Effect of Retrogression with Different Cooling Ways on the Microstructure and Properties of T’/η’ Strengthened Al-Zn-Mg-Cu Alloys
Abstract
1. Introduction
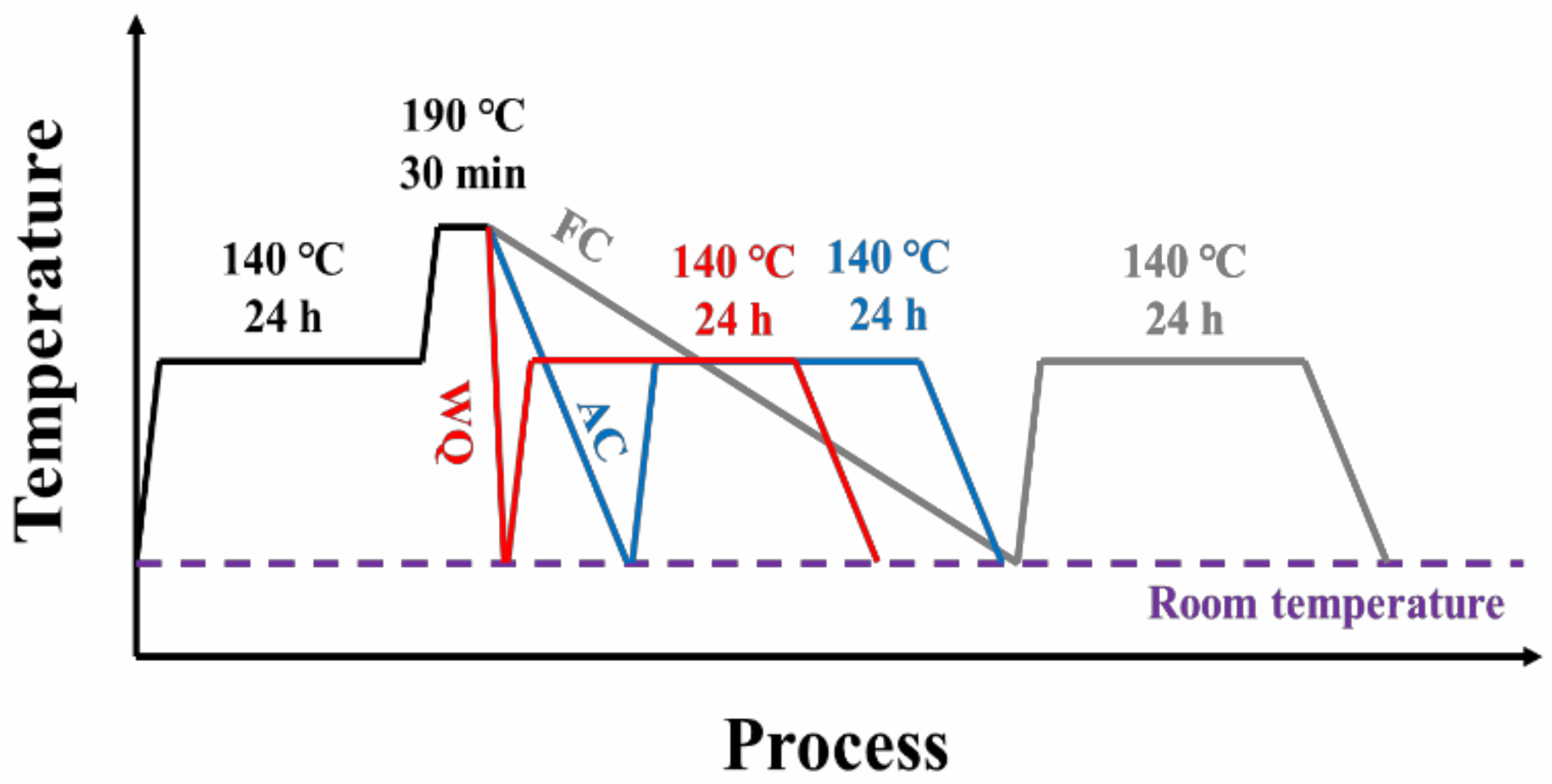
2. Materials and Methods
2.1. Materials
2.2. Mechanical Tests
2.3. IGC Test
2.4. Electrochemical Test
2.5. Microstructure Observation
3. Results
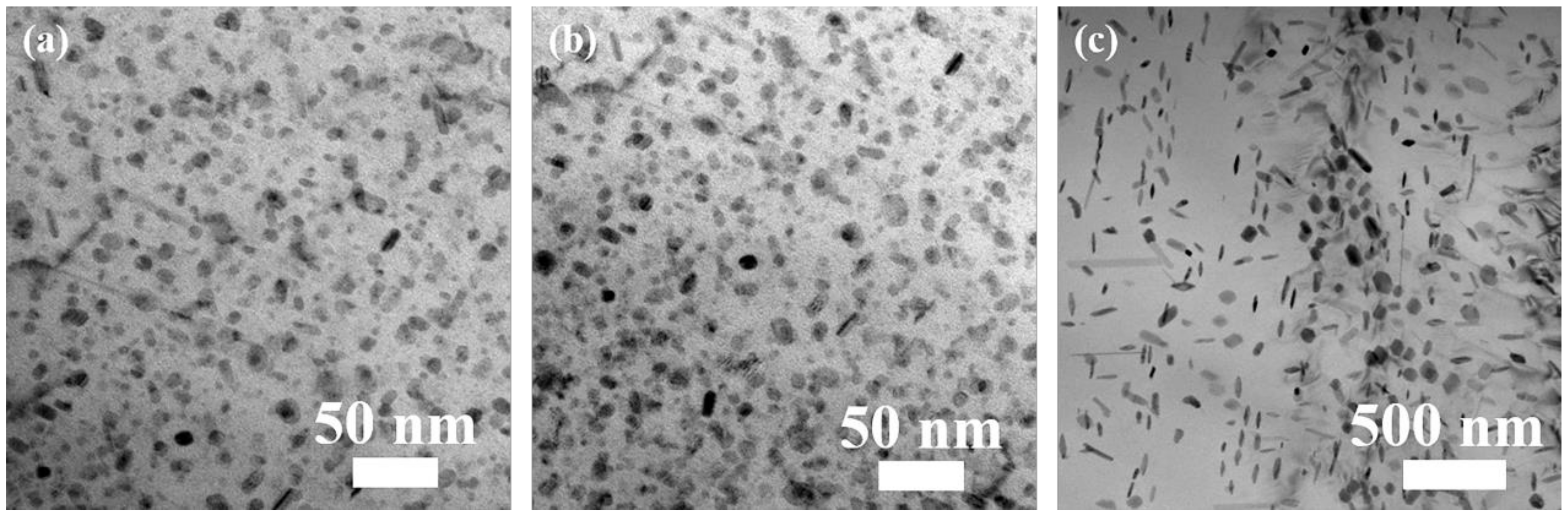
3.1. Microstructure
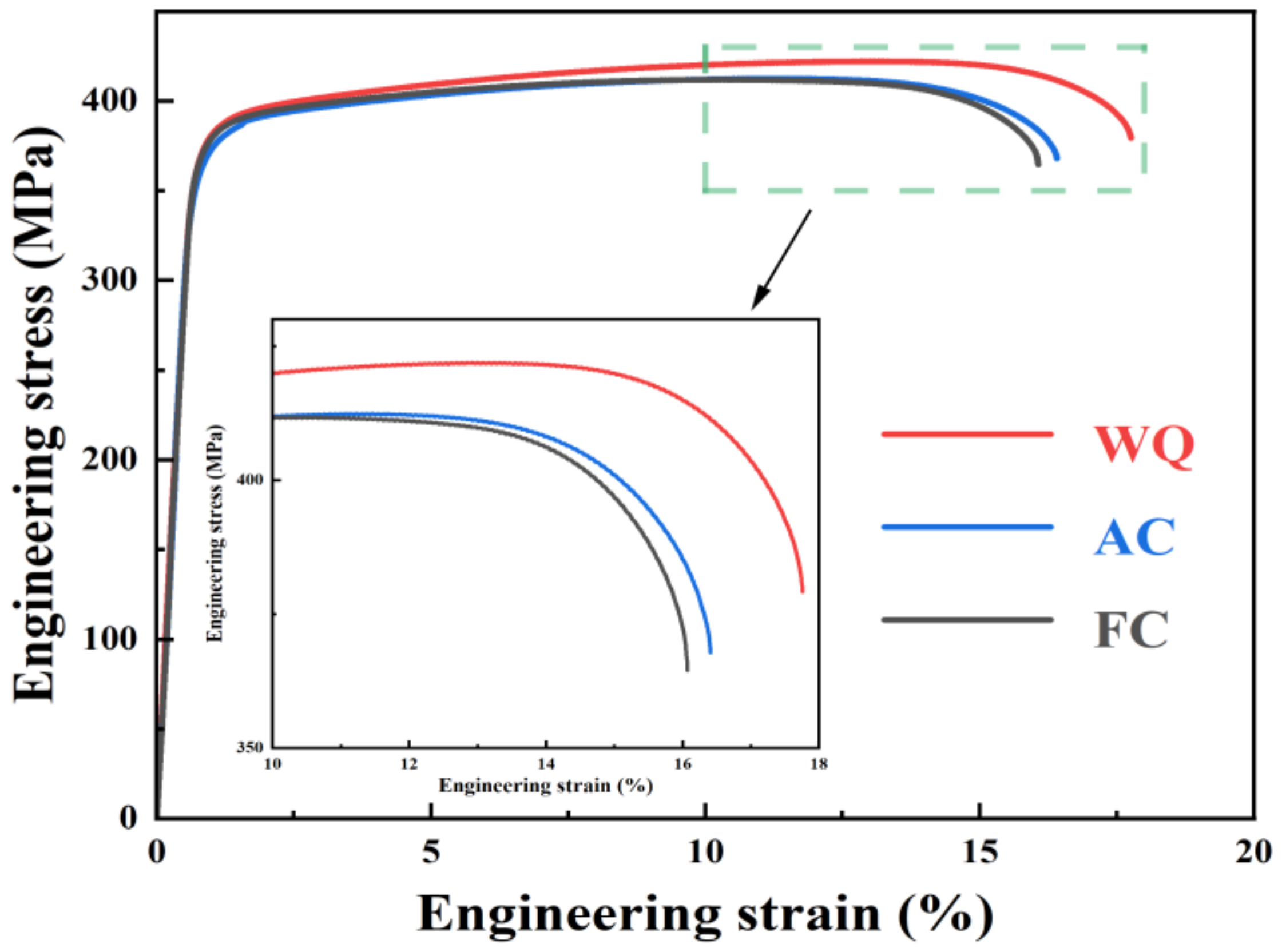
3.2. Mechanical Properties
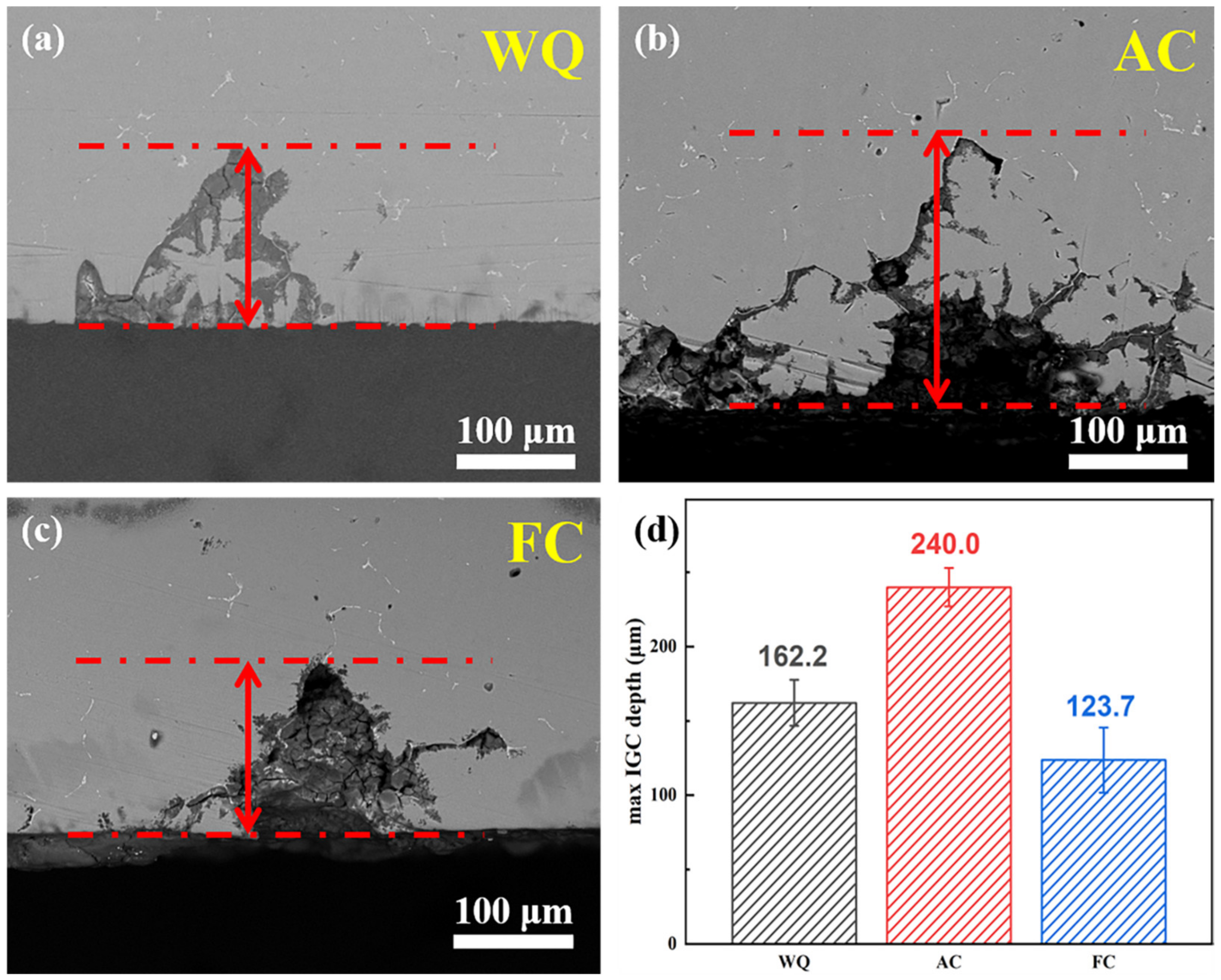
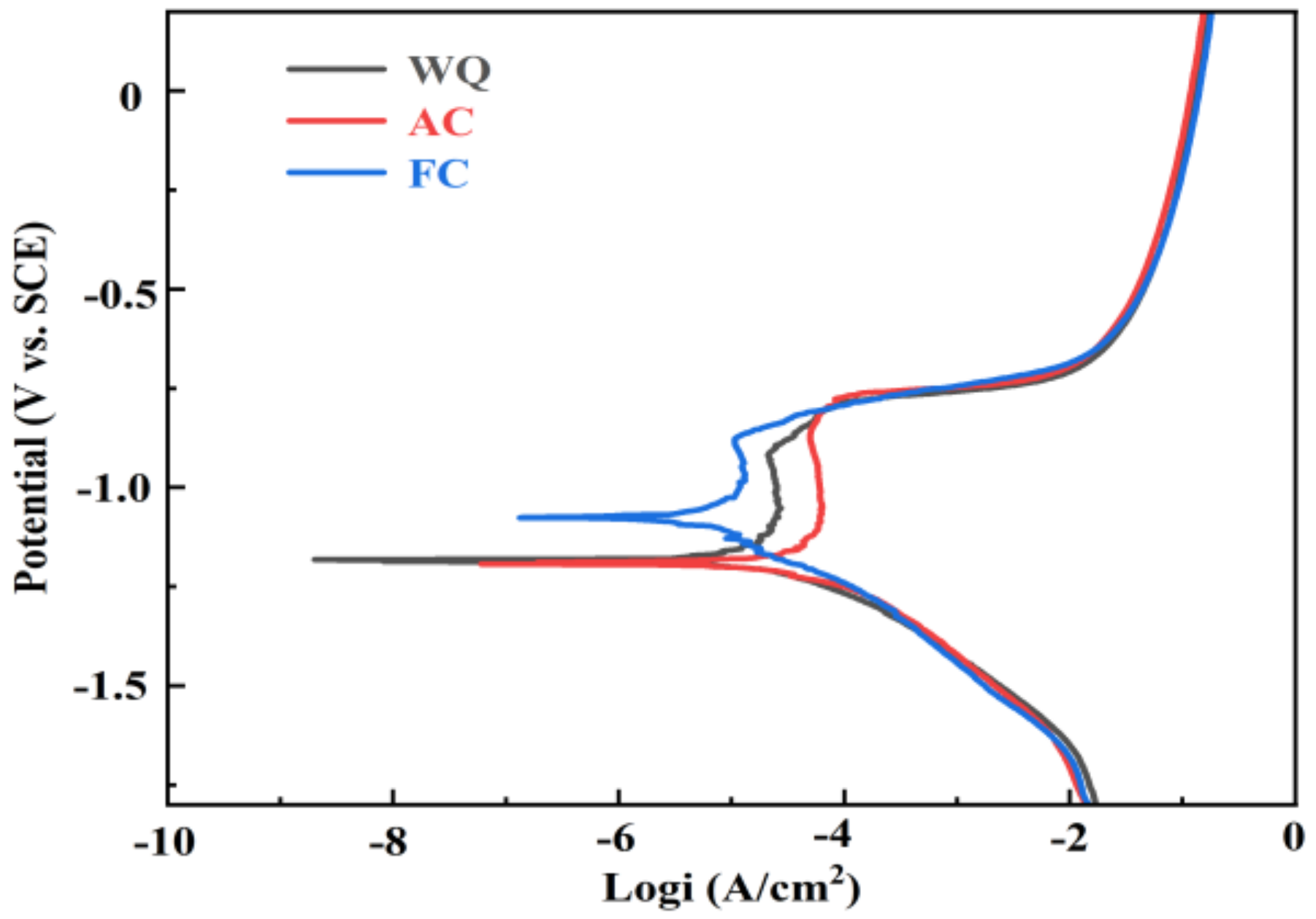
3.3. IGC and Electrochemical Tests
4. Discussion
4.1. Effect of Retrogression with Different Cooling Ways on Mechanical Properties
4.2. Effect of Retrogression with Different Cooling Ways on Corrosion Behavior
5. Conclusions
- (1)
- Samples after RRA treatment with water-quenching retrogression exhibit better mechanical properties than those of air-cooled and furnace-cooled samples. The ultimate tensile strength, yield strength, and elongation of samples after RRA treatment with water-quenching retrogression are 419.2 MPa, 370.2 MPa, and 15.9, respectively. The coarsening of precipitates within grains counts for the more decreased strength of air-cooled samples than that of water-quenching samples. The similar strength of air-cooled and furnace-cooled samples is related to the size of T’/η’ precipitates within grains as well as the proportion of T’ and η’ phases;
- (2)
- The corrosion resistance of water-quenching and furnace-cooled samples is better than that of air-cooled samples, manifested as a reduction in IGC depth and corrosion current density. The minimum IGC depth (123.7 μm) and the lowest corrosion current density (0.712 × 10−5 A/cm2) indicate the best corrosion resistance of furnace-cooled samples. The difference in IGC resistance depends on the continuity of GBPs;
- (3)
- Taking into account the mechanical properties, corrosion resistance, and time cost, water quenching appears as a suitable retrogression way for T’/η’ strengthened Al-Zn-Mg-Cu alloys.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trink, B.; Weißensteiner, I.; Uggowitzer, P.J.; Strobel, K.; Hofer-Roblyek, A.; Pogatscher, S. Processing and microstructure-property relations of Al-Mg-Si-Fe crossover alloys. Acta Mater. 2023, 247, 119160. [Google Scholar] [CrossRef]
- Zarif, M.; Spacil, I.; Pabel, T.; Schumacher, P.; Li, J.H. Effect of Ca and P on the size and morphology of eutectic Mg2Si in high-purity Al-Mg-Si alloys. Metals 2023, 13, 784. [Google Scholar] [CrossRef]
- Spacil, I.; Gehringer, D.; Holec, D.; Albu, M.; Li, J.H. Elucidating effects of Eu and P on solidification and precipitation of Al-7Si-0.3Mg based alloys refined by Ta and TiB2. J. Alloys Compd. 2024, 978, 173343. [Google Scholar] [CrossRef]
- Morais, P.J.; Gomes, B.; Santos, P.; Gomes, M.; Gradinger, R.; Schnall, M.; Bozorgi, S.; Klein, T.; Fleischhacker, D.; Warczok, P.; et al. Characterisation of a high-performance Al–Zn–Mg–Cu Alloy designed for wire arc additive manufacturing. Materials 2020, 13, 1610. [Google Scholar] [CrossRef] [PubMed]
- Raabe, D.; Ponge, D.; Uggowitzer, P.J.; Roscher, M.; Paolantonio, M.; Liu, C.L.; Antrekowitsch, H.; Kozeschnik, E.; Seidmann, D.; Gault, B.; et al. Making sustainable aluminum by recycling scrap: The science of “dirty” alloys. Prog. Mater. Sci. 2022, 128, 100947. [Google Scholar]
- Dumont, D.; Deschamps, A.; Brechet, Y. On the relationship between microstructure, strength and toughness in AA7050 aluminum alloy. Mater. Sci. Eng. A 2003, 356, 326–336. [Google Scholar] [CrossRef]
- Marlaud, T.; Deschamps, A.; Bley, F.; Lefebvre, W.; Baroux, B. Influence of alloy composition and heat treatment on precipitate composition in Al–Zn–Mg–Cu alloys. Acta Mater. 2010, 58, 248–260. [Google Scholar] [CrossRef]
- Knight, S.P.; Birbilis, N.; Muddle, B.C.; Trueman, A.R.; Lynch, S.P. Correlations between intergranular stress corrosion cracking, grain-boundary microchemistry, and grain-boundary electrochemistry for Al–Zn–Mg–Cu alloys. Corros. Sci. 2010, 52, 4073–4080. [Google Scholar] [CrossRef]
- Willenshofer, P.D.; Tunes, M.A.; Kainz, C.; Renk, O.; Kremmer, T.M.; Gneiger, S.; Uggowitzer, P.J.; Pogatscher, S. Precipitation behaviour in AlMgZnCuAg crossover alloy with coarse and ultrafine grains. Mater. Res. Lett. 2023, 11, 1063–1072. [Google Scholar] [CrossRef]
- Mukherjee, D.; Roy, H.; Chandrakanth, B.; Mandal, N.; Samanta, S.K.; Mukherjee, M. Enhancing properties of Al-Zn-Mg-Cu alloy through microalloying and heat treatment. Mater. Chem. Phys. 2024, 314, 128881. [Google Scholar] [CrossRef]
- Senkov, O.N.; Shagiev, M.R.; Senkova, S.V.; Miracle, D.B. Precipitation of Al3(Sc, Zr) particles in an Al-Zn-Mg-Cu-Sc-Zr alloy during conventional solution heat treatment and its effect on tensile properties. Acta Mater. 2008, 56, 3723–3738. [Google Scholar] [CrossRef]
- Takaya, M.; Ichitani, K.; Minoda, T. Effects of Sc and Zr addition on the mechanical properties of 7000 series aluminum alloys. Mater. Trans. 2023, 64, 443–447. [Google Scholar] [CrossRef]
- Wang, Y.C.; Wu, X.D.; Cao, L.F.; Tong, X.; Couper, M.J.; Liu, Q. Effect of trace Er on the microstructure and properties of Al-Zn-Mg-Cu-Zr alloys during heat treatments. Mater. Sci. Eng. A 2020, 792, 139807. [Google Scholar] [CrossRef]
- Glavatskikh, M.V.; Barkov, R.Y.; Gorlov, L.E.; Khomutov, M.G.; Pozdniakov, A.V. Novel cast and wrought Al-3Zn-3Mg-3Cu-Zr-Y(Er) alloys with improved heat resistance. Metals 2023, 13, 909. [Google Scholar] [CrossRef]
- Ghosh, A.; Ghosh, M.; Kalsar, R. Influence of homogenization time on evolution of eutectic phases, dispersoid behavior and crystallographic texture for Al-Zn-Mg-Cu-Ag alloy. J. Alloys Compd. 2019, 802, 276–289. [Google Scholar] [CrossRef]
- Pan, T.A.; Lian, Y.J.; Tzeng, Y.C.; Bor, H.Y.; Lee, S.L. Effects of trace Ag and heat treatment on the mechanical properties and corrosion resistance of Al-Zn-Mg-Cu alloys. JOM 2022, 74, 3877–3886. [Google Scholar] [CrossRef]
- Afify, N.; Gaber, A.F.; Abbady, G. Fine scale precipitates in Al-Mg-Zn alloys after various aging temperatures. Mater. Sci. Appl. 2011, 2, 427–434. [Google Scholar] [CrossRef]
- Stemper, L.; Tunes, M.A.; Oberhauser, P.; Uggowitzer, P.J.; Pogatscher, S. Age-hardening response of AlMgZn alloys with Cu and Ag additions. Acta Mater. 2020, 195, 541–554. [Google Scholar] [CrossRef]
- Stemper, L.; Tunes, M.A.; Dumitraschkewitz, P.; Mendez-Martin, F.; Tosone, R.; Marchand, D.; Curtin, W.A.; Uggowitzer, P.J.; Pogatscher, S. Giant hardening response in AlMgZn(Cu) alloys. Acta Mater. 2021, 206, 116617. [Google Scholar] [CrossRef]
- Stemper, L.; Tunes, M.A.; Tosone, R.; Uggowitzer, P.J.; Pogatscher, S. On the potential of aluminum crossover alloys. Prog. Mater. Sci. 2022, 124, 100873. [Google Scholar] [CrossRef]
- Shen, G.W.; Chen, X.L.; Yan, J.; Fan, L.Y.; Yang, Z.; Zhang, J.; Guan, R.G. A review of progress in the study of Al-Mg-Zn(-Cu) wrought alloys. Metals 2023, 13, 345. [Google Scholar] [CrossRef]
- Meng, C.Y.; Zhang, D.; Cui, H.; Zhuang, L.Z.; Zhang, J.S. Mechanical properties, intergranular corrosion behavior and microstructure of Zn modified Al-Mg alloys. J. Alloys Compd. 2014, 617, 925–932. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, D.; Wang, X.; Ma, Q.B.; Zhuang, L.Z.; Zhang, J.S. Effects of Cu addition on the precipitation hardening response and intergranular corrosion of Al-5.2Mg-2.0Zn (wt.%) alloy. Mater. Charact. 2016, 122, 177–182. [Google Scholar] [CrossRef]
- Pan, Y.L.; Zhang, D.; Liu, H.R.; Zhuang, L.Z.; Zhang, J.S. Precipitation hardening and intergranular corrosion behavior of novel Al-Mg-Zn(-Cu) alloys. J. Alloys Compd. 2021, 853, 157199. [Google Scholar] [CrossRef]
- Zou, Y.; Wu, X.D.; Tang, S.B.; Zhu, Q.Q.; Song, H.; Guo, M.X.; Cao, L.F. Investigation on microstructure and mechanical properties of Al-Zn-Mg-Cu alloys with various Zn/Mg ratios. J. Mater. Sci. Technol. 2021, 85, 106–117. [Google Scholar] [CrossRef]
- Zou, Y.; Wu, X.D.; Tang, S.B.; Zhao, K.; Cao, L.F. Tailoring phase fractions of T’ and η’ phases in dual-phase strengthened Al-Zn-Mg-Cu alloy via ageing treatment. Trans. Nonferrous Met. Soc. China 2022, 32, 3182–3196. [Google Scholar] [CrossRef]
- Kumar, A.; Chaudhari, G.P.; Nath, S.K. Correlation of microstructure with corrosion performance in high zinc 7068 aluminum alloy aged using different T6 conditions. Mater. Charact. 2022, 191, 112133. [Google Scholar] [CrossRef]
- Teichmann, K.; Marioara, C.D.; Andersen, S.J.; Marthinsen, K. The effect of preaging deformation on the precipitation behavior of an Al-Mg-Si alloy. Metall. Mater. Trans. A 2012, 43, 4006–4014. [Google Scholar] [CrossRef]
- Ghiaasiaan, S.R.; Amirkhiz, B.S.; Shankar, S. High-resolution electron microscopy and kinetic studies of precipitation hardening reactions in cast Al-5.8Zn-2.2Mg-2.5Cu. J. Mater. Eng. Perform. 2019, 28, 4630–4646. [Google Scholar] [CrossRef]
- Rometsch, P.A.; Zhang, Y.; Knight, S. Heat treatment of 7xxx series aluminium alloys—Some recent developments. Trans. Nonferrous Met. Soc. China 2014, 24, 2003–2017. [Google Scholar] [CrossRef]
- Azarniya, A.; Taheri, A.K.; Taheri, K.K. Recent advances in ageing of 7xxx series aluminum alloys: A physical metallurgy perspective. J. Alloys Compd. 2019, 781, 945–983. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, D.M.; Li, W.J. The effect of multistage ageing on microstructure and mechanical properties of 7050 alloy. J. Alloys Compd. 2016, 671, 408–418. [Google Scholar] [CrossRef]
- Xiong, H.Q.; Zhou, Y.X.; Kong, C.; Yu, H.L. Effect of retrogression treatment with different heating rates on microstructure, strength and corrosion behaviors of 7050 alloy. Mater. Charact. 2022, 186, 111819. [Google Scholar] [CrossRef]
- Li, J.F.; Birbilis, N.; Li, C.X.; Jia, Z.Q.; Cai, B.; Zheng, Z.Q. Influence of retrogression temperature and time on the mechanical properties and exfoliation corrosion behavior of aluminium alloy AA7150. Mater. Charact. 2009, 60, 1334–1341. [Google Scholar] [CrossRef]
- Ranganatha, R.; Kumar, V.A.; Nandi, V.S.; Bhat, R.R.; Muralidhara, B.K. Multi-stage heat treatment of aluminum alloy AA7049. Trans. Nonferrous Met. Soc. China 2013, 23, 1570–1575. [Google Scholar] [CrossRef]
- Oliveira, A.F.; de Barros, M.C.; Cardoso, K.R.; Travessa, D.N. The effect of RRA on the strength and SCC resistance on AA7050 and AA7150 aluminium alloys. Mater. Sci. Eng. A 2004, 379, 321–326. [Google Scholar] [CrossRef]
- Angappan, M.; Sampath, V.; Ashok, B.; Deepkumar, V.P. Retrogression and re-aging treatment on short transverse tensile properties of 7010 aluminium alloy extrusions. Mater. Des. 2011, 32, 4050–4053. [Google Scholar] [CrossRef]
- GB/T 7998-2005; Test Method for Intergranular Corrosion of Aluminium Alloy. Standardization Administration of the People’s Republic of China: Beijing, China, 2005.
- Berg, L.K.; Gjønnes, J.; Hansen, V.; Li, X.Z.; Knutson Wedel, M.; Waterloo, G.; Schryvers, D.; Wallenberg, L.R. GP-zones in Al-Zn-Mg alloys and their role in artificial aging. Acta Mater. 2001, 49, 3443–3451. [Google Scholar] [CrossRef]
- Yang, W.C.; Ji, S.X.; Wang, M.P.; Li, Z. Precipitation behaviour of Al-Zn-Mg-Cu alloy and diffraction analysis from η′ precipitates in four variants. J. Alloys Compd. 2014, 610, 623–629. [Google Scholar] [CrossRef]
- Bergman, G.; Waugh, J.L.T.; Pauling, L. The crystal structure of the metallic phase Mg32(Al, Zn)49. Acta Crystallogr. 1957, 10, 254–259. [Google Scholar] [CrossRef]
- Bigot, A.; Auger, P.; Chambreland, S.; Blavette, D.; Reeves, A. Atomic scale imaging and analysis of T′ precipitates in Al-Mg-Zn alloys. Microsc. Microanal. Microstruct. 1997, 8, 103–113. [Google Scholar] [CrossRef]
- Shen, G.W.; Chen, X.L.; Yan, J.; Fan, L.Y.; Yang, Z.; Zhang, J.; Guan, R.G. Effects of heat treatment processes on the mechanical properties, microstructure evolution, and strengthening mechanisms of Al-Mg-Zn-Cu alloy. J. Mater. Res. Technol. 2023, 27, 5380–5388. [Google Scholar] [CrossRef]
- Liu, J.Z.; Chen, J.H.; Yuan, D.W.; Wu, C.L.; Zhu, J.; Cheng, Z.Y. Fine precipitation scenarios of AlZnMg(Cu) alloys revealed by advanced atomic-resolution electron microscopy study Part I: Structure determination of the precipitates in AlZnMg(Cu) alloys. Mater. Charact. 2015, 99, 277–286. [Google Scholar] [CrossRef]
- Chung, T.F.; Yang, Y.L.; Huang, B.M.; Shi, Z.S.; Lin, J.G.; Ohmura, T.; Yang, J.R. Transmission electron microscopy investigation of separated nucleation and in-situ nucleation in AA7050 aluminium alloy. Acta Mater. 2018, 149, 377–387. [Google Scholar] [CrossRef]
- Chung, T.F.; Yang, Y.L.; Shiojiri, M.; Hsiao, C.N.; Li, W.C.; Tsao, C.S.; Shi, Z.S.; Lin, J.G.; Yang, J.R. An atomic scale structural investigation of nanometre-sized η precipitates in the 7050 aluminium alloy. Acta Mater. 2019, 174, 351–368. [Google Scholar] [CrossRef]
- Yang, X.B.; Chen, J.H.; Liu, J.Z.; Qin, F.; Xie, J.; Wu, C.L. A high-strength AlZnMg alloy hardened by the T-phase precipitates. J. Alloys Compd. 2014, 610, 69–73. [Google Scholar] [CrossRef]
- Zou, Y.; Wu, X.D.; Tang, S.B.; Zhu, Q.Q.; Song, H.; Cao, L.F. Co-precipitation of T′ and η′ phase in Al-Zn-Mg-Cu alloys. Mater. Charact. 2020, 169, 110610. [Google Scholar] [CrossRef]
- Han, H.M.; Jiang, L.T.; Chao, Z.L.; Xue, W.; Zhu, M.; Han, B.Z.; Zhang, R.W.; Du, S.Q.; Luo, T.; Mei, Y. Revealing corrosion behavior of B4C/pure Al composite at different interfaces between B4C particles and Al matrix in NaCl electrolyte. J. Mater. Res. Technol. 2023, 27, 7213–7227. [Google Scholar] [CrossRef]
- Viana, F.; Pinto, A.M.P.; Santos, H.M.C.; Lopes, A.B. Retrogression and re-ageing of 7075 aluminium alloy: Microstructural characterization. J. Mater. Process. Technol. 1999, 92–93, 54–59. [Google Scholar] [CrossRef]
- Feng, C.; Liu, Z.Y.; Ning, A.L.; Liu, Y.B.; Zeng, S.M. Retrogression and re-aging treatment of Al-9.99%Zn-1.72%Cu-2.5%Mg-0.13%Zr aluminum alloy. Trans. Nonferrous Met. Soc. China 2006, 16, 1163–1170. [Google Scholar] [CrossRef]
- Hou, L.G.; Yu, H.; Wang, Y.W.; You, L.; He, Z.B.; Wu, C.M.; Eskin, D.G.; Katgerman, L.; Zhuang, L.Z.; Zhang, J.S. Tailoring precipitation/properties and related mechanisms for a high-strength aluminum alloy plate via low-temperature retrogression and re-aging processes. J. Mater. Sci. Technol. 2022, 120, 15–35. [Google Scholar] [CrossRef]
- Huang, Z.W.; Loretto, M.H.; White, J. Influence of lithium additions on precipitation and age hardening of 7075 alloy. Mater. Sci. Technol. 1993, 9, 967–980. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, D.; Zhuang, L.Z.; Zhang, J.S. Improved age-hardening response and altered precipitation behavior of Al-5.2Mg-0.45Cu-2.0Zn (wt%) alloy with pre-aging treatment. J. Alloys Compd. 2017, 691, 40–43. [Google Scholar] [CrossRef]
- Tang, H.P.; Wang, Q.D.; Luo, C.; Lei, C.; Liu, T.W.; Li, Z.Y.; Jiang, H.Y.; Ding, W.J.; Fang, J.; Zhang, J.W. Effects of aging treatment on the precipitation behaviors and mechanical properties of Al-5.0Mg-3.0Zn-1.0Cu cast alloys. J. Alloys Compd. 2020, 842, 155707. [Google Scholar] [CrossRef]
- Ma, Q.B.; Zhang, D.; Zhuang, L.Z.; Zhang, J.S. Intergranular corrosion resistance of Zn modified 5××× series Al alloy during retrogression and re-aging treatment. Mater. Charact. 2018, 144, 264–273. [Google Scholar] [CrossRef]
- Fang, H.C.; Chao, H.; Chen, K.H. Effect of recrystallization on intergranular fracture and corrosion of Al-Zn-Mg-Cu-Zr alloy. J. Alloys Compd. 2015, 622, 166–173. [Google Scholar] [CrossRef]
- Marlaud, T.; Deschamps, A.; Bley, F.; Lefebvre, W.; Baroux, B. Evolution of precipitate microstructures during the retrogression and re-ageing heat treatment of an Al-Zn-Mg-Cu alloy. Acta Mater. 2010, 58, 4814–4826. [Google Scholar] [CrossRef]
- Zhong, H.L.; Li, S.C.; Wu, J.L.; Deng, H.L.; Chen, J.Q.; Yan, N.; Chen, Z.X.; Duan, L.B. Effects of retrogression and re-aging treatment on precipitation behavior, mechanical and corrosion properties of a Zr+Er modified Al-Zn-Mg-Cu alloy. Mater. Charact. 2022, 183, 111617. [Google Scholar] [CrossRef]
- Han, N.M.; Feng, D.; Zhang, X.M.; Liu, S.D. The precipitates and properties evolution behaviors of AlZnMgCu alloy during the retrogression process with slow heating. J. Mater. Res. Technol. 2023, 26, 3544–3557. [Google Scholar] [CrossRef]



| Zn | Zn/Mg | Cu | Mn | Zr | Ti | Fe | Si | Al |
|---|---|---|---|---|---|---|---|---|
| 5.5~6.0 | 2.0~2.5 | ≤0.5 | ≤0.2 | 0.2 | 0.1 | ≤0.5 | ≤0.5 | Balance |
| Sample | Pre-Aging | Retrogression | Cooling Environment | Re-Aging |
|---|---|---|---|---|
| WQ | 120 °C/24 h | 190 °C/30 min | water | 120 °C/24 h |
| AC | air | |||
| FC | furnace |
| Sample | Plate-Like Precipitates | Round-Shape Precipitates | ||
|---|---|---|---|---|
| Diameter/nm | Thickness/nm | Aspect Ratio | Diameter/nm | |
| WQ | 19.4 ± 7.4 | 7.9 ± 2.9 | 2.6 ± 0.9 | 7.9 ± 1.8 |
| AC | 14.3 ± 2.1 | 4.8 ± 0.7 | 3.0 ± 0.6 | 9.2 ± 1.4 |
| FC | 126.8 ± 19.5 | 32.6 ± 6.7 | 4.0 ± 0.9 | 61.9 ± 8.9 |
| Sample | UTS (MPa) | YS (MPa) | EL (%) |
|---|---|---|---|
| WQ | 419.2 ± 3.0 | 370.2 ± 2.7 | 15.9 ± 1.7 |
| AC | 408.3 ± 6.5 | 356.2 ± 1.9 | 15.0 ± 1.2 |
| FC | 409.8 ± 1.7 | 366.5 ± 5.6 | 15.0 ± 1.0 |
| Ecorr (V) | Icorr (10−5 A/cm2) | |
|---|---|---|
| WQ | −1.001 ± 0.171 | 0.833 ± 0.394 |
| AC | −1.165 ± 0.038 | 2.363 ± 0.805 |
| FC | −1.128 ± 0.030 | 0.712 ± 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Shen, G.; Han, B.; Li, D.; Xu, Z.; Chao, Z.; Chen, G.; Jiang, L. Effect of Retrogression with Different Cooling Ways on the Microstructure and Properties of T’/η’ Strengthened Al-Zn-Mg-Cu Alloys. Materials 2024, 17, 1746. https://doi.org/10.3390/ma17081746
Zhang J, Shen G, Han B, Li D, Xu Z, Chao Z, Chen G, Jiang L. Effect of Retrogression with Different Cooling Ways on the Microstructure and Properties of T’/η’ Strengthened Al-Zn-Mg-Cu Alloys. Materials. 2024; 17(8):1746. https://doi.org/10.3390/ma17081746
Chicago/Turabian StyleZhang, Jianlei, Guwei Shen, Bingzhuo Han, Dayong Li, Zhenyu Xu, Zhenlong Chao, Guoqin Chen, and Longtao Jiang. 2024. "Effect of Retrogression with Different Cooling Ways on the Microstructure and Properties of T’/η’ Strengthened Al-Zn-Mg-Cu Alloys" Materials 17, no. 8: 1746. https://doi.org/10.3390/ma17081746
APA StyleZhang, J., Shen, G., Han, B., Li, D., Xu, Z., Chao, Z., Chen, G., & Jiang, L. (2024). Effect of Retrogression with Different Cooling Ways on the Microstructure and Properties of T’/η’ Strengthened Al-Zn-Mg-Cu Alloys. Materials, 17(8), 1746. https://doi.org/10.3390/ma17081746







