Influence of Curing Temperature on the Performance of Calcined Coal Gangue–Limestone Blended Cements
Abstract
:1. Introduction
2. Materials and Methods
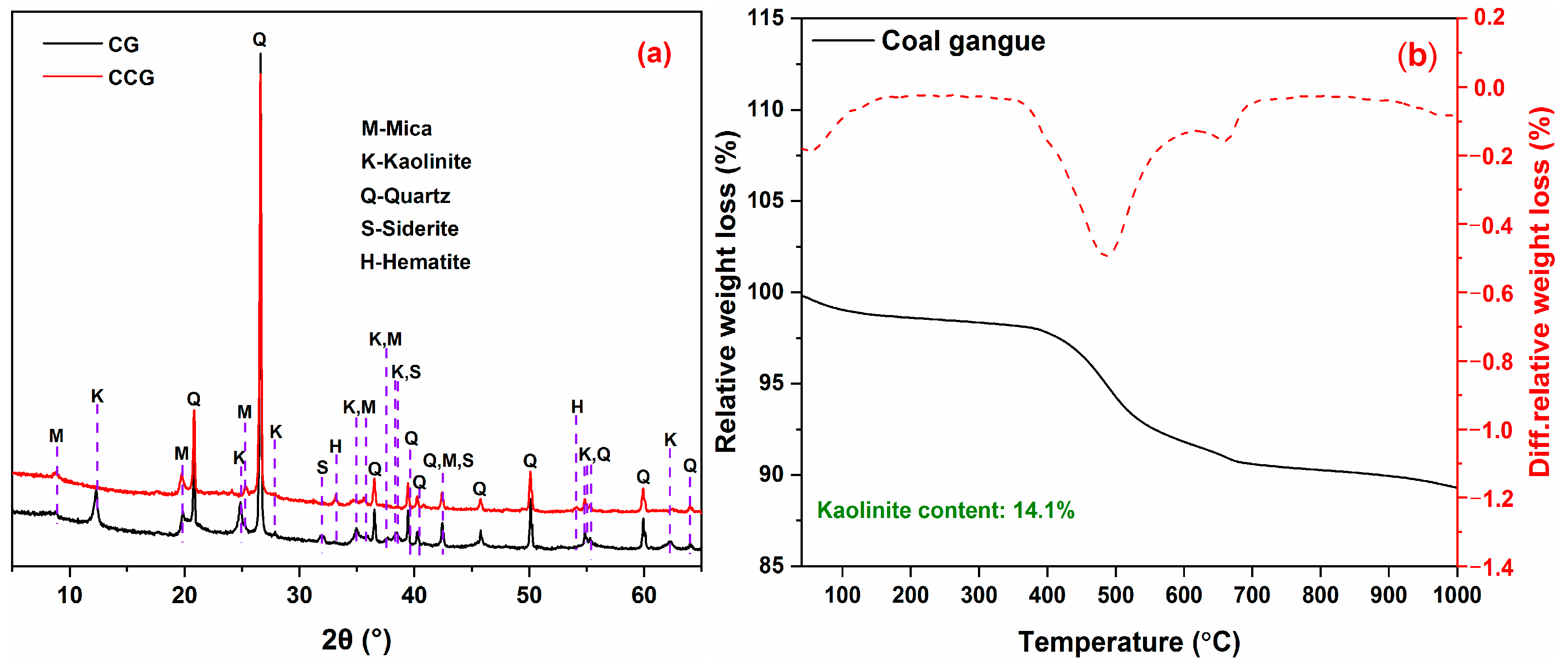
2.1. Starting Materials
2.2. Mixture Proportions
2.3. Mortars and Pastes Preparation
2.4. Analytical Techniques
3. Results and Discussion
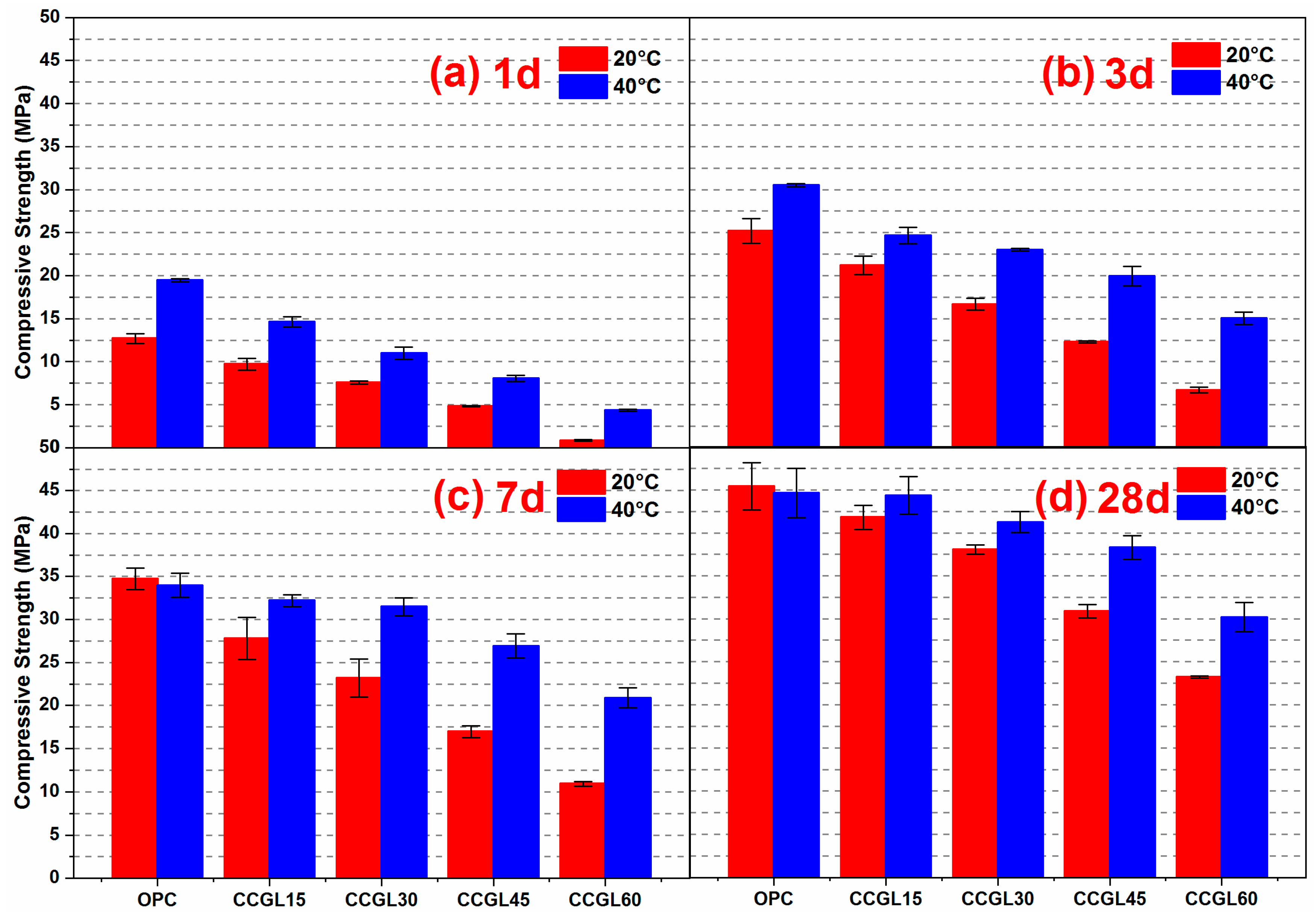
3.1. Compressive Strength
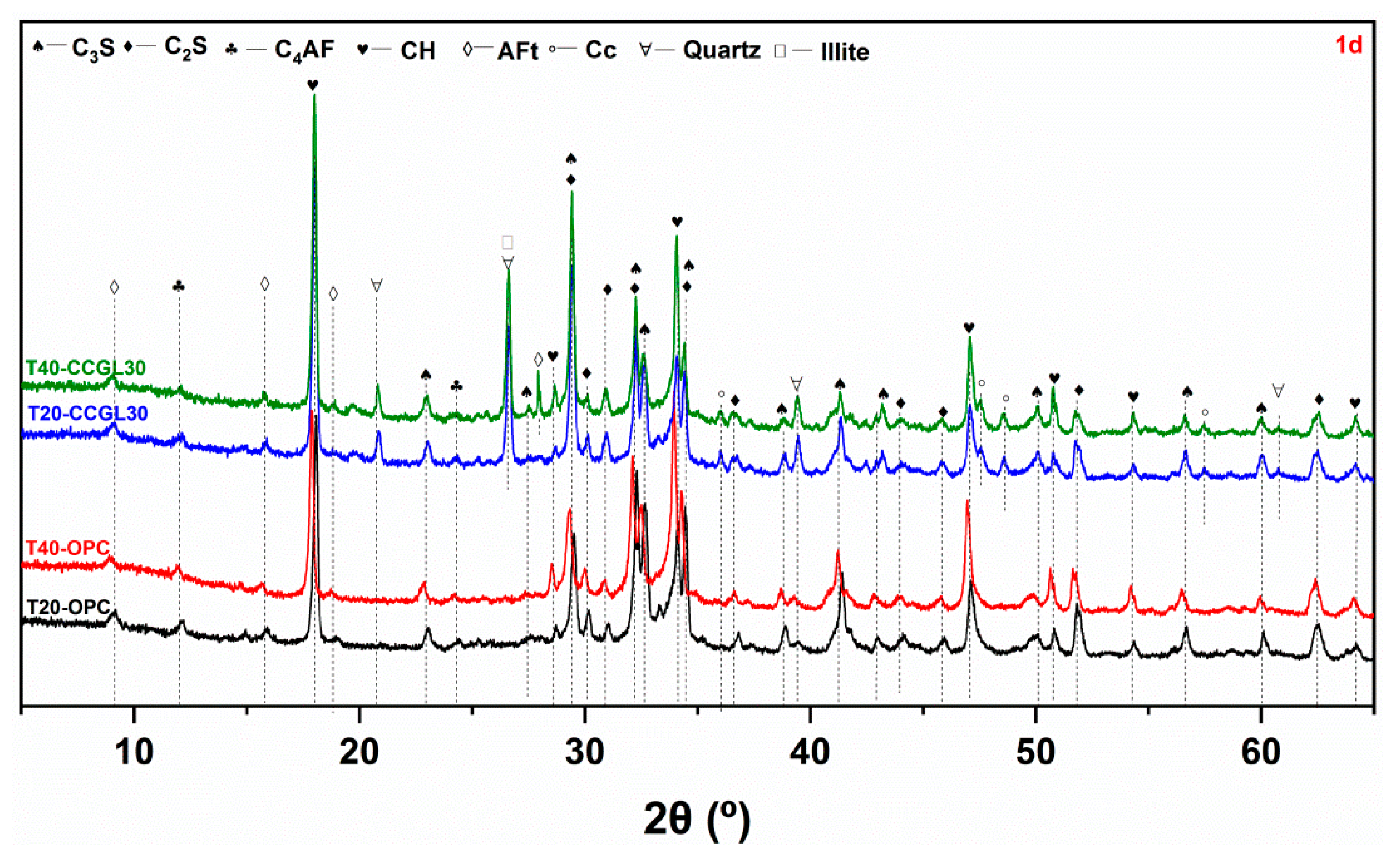
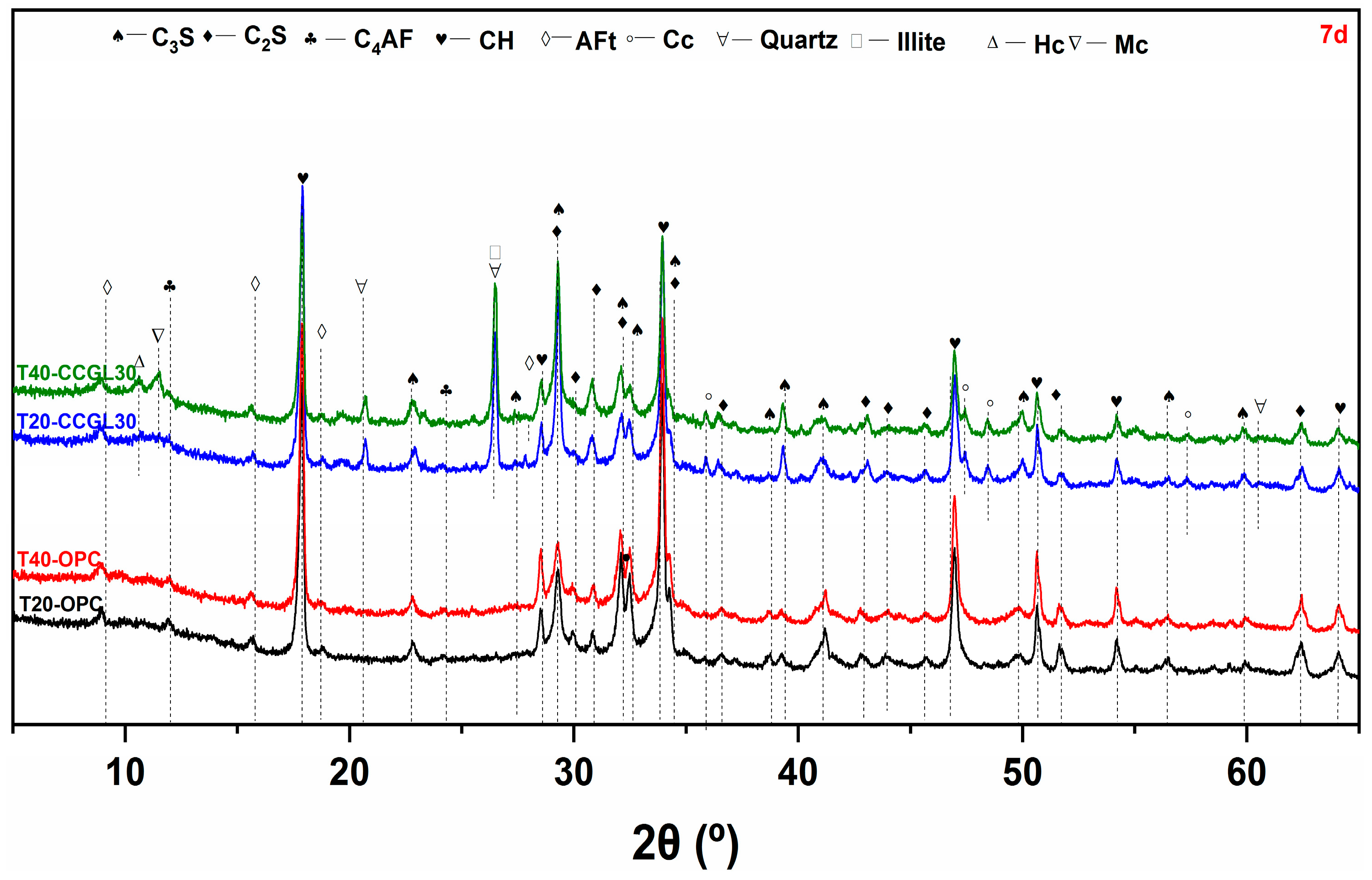
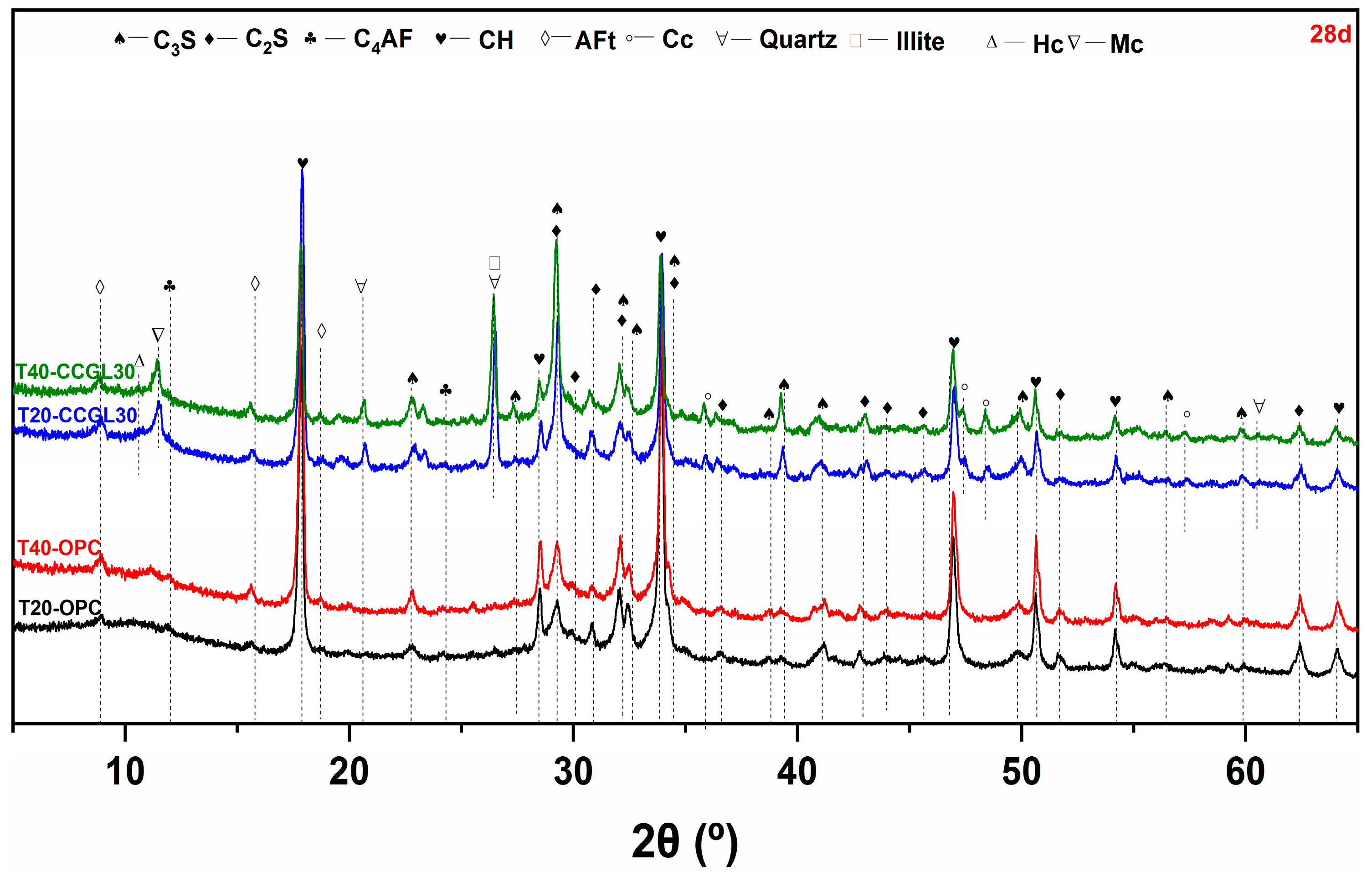
3.2. XRD Analysis
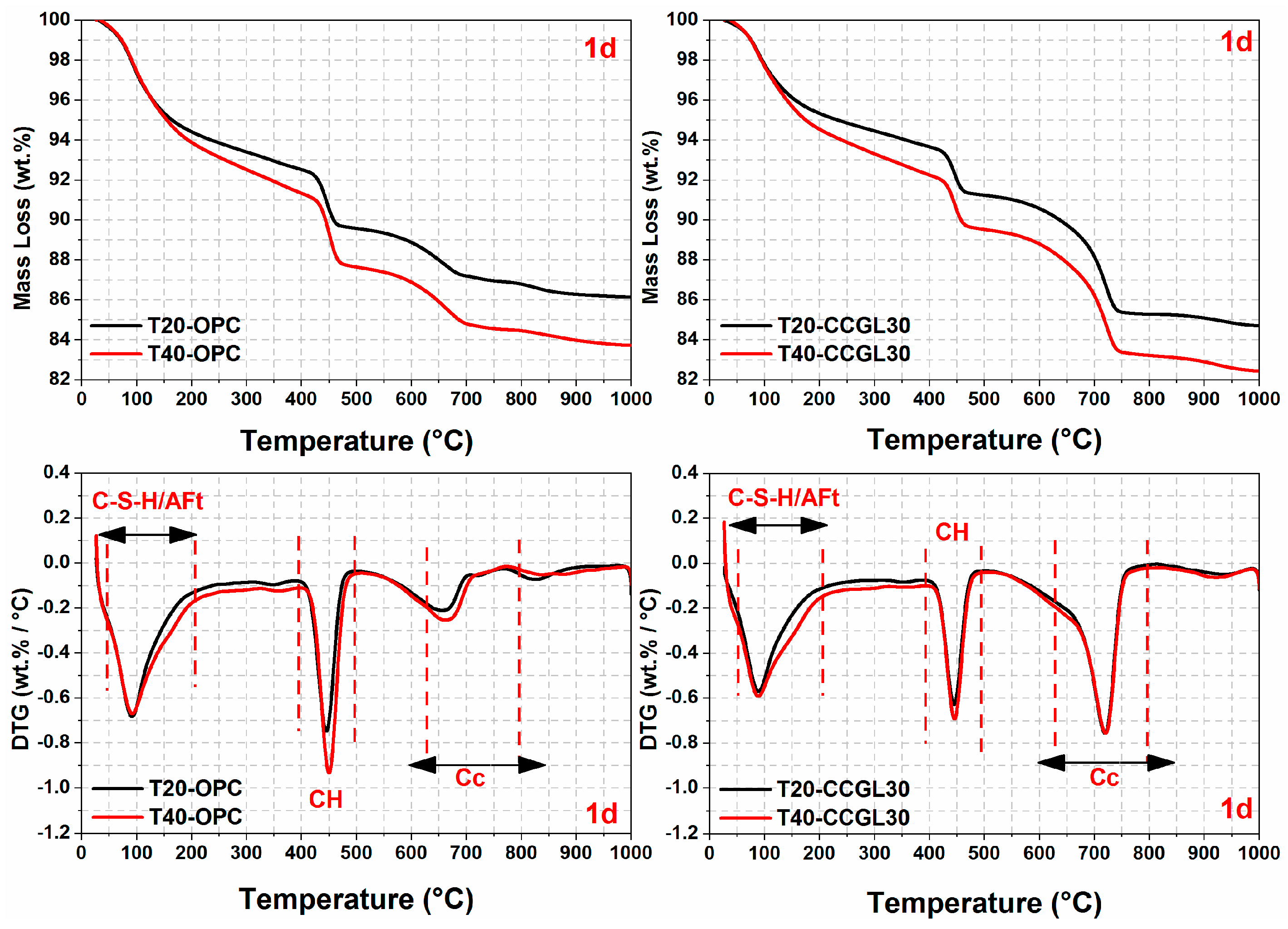
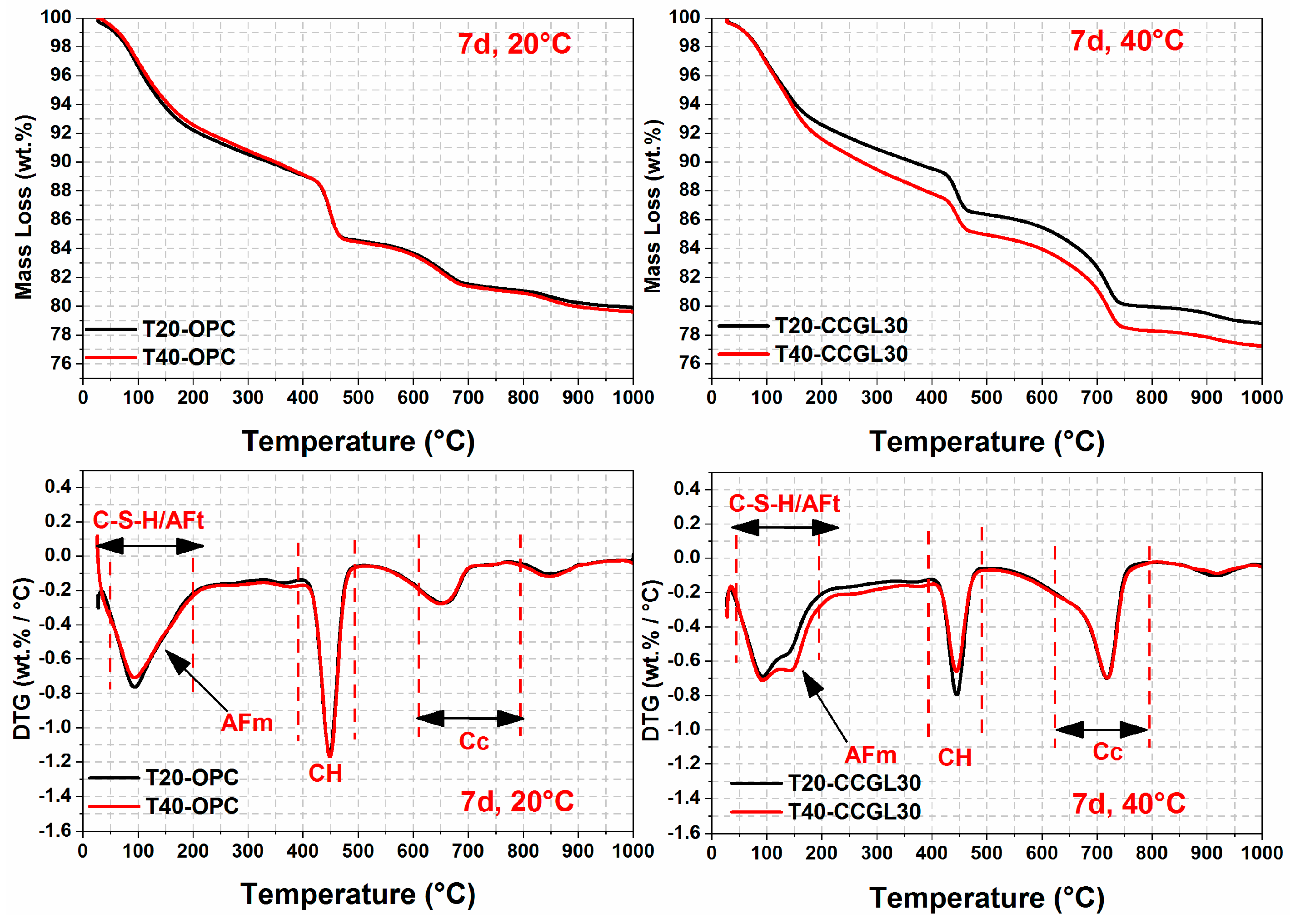
3.3. Thermal Analysis
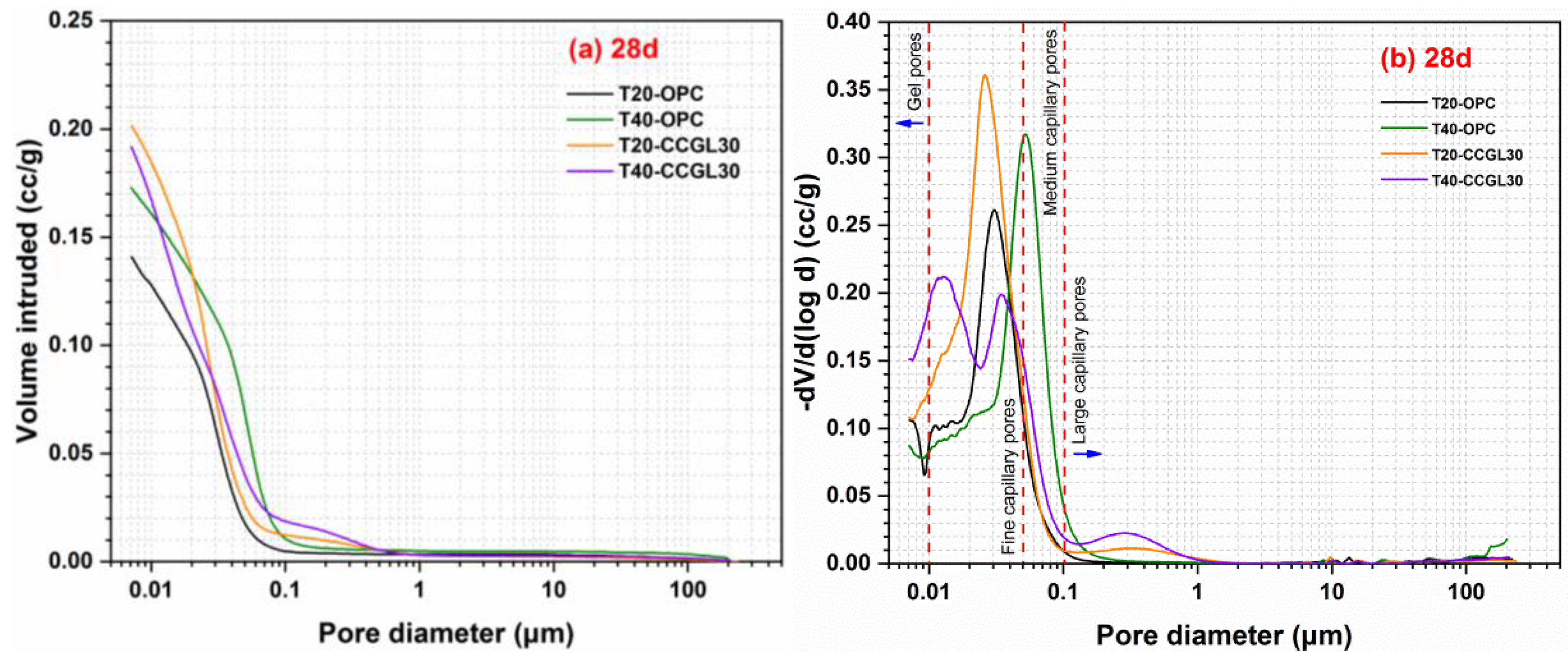
3.4. Pore Structure Analysis
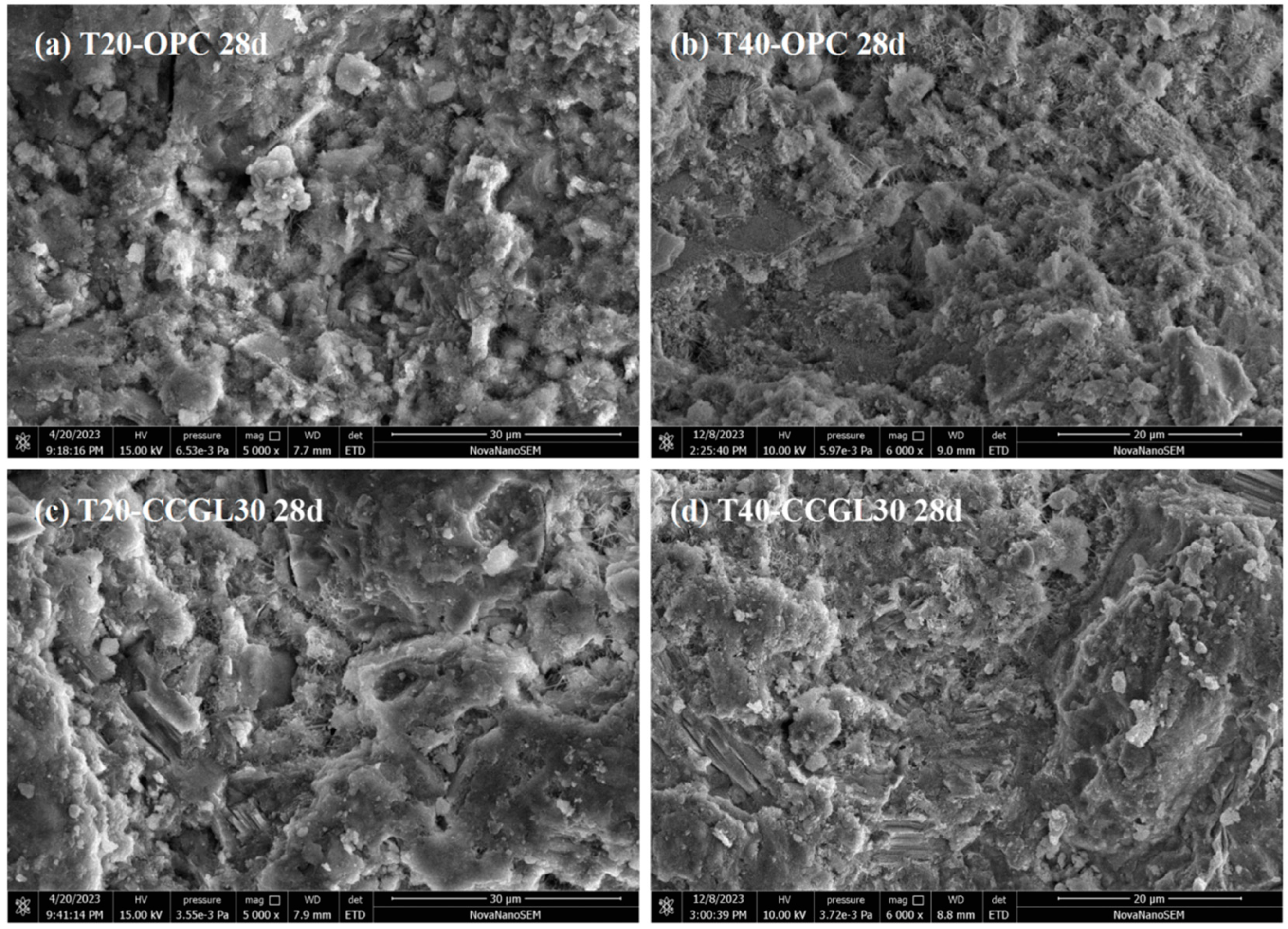
3.5. SEM Analysis
4. Conclusions
- The compressive strength of the calcined coal gangue–limestone blended cements is effectively enhanced by elevating the curing temperature to 40 °C, particularly in low clinker systems which exhibit greater sensitivity toward temperature.
- In calcined coal gangue–limestone blended cements, increasing the curing temperature not only promotes cement hydration but also facilitates the reaction of CCG, which precipitated more hydrates such as C-A-S-H gel, Hc and Mc.
- The total porosity of the calcined coal gangue–limestone blended cements decreases with increasing curing temperature. At 20 °C, the pore size distribution is mainly concentrated in fine capillary pores. At 40 °C, there is a more pronounced shift toward gel pores observed in the pore size distribution curve.
- The calcined coal gangue–limestone blended cements will exhibit a denser microscopic morphology when the curing temperature is increased.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, S.B.; Haskin, N.; Dastgheib, S.A. Coal-based graphene oxide-like materials: A comprehensive review. Carbon 2022, 215, 118447. [Google Scholar] [CrossRef]
- Hao, Y.; Guo, X.; Yao, X.; Han, R.; Li, L.; Zhang, M. Using Chinese coal gangue as an ecological aggregate and its modification: A review. Materials 2022, 15, 4495. [Google Scholar] [CrossRef]
- Li, J.; Wang, J. Comprehensive utilization and environmental risks of coal gangue: A review. J. Clean. Prod. 2019, 239, 117946. [Google Scholar] [CrossRef]
- Zhang, Y.; Ling, T.-C. Reactivity activation of waste coal gangue and its impact on the properties of cement-based materials—A review. Constr. Build. Mater. 2020, 234, 117424. [Google Scholar] [CrossRef]
- Li, L.; Shao, X.; Ling, T.-C. Life cycle assessment of coal gangue composite cements: From sole OPC towards low-carbon quaternary binder. J. Clean. Prod. 2023, 414, 137674. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Q.; Zhang, S.; Li, Y.; Yang, L. The thermal transformation behavior and products of pyrite during coal gangue combustion. Fuel 2022, 324, 124803. [Google Scholar] [CrossRef]
- Jiu, S.; Cheng, S.; Li, H.; Wang, L. Reaction mechanism of metakaolin materials prepared by calcining coal gangue. Mater. Res. Express 2021, 8, 015508. [Google Scholar] [CrossRef]
- Wang, A.; Liu, P.; Mo, L.; Liu, K.; Ma, R.; Guan, Y.; Sun, D. Mechanism of thermal activation on granular coal gangue and its impact on the performance of cement mortars. J. Build. Eng. 2022, 45, 103616. [Google Scholar] [CrossRef]
- Sanchez, M.A.; Garcia, H.L.C.; Molina, W.M.; Guzman, E.M.A.; Acosta, A.A.T.; Ortega, J.M.P. Use of metakaolin or coal gangue as a partial substitution of cement in mechanical performance of PC mortars. Eur. J. Environ. Civ. Eng. 2021, 25, 502–515. [Google Scholar] [CrossRef]
- Guo, Z.; Xu, J.; Xu, Z.; Gao, J.; Zhu, X. Performance of cement-based materials containing calcined coal gangue with different calcination regimes. J. Build. Eng. 2022, 56, 104821. [Google Scholar] [CrossRef]
- Zhang, M.; Li, L.; Yang, F.; Zhang, S.; Zhang, H.; Zhu, Y.; An, J. Thermal activation of high-alumina coal gangue auxiliary cementitious admixture: Thermal transformation, calcining product formation and mechanical properties. Materials 2024, 17, 415. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Dong, J.; Yu, L.; Xu, C.; Jiao, X.; Wang, M. Effect of activated coal gangue in North China on the compressive strength and hydration process of cement. J. Mater. Civ. Eng. 2019, 31, 04019022. [Google Scholar] [CrossRef]
- Yang, J.; Su, Y.; He, X.; Tan, H.; Jiang, Y.; Zeng, L.; Strnadel, B. Pore structure evaluation of cementing composites blended with coal by-products: Calcined coal gangue and coal fly ash. Fuel Process. Technol. 2018, 181, 75–90. [Google Scholar] [CrossRef]
- Yu, L.; Xia, J.; Gu, J.; Zhang, S.; Zhou, Y. Degradation mechanism of coal gangue concrete suffering from sulfate attack in the mine environment. Materials 2023, 16, 1234. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Hao, F.; Liu, P.; Mo, L.; Liu, K.; Li, Y.; Cao, J.; Sun, D. Separation of calcined coal gangue and its influence on the performance of cement-based materials. J. Build. Eng. 2022, 51, 104293. [Google Scholar] [CrossRef]
- Ren, B.; Chai, L.; Liu, Y.; Wang, Y. Preparation of high-ductility cement-calcined coal-cangue-powder-composite-based rapid repair material. Materials 2023, 16, 6049. [Google Scholar] [CrossRef] [PubMed]
- Jiu, S.; Wang, M.; Chen, Y.; Chen, J.; Gao, Q. Synthesis and characterization of low-carbon cementitious materials from suspended calcined coal gangue. Front. Mater. 2022, 9, 982861. [Google Scholar] [CrossRef]
- Liu, Y.; Ling, T.-C.; Wang, M.; Wu, Y.-Y. Synergic performance of low-kaolinite calcined coal gangue blended with limestone in cement mortars. Constr. Build. Mater. 2021, 300, 124012. [Google Scholar] [CrossRef]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined clay limestone cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- Antoni, M.; Rossen, J.; Martirena, F.; Scrivener, K. Cement substitution by a combination of metakaolin and limestone. Cem. Concr. Res. 2012, 42, 1579–1589. [Google Scholar] [CrossRef]
- Avet, F.; Scrivener, K. Investigation of the calcined kaolinite content on the hydration of limestone calcined clay cement (LC3). Cem. Concr. Res. 2018, 107, 124–135. [Google Scholar] [CrossRef]
- Lin, R.-S.; Oh, S.; Du, W.; Wang, X.-Y. Strengthening the performance of limestone-calcined clay cement (LC3) using nano silica. Constr. Build. Mater. 2022, 340, 127723. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, M.; Yang, Z.; Guo, R.; Wang, X.-Y.; Lin, R.-S. Properties of red sandstone-limestone-cement ternary com-posites: Hydration mechanism, microstructure, and high-temperature damage. Dev. Built Environ. 2024, 17, 100346. [Google Scholar] [CrossRef]
- Herath, C.; Gunasekara, C.; Law, D.W.; Setunge, S. Performance of high volume fly ash concrete incorporating additives: A systematic literature review. Constr. Build. Mater. 2020, 258, 120606. [Google Scholar] [CrossRef]
- Emmanuel, A.C.; Bishnoi, S. Influence of clinker replacement and curing temperature on hydration kinetics, strength development, and phase assemblage of fly ash–blended cements. J. Mater. Civ. Eng. 2022, 34, 04022107. [Google Scholar] [CrossRef]
- Pang, X.; Sun, L.; Chen, M.; Xian, M.; Cheng, G.; Liu, Y.; Qin, J. Influence of curing temperature on the hydration and strength development of Class G Portland cement. Cem. Concr. Res. 2022, 156, 106776. [Google Scholar] [CrossRef]
- Bai, S.; Guan, X.; Li, G. Early-age hydration heat evolution and kinetics of Portland cement containing nano-silica at different temperatures. Constr. Build. Mater. 2022, 334, 127363. [Google Scholar] [CrossRef]
- Narmluk, M.; Nawa, T. Effect of fly ash on the kinetics of Portland cement hydration at different curing temperatures. Cem. Concr. Res. 2011, 41, 579–589. [Google Scholar] [CrossRef]
- Shirani, S.; Cuesta, A.; Morales-Cantero, A.; De la Torre, A.G.; Olbinado, M.P.; Aranda, M.A. Influence of curing temperature on belite cement hydration: A comparative study with Portland cement. Cem. Concr. Res. 2021, 147, 106499. [Google Scholar] [CrossRef]
- Shi, M.; Wang, Q.; Zhou, Z. Comparison of the properties between high-volume fly ash concrete and high-volume steel slag concrete under temperature matching curing condition. Constr. Build. Mater. 2015, 98, 649–655. [Google Scholar]
- Bentz, D.P. Activation energies of high-volume fly ash ternary blends: Hydration and setting. Cem. Concr. Compos. 2014, 53, 214–223. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Z.; Chen, Z. Hydration mechanism of composite binders containing blast furnace ferronickel slag at different curing temperatures. J. Therm. Anal. Calorim. 2018, 131, 2291–2301. [Google Scholar] [CrossRef]
- Snellings, R.; Machner, A.; Bolte, G.; Kamyab, H.; Durdzinski, P.; Teck, P.; Zajac, M.; Muller, A.; de Weerdt, K.; Ben Haha, M. Hydration kinetics of ternary slag-limestone cements: Impact of water to binder ratio and curing temperature. Cem. Concr. Res. 2022, 151, 106647. [Google Scholar] [CrossRef]
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). China Standard Press: Beijing, China, 2021.
- GB/T 50081-2019; Standard for Test Methods of Concrete Physical and Mechanical Properties. China Construction Industry Press: Beijing, China, 2019.





| CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | Na2O | K2O | TiO2 | P2O5 | LOI | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cement | 54.0 | 17.6 | 3.7 | 3.3 | 2.4 | 2.9 | 0.1 | 0.7 | 0.3 | 0.1 | 14.6 |
| Limestone | 55.6 | 5.2 | 2.4 | 1.3 | 2.7 | 0.2 | 0.03 | 0.6 | 0.1 | 0.3 | 31.6 |
| CG | 1.2 | 43.6 | 19.3 | 2.6 | 0.4 | 0.1 | 0.3 | 1.1 | 0.7 | 0.1 | 16.5 |
| T | Notation | OPC | CCG | LS | Gypsum | Water | Sand |
|---|---|---|---|---|---|---|---|
| 20 °C | T20-OPC | 100 | 0 | 0 | 0 | 50 | 300 |
| 20 °C | T20CCGL15 | 84.8 | 10 | 5 | 0.2 | 50 | 300 |
| 20 °C | T20CCGL30 | 69.6 | 20 | 10 | 0.4 | 50 | 300 |
| 20 °C | T20CCGL45 | 54.4 | 30 | 15 | 0.6 | 50 | 300 |
| 20 °C | T20CCGL60 | 39.2 | 40 | 20 | 0.8 | 50 | 300 |
| 40 °C | T40-OPC | 100 | 0 | 0 | 0 | 50 | 300 |
| 40 °C | T40-CCGL15 | 84.8 | 10 | 5 | 0.2 | 50 | 300 |
| 40 °C | T40-CCGL30 | 69.6 | 20 | 10 | 0.4 | 50 | 300 |
| 40 °C | T40-CCGL45 | 54.4 | 30 | 15 | 0.6 | 50 | 300 |
| 40 °C | T40-CCGL60 | 39.2 | 40 | 20 | 0.8 | 50 | 300 |
| Curing Age | Stage | OPC | CCGL30 | ||
|---|---|---|---|---|---|
| 20 °C | 40 °C | 20 °C | 40 °C | ||
| 1 d | 40–200 °C | 5.41 | 5.82 | 4.89 | 5.93 |
| 400–500 °C | 2.97 | 3.74 | 2.47 | 2.79 | |
| 40–1000 °C | 9.69 | 11.17 | 13.9 | 15.81 | |
| 3 d | 40–200 °C | 6.63 | 6.59 | 6.58 | 7.82 |
| 400–500 °C | 3.69 | 4.23 | 3.04 | 4.61 | |
| 40–1000 °C | 11.91 | 12.17 | 16.69 | 17.08 | |
| 7 d | 40–200 °C | 6.84 | 6.23 | 7.66 | 8.78 |
| 400–500 °C | 4.58 | 4.73 | 3.26 | 2.96 | |
| 40–1000 °C | 13.13 | 12.05 | 18.49 | 19.48 | |
| 28 d | 40–200 °C | 7.94 | 8.01 | 9.65 | 9.82 |
| 400–500 °C | 4.42 | 4.59 | 3.18 | 3.01 | |
| 40–1000 °C | 14.21 | 13.85 | 20.62 | 20.44 | |
| 1 d | 28 d | |
|---|---|---|
| T20-OPC | 27.91 | 14.07 |
| T40-OPC | 25.88 | 19.17 |
| T20-CCGL30 | 34.18 | 20.10 |
| T40-CCGL30 | 29.47 | 19.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zhou, H.; Hu, Y.; Wang, J.; Ma, J.; Jiang, R.; Sun, J. Influence of Curing Temperature on the Performance of Calcined Coal Gangue–Limestone Blended Cements. Materials 2024, 17, 1721. https://doi.org/10.3390/ma17081721
Zhang W, Zhou H, Hu Y, Wang J, Ma J, Jiang R, Sun J. Influence of Curing Temperature on the Performance of Calcined Coal Gangue–Limestone Blended Cements. Materials. 2024; 17(8):1721. https://doi.org/10.3390/ma17081721
Chicago/Turabian StyleZhang, Wenguang, Hao Zhou, Yueyang Hu, Jiaqing Wang, Jian Ma, Ruiyu Jiang, and Jinfeng Sun. 2024. "Influence of Curing Temperature on the Performance of Calcined Coal Gangue–Limestone Blended Cements" Materials 17, no. 8: 1721. https://doi.org/10.3390/ma17081721






