Properties of Geopolymer Mixtures Incorporating Recycled Ceramic Fines
Abstract
1. Introduction
2. Materials and Research Methodology
2.1. Characteristics of the Raw Materials
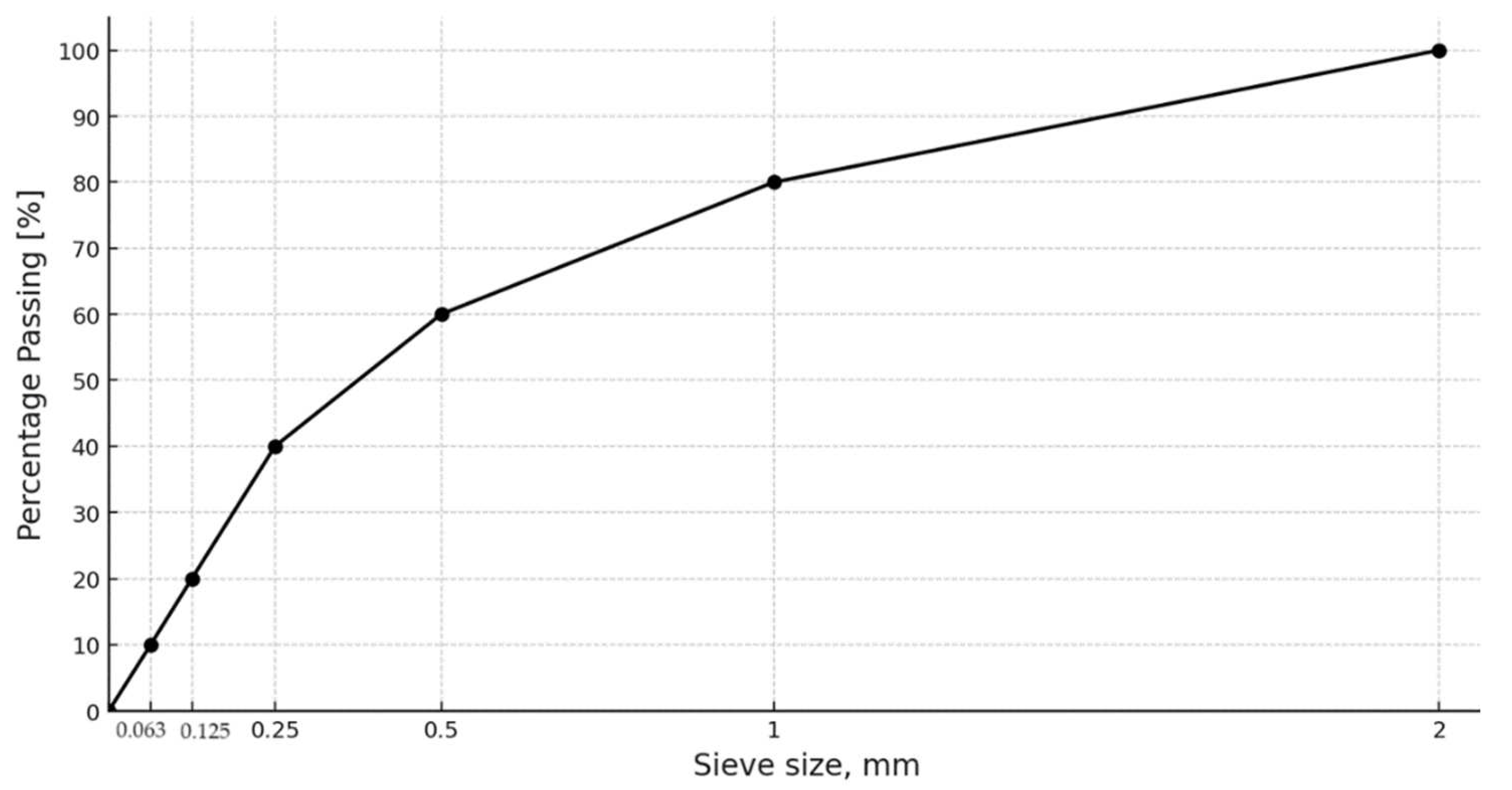
Ceramic Fines
2.2. Research Methods
2.3. Pore Distribution—Mercury Intrusion Porosimetry (MIP)
2.4. Scanning Electron Microscopy (SEM)
3. Design of the Experiment
Selection of Variables and Development of the Experimental Plan—With Ceramic Fines
4. Test Results and Analysis
4.1. Flexural Strength of Geopolymer Mortars after 28 Days
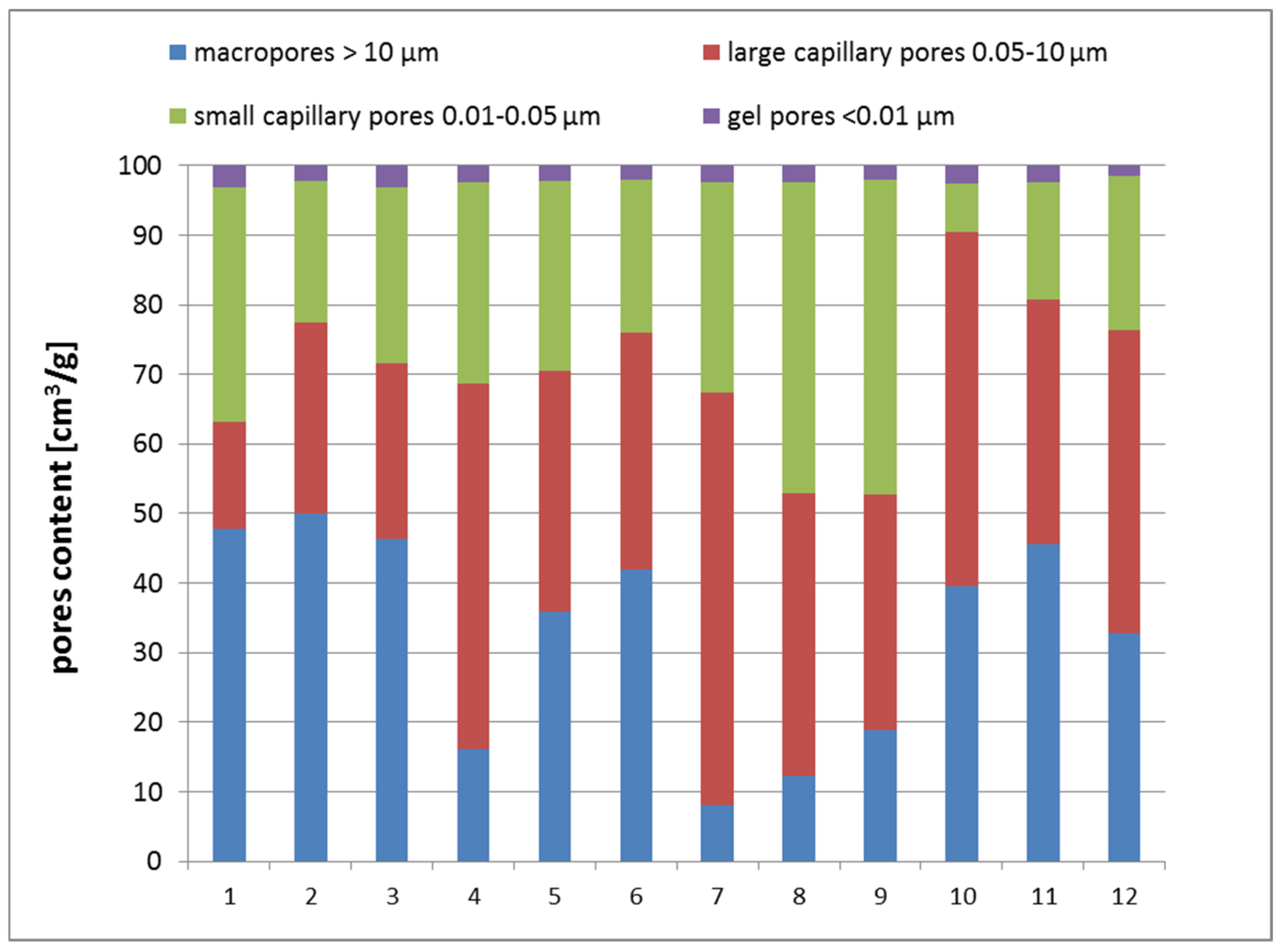
4.2. Porosimetry Test Results
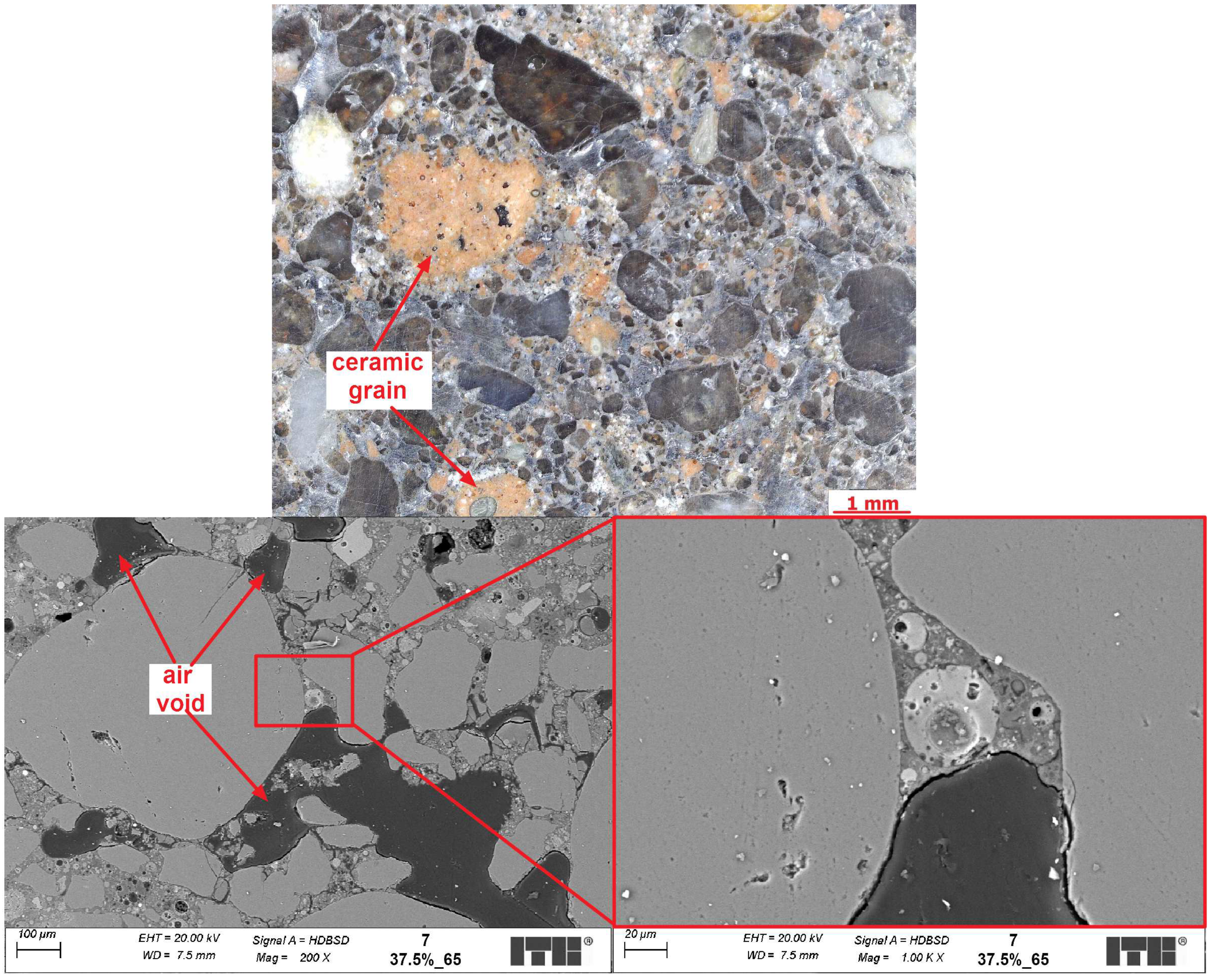
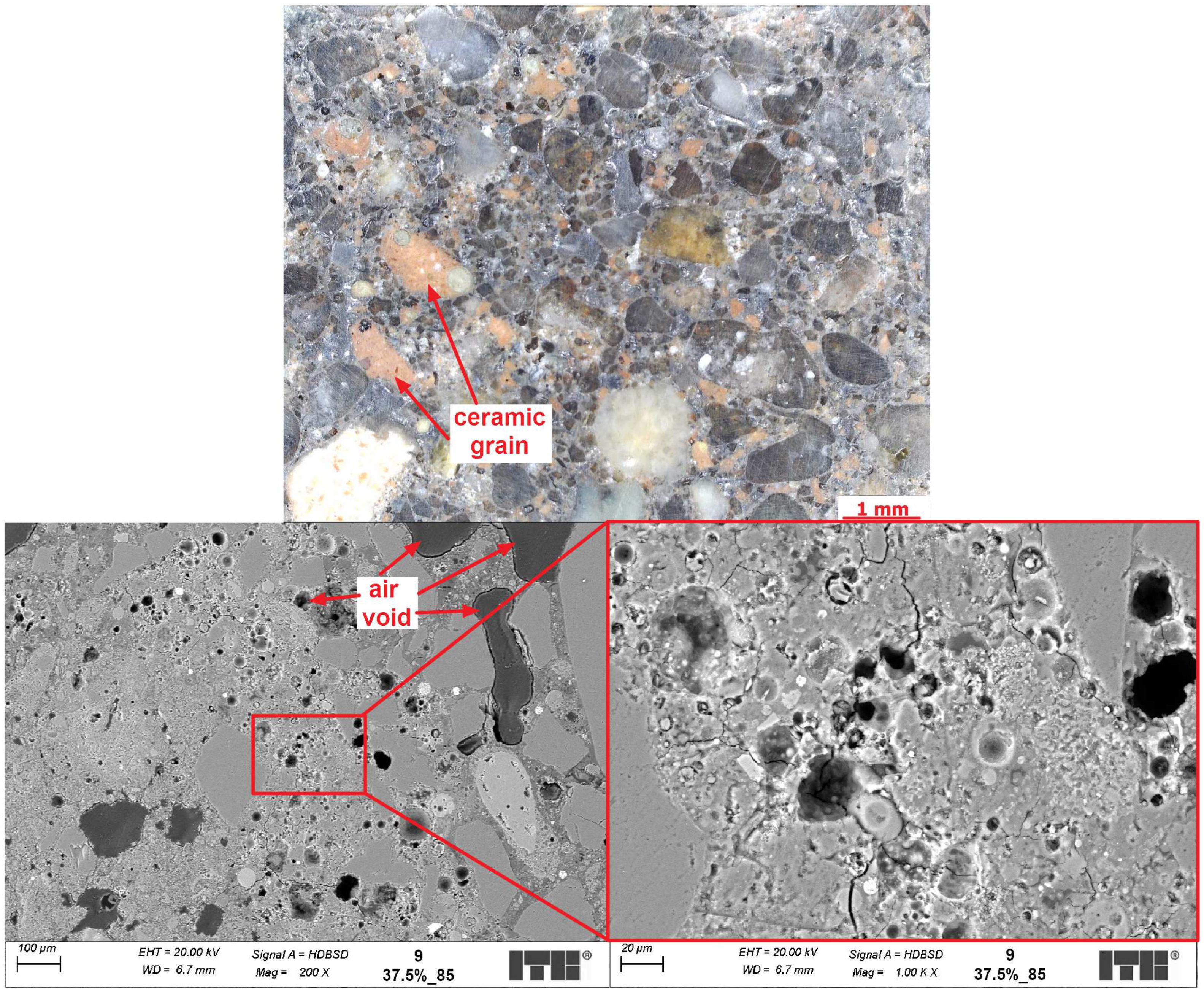
4.3. Microstructure
5. Conclusions
- CFs can serve as an effective additive for geopolymer mixtures by partially replacing FA, provided the appropriate replacement level and curing temperature are implemented.
- Enhancement in the bending and compressive strength of geopolymer mixtures was noted, with an increase in heating temperature from 65 °C to 85 °C using 25% CFs and 37.5% FA. However, employing 50% CFs alongside elevated heating temperatures resulted in diminished strength properties.
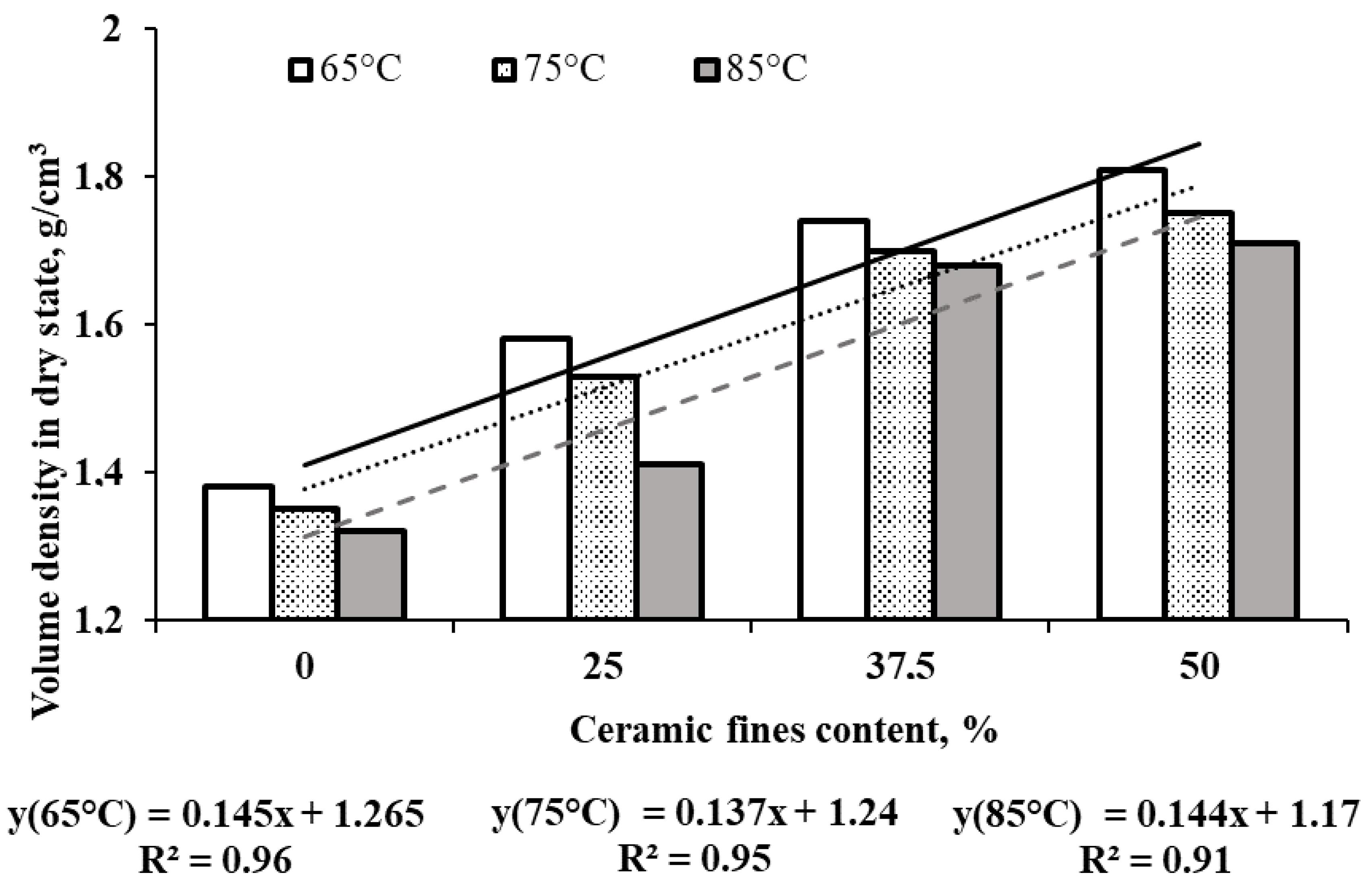
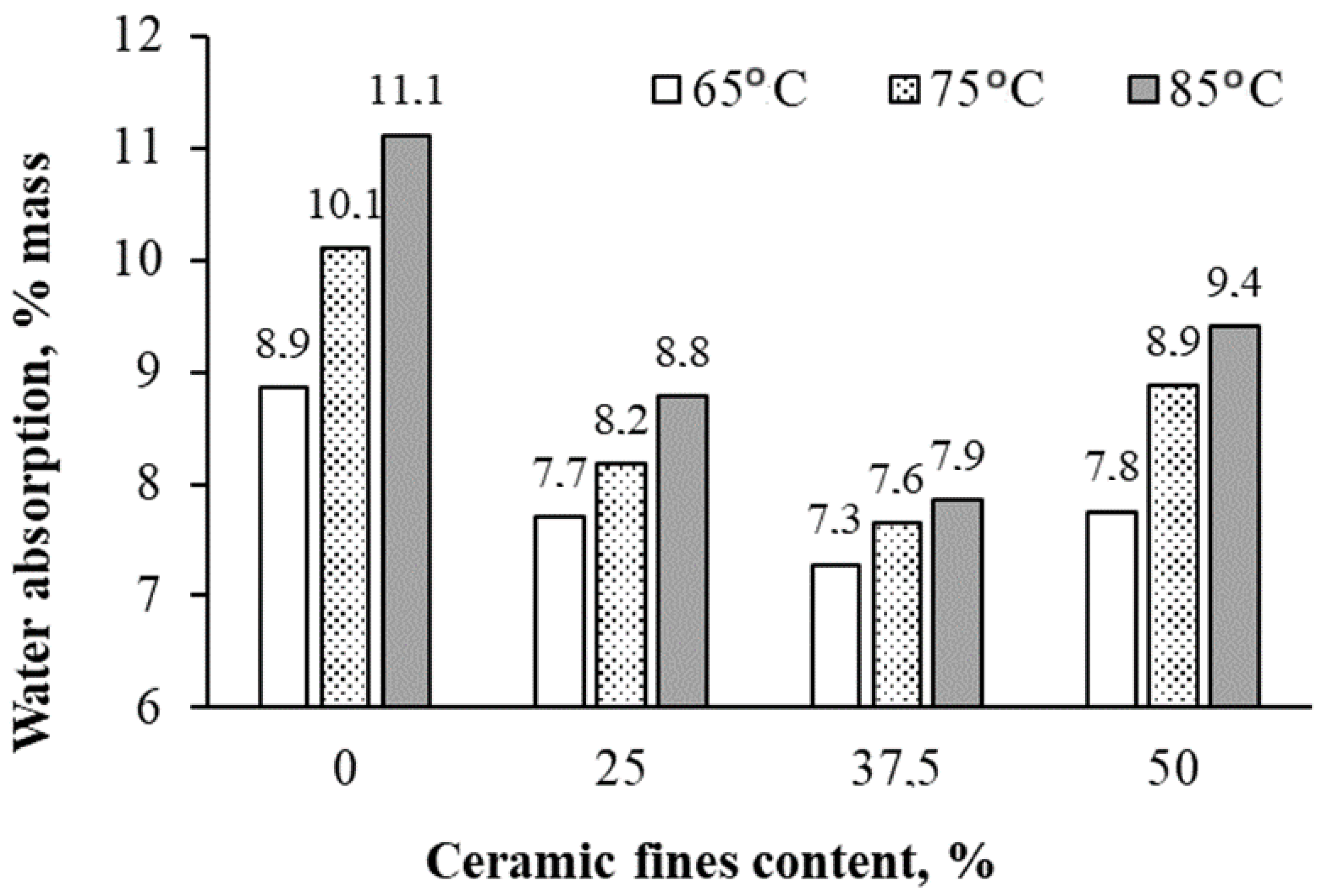
- The incorporation of CFs generally led to reduced water absorption in geopolymers, attributed to additional sealing of their porous structure, consequently increasing bulk density.
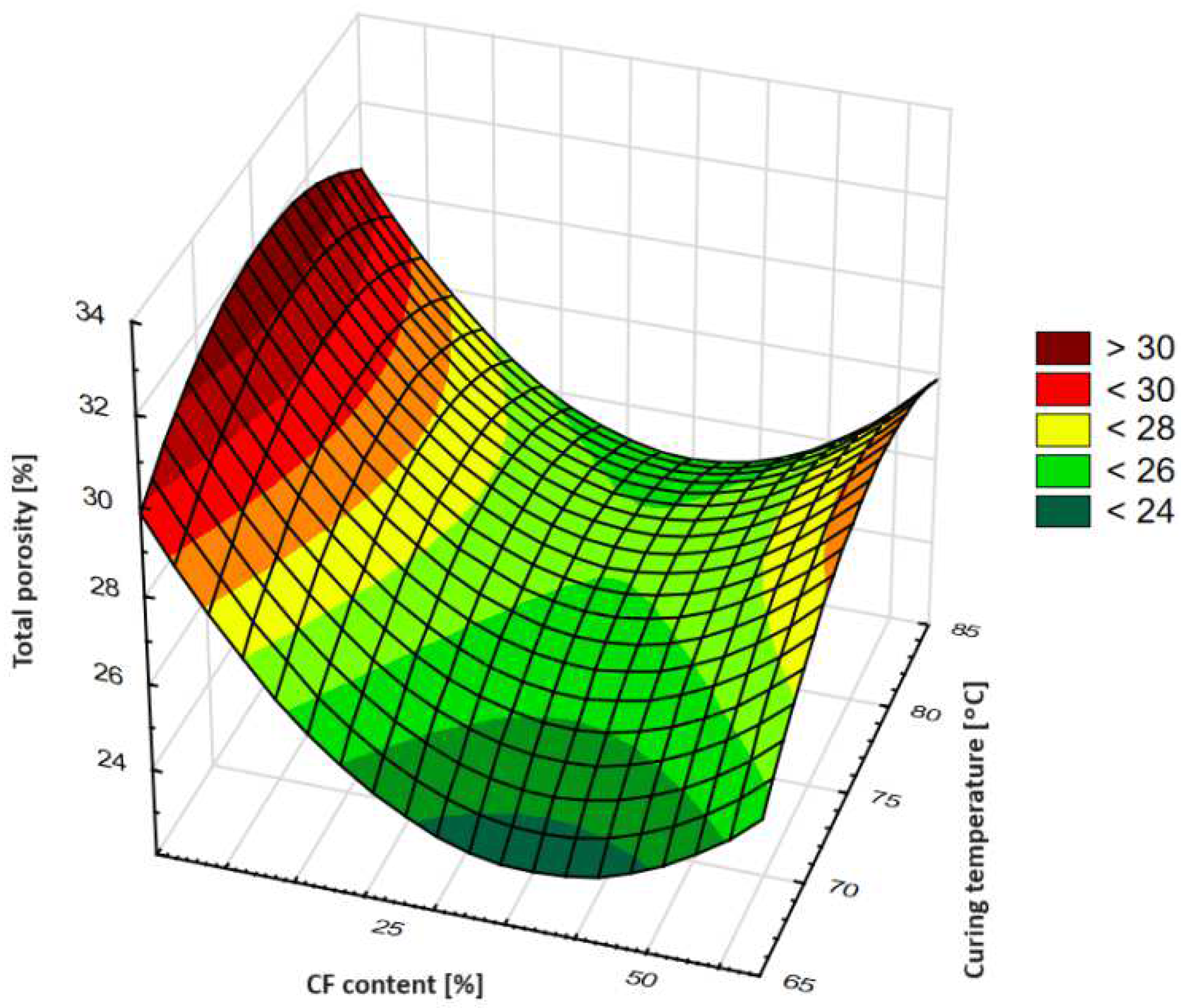
- The total porosity of the tested geopolymer mixtures as a function of ceramic fines content and temperature of curing has a local extremum–minimum, at a CF content of about 35%. Curing the geopolymer samples at 75 °C seems to have the strongest effect in lowering the total porosity of geopolymers. Observed changes in the total porosity are mostly caused by the amount of macropores and capillary pores, but the amount of gel pores seems to be almost constant.
- Increasing the temperature of curing would cause the sealing up of geopolymer microstructures initially, but a further increase in the temperature causes the formation of new macropores, which might be due to the formation of a denser C-A-S-H phase. However, this hypothesis needs further investigation to confirm.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal/delivering-european-green-deal_en (accessed on 7 January 2024).
- Belaïd, F. How does concrete and cement industry transformation contribute to mitigating climate change challenges? RCRA 2022, 15, 200084. [Google Scholar] [CrossRef]
- Cao, Z.; Masanet, E.; Tiwari, A.; Akolawala, S. Decarbonizing Concrete: Deep Decarbonization Pathways for the Cement and Concrete Cycle in the United States, India, and China; Climateworks Foundation: San Francisco, CA, USA, 2021. [Google Scholar]
- Antunes, M.; Santos, R.L.; Pereira, J.; Rocha, P.; Horta, R.B.; Colaço, R. Alternative Clinker Technologies for Reducing Carbon Emissions in Cement Industry: A Critical Review. Materials 2022, 15, 209. [Google Scholar] [CrossRef] [PubMed]
- Alturki, A. The Global Carbon Footprint and How New Carbon Mineralization Technologies Can Be Used to Reduce CO2 Emissions. ChemEngineering 2022, 6, 44. [Google Scholar] [CrossRef]
- Luhar, I.; Luhar, S.; Abdullah, M.M.A.B.; Nabiałek, M.; Sandu, A.V.; Szmidla, J.; Jurczyńska, A.; Razak, R.A.; Aziz, I.H.A.; Jamil, N.H.; et al. Assessment of the Suitability of Ceramic Waste in Geopolymer Composites: An Appraisal. Materials 2021, 14, 3279. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Torgal, F.; Moura, D.; Ding, Y.; Jalali, S. Composition, strength and workability of alkali-activated metakaolin based mortars. Constr. Build. Mater. 2011, 25, 3732–3745. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Hardjito, D.; Wallah, S.E.; Sumajouw, D.M.J.; Rangan, B.V. On the development of fly ash-based geopolymer concrete. ACI Mater. J. 2005, 101, 467–472. [Google Scholar]
- Barbosa, V.F.F.; MacKenzie, K.J.D. Thermal behaviour of inorganic geopolymers and composites derived from sodium polysialate. Mater. Res. Buil 2003, 38, 319–331. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. The role of inorganic polymer technology in development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- McLellan, B.C.; Williams, R.P.; Lay, J.; van Riessen, A.; Corder, G. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Toniolo, N.; Rincón, A.; Roether, J.A.; Ercole, P.; Bernardo, E.; Boccaccini, A.R. Extensive reuse of soda-lime waste glass in fly ash-based geopolymers. Constr. Build. Mater. 2018, 188, 1077–1084. [Google Scholar] [CrossRef]
- Alhawat, M.; Ashour, A.; Yildirim, G.; Aldemir, A.; Sahmaran, M. Properties of geopolymers sourced from construction and demolition waste: A review. J. Build. Eng. 2022, 50, 104104. [Google Scholar] [CrossRef]
- Oikonomou, N. Recycled concrete aggregates. Cem. Concr. Compos. 2005, 27, 315–318. [Google Scholar] [CrossRef]
- Fatta, D.; Papadopoulos, A.; Avramikos, E.; Sgourou, E.; Moustakas, K.; Kourmoussis, F.; Mentzis, A.; Loizidou, M. Generation and management of construction and demolition waste in Greece—An existing challenge. Resour. Conserv. Recycl. 2003, 40, 81–91. [Google Scholar] [CrossRef]
- Reig, L.; Tashima, M.M.; Borrachero, M.; Monzó, J.; Cheeseman, C.; Payá, J. Properties and microstructure of alkali-activated red clay brick waste. Constr. Build. Mater. 2013, 43, 98–106. [Google Scholar] [CrossRef]
- Daniyal, M.; Ahmad, S. Application of waste ceramic tile aggregates in concrete. Int. J. Innov. Res. Sci. Eng. Technol. 2015, 4, 12808–12815. [Google Scholar]
- Samadi, M.; Hussin, M.W.; Lee, H.S.; Sam, A.R.M.; Ismail, M.; Lim, N.H.A.S.; Ariffin, N.F.; Khalid, N.H.A. Poperties of mortar containing ceramic powder waste as cement replacement. J. Technol. 2015, 77, 93–97. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Mirza, J.; Tahir, M.M.; Asaad, M.A.; Ismail, M.; Shah, K.W. Waste ceramic powder incorporated alkali activated mortars exposed to elevated Temperatures: Performance evaluation. Constr. Build. Mater. 2018, 187, 307–317. [Google Scholar] [CrossRef]
- Ricciotti, L.; Molino, A.J.; Roviello, V.; Chianese, E.; Cennamo, P.; Roviello, G. Geopolymer Composites for Potential Applications in Cultural Heritage. Environments 2017, 4, 91. [Google Scholar] [CrossRef]
- Aly, S.T.; Kanaan, D.M.; El-Dieb, A.S.; Abu-Eishah, S.I. Properties of Ceramic Waste Powder-Based Geopolymer Concrete. In Proceedings of the International Congress on Polymers in Concrete: Polymers for Resilient and Sustainable Concrete Infrastructure (ICPIC 2018), Washington, DC, USA, 29 April–1 May 2018; pp. 429–435. [Google Scholar] [CrossRef]
- Bhavsar, J.K.; Panchal, V. Ceramic Waste Powder as a Partial Substitute of Fly Ash for Geopolymer Concrete Cured at Ambient Temperature. Civ. Eng. J. 2022, 8, 1369–1387. [Google Scholar] [CrossRef]
- Shehata, N.; Mohamed, O.A.; Sayed, E.T.; Abdelkareem, M.A.; Olabi, A.G. Geopolymer concrete as green building materials: Recent applications, sustainable development and circular economy potentials. Sci. Total Environ. 2022, 836, 155577. [Google Scholar] [CrossRef] [PubMed]
- Chindaprasirt, P.; Rattanasak, U. Characterization of the high-calcium fly ash geopolymer mortar with hot-weather curing systems for sustainable application. Adv. Powder Technol. 2017, 28, 2317–2324. [Google Scholar] [CrossRef]
- Silva, G.; Castañeda, D.; Kim, S.; Castañeda, A.; Bertolotti, B.; Ortega-San-Martin, L.; Nakamatsu, J.; Aguilar, R. Analysis of the production conditions of geopolymer matrices from natural pozzolana and fired clay brick wastes. Constr. Build. Mater. 2019, 215, 633–643. [Google Scholar] [CrossRef]
- EN 450-1:2012; Fly Ash for Concrete—Part 1: Definitions, Specifications, and Compliance Criteria. European Committee for Standardization: Brussels, Belgium, 2012.
- Okoye, F.; Durgaprasad, J.; Singh, N. Mechanical properties of alkali activated flyash/Kaolin based geopolymer concrete. Constr. Build. Mater. 2015, 98, 685–691. [Google Scholar] [CrossRef]
- EN 196-1:2016; Methods of Testing Cement. Determination of Strength. European Committee for Standardization: Brussels, Belgium, 2016.
- EN 1015-10:1999; Methods of test for Mortar for Masonry—Part 10: Determination of Dry Bulk Density Of Hardened Mortar. European Committee for Standardization: Brussels, Belgium, 1999.
- Chyliński, F.; Kuczyński, K. Ilmenite Mud Waste as an Additive for Frost Resistance in Sustainable Concrete. Materials 2020, 13, 2904. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Koksa, F.; Gence, O.I.; Junaid, M.M.; Kazmi, S.M.S. Influence of micro Fe2O3 and MgO on the physical and mechanical properties of the zeolite and kaolin based geopolymer mortar. J. Build. Eng. 2022, 52, 104443. [Google Scholar] [CrossRef]
- Kaze, R.C.; Moungam, L.M.B.; Cannio, M.; Rosa, R.; Kamseu, E.; Melo, U.C.; Leonelli, C. Microstructure and engineering properties of Fe2O3(FeO)-Al2O3-SiO2 based geopolymer composites. J. Clean. Prod. 2018, 199, 849–859. [Google Scholar] [CrossRef]
- Aredes, F.G.M.; Campos, T.M.B.; Machado, J.P.B.; Sakane, K.K.; Thim, G.P.; Brunelli, D.D. Effect of cure temperature on the formation of metakaolinite-based geopolymer. Ceram. Int. 2015, 41, 7302–7311. [Google Scholar] [CrossRef]







| Composition Percentage, % | CaO | Fe2O3 | SiO2 | Al2O3 | MgO | SO3 | Na2O | K2O | LOI |
|---|---|---|---|---|---|---|---|---|---|
| CFs | 10.22 | 13.69 | 38.56 | 18.94 | 4.06 | 0.15 | 1.58 | 6.29 | - |
| FA | 2.14 | 4.97 | 54.60 | 25.30 | 1.80 | 0.37 | 0.84 | 2.80 | 4.37 |
| Properties | Skeletal Density, g/cm3 | Bulk Density, g/cm3 | Bulk Density in a Loose State, g/cm3 |
|---|---|---|---|
| CFs | 2.82 | 0.76 | 0.71 |
| FA | 2.35 | 0.85 | 0.79 |
| X1 | Amount of CFs | 0 | 25 | 37.5 | 50 | % of FA |
| X2 | Temperature of curing | - | 65 | 75 | 85 | °C |
| Series | Variables | |
|---|---|---|
| X1, % | X2, °C | |
| 1 | 0 | 65 |
| 2 | 0 | 75 |
| 3 | 0 | 85 |
| 4 | 25 | 65 |
| 5 | 25 | 75 |
| 6 | 25 | 85 |
| 7 | 37.5 | 65 |
| 8 | 37.5 | 75 |
| 9 | 37.5 | 85 |
| 10 | 50 | 65 |
| 11 | 50 | 75 |
| 12 | 50 | 85 |
| Series | Percentage of CF Content, % | CF, g | FA, g | Activator 10 M, g | Sand, g | Curing Temperature, °C |
|---|---|---|---|---|---|---|
| 1CF0_65 | 0 | 0 | 450 | 250 | 1350 | 65 |
| 2CF0_75 | 0 | 0 | 450 | 250 | 1350 | 75 |
| 3CF0_85 | 0 | 0 | 450 | 250 | 1350 | 85 |
| 4CF25_65 | 25 | 112.5 | 337.5 | 250 | 1350 | 65 |
| 5CF25_75 | 25 | 112.5 | 337.5 | 250 | 1350 | 75 |
| 6CF25_85 | 25 | 112.5 | 337.5 | 250 | 1350 | 85 |
| 7CF37.5_65 | 37.5 | 168.75 | 281.25 | 250 | 1350 | 65 |
| 8CF37.5_75 | 37.5 | 168.75 | 281.25 | 250 | 1350 | 75 |
| 9CF37.5_85 | 37.5 | 168.75 | 281.25 | 250 | 1350 | 85 |
| 10CF50_65 | 50 | 225 | 225 | 250 | 1350 | 65 |
| 11CF50_75 | 50 | 225 | 225 | 250 | 1350 | 75 |
| 12CF50_85 | 50 | 225 | 225 | 250 | 1350 | 85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinowska-Wichrowska, K.; Pawluczuk, E.; Chyliński, F.; Chai, H.K.; Joka Yildiz, M.; Chuczun, A.; Łuniewski, S. Properties of Geopolymer Mixtures Incorporating Recycled Ceramic Fines. Materials 2024, 17, 1740. https://doi.org/10.3390/ma17081740
Kalinowska-Wichrowska K, Pawluczuk E, Chyliński F, Chai HK, Joka Yildiz M, Chuczun A, Łuniewski S. Properties of Geopolymer Mixtures Incorporating Recycled Ceramic Fines. Materials. 2024; 17(8):1740. https://doi.org/10.3390/ma17081740
Chicago/Turabian StyleKalinowska-Wichrowska, Katarzyna, Edyta Pawluczuk, Filip Chyliński, Hwa Kian Chai, Magdalena Joka Yildiz, Aleksandra Chuczun, and Stanisław Łuniewski. 2024. "Properties of Geopolymer Mixtures Incorporating Recycled Ceramic Fines" Materials 17, no. 8: 1740. https://doi.org/10.3390/ma17081740
APA StyleKalinowska-Wichrowska, K., Pawluczuk, E., Chyliński, F., Chai, H. K., Joka Yildiz, M., Chuczun, A., & Łuniewski, S. (2024). Properties of Geopolymer Mixtures Incorporating Recycled Ceramic Fines. Materials, 17(8), 1740. https://doi.org/10.3390/ma17081740










