Textile-Based Adsorption Sensor via Mixed Solvent Dyeing with Aggregation-Induced Emission Dyes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
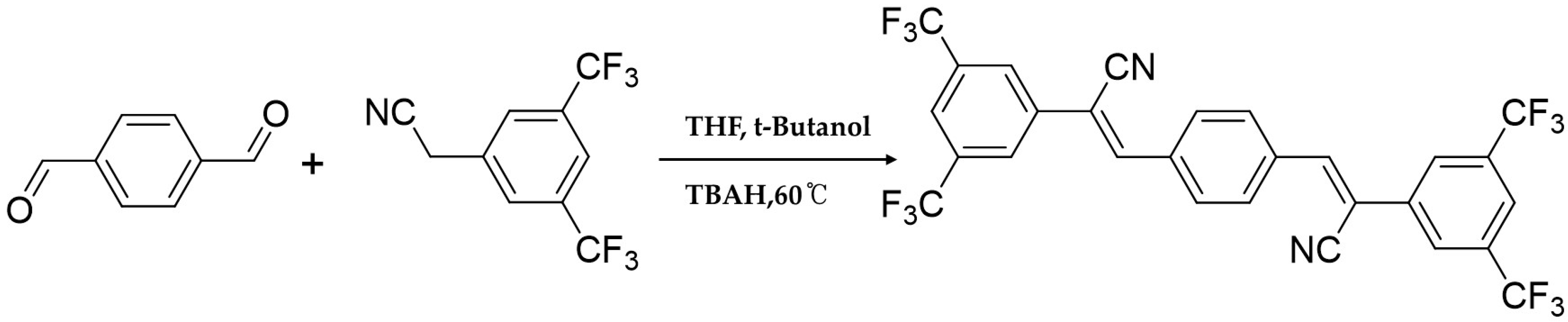
2.2. Synthesis of AIE Dye
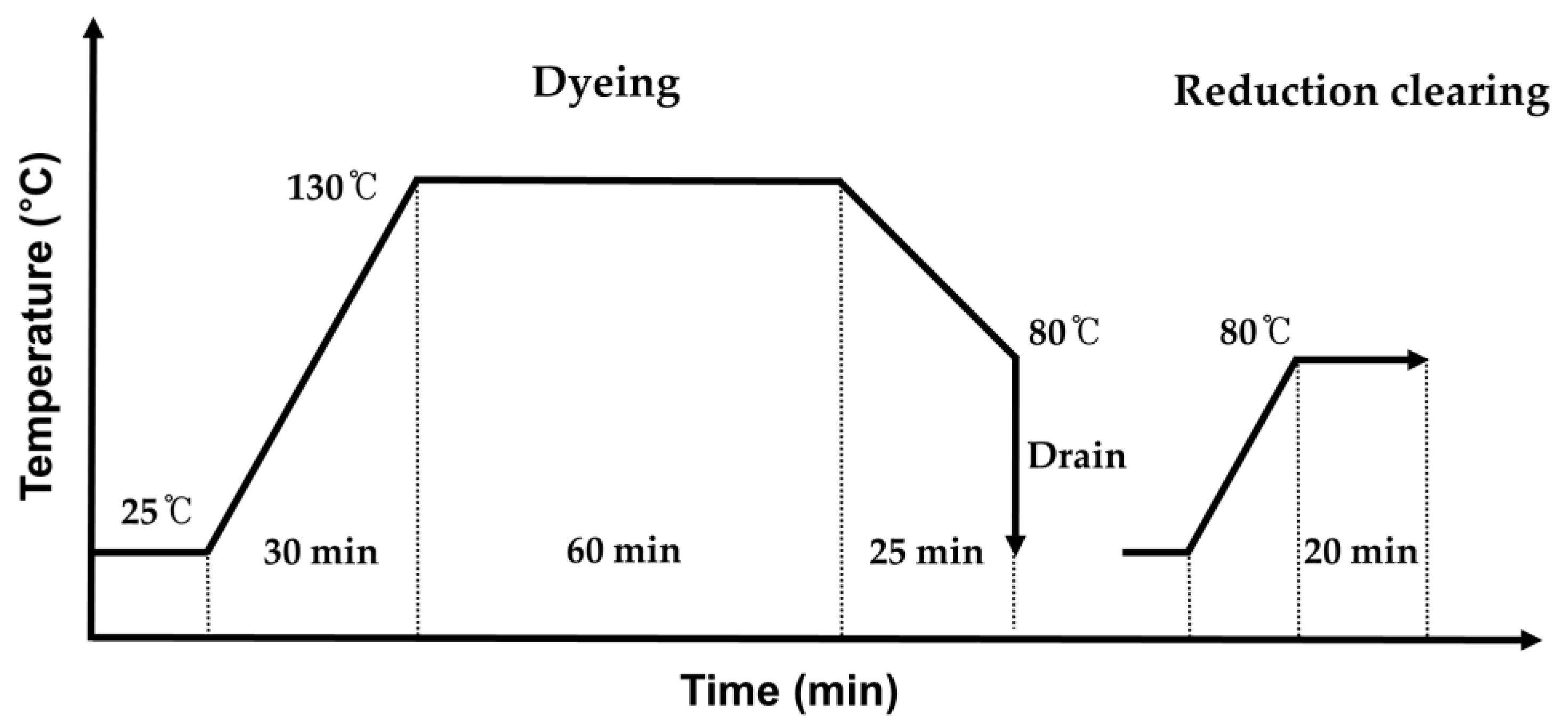
2.3. Preparation of AIE-Dyed Fabric
2.4. Characterizations
3. Results and Discussion
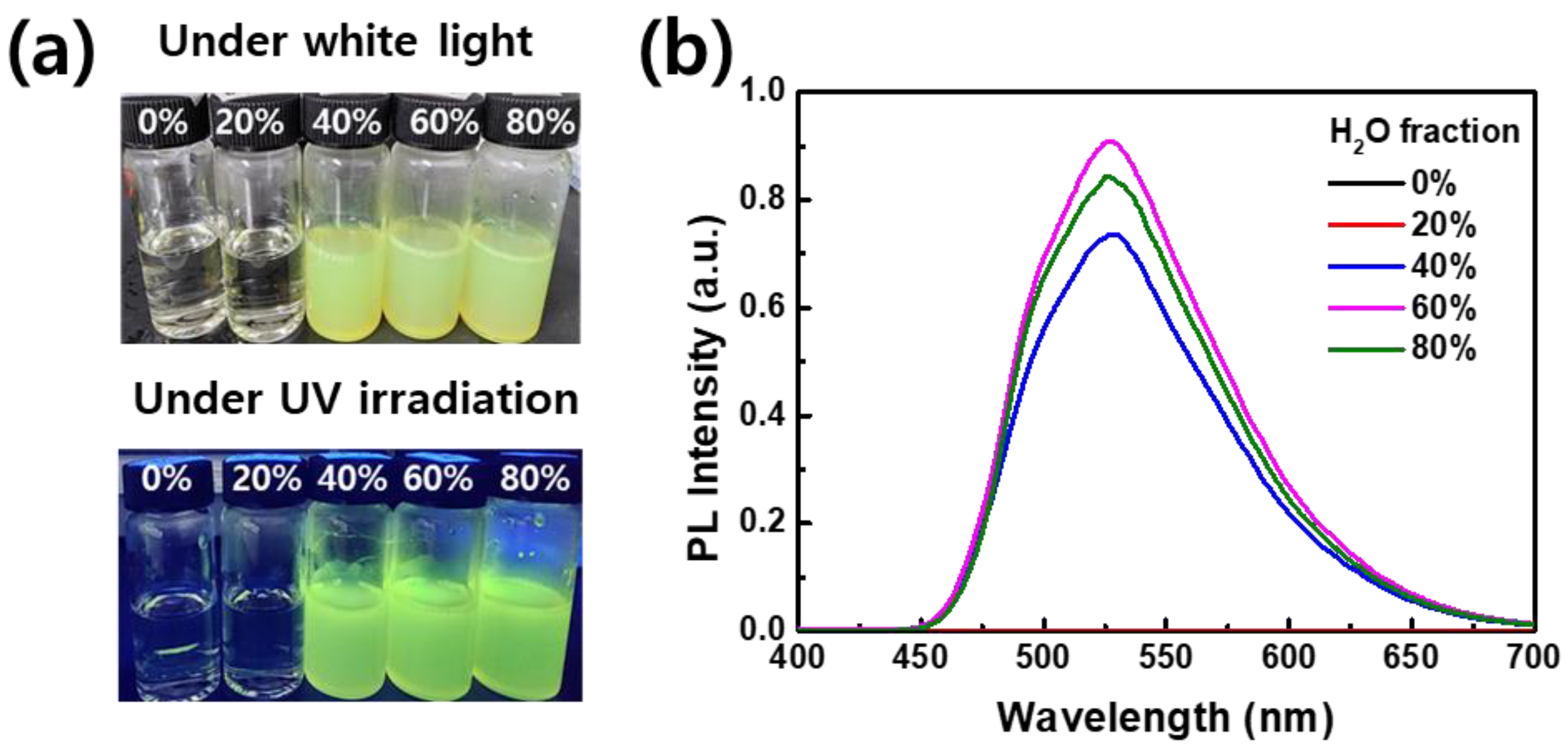
3.1. Fluorescence Properties of AIE Dye in Solution
3.2. Fluorescence Properties of Fabrics Dyed with AIE Dye
3.3. Microstructural Analysis of AIE Dye and AIE-Dyed Fabrics
3.4. Sensing Properties of AIE-Dyed Fabrics
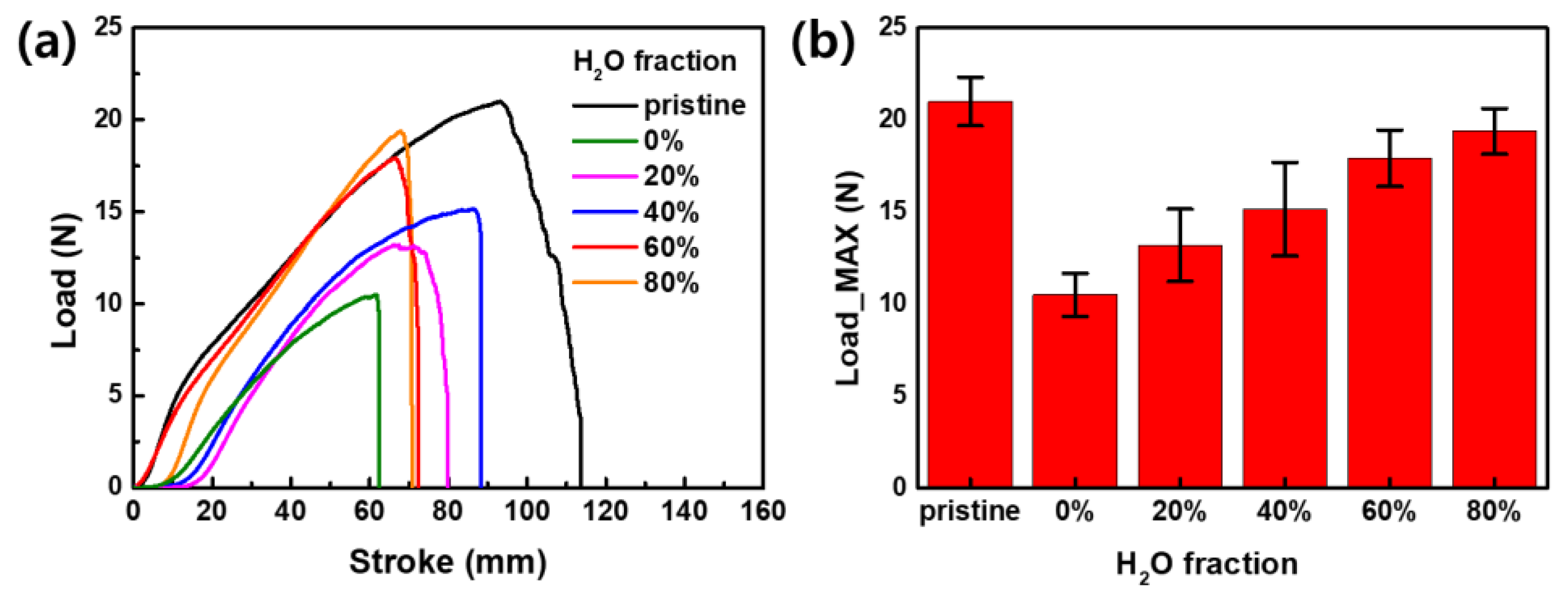
3.5. Mechanical Properties of AIE-Dyed Fabrics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Q.Q.; Tang, Y.H.; Hu, W.P.; Li, Z. Fluorescence of Nonaromatic Organic Systems and Room Temperature Phosphorescence of Organic Luminogens: The Intrinsic Principle and Recent Progress. Small 2018, 14, 1801560. [Google Scholar] [CrossRef]
- Yang, J.; Fang, M.M.; Li, Z. Organic luminescent materials: The concentration on aggregates from aggregation-induced emission. Aggregate 2020, 1, 6–18. [Google Scholar] [CrossRef]
- Xu, L.J.; Jiang, X.P.; Liang, K.; Gao, M.; Kong, B. Frontier luminous strategy of functional silica nanohybrids in sensing and bioimaging: From ACQ to AIE. Aggregate 2022, 3, e121. [Google Scholar] [CrossRef]
- Xu, S.D.; Duan, Y.K.; Liu, B. Precise Molecular Design for High-Performance Luminogens with Aggregation-Induced Emission. Adv. Mater. 2020, 32, 1903530. [Google Scholar] [CrossRef] [PubMed]
- Voskuhl, J.; Giese, M. Mesogens with aggregation-induced emission properties: Materials with a bright future. Aggregate 2022, 3, e124. [Google Scholar] [CrossRef]
- Jiang, Q.; Yuan, H.L.; Dong, K.; Lin, J.H.; Wu, L.W.; Tang, Y.H. Continuous and scalable manufacture of aggregation induced emission luminogen fibers for anti-counterfeiting and hazardous gas detecting smart textiles. Mater. Des. 2022, 205, 109761. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, H.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: New Vistas at the Aggregate Level. Angew. Chem.-Int. Ed. 2020, 59, 9888–9907. [Google Scholar] [CrossRef] [PubMed]
- Long, X.T.; Wu, J.Y.; Yang, S.R.; Deng, Z.Q.; Zheng, Y.S.; Zhang, W.Y.; Jiang, X.F.; Lu, F.S.; Li, M.D.; Xu, L. Discovery of and insights into one-photon and two-photon excited ACQ-to-AIE conversion positional isomerization. J. Mater. Chem. C 2021, 9, 11679–11689. [Google Scholar] [CrossRef]
- Würthner, F. Aggregation-Induced Emission (AIE): A Historical Perspective. Angew. Chem.-Int. Ed. 2020, 59, 14192–14196. [Google Scholar] [CrossRef]
- Chatterjee, A.; Chatterjee, J.; Sappati, S.; Sheikh, T.; Umesh, R.M.; Ambhore, M.D.; Lahiri, M.; Hazra, P. Emergence of Aggregation Induced Emission (AIE), Room-Temperature Phosphorescence (RTP), and Multistimuli Response from a Single Organic Luminogen by Directed Structural Modification. J. Phys. Chem. B 2021, 125, 12832–12846. [Google Scholar] [CrossRef]
- Guo, M.C.; Song, H.; Li, K.; Ma, M.C.; Liu, Y.; Fu, Q.; He, Z.G. A new approach to developing diagnostics and therapeutics: Aggregation-induced emission-based fluorescence turn-on. Med. Res. Rev. 2020, 40, 27–53. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhang, T.F.; Bai, H.T.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Recent Advances in Aggregation-Induced Emission Materials and Their Biomedical and Healthcare Applications. Adv. Healthc. Mater. 2021, 10, 2101055. [Google Scholar] [CrossRef]
- Kachwal, V.; Tan, J.C. Stimuli-Responsive Electrospun Fluorescent Fibers Augmented with Aggregation-Induced Emission (AIE) for Smart Applications. Adv. Sci. 2023, 10, 2204848. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.X.; Li, H.X.; Song, N.; Wang, D.; Tang, B.Z. Supramolecular materials based on AIE luminogens (AIEgens): Construction and applications. Chem. Soc. Rev. 2020, 49, 1144–1172. [Google Scholar] [CrossRef]
- Yu, W.T.; Yu, X.X.; Qiu, Z.D.; Xu, C.J.; Gao, M.Y.; Zheng, J.J.; Zhang, J.Y.; Wang, G.; Cheng, Y.H.; Zhu, M.F. 1+1>2: Fiber Synergy in Aggregation-Induced Emission. Chem.—A Eur. J. 2022, 28, e202201664. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Jin, B.Q.; Wang, Y.W.; Deng, B.Y.; Wang, D.; Tang, B.Z. As Fiber Meets with AIE: Opening a Wonderland for Smart Flexible Materials. Adv. Mater. 2023, 35, 2210085. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.F.; Sun, J.M.; Chen, G.Y.; Zhou, L.Y.; Wang, J.; Gu, X.G.; Wan, J.M.; Pu, X.; Tang, B.Z.; Wang, Z.L. Stretchable multi-luminescent fibers with AIEgens. J. Mater. Chem. C 2019, 7, 10769–10776. [Google Scholar] [CrossRef]
- Zhao, S.K.; Sun, J.M.; Qin, Z.; Li, Y.F.; Yu, H.; Wang, G.; Gu, X.G.; Pan, K. Janus-Structural AIE Nanofiber with White Light Emission and Stimuli-Response. Small 2022, 18, 2201117. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, Z.L.; Chen, M.H.; Liu, Y.; Wang, T.; Li, X.H. Fluorescent fibrous mats assembled with self-propagating probes for visual sensing of hydrogen peroxide and choline. Analyst 2019, 144, 5624–5636. [Google Scholar] [CrossRef]
- Liu, F.K.; Cui, M.X.; Ma, J.J.; Zou, G.; Zhang, Q.J. An optical fiber taper fluorescent probe for detection of nitro-explosives based on tetraphenylethylene with aggregation-induced emission. Opt. Fiber Technol. 2017, 36, 98–104. [Google Scholar] [CrossRef]
- Xu, S.Q.; Lin, Y.; Huang, J.; Li, Z.; Xu, X.J.; Zhang, L.N. Construction of high strength hollow fibers by self-assembly of a stiff polysaccharide with short branches in water. J. Mater. Chem. A 2013, 1, 4198–4206. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, T.; Wu, Q.; Liu, Y.; Chen, Z.J.; Li, X.H. Fluorescent Strips of Electrospun Fibers for Ratiometric Sensing of Serum Heparin and Urine Trypsin. ACS Appl. Mater. Interfaces 2017, 9, 3400–3410. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.F.; Jiang, Q.J.; Qiu, Y.Q.; Chen, X.C.; Fan, B.; Wang, Y.; Wang, D.N. Hollow-Core-Photonic-Crystal-Fiber-Based Miniaturized Sensor for the Detection of Aggregation-Induced-Emission Molecules. Anal. Chem. 2019, 91, 780–784. [Google Scholar] [CrossRef]
- Jiang, Y.M.; Cheng, Y.H.; Liu, S.J.; Zhang, H.K.; Zheng, X.Y.; Chen, M.; Khorloo, M.; Xiang, H.X.; Tang, B.Z.; Zhu, M.F. Solid-state intramolecular motions in continuous fibers driven by ambient humidity for fluorescent sensors. Natl. Sci. Rev. 2021, 8, nwaa135. [Google Scholar] [CrossRef]
- Kachwal, V.; Mollick, S.; Tan, J.C. Tailored Broad-Spectrum Emission in Hybrid Aggregation Induced Emission (AIE)-MOFs: Boosting White Light Efficiency in Electrospun Janus Microfibers. Adv. Funct. Mater. 2023, 34, 2308062. [Google Scholar] [CrossRef]
- Payne, J.; Jones, M.D. The Chemical Recycling of Polyesters for a Circular Plastics Economy: Challenges and Emerging Opportunities. ChemSusChem 2021, 14, 4041–4070. [Google Scholar] [CrossRef]
- Satti, S.M.; Shah, A.A. Polyester-based biodegradable plastics: An approach towards sustainable development. Lett. Appl. Microbiol. 2020, 70, 413–430. [Google Scholar] [CrossRef]
- Telli, A.; Özdil, N. Effect of Recycled PET Fibers on the Performance Properties of Knitted Fabrics. J. Eng. Fibers Fabr. 2015, 10, 47–60. [Google Scholar] [CrossRef]
- An, B.K.; Gihm, S.H.; Chung, J.W.; Park, C.R.; Kwon, S.K.; Park, S.Y. Color-Tuned Highly Fluorescent Organic Nan-owires/Nanofabrics: Easy Massive Fabrication and Molecular Structural Origin. J. Am. Chem. Soc. 2009, 131, 3950–3957. [Google Scholar] [CrossRef]
- Park, S.K.; Kim, J.H.; Yoon, S.J.; Kwon, O.K.; An, B.K.; Park, S.Y. High-Performance-Type Organic Transistor with a Solution-Processed and Exfoliation-Transferred Two-Dimensional Crystalline Layered Film. Chem. Mater. 2012, 24, 3263–3268. [Google Scholar] [CrossRef]
- An, B.K.; Kwon, S.K.; Jung, S.D.; Park, S.Y. Enhanced emission and its switching in fluorescent organic nanoparticles. J. Am. Chem. Soc. 2002, 124, 14410–14415. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Prlj, A.; Lin, K.H.; Hollas, D.; Corminboeuf, C. Mechanisms of fluorescence quenching in prototypical aggregation-induced emission systems: Excited state dynamics with TD-DFTB. Phys. Chem. Chem. Phys. 2019, 21, 9026–9035. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chang, J.Y. Reversible Thermochromic Polymer Film Embedded with Fluorescent Organogel Nanofibers. Langmuir 2014, 30, 13673–13679. [Google Scholar] [CrossRef]
- Chung, J.W.; Yoon, S.J.; Lim, S.J.; An, B.K.; Park, S.Y. Dual-Mode Switching in Highly Fluorescent Organogels: Binary Logic Gates with Optical/Thermal Inputs. Angew. Chem.-Int. Ed. 2009, 48, 7030–7034. [Google Scholar] [CrossRef]
- Dou, C.D.; Han, L.A.; Zhao, S.S.; Zhang, H.Y.; Wang, Y. Multi-Stimuli-Responsive Fluorescence Switching of a Donor-Acceptor π-Conjugated Compound. J. Phys. Chem. Lett. 2011, 2, 666–670. [Google Scholar] [CrossRef]
- Xue, P.C.; Yao, B.Q.; Wang, P.P.; Gong, P.; Zhang, Z.Q.; Lu, R. Strong Fluorescent Smart Organogel as a Dual Sensing Material for Volatile Acid and Organic Amine Vapors. Chem.—A Eur. J. 2015, 21, 17508–17515. [Google Scholar] [CrossRef]
- An, B.K.; Gierschner, J.; Park, S.Y. π-Conjugated Cyanostilbene Derivatives: A Unique Self-Assembly Motif for Molecular Nanostructures with Enhanced Emission and Transport. Acc. Chem. Res. 2012, 45, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.L.; Jiao, Z.; Guo, Z.N.; Yang, J.L.; Alam, P.; Liu, Y.; Men, Y.F.; Zhang, P.F.; Feng, H.T.; Yao, S.H.; et al. Development of Reaction-Based AIE Handy Pen for Visual Detection of Toxic Vapors. Acs Mater. Lett. 2021, 3, 249–254. [Google Scholar] [CrossRef]
- Bard, S.; Tran, T.; Schönl, F.; Rosenfeldt, S.; Demleitner, M.; Ruckdäschel, H.; Retsch, M.; Altstädt, V. Relationship between the tensile modulus and the thermal conductivity perpendicular and in the fiber direction of PAN-based carbon fibers. Carbon Lett. 2023, 34, 361–369. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Guan, X.Y.; Fang, Q.R. Recent advances in AIEgen-based crystalline porous materials for chemical sensing. Aggregate 2021, 2, e34. [Google Scholar] [CrossRef]
- La, D.D.; Bhosale, S.V.; Jones, L.A.; Bhosale, S.V. Tetraphenylethylene-Based AIE-Active Probes for Sensing Applications. Acs Appl. Mater. Interfaces 2018, 10, 12189–12216. [Google Scholar] [CrossRef] [PubMed]
- Linson, N.; Jacob, J.; Kuriakose, S. Synthesis and characterization of iron-doped carbon nanoparticles encapsulated within functionally modified hyperbranched polyglycerol and study of their chromium (VI) ion sensing and antimicrobial applications. Carbon Lett. 2023, 33, 1847–1862. [Google Scholar] [CrossRef]
- Horak, E.; Hranjec, M.; Vianello, R.; Steinberg, I.M. Reversible pH switchable aggregation-induced emission of self assembled benzimidazole-based acrylonitrile dye in aqueous solution. Dye Pigment. 2017, 142, 108–115. [Google Scholar] [CrossRef]
- Sasikumar, K.; Rajamanikandan, R.; Ju, H. Inner filter effect-based highly sensitive quantification of 4-nitrophenol by strong fluorescent N, S co-doped carbon dots. Carbon Lett. 2024, 34, 851–863. [Google Scholar] [CrossRef]
- Baatout, K.; Saad, F.; Baffoun, A.; Mahltig, B.; Kreher, D.; Jaballah, N.; Majdoub, M. Luminescent cotton fibers coated with fluorescein dye for anti-counterfeiting applications. Mater. Chem. Phys. 2019, 234, 304–310. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.G.; Oh, B.M.; Kim, J.H.; Lee, J.U. Textile-Based Adsorption Sensor via Mixed Solvent Dyeing with Aggregation-Induced Emission Dyes. Materials 2024, 17, 1745. https://doi.org/10.3390/ma17081745
Hong SG, Oh BM, Kim JH, Lee JU. Textile-Based Adsorption Sensor via Mixed Solvent Dyeing with Aggregation-Induced Emission Dyes. Materials. 2024; 17(8):1745. https://doi.org/10.3390/ma17081745
Chicago/Turabian StyleHong, Seong Gyun, Byeong M. Oh, Jong H. Kim, and Jea Uk Lee. 2024. "Textile-Based Adsorption Sensor via Mixed Solvent Dyeing with Aggregation-Induced Emission Dyes" Materials 17, no. 8: 1745. https://doi.org/10.3390/ma17081745
APA StyleHong, S. G., Oh, B. M., Kim, J. H., & Lee, J. U. (2024). Textile-Based Adsorption Sensor via Mixed Solvent Dyeing with Aggregation-Induced Emission Dyes. Materials, 17(8), 1745. https://doi.org/10.3390/ma17081745





