The Precursors Used for Developing Geopolymer Composites for Circular Economy—A Review
Abstract
1. Introduction
2. Geopolymers and Their Most Common Precursors
2.1. Fly Ash
2.2. Metakaolin
2.3. Blast Furnace Slag
2.4. Glass Waste
2.5. Silica Fume
2.6. Ceramic Waste Powders
3. Alkaline Activators
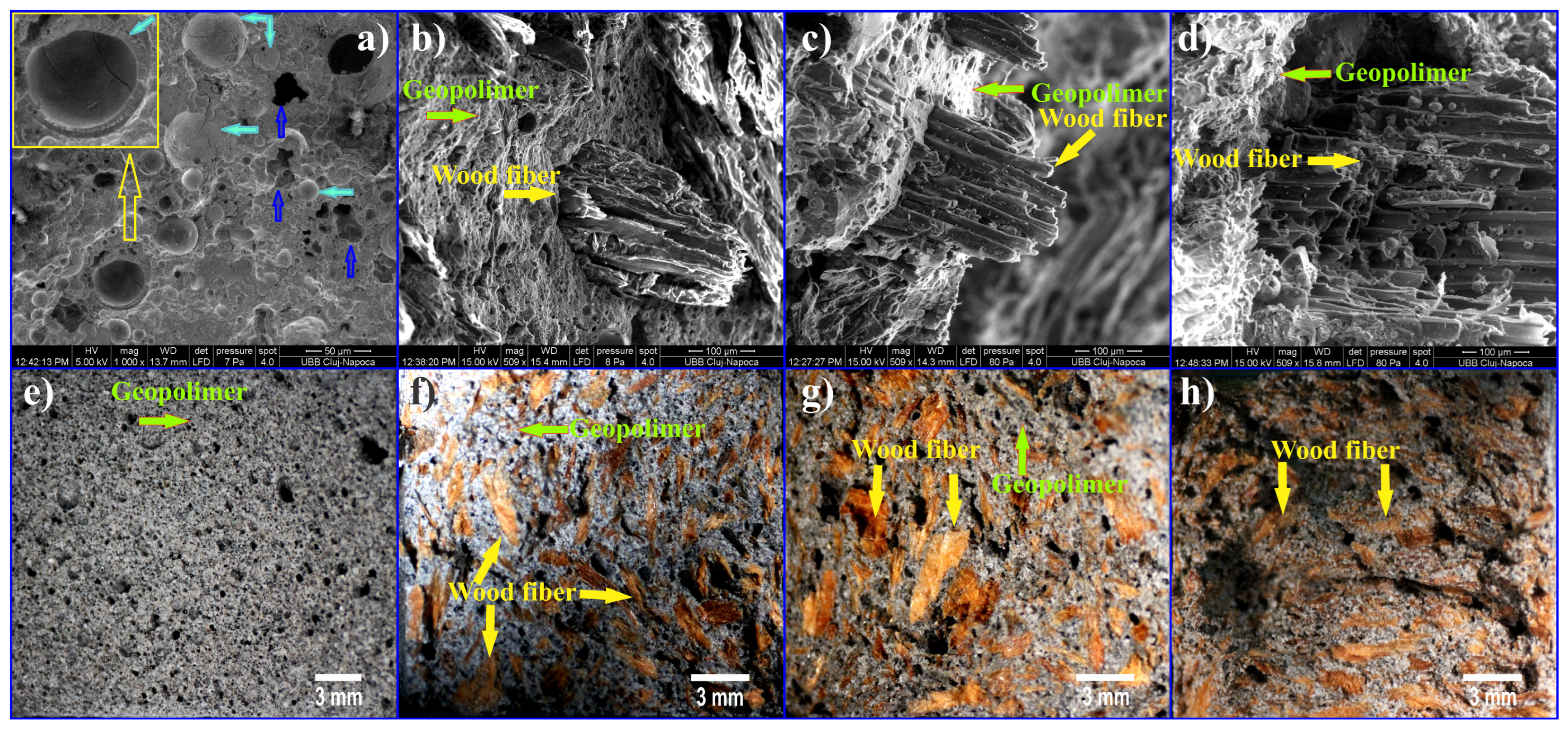
4. Fibers Used as Reinforcement of Geopolymers
5. The Mechanism Used to Obtain Geopolymer Cement
6. The Addition of Fibers, Expansion Agent or Polymers in the Building Materials
7. The Influence of Different Precursors on Geopolymer Properties
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tesanasin, T.; Suksiripattanapong, C.; Van Duc, B.; Tabyang, W.; Phetchuay, C.; Phoo-ngernkham, T.; Chindaprasirt, P. Engineering properties of marginal lateritic soil stabilized with one-part high calcium fly ash geopolymer as pavement materials. Case Stud. Constr. Mater. 2022, 17, e01328. [Google Scholar] [CrossRef]
- Strzałkowska, E. Fly ash—A valuable material for the circular economy. Gospod. Surow. Miner. 2021, 37, 49–62. [Google Scholar] [CrossRef]
- Łach, M.; Grela, A.; Kozub, B.; Korniejenko, K.; Azzopardi, B. The Fly-Ash Based Geopolymer Composites as an Innovative Material for Circular. MATEC Web Conf. 2020, 322, 01016. [Google Scholar] [CrossRef]
- Siddika, A.; Hajimohammadi, A.; Mamun, M.A.A.; Alyousef, R.; Ferdous, W. Waste glass in cement and geopolymer concretes: A review on durability and challenges. Polymers 2021, 13, 2071. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yao, W.; Zheng, K.; Cui, N.; Xie, N. Synergistically Using Bauxite Residue (Red Mud) and Other Solid Wastes to Manufacture Eco-Friendly Cementitious Materials. Buildings 2022, 12, 117. [Google Scholar] [CrossRef]
- Davidovits, J.; Izquierdo, M.; Querol, X.; Antennuci, D.; Nugteren, H.; Butselaar-Orthlieb, V.; Fernández-Pereira, C.; Luna, Y. The European Research Project Geoash: Geopolymeric Cement Based on European Fly-Ashes; Geopolymer Institute Library: Saint-Quentin, France, 2014. [Google Scholar]
- Cong, P.; Cheng, Y. Advances in geopolymer materials: A comprehensive review. J. Traffic Transp. Eng. 2021, 8, 283–314. [Google Scholar] [CrossRef]
- Liu, J.; Doh, J.H.; Ong, D.E.; Liu, Z.; Hadi, M.N. Methods to evaluate and quantify the geopolymerization reactivity of waste-derived aluminosilicate precursor in alkali-activated material: A state-of-the-art review. Constr. Build. Mater. 2023, 362, 129784. [Google Scholar] [CrossRef]
- Sharko, A.; Louda, P.; Nguyen, V.V.; Buczkowska, K.E.; Stepanchikov, D.; Ercoli, R.; Kascak, P.; Le, V.S. Multicriteria Assessment for Calculating the Optimal Content of Calcium-Rich Fly Ash in Metakaolin-Based Geopolymers. Ceramics 2023, 6, 525–537. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, D.C.H.; Tong, S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Solouki, A.; Viscomi, G.; Lamperti, R.; Tataranni, P. Quarry waste as precursors in geopolymers for civil engineering applications: A decade in review. Materials 2020, 13, 3146. [Google Scholar] [CrossRef]
- Furtos, G.; Silaghi-Dumitrescu, L.; Pascuta, P.; Sarosi, C.; Korniejenko, K. Mechanical Properties of Wood Fiber Reinforced Geopolymer Composites with Sand Addition. J. Nat. Fibers 2021, 18, 285–296. [Google Scholar] [CrossRef]
- Furtos, G.; Molnar, L.; Silaghi-Dumitrescu, L.; Pascuta, P.; Korniejenko, K. Mechanical and thermal properties of wood fiber reinforced geopolymer composites. J. Nat. Fibers 2022, 19, 6676–6691. [Google Scholar] [CrossRef]
- Manjarrez, L.; Nikvar-Hassani, A.; Shadnia, R.; Zhang, L. Experimental Study of Geopolymer Binder Synthesized with Copper Mine Tailings and Low-Calcium Copper Slag. J. Mater. Civ. Eng. 2019, 31, 04019156. [Google Scholar] [CrossRef]
- Slaný, M.; Kuzielová, E.; Žemlička, M.; Matejdes, M.; Struhárová, A.; Palou, M.T. Metabentonite and metakaolin-based geopolymers/zeolites: Relation between kind of clay, calcination temperature and concentration of alkaline activator. J. Therm. Anal. Calorim. 2023, 148, 10531–10547. [Google Scholar] [CrossRef]
- Abdullah, M.M.A.; Hussin, K.; Bnhussain, M.; Ismail, K.N.; Ibrahim, W.M.W. Mechanism and chemical reaction of fly ash geopolymer cement—A review. Int. J. Pure Appl. Sci. Technol. 2011, 6, 35–44. [Google Scholar]
- Palomo, A.; Alonso, S.; Fernández-Jiménez, A. Alkaline activation of fly ashes: A NMR study of the reaction products. J. Am. Ceram. Soc. 2004, 87, 1141–1145. [Google Scholar] [CrossRef]
- Mohapatra, S.S.; Mishra, J.; Nanda, B.; Patro, S.K. A review on waste-derived alkali activators for preparation of geopolymer composite. Mater. Today Proc. 2022, 56, 440–446. [Google Scholar] [CrossRef]
- Farhan, K.Z.; Johari, M.A.M.; Demirboğa, R. Assessment of important parameters involved in the synthesis of geopolymer composites: A review. Constr. Build. Mater. 2020, 264, 120276. [Google Scholar] [CrossRef]
- ASTM C618-19; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PA, USA, 2019.
- Castillo, H.; Collado, H.; Droguett, T.; Vesely, M.; Garrido, P.; Palma, S. State of the art of geopolymers: A review. E-Polymers 2022, 22, 108–124. [Google Scholar] [CrossRef]
- Venkatesan, M.; Zaib, Q.; Shah, I.H.; Park, H.S. Optimum utilization of waste foundry sand and fly ash for geopolymer concrete synthesis using D-optimal mixture design of experiments. Resour. Conserv. Recycl. 2019, 148, 114–123. [Google Scholar] [CrossRef]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilization of Bayer red mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Li, Z.; Fei, M.E.; Huyan, C.; Shi, X. Nano-engineered, fly ash-based geopolymer composites: An overview. Resour. Conserv. Recycl. 2021, 168, 105334. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Nanostructure/microstructure of fly ash geopolymers. In Geopolymers—Structure, Processing, Properties and Industrial Applications; Provis, J.L., Van Deventer, J.S.J., Eds.; Woodhead Publishing Limited: New Delhi, India, 2009; pp. 89–117. [Google Scholar]
- Davidovits, R.; Plelegris, C.; Davidovits, J. Standardized Method in Testing Commercial Metakaolins for Geopolymer Formulations; Technical Paper #26 MK-testing; Geopolymer Institute Library: Saint-Quentin, France, 2019. [Google Scholar] [CrossRef]
- Luo, Y.; Bao, S.; Zhang, Y.; Yuan, Y. Recycling vanadium-bearing shale leaching residue for the production of one-part geopolymers. Mater. Res. Express. 2019, 6, 105203. [Google Scholar] [CrossRef]
- Tan, J.; Cai, J.; Li, X.; Pan, J.; Li, J. Development of eco-friendly geopolymers with ground mixed recycled aggregates and slag. J. Clean. Prod. 2020, 256, 120369. [Google Scholar] [CrossRef]
- Kiventerä, J.; Lancellotti, I.; Catauro, M.; Poggetto, F.D.; Leonelli, C.; Illikainen, M. Alkali activation as new option for gold mine tailings inertization. J. Clean. Prod. 2018, 187, 76–84. [Google Scholar] [CrossRef]
- Sajid, M.; Bai, C.; Aamir, M.; You, Z.; Yan, Z.; Lv, X. Understanding the structure and structural effects on the properties of blast furnace slag (BFS). Iron Steel Inst. Jpn. 2019, 59, 1153–1166. [Google Scholar] [CrossRef]
- Marvila, M.T.; de Azevedo, A.R.G.; Júnior, J.A.T.L.; Vieira, C.M.F. Activated alkali cement based on blast furnace slag: Effect of curing type and concentration of Na2O. J. Mater. Res. Technol. 2023, 23, 4551–4565. [Google Scholar] [CrossRef]
- Perná, I.; Hanzlíček, T. The setting time of a clay-slag geopolymer matrix: The influence of blast-furnace-slag addition and the mixing method. J. Clean. Prod. 2016, 112, 1150–1155. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Q.; Zuo, Z.; Yang, F.; Han, Z.; Qin, Q. Reactivity and performance of dry granulation blast furnace slag cement. Cem. Concr. Compos. 2019, 95, 19–24. [Google Scholar] [CrossRef]
- BS EN 197-1; Cement—Composition, Specifications and Conformity Criteria for Common Cements. British Standards Institution: London, UK, 1992.
- Pal, S.C.; Mukherjee, A.; Pathak, S.R. Investigation of hydraulic activity of ground granulated blast furnace slag in concrete. Cem. Concr. Res. 2003, 33, 1481–1486. [Google Scholar] [CrossRef]
- Escalante-Garcia, J.I.; Gomez-Zamorano, L.Y.; Johal, K.K.; Mendoza, G.; Mancha, H.; Mendez, J. Reactivity of blast-furnace slag in Portland cement blends hydrated under different conditions. Cem. Concr. Res. 2001, 31, 1403–1409. [Google Scholar] [CrossRef]
- Wang, P.Z.; Trettin, R.; Rudert, V. Effect of fineness and particle size distribution of granulated blast-furnace slag on the hydraulic reactivity in cement systems. Adv. Cem. Res. 2005, 17, 161–167. [Google Scholar] [CrossRef]
- Xiao, R.; Ma, Y.; Jiang, X.; Zhang, M.; Zhang, Y.; Wang, Y.; He, Q. Strength, microstructure, efflorescence behavior and environmental impacts of waste glass geopolymers cured at ambient temperature. J. Clean. Prod. 2020, 252, 119610. [Google Scholar] [CrossRef]
- Caldas, P.H.C.H.; de Azevedo, A.R.G.; Marvila, M.T. Silica fume activated by NaOH and KOH in cement mortars: Rheological and mechanical study. Constr. Build. Mater. 2023, 400, 132623. [Google Scholar] [CrossRef]
- Luhar, I.; Luhar, S.; Abdullah, M.M.A.B.; Nabiałek, M.; Sandu, A.V.; Szmidla, J.; Deraman, L.M. Assessment of the suitability of ceramic waste in geopolymer composites: An appraisal. Materials 2021, 14, 3279. [Google Scholar] [CrossRef] [PubMed]
- Abdul Rahim, R.H.; Rahmiati, T.; Azizli, K.A.; Man, Z.; Nuruddin, M.F.; Ismail, L. Comparison of using NaOH and KOH activated fly ash-based geopolymer on the mechanical properties. Mater. Sci. Forum 2015, 803, 179–184. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Geopolymerisation: A review and prospects for the minerals industry. Miner. Eng. 2007, 20, 1261–1277. [Google Scholar] [CrossRef]
- Risdanareni, P.; Ekaputri, J.J. The influence of alkali activator concentration to mechanical properties of geopolymer concrete with trass as a filler. Mater. Sci. Forum 2015, 803, 125–134. [Google Scholar] [CrossRef]
- Adesanya, E.; Perumal, P.; Luukkonen, T.; Yliniemi, J.; Ohenoja, K.; Kinnunen, P.; Illikainen, M. Opportunities to improve sustainability of alkali-activated materials: A review of side-stream based activators. J. Clean. Prod. 2021, 286, 125558. [Google Scholar] [CrossRef]
- Rattanasak, U.; Chindaprasirt, P. Influence of NaOH solution on the synthesis of fly ash geopolymer. Miner. Eng. 2009, 22, 1073–1078. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; Van Deventer, J.S. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Gharzouni, A.; Joussein, E.; Samet, B.; Baklouti, S.; Rossignol, S. Effect of the reactivity of alkaline solution and metakaolin on geopolymer formation. J. Non-Cryst. Solids 2015, 410, 127–134. [Google Scholar] [CrossRef]
- Shehata, N.; Sayed, E.T.; Abdelkareem, M.A. Recent progress in environmentally friendly geopolymers: A review. Sci. Total Environ. 2021, 762, 143166. [Google Scholar] [CrossRef]
- Siyal, A.A.; Mohamed, R.M.S.R.; Shamsuddin, R.; Ridzuan, M.B. A comprehensive review of synthesis kinetics and formation mechanism of geopolymers. RSC Adv. 2024, 14, 446–462. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Huang, M.; Yin, H.; Jiang, K.; Xiao, K.; Tang, S. Influence of different alkali sulfates on the shrinkage, hydration, pore structure, fractal dimension and microstructure of low-heat Portland cement, medium-heat Portland cement and ordinary Portland cement. Fractal Fract. 2021, 5, 79. [Google Scholar] [CrossRef]
- Cho, Y.K.; Yoo, S.W.; Jung, S.H.; Lee, K.M.; Kwon, S.J. Effect of Na2O content, SiO2/Na2O molar ratio, and curing conditions on the compressive strength of FA-based geopolymer. Constr. Build. Mater. 2017, 145, 253–260. [Google Scholar] [CrossRef]
- Purbasari, A.; Samadhi, T.W.; Bindar, Y. The effect of alkaline activator types on strength and microstructural properties of geopolymer from co-combustion residuals of bamboo and kaolin. Indones. J. Chem. 2018, 18, 397–402. [Google Scholar] [CrossRef]
- Giacobello, F.; Ielo, I.; Belhamdi, H.; Plutino, M.R. Geopolymers and functionalization strategies for the development of sustainable materials in construction industry and cultural heritage applications: A Review. Materials 2022, 15, 1725. [Google Scholar] [CrossRef]
- Buczkowska, K.E.; Louda, P.; Sharko, A.; Sharko, O.; Stepanchikov, D.; Jancik, L.; Los, P.; Plawecka, K.; Le, V.S. Maximizing Performance of Geopolymer Mortar: Optimizing Basalt and Carbon Fiber Content Composition. J. Nat. Fibers 2024, 21, 2293047. [Google Scholar] [CrossRef]
- Guo, G.; Lv, C.; Liu, J.; Wang, L. Properties of Fiber-Reinforced One-Part Geopolymers: A Review. Polymers 2022, 14, 3333. [Google Scholar] [CrossRef] [PubMed]
- Al Faruque, M.A.; Salauddin, M.; Raihan, M.M.; Chowdhury, I.Z.; Ahmed, F.; Shimo, S.S. Bast fiber reinforced green polymer composites: A review on their classification, properties, and applications. J. Nat. Fibers 2022, 19, 8006–8021. [Google Scholar] [CrossRef]
- Silva, G.; Kim, S.; Aguilar, R.; Nakamatsu, J. Natural fibers as reinforcement additives for geopolymers—A review of potential eco-friendly applications to the construction industry. Sustain. Mater. Technol. 2020, 23, e00132. [Google Scholar] [CrossRef]
- Addis, L.B.; Sendekie, Z.B.; Satheesh, N. Degradation kinetics and durability enhancement strategies of cellulosic fiber-reinforced geopolymers and cement composites. Adv. Mater. Sci. Eng. 2022, 2022, 1981755. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated binders: A review: Part Historical background, terminology, reaction mechanisms and hydration products. Constr. Build. Mater. 2008, 22, 1305–1314. [Google Scholar] [CrossRef]
- Van Deventer, J.S.J.; Provis, J.L.; Duxson, P.; Lukey, G.C. Reaction mechanisms in the geopolymeric conversion of inorganic waste to useful products. J. Hazard. Mater. 2007, 139, 506–513. [Google Scholar] [CrossRef]
- Davidovits, J.; Davidovits, R.; Davidovits, M. Geopolymeric Cement Based on Fly Ash and Harmless to Use. U.S. Patent No. 8,202,362, 19 June 2012. [Google Scholar]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Lukey, G.C. The characterisation of source materials in fly ash-based geopolymers. Mater. Lett. 2003, 57, 1272–1280. [Google Scholar] [CrossRef]
- Tiyasangthong, S.; Yoosuk, P.; Krosoongnern, K.; Krittacom, B.; Nachaisit, P.; Suksiripattanapong, C. Unit Weight, Strengths and Thermal Conductivity of Cellular Lightweight Fly Ash Geopolymer Mortar Reinforced with Polyvinyl Alcohol. Civ. Eng. Archit. 2022, 10, 2943–2952. [Google Scholar] [CrossRef]
- Wang, L.; Song, X.; Yang, H.; Wang, L.; Tang, S.; Wu, B.; Mao, W. Pore Structural and Fractal Analysis of the Effects of MgO Reactivity and Dosage on Permeability and F–T Resistance of Concrete. Fractal Fract. 2022, 6, 113. [Google Scholar] [CrossRef]
- Wang, L.E.I.; Guo, F.; Yang, H.; Wang, Y.A.N.; Tang, S. Comparison of fly ash, PVA fiber, MgO and shrinkage-reducing admixture on the frost resistance of face slab concrete via pore structural and fractal analysis. Fractals 2021, 29, 2140002. [Google Scholar] [CrossRef]
- Yoosuk, P.; Suksiripattanapong, C.; Sukontasukkul, P.; Chindaprasirt, P. Properties of polypropylene fiber reinforced cellular lightweight high calcium fly ash geopolymer mortar. Case Stud. Constr. Mater. 2021, 15, e00730. [Google Scholar] [CrossRef]
- Tabyang, W.; Suksiripattanapong, C.; Wonglakorn, N.; Laksanakit, C.; Chusilp, N. Utilization of municipal solid waste incineration fly ash for non-bearing masonry units containing coconut fiber. J. Nat. Fibers 2022, 19, 12522–12535. [Google Scholar] [CrossRef]
- Nawaz, M.; Heitor, A.; Sivakumar, M. Geopolymers in construction-recent developments. Constr. Build. Mater. 2020, 260, 120472. [Google Scholar] [CrossRef]
- Wattimena, O.K.; Antoni; Hardjito, D. A review on the effect of fly ash characteristics and their variations on the synthesis of fly ash based geopolymer. AIP Conf. Proc. 2017, 1887, 020041. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Xiao, R.; Polaczyk, P.; Zhang, M.; Hu, W.; Huang, B. A comparative study on geopolymers synthesized by different classes of fly ash after exposure to elevated temperatures. J. Clean. Prod. 2020, 270, 122500. [Google Scholar] [CrossRef]
- Kirgiz, M.S. Advance treatment by nanographite for Portland pulverised fly ash cement (the class F) systems. Compos. Part B Eng. 2015, 82, 59–71. [Google Scholar] [CrossRef]
- Wang, H.; Wu, H.; Xing, Z.; Wang, R.; Dai, S. The effect of various Si/Al, Na/Al molar ratios and free water on micromorphology and macro-strength of metakaolin-based geopolymer. Material 2021, 14, 3845. [Google Scholar] [CrossRef] [PubMed]
- Suksiripattanapong, C.; Horpibulsuk, S.; Yeanyong, C.; Arulrajah, A. Evaluation of polyvinyl alcohol and high calcium fly ash based geopolymer for the improvement of soft Bangkok clay. Transp. Geotech. 2021, 27, 100476. [Google Scholar] [CrossRef]
- Provis, J.L.; Duxson, P.; Kavalerova, E.; Krivenko, P.V.; Pan, Z.; Puertas, F.; van Deventer, J.S. Historical aspects and overview. In Alkali Activated Materials; State-of-the-Art Report, RILEM TC 224-AAM; Springer: Dordrecht, The Netherlands, 2014; pp. 11–57. [Google Scholar]
- Aziz, I.H.; Abdullah, M.M.A.B.; Heah, C.Y.; Liew, Y.M. Behaviour changes of ground granulated blast furnace slag geopolymers at high temperature. Adv. Cem. Res. 2020, 32, 465–475. [Google Scholar] [CrossRef]
- Zhuguo, L.I.; Sha, L.I. Carbonation resistance of fly ash and blast furnace slag based geopolymer concrete. Constr. Build. Mater. 2018, 163, 668–680. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furtos, G.; Prodan, D.; Sarosi, C.; Popa, D.; Moldovan, M.; Korniejenko, K. The Precursors Used for Developing Geopolymer Composites for Circular Economy—A Review. Materials 2024, 17, 1696. https://doi.org/10.3390/ma17071696
Furtos G, Prodan D, Sarosi C, Popa D, Moldovan M, Korniejenko K. The Precursors Used for Developing Geopolymer Composites for Circular Economy—A Review. Materials. 2024; 17(7):1696. https://doi.org/10.3390/ma17071696
Chicago/Turabian StyleFurtos, Gabriel, Doina Prodan, Codruta Sarosi, Dorin Popa, Marioara Moldovan, and Kinga Korniejenko. 2024. "The Precursors Used for Developing Geopolymer Composites for Circular Economy—A Review" Materials 17, no. 7: 1696. https://doi.org/10.3390/ma17071696
APA StyleFurtos, G., Prodan, D., Sarosi, C., Popa, D., Moldovan, M., & Korniejenko, K. (2024). The Precursors Used for Developing Geopolymer Composites for Circular Economy—A Review. Materials, 17(7), 1696. https://doi.org/10.3390/ma17071696











