu, Y.; Gong, M.; Shi, R.; Chen, D.; Zhang, L.; Lvov, Y. Electrospun Microfiber Membranes Embedded with Drug-Loaded Clay Nanotubes for Sustained Antimicrobial Protection. ACS Nano 2015, 9, 1600–1612. [Google Scholar] [CrossRef]
- Huang, Z.; Liao, J.; Zhang, Y.; Yuan, F.; Liu, S.; Ouyang, J.; Yang, H. Electrospinning with a Spindle-Knot Structure for Effective PM2.5 Capture. Sci. China Mater. 2021, 64, 1278–1290. [Google Scholar] [CrossRef]
- Long, M.; Liu, Q.; Wang, D.; Wang, J.; Zhang, Y.; Tang, A.; Liu, N.; Bui, B.; Chen, W.; Yang, H. A New Nanoclay-Based Bifunctional Hybrid Fiber Membrane with Hemorrhage Control and Wound Healing for Emergency Self-Rescue. Mater. Today Adv. 2021, 12, 100190. [Google Scholar] [CrossRef]
- Wali, A.; Gorain, M.; Inamdar, S.; Kundu, G.; Badiger, M. In Vivo Wound Healing Performance of Halloysite Clay and Gentamicin-Incorporated Cellulose Ether-PVA Electrospun Nanofiber Mats. ACS Appl. Bio Mater. 2019, 2, 4324–4334. [Google Scholar] [CrossRef] [PubMed]
- Electrospun, A.; Nanofibers, P. Antibacterial Electrospun Polycaprolactone Nanofibers. Polymers 2022, 14, 746. [Google Scholar] [CrossRef]
- Rozhina, E.; Batasheva, S.; Miftakhova, R.; Yan, X.; Vikulina, A.; Volodkin, D.; Fakhrullin, R. Comparative Cytotoxicity of Kaolinite, Halloysite, Multiwalled Carbon Nanotubes and Graphene Oxide. Appl. Clay Sci. 2021, 205, 106041. [Google Scholar] [CrossRef]
- Gorbachevskii, M.; Stavitskaya, A.; Novikov, A.; Fakhrullin, R.; Rozhina, E.; Naumenko, E.; Vinokurov, V. Fluorescent Fold Nanoclusters Stabilized on Halloysite Nanotubes: In Vitro Study on Cytotoxicity. Appl. Clay Sci. 2021, 207, 106106. [Google Scholar] [CrossRef]
- Stavitskaya, A.; Sitmukhanova, E.; Sayfutdinova, A.; Khusnetdenova, E.; Mazurova, K.; Cherednichenko, K.; Naumenko, E.; Fakhrullin, R. Photoinduced Antibacterial Activity and Cytotoxicity of CdS Stabilized on Mesoporous Aluminosilicates and Silicates. Pharmaceutics 2022, 14, 1309. [Google Scholar] [CrossRef]
- Dário, G.; Silva, G.; Gonçalves, D.; Silveira, P.; Junior, A.; Angioletto, E.; Bernardi, A. Evaluation of the Healing Activity of Therapeutic Clay in Rat Skin Wounds. Mater. Sci. Eng. C-Mater. Biol. Appl. 2024, 43, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiang, Y.; Tian, G.; Zhu, C.; Zhang, Y. Toxicological Evaluation toward Refined Montmorillonite with Human Colon Associated Cells and Human Skin Associated Cells. J. Funct. Biomater. 2024, 15, 75. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, G.; Wang, Z.; Huang, Z.; Xie, Z.; Xia, L.; Zhang, Y. Clays and Wound Healing. Materials 2024, 17, 1691. https://doi.org/10.3390/ma17071691
Tian G, Wang Z, Huang Z, Xie Z, Xia L, Zhang Y. Clays and Wound Healing. Materials. 2024; 17(7):1691. https://doi.org/10.3390/ma17071691
Chicago/Turabian StyleTian, Guangjian, Zhou Wang, Zongwang Huang, Zuyan Xie, Lu Xia, and Yi Zhang. 2024. "Clays and Wound Healing" Materials 17, no. 7: 1691. https://doi.org/10.3390/ma17071691
APA StyleTian, G., Wang, Z., Huang, Z., Xie, Z., Xia, L., & Zhang, Y. (2024). Clays and Wound Healing. Materials, 17(7), 1691. https://doi.org/10.3390/ma17071691





