Preparation and Performance of Ceramic Tiles with Steel Slag and Waste Clay Bricks
Abstract
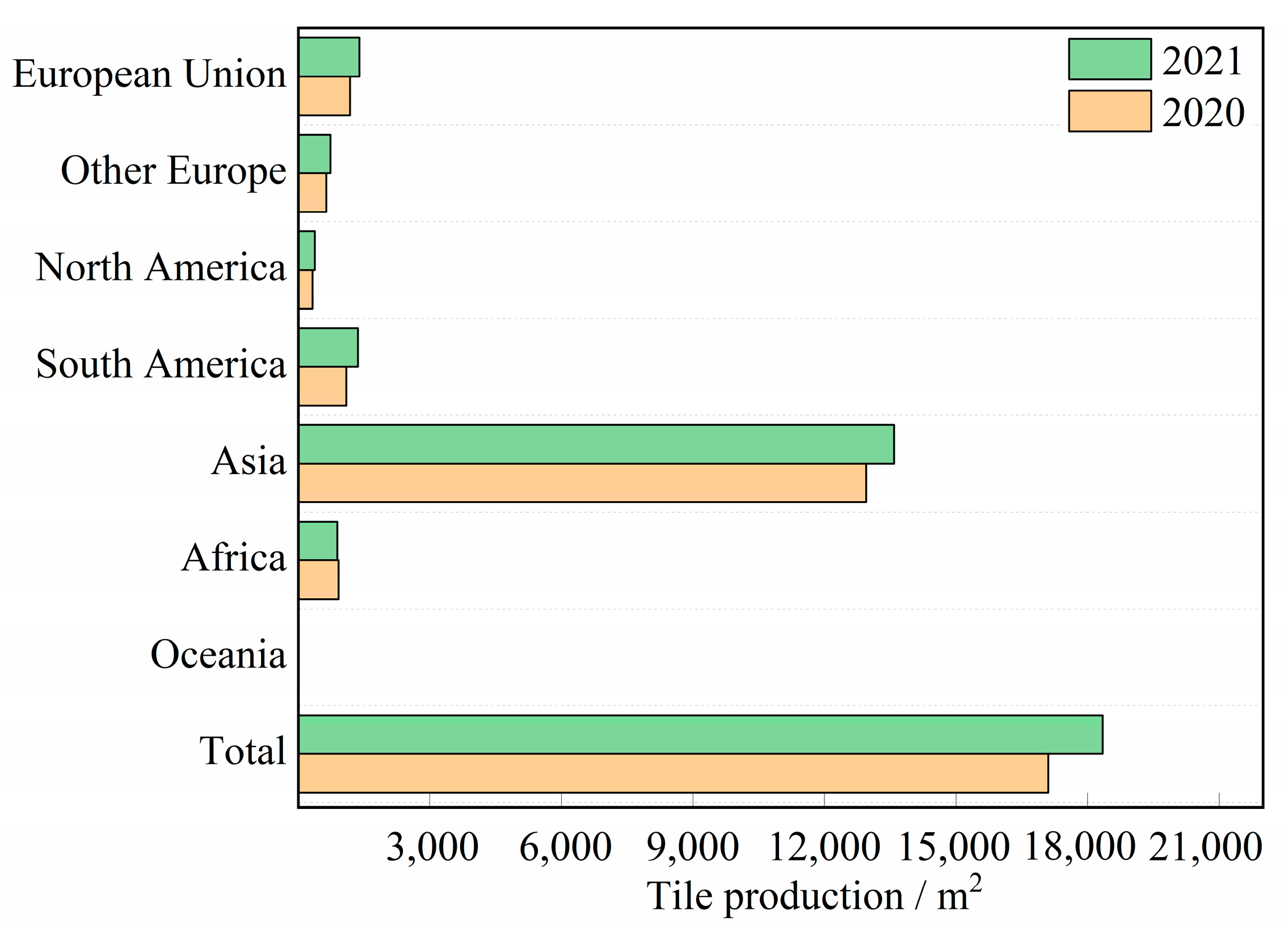
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Orthogonal Experimental Design
2.3. Single Factor Experiment of Process Parameters
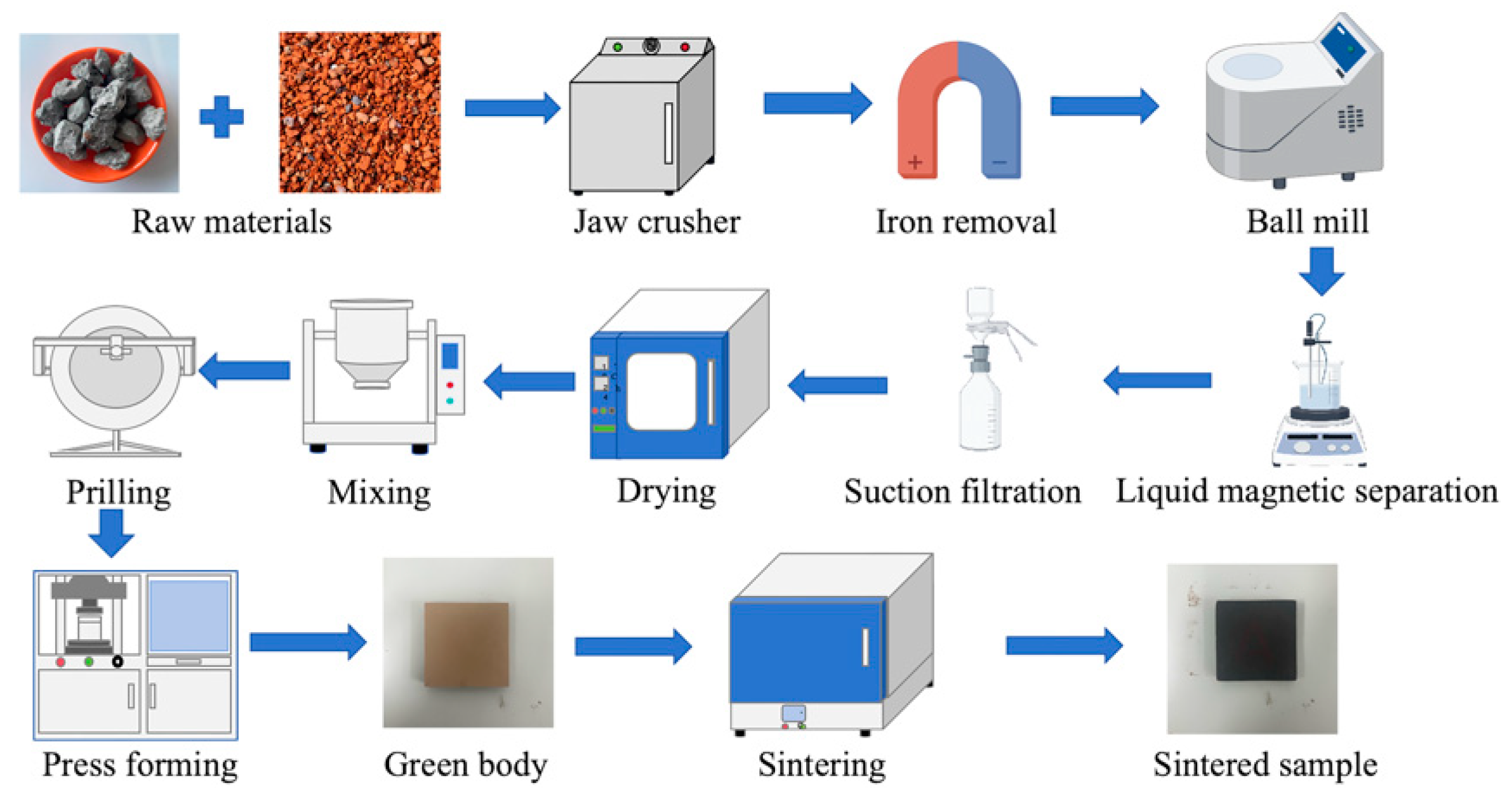
2.4. Raw Material Pretreatment
2.5. Sample Preparation
2.6. Sample Performance Measurements
2.6.1. Macroscopic Performance Measurements
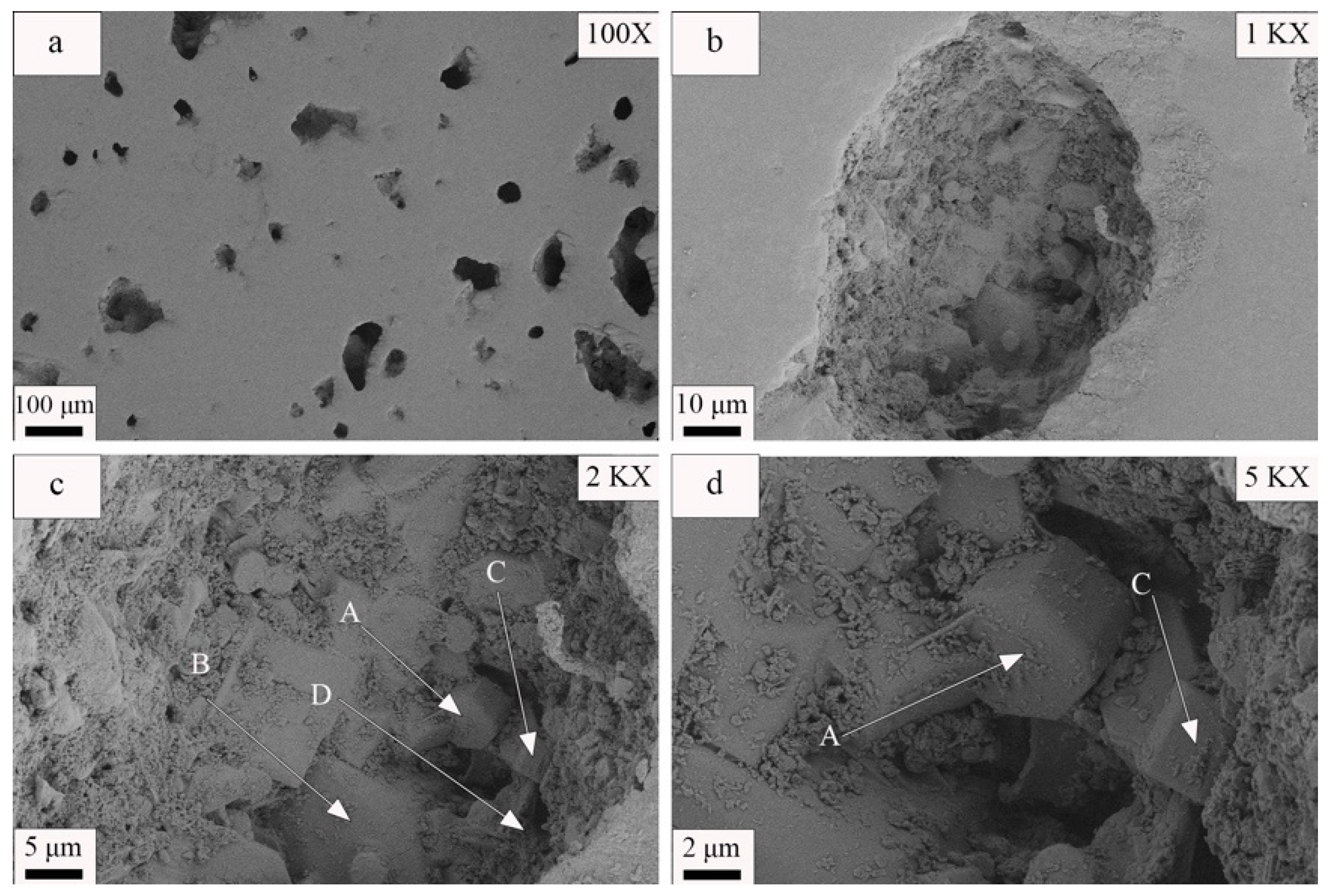
2.6.2. Microscopic Performance Measurements
3. Results and Discussion
3.1. Analysis of the Orthogonal Experiment Results
3.2. Single-Factor Experiment Results and Analysis
3.2.1. Effects of the Molding Pressure
3.2.2. Effects of the Sintering Temperature
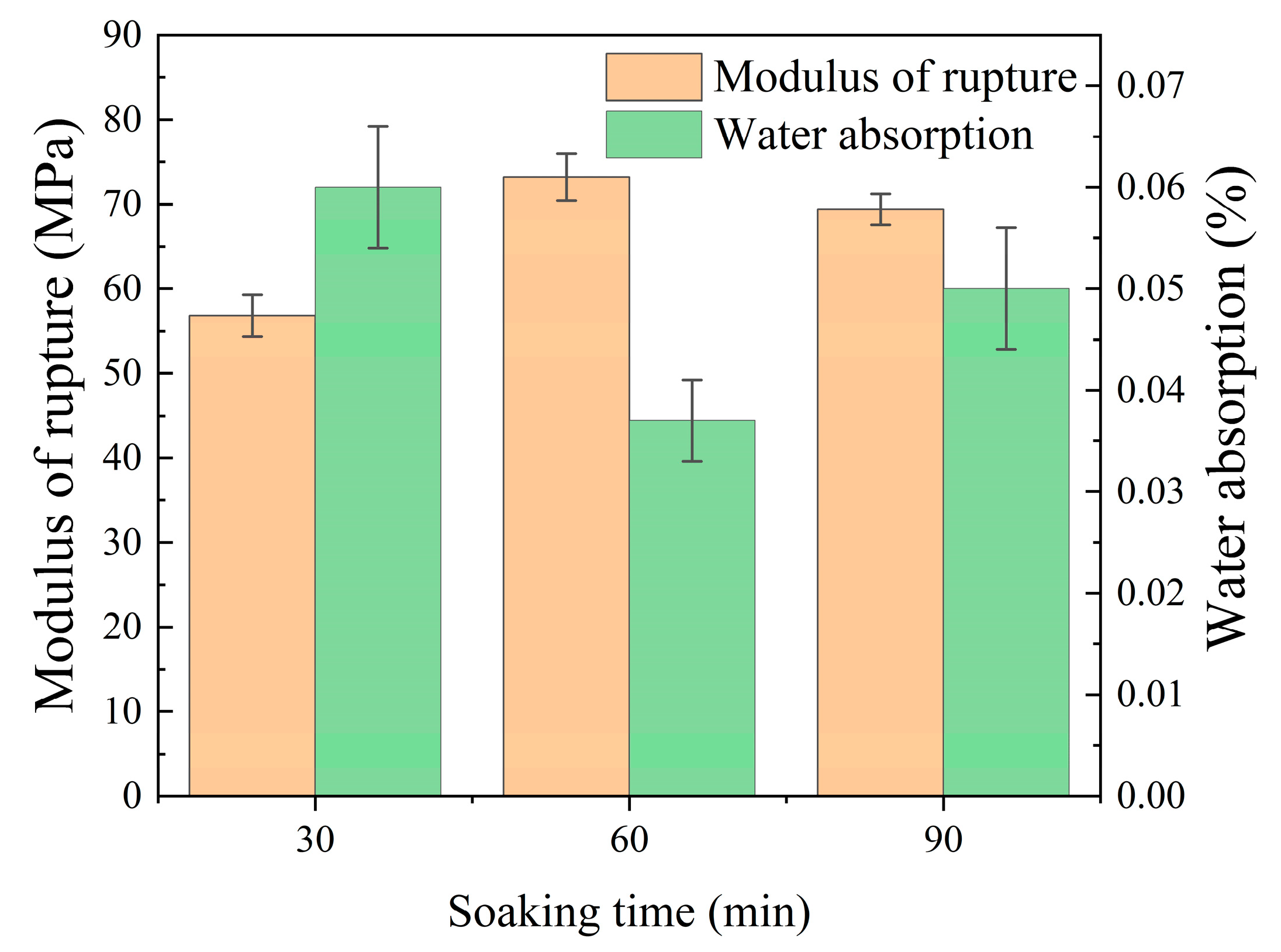
3.2.3. Effects of the Soaking Time
3.2.4. Economic Analysis
4. Conclusions
- (1)
- Using an L9 (33) orthogonal experiment and range analyses, an optimal formula was obtained for a ceramic tile material that used akermanite as the primary crystalline phase. The formula consisted of 45% steel slag, 35% waste clay brick powder, and 25% talc; thus, 76% of its composition was solid waste. The material was sintered at a temperature of 1190 °C. The ceramic tile samples produced using this formula exhibited a modulus of rupture of 63.68 MPa and a water absorption rate of 0.08%; thus, they satisfied the requirements of the ISO 10545-3 standard and ISO 10545-4 standard (which specified that the modulus of rupture should be greater than or equal to 35 MPa, while the water absorption rate should be less than or equal to 0.5%).
- (2)
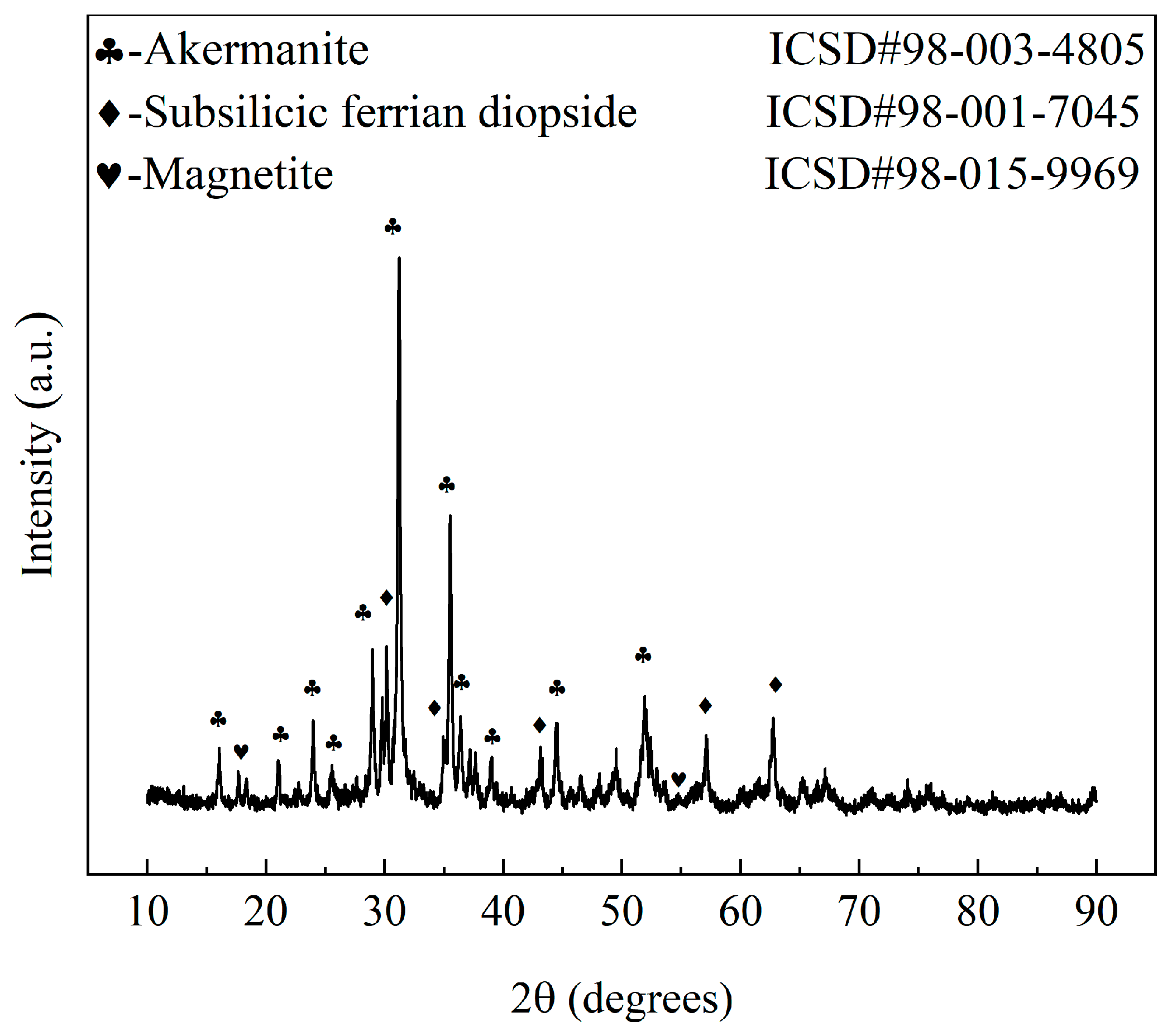
- XRD patterns obtained for the samples demonstrated that akermanite, diopside, and magnetite were the primary crystalline phases present in the samples fabricated with this particular composition.
- (3)
- The ideal process parameters to use when producing ceramic samples with this composition were determined from a single-factor experiment. They included a molding pressure of 25 MPa, a sintering temperature of 1190 °C, and a soaking time of 60 min. The ceramic tiles, produced with the optimal composition and process parameters, had a modulus of rupture of 73.2 MPa and a water absorption rate of only 0.04%, surpassing the requirements of the ISO 10545 standard.
- (4)
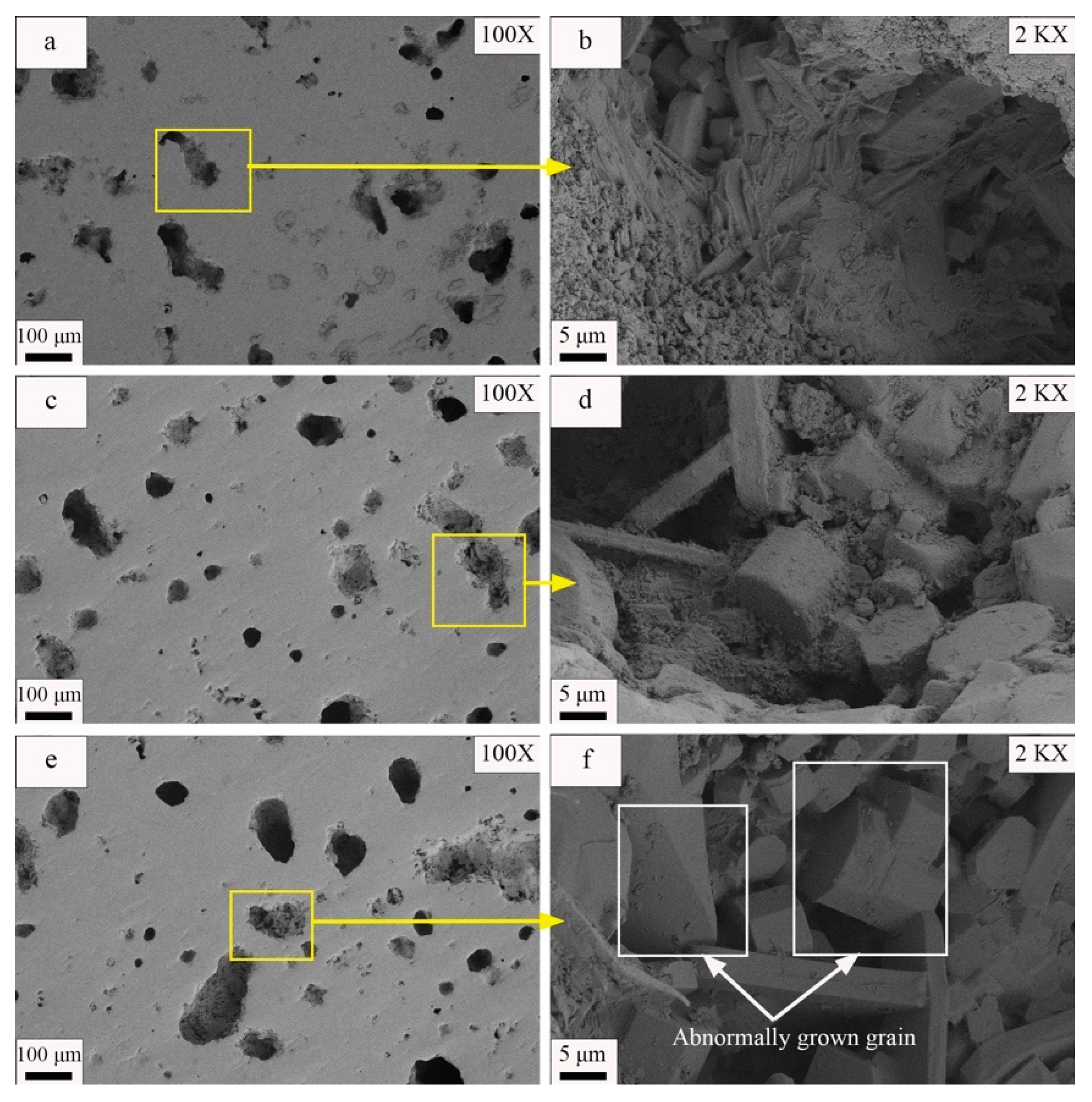
- The appropriate increase in molding pressure promoted particle rearrangement, reduced the required melt volume, improved ceramic density, enhanced crystal development, and optimized material performance. Excessive molding pressure led to hardening defects in the green body, resulting in decreased density, incomplete crystal development, and deteriorated material performance. In this experiment, the fracture modulus at the optimal molding pressure was 69.21 MPa, with a water absorption rate of 0.05.
- (5)
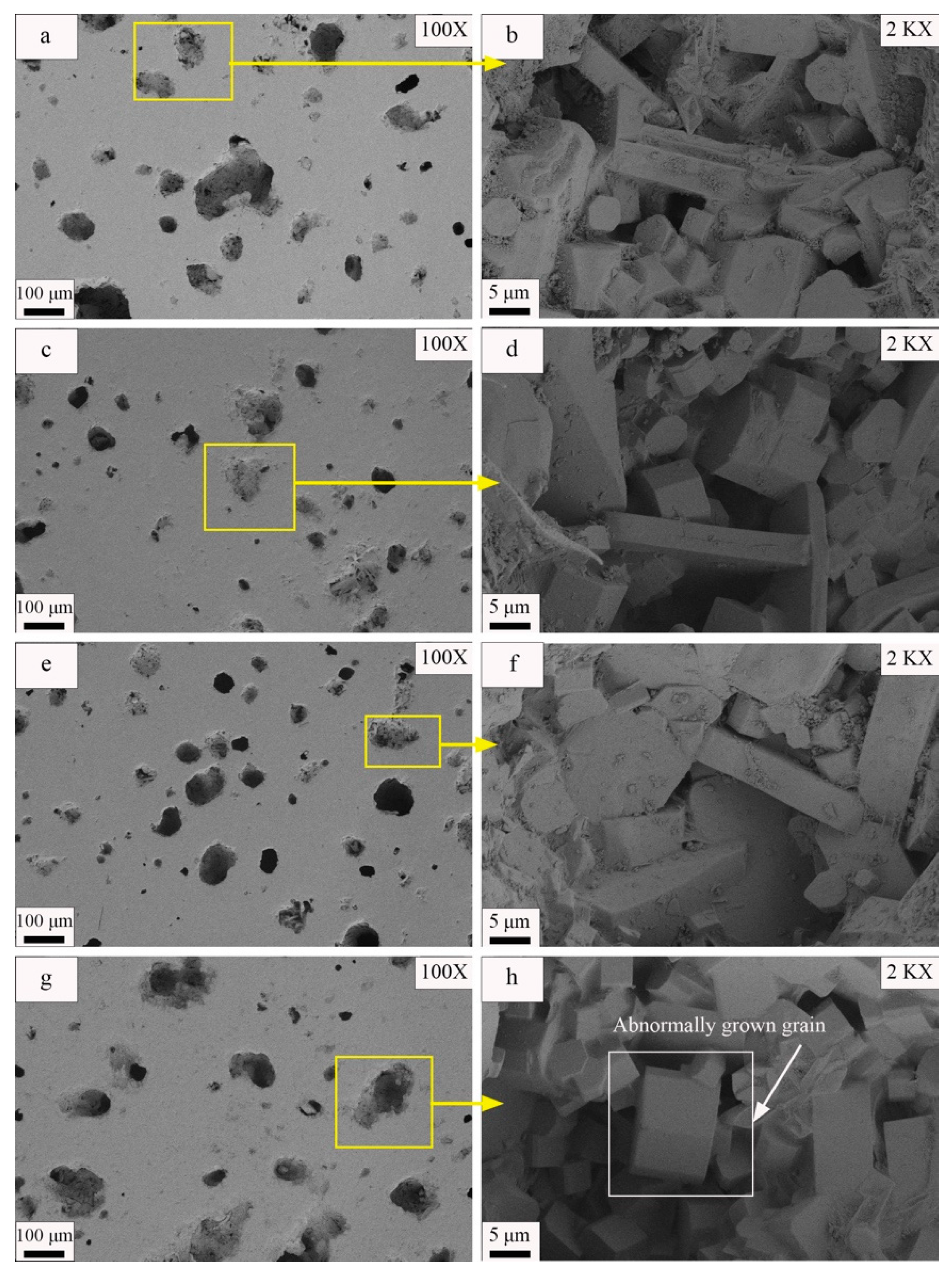
- Appropriate increases in sintering temperature facilitated the formation of a liquid phase, promoting ceramic densification, crystal growth, and optimization of material properties. However, excessive sintering temperature could lead to an excess of liquid phase formation, causing ceramic deformation, abnormal grain growth, and deterioration of material properties. In this experiment, the optimal sintering temperature was determined as 1190 °C, resulting in a modulus of rupture of 69.21 MPa and a water absorption of 0.05.
- (6)
- Appropriate increases in soaking time promoted volume expansion, densification, crystal development, and optimization of ceramic material performance at high temperatures. Excessive soaking time led to non-uniform growth of secondary grains in ceramics, resulting in the deterioration of material performance. In this experiment, the fracture modulus obtained at the optimal sintering temperature of 1190 °C was 73.2 MPa, with a water absorption of 0.04%.
- (7)
- The ceramic tiles exhibited a water absorption rate of only 0.04% and possessed a fracture modulus of 73.2 MPa, surpassing that of commercially available samples. Furthermore, the cost per metric ton of raw materials was only CNY 226, representing a remarkable reduction by 78% compared with traditional materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baraldi, L. World production and consumption of ceramic tiles. Ceram. World Rev. 2022, 32, 36–50. [Google Scholar]
- Zhang, X.; Jiao, Y.; Yu, L.H.; Liu, L.L.; Wang, X.D.; Zhang, Y.Y. Effect of Sewage Sludge Addition on Microstructure and Mechanical Properties of Kaolin-Sewage Sludge Ceramic Bricks. Coatings 2022, 12, 944. [Google Scholar] [CrossRef]
- Zhang, J.; Li, R.; Nie, D.P.; Zhang, Y. Preparation of building ceramic bricks using waste residue obtained by mutual treatment of electrolytic manganese residue and red mud. Ceram. Int. 2023, 49, 22492–22505. [Google Scholar] [CrossRef]
- Guo, Y.C.; Wang, C.; Li, S.; He, Y.Y.; Liu, H. Preparation of permeable ceramic bricks with tungsten tailings by two-stage calcination technology. Constr. Build. Mater. 2024, 411, 134382. [Google Scholar] [CrossRef]
- Cetin, S. Production of sintered glass-ceramic composites from low-cost materials. Ceram. Int. 2023, 49, 22386–22392. [Google Scholar] [CrossRef]
- Shichalin, O.O.; Yarusova, S.B.; Ivanov, N.P.; Papynov, E.K.; Belov, A.A.; Azon, S.A.; Buravlev, I.Y.; Myagchilov, A.V.; Fedorets, A.N.; Rastorguev, V.L.; et al. Calcium silicate solid-state matrices from boric acid production waste for 60Co removal and immobilization by spark plasma sintering. J. Water Process. Eng. 2024, 59, 105042. [Google Scholar] [CrossRef]
- López-García, A.B.; Cotes-Palomino, T.; Uceda-Rodríguez, M.; Moreno-Maroto, J.M.; Cobo-Ceacero, C.J.; Andreola, N.M.F.; Martínez-García, C. Application of Life Cycle Assessment in the Environmental Study of Sustainable Ceramic Bricks Made with ‘alperujo’ (Olive Pomace). Appl. Sci. 2021, 11, 2278. [Google Scholar] [CrossRef]
- Gao, W.H.; Zhou, W.T.; Lyu, X.; Liu, X.; Su, H.L.; Li, C.M.; Wang, H. Comprehensive utilization of steel slag: A review. Powder Technol. 2023, 422, 118449. [Google Scholar] [CrossRef]
- Omer, M.M.; Rahman, R.A.; Almutairi, S. Construction waste recycling: Enhancement strategies and organization size. Phys. Chem. Earth 2022, 126, 103114. [Google Scholar] [CrossRef]
- Liu, T.J.; Wang, Y.T.; Li, J.G.; Yu, Q.; Wang, X.M.; Gao, D.; Wang, F.P.; Cai, S.; Zeng, Y.A. Effects from Fe, P, Ca, Mg, Zn and Cu in Steel Slag on Growth and Metabolite Accumulation of Microalgae: A Review. Appl. Sci. 2021, 11, 6589. [Google Scholar] [CrossRef]
- Pang, L.; Liao, S.C.; Wang, D.Q.; An, M.Z. Influence of steel slag fineness on the hydration of cement-steel slag composite pastes. J. Build. Eng. 2022, 57, 104866. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.Q.; Hou, G.H.; Yan, P.Y. Preparation of sustainable and green cement-based composite binders with high -volume steel slag powder and ultra fine blast furnace slag powder. J. Clean. Prod. 2021, 289, 125133. [Google Scholar] [CrossRef]
- Wang, P.; Xie, M.; Liu, L. Study on Early Shrinkage and Mechanical Properties of Concrete with Various Cementitious Materials. Buildings 2022, 12, 1543. [Google Scholar] [CrossRef]
- Ning, D.F.; Song, A.L.; Fan, F.L.; Li, Z.J.; Liang, Y.C. Effects of Slag-Based Silicon Fertilizer on Rice Growth and Brown-Spot Resistance. PLoS ONE 2014, 9, e102681. [Google Scholar] [CrossRef]
- Gao, D.; Wang, F.P.; Wang, Y.T.; Zeng, Y.N. Sustainable Utilization of Steel Slag from Traditional Industry and Agriculture to Catalysis. Sustainability 2020, 12, 9295. [Google Scholar] [CrossRef]
- Yu, H.F.; Lu, X.; Miki, T.; Matsubae, K.; Sasaki, Y.; Nagasaka, T. Sustainable phosphorus supply by phosphorus recovery from steelmaking slag: A critical review. Resour. Conserv. Recycl. 2022, 180, 106203. [Google Scholar] [CrossRef]
- Chen, Z.M.; Li, R.; Zheng, X.M.; Liu, J.X. Carbon sequestration of steel slag and carbonation for activating RO phase. Cem. Concr. Res. 2021, 139, 106271. [Google Scholar] [CrossRef]
- Li, J.J.; Zhao, S.W.; Song, X.Q.; Ni, W.; Mao, S.L.; Du, H.H.; Zhu, S.T.; Jiang, F.X.; Zeng, H.; Deng, X.J.; et al. Carbonation Curing on Magnetically Separated Steel Slag for the Preparation of Artificial Reefs. Materials 2022, 15, 2055. [Google Scholar] [CrossRef]
- Nielsen, P.; Boone, M.A.; Horckmans, L.; Snellings, R.; Quaghebeur, M. Accelerated carbonation of steel slag monoliths at low CO2 pressure-microstructure and strength development. J. CO2 Util. 2020, 36, 124–134. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, B.M.; Chang, J. Effect of zeolite calcination temperature on the carbonation degree and strength of steel slag compacts. Constr. Build. Mater. 2022, 343, 127987. [Google Scholar] [CrossRef]
- Lin, S.N.; Zhang, T.A.; Cao, X.J.; Liu, X.Q. Recovery of converter steel slag to prepare catalytic H2O2 degradation of dye wastewater as a catalyst. J. Mater. Sci.-Mater. Electron. 2021, 32, 24889–24901. [Google Scholar] [CrossRef]
- Shu, Y.R.; Ji, B.; Li, Y.X.; Zhang, W.; Zhang, H.N.; Zhang, J. Natural pyrite improved steel slag towards environmentally sustainable chromium reclamation from hexavalent chromium-containing wastewater. Chemosphere 2021, 282, 130974. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.H.; Wang, X.C.; Zhou, S.; Zuo, X.M.; Wang, C. Mechanism, application, influencing factors and environmental benefit assessment of steel slag in removing pollutants from water: A review. J. Water Process. Eng. 2022, 47, 102666. [Google Scholar] [CrossRef]
- Lu, H.B.; Xiao, L.P.; Wang, T.; Lu, S.Y.; Wang, H.H.; Guo, X.C.; Li, J.X. The application of steel slag in a multistage pond constructed wetland to purify low-phosphorus polluted river water. J. Environ. Manag. 2021, 292, 112578. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.X.; Peng, Z.W.; Huang, Y.W.; Gu, F.Q.; Zhang, J.; Tang, H.M.; Yang, L.; Tian, W.G.; Rao, M.J.; Li, G.H.; et al. Production of glass-ceramics from metallurgical slags. J. Clean. Prod. 2021, 317, 128220. [Google Scholar] [CrossRef]
- Wu, Q.S.; Huang, Z.C. Preparation and performance of lightweight porous ceramics using metallurgical steel slag. Ceram. Int. 2021, 47, 25169–25176. [Google Scholar] [CrossRef]
- Luo, Z.H.; He, F.; Zhang, W.T.; Xiao, Y.L.; Xie, J.L.; Sun, R.J.; Xie, M.Q. Effects of fluoride content on structure and properties of steel slag glass-ceramics. Mater. Chem. Phys. 2020, 242, 122531. [Google Scholar] [CrossRef]
- Dun, Z.L.; Wang, M.Q.; Zou, Y.F.; Ren, L.W. Laboratory Tests on Performance of Waste-Clay-Brick-Powder Cement Grouting Materials for Ground Improvement in Mine Goaf. Adv. Eng. Mater. 2022, 24, 2101575. [Google Scholar] [CrossRef]
- Beldjilali, S.; Bougara, A.; Aguiar, J.; Bouhamou, N.E.; Dabbebi, R. Properties of a new material based on a gypsum matrix incorporating waste brick. Constr. Build. Mater. 2020, 259, 120416. [Google Scholar] [CrossRef]
- Naciri, K.; Aalil, I.; Chaaba, A. Eco-friendly gypsum-lime mortar with the incorporation of recycled waste brick. Constr. Build. Mater. 2022, 325, 126770. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.Q.; Wang, H.; Dun, Z.; Ren, L.W. Experimental Research on Application of Waste Concrete Powder-Waste Brick Powder-Cement Grout for Foundation Reinforcement in Mining Goaf. Materials 2023, 16, 6075. [Google Scholar] [CrossRef]
- Lam, M.N.T.; Nguyen, D.T.; Nguyen, D.L. Potential use of clay brick waste powder and ceramic waste aggregate in mortar. Constr. Build. Mater. 2021, 313, 125516. [Google Scholar] [CrossRef]
- Rahou, J.; Rezqi, H.; El Ouahabi, M.; Fagel, N. Characterization of Moroccan steel slag waste: The potential green resource for ceramic production. Constr. Build. Mater. 2022, 314, 125663. [Google Scholar] [CrossRef]
- Zhao, L.H.; Li, Y.; Zhou, Y.Y.; Cang, D.Q. Preparation of novel ceramics with high CaO content from steel slag. Mater. Des. 2014, 64, 608–613. [Google Scholar] [CrossRef]
- Zhao, L.H.; Wei, W.; Bai, H.; Zhang, X.; Cang, D.Q. Synthesis of steel slag ceramics: Chemical composition and crystalline phases of raw materials. Int. J. Miner. Metall. Mater. 2015, 22, 325–333. [Google Scholar] [CrossRef]
- Liu, H.; Dang, H.; Wang, W. A Novel Fabrication Approach for Ceramic Tiles from Red Mud and Steel Slag. Russ. J. Non-Ferrous Metals 2022, 63, 441–447. [Google Scholar] [CrossRef]
- Tabit, K.; Hajjou, H.; Waqif, M.; Saâdi, L. Effect of CaO/SiO2 ratio on phase transformation and properties of anorthite-based ceramics from coal fly ash and steel slag. Ceram. Int. 2020, 46, 7550–7558. [Google Scholar] [CrossRef]
- Teo, P.T.; Zakaria, S.K.; Sharif, N.M.; Abu Seman, A.; Taib, M.A.A.; Mohamed, J.J.; Yusoff, M.; Yusoff, A.H.; Mohamad, M.; Ali, A.; et al. Application of General Full Factorial Statistical Experimental Design’s Approach for the Development of Sustainable Clay-Based Ceramics Incorporated with Malaysia’s Electric Arc Furnace Steel Slag Waste. Crystals 2021, 11, 442. [Google Scholar] [CrossRef]
- Mymrin, V.; Guidolin, M.A.; Klitzke, W.; Alekseev, K.; Guidolin, R.H.; Avanci, M.A.; Pawlowsky, U.; Winter, E.; Catai, R.E. Environmentally clean ceramics from printed circuit board sludge, red mud of bauxite treatment and steel slag. J. Clean. Prod. 2017, 164, 831–839. [Google Scholar] [CrossRef]
- Xu, X.H.; Zhang, J.Y.; Wu, J.F.; Chen, Z.C.; Wang, Y.Y. Preparation of foamed ceramics from steel slag with high calcium and iron content. Int. J. Appl. Ceram. Technol. 2023, 20, 2993–3003. [Google Scholar] [CrossRef]
- Tang, Z.J.; Liang, J.; Jiang, W.H.; Liu, J.M.; Jiang, F.; Feng, G.; Lao, X.B.; Tang, H.D.; Wang, T. Preparation of high strength foam ceramics from sand shale and steel slag. Ceram. Int. 2020, 46, 9256–9262. [Google Scholar] [CrossRef]
- Zadehnajar, P.; Mirmusavi, M.H.; Bakhtiari, S.S.E.; Bakhsheshi-Rad, H.R.; Karbasi, S.; RamaKrishna, S.; Berto, F. Recent advances on akermanite calcium-silicate ceramic for biomedical applications. Int. J. Appl. Ceram. Technol. 2021, 18, 1901–1920. [Google Scholar] [CrossRef]
- Ismail, H.; Ahmad, A.; Mohamad, H. Characteristics of akermanite-gehlenite and diopside bioceramics derived from CaO-MgO-Al2O3-SiO2 system as a potential bone substitute material. J. Am. Ceram. Soc. 2023, 59, 1361–1371. [Google Scholar] [CrossRef]
- ISO 10545-3; Ceramic Tiles—Part 3: Determination of Water Absorption, Apparent Porosity, Apparent Relative Density and Bulk Density (Second Edition). International Organization for Standardization: Geneva, Switzerland, 2018.
- ISO 10545-4; Ceramic Tiles—Part 4: Determination of Modulus of Rupture and Breaking Strength (Fourth Edition). International Organization for Standardization: Geneva, Switzerland, 2019.
- Sánchez-Soto, P.J.; Garzón, E.; Pérez-Villarejo, L.; Angelopoulos, G.N.; Eliche-Quesada, D. Mining Wastes of an Albite Deposit as Raw Materials for Vitrified Mullite Ceramics. Minerals 2021, 11, 232. [Google Scholar] [CrossRef]
- Zong, Y.B.; Chen, W.H.; Fan, Y.; Yang, T.L.; Liu, Z.B.; Cang, D.Q. Complementation in the composition of steel slag and red mud for preparation of novel ceramics. Int. J. Miner. Metall. Mater. 2018, 25, 1010–1017. [Google Scholar] [CrossRef]
- Cook, R.F.; Pharr, G.M. Mechanical Properties of Ceramics. In Materials Science and Technology; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2006. [Google Scholar]
- Zhang, X.D.; Zheng, C.Z.; Liu, S.P.; Zong, Y.B.; Zhou, Q.F.; Qin, S.C. Preparation of Steel Slag Ceramics with Different MgO/Al2O3 Ratios. Appl. Sci. 2019, 9, 4741. [Google Scholar] [CrossRef]
- Yu, J.W.; Han, Y.X.; Li, Y.J.; Gao, P. Growth behavior of the magnetite phase in the reduction of hematite via a fluidized bed. Int. J. Miner. Metall. Mater. 2019, 26, 1231–1238. [Google Scholar] [CrossRef]
- Erdogmus, E.; Sutcu, M.; Hossain, S.; Bayram, M.; Sari, A.; Gencel, O.; Ozbakkaloglu, T. Effect of molding pressure and firing temperature on the properties of ceramics from natural zeolite. Constr. Build. Mater. 2023, 402, 132960. [Google Scholar] [CrossRef]
- Choi, Y.M.; Lee, Y.L.; Lim, E.S.; Trimzi, M.A.; Hwangbo, S.A.; Ham, Y.B. Performance Improvement of Ring-Type PZT Ceramics for Ultrasonic Dispersion System. Micromachines 2020, 11, 144. [Google Scholar] [CrossRef]
- Pérez, J.M.; Romero, M. Microstructure and technological properties of porcelain stoneware tiles moulded at different pressures and thicknesses. Ceram. Int. 2014, 40, 1365–1377. [Google Scholar] [CrossRef]
- Heady, R.B.; Cahn, J.W. An analysis of the capillary forces in liquid-phase sintering of spherical particles. Metall. Mater. 1970, 1, 185–189. [Google Scholar] [CrossRef]
- Xu, K.; Mehrabadi, M.M. A micromechanical model for the initial rearrangement stage of liquid phase sintering. Mech. Mater. 1997, 25, 137–157. [Google Scholar] [CrossRef]
- Tahir, A.M.; Malik, A.; Amberg, G. Modeling of the primary rearrangement stage of liquid phase sintering. Model. Simul. Mater. Sci. Eng. 2016, 24, 075009. [Google Scholar] [CrossRef]
- Yin, Z.B.; Ye, J.D.; Hong, D.B.; Xu, W.W. Effect of molding pressure on structural and densification behavior of microwave-sintered ceramic tool material. Int. J. Appl. Ceram. Technol. 2019, 16, 2085–2093. [Google Scholar] [CrossRef]
- Ding, J.; Tian, Y.; Wang, L.-S.; Huang, X.; Zheng, H.-R.; Song, K.; Zeng, X.-G. Micro-mechanism of the effect of grain size and temperature on the mechanical properties of polycrystalline TiAl. Comput. Mater. Sci. 2019, 158, 76–87. [Google Scholar] [CrossRef]
- Maejima, T.; Yonemura, M.; Kawano, K.; Miyamoto, G. Lattice Strain and Strength Evaluation on V Microalloyed Pearlite Steel. Tetsu To Hagane-J. Iron Steel Inst. Jpn. 2020, 60, 1810–1818. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Yang, Z.G.; Yin, Z.Q.; Chen, B.; Yu, J.B.; Ren, Z.M.; Yu, G.; Zhang, G.L. Investigation of the properties and leaching characteristics of ceramic cores fabricated using BaZrO3 as the raw material. Mater. Chem. Phys. 2021, 272, 124925. [Google Scholar] [CrossRef]
- Jana, D.C.; Barick, P.; Saha, B.P. Effect of Sintering Temperature on Density and Mechanical Properties of Solid-State Sintered Silicon Carbide Ceramics and Evaluation of Failure Origin. J. Mater. Eng. Perform. 2018, 27, 4978. [Google Scholar] [CrossRef]
- Le, P.G.; Jo, G.Y.; Ko, S.Y.; Fisher, J.G. The effect of sintering temperature and time on the growth of single crystals of 0.75 (Na0.5Bi0.5)TiO3-0.25 SrTiO3 by solid state crystal growth. J. Electroceram. 2018, 40, 122–137. [Google Scholar] [CrossRef]
- Arcaro, S.; Maia, B.G.D.; Souza, M.T.; Cesconeto, F.R.; Granados, L.; de Oliveira, A.P.N. Thermal Insulating Foams Produced from Glass Waste and Banana Leaves. Mater. Res. 2016, 19, 1064–1069. [Google Scholar] [CrossRef]
- Bordia, R.K.; Kang, S.J.L.; Olevsky, E.A. Current understanding and future research directions at the onset of the next century of sintering science and technology. J. Am. Ceram. Soc. 2017, 100, 2314–2352. [Google Scholar] [CrossRef]
- Dunce, M.; Birks, E.; Antonova, M.; Bikse, L.; Dutkevica, S.; Freimanis, O.; Livins, M.; Eglite, L.; Smits, K.; Sternberg, A. Influence of sintering temperature on microstructure of Na0.5Bi0.5TiO3 ceramics. J. Alloys Compd. 2021, 884, 160955. [Google Scholar] [CrossRef]
- Fisher, J.G.; Kang, S.J.L. Strategies and practices for suppressing abnormal grain growth during liquid phase sintering. J. Am. Ceram. Soc. 2019, 102, 717–735. [Google Scholar] [CrossRef]
- Wang, W.; Liu, H.T.; Gu, W.D. A novel fabrication approach for improving the mechanical and sound absorbing properties of porous sound-absorbing ceramics. J. Alloys Compd. 2017, 695, 2477–2482. [Google Scholar] [CrossRef]
- Pan, Y.; Li, H.; Liu, Y.S.; Liu, Y.S.; Hu, K.H.; Wang, N.; Lu, Z.G.; Liang, J.J.; He, S.J. Effect of Holding Time During Sintering on Microstructure and Properties of 3D Printed Alumina Ceramics. Front. Mater. 2020, 7, 54. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Tian, Y.M.; Feng, M.; Qing, M.; Wu, Y.Q. Effects of holding time on structure and the properties of spodumene/mullite composite ceramics. Int. J. Appl. Ceram. Technol. 2022, 19, 1300–1308. [Google Scholar] [CrossRef]
- Wakai, F.; Guillon, O.; Okuma, G.; Nishiyama, N. Sintering forces acting among particles during sintering by grain-boundary/surface diffusion. J. Am. Ceram. Soc. 2019, 102, 538–547. [Google Scholar] [CrossRef]
- Yan, S.Y.; Yin, Z.B.; Yuan, J.T.; Xu, W.W.; Chen, M.D.; Ye, J.D. Microstructure and properties of submicron grained alumina ceramic tool material prepared by two-step microwave sintering. Ceram. Int. 2018, 44, 17479–17485. [Google Scholar] [CrossRef]
- Manan, A.; Ahmad, A.S.; Ullah, A.; Wazir, A.H.; Shah, A.A. The Effects of Dwell Sintering Time on the Densification and Microwave Dielectric Properties of Sr0.5Ca0.5La4Ti5O17 Ceramic. Iran. J. Sci. Technol. Trans. A-Sci. 2019, 43, 2699–2703. [Google Scholar] [CrossRef]










| SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | MnO | P2O5 | TiO2 | LOI | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SS | 14.53 | 3.40 | 20.49 | 5.41 | 34.92 | 0.21 | 0.07 | 6.00 | 1.98 | 1.39 | 1.3 |
| WCB | 47.03 | 12.97 | 5.72 | 2.32 | 8.78 | 1.61 | 2.27 | 0.13 | 0.19 | 0.72 | 2.1 |
| Talc | 64.07 | 0.55 | 0.08 | 31.7 | 0.32 | - | - | - | - | - | 4.8 |
| Level | Experimental Factor | ||
|---|---|---|---|
| SS (%) | WCB (%) | Temperature (°C) | |
| 1 | 35 | 40 | 1180 |
| 2 | 40 | 35 | 1190 |
| 3 | 45 | 30 | 1200 |
| No. | Experimental Factor | ||
|---|---|---|---|
| SS (%) | WCB (%) | Temperature (°C) | |
| Zj-1 | 35 | 40 | 1180 |
| Zj-2 | 35 | 35 | 1190 |
| Zj-3 | 35 | 30 | 1200 |
| Zj-4 | 40 | 40 | 1200 |
| Zj-5 | 40 | 35 | 1180 |
| Zj-6 | 40 | 30 | 1190 |
| Zj-7 | 45 | 40 | 1190 |
| Zj-8 | 45 | 35 | 1200 |
| Zj-9 | 45 | 30 | 1180 |
| No. | SS (%) | WCB (%) | Talc (%) | Temperature (°C) |
|---|---|---|---|---|
| Y-1 | 40 | 35 | 25 | 1200 |
| Y-2 | 45 | 35 | 25 | 1190 |
| No. | Molding Pressure (MPa) | Temperature (°C) | Soaking Time (min) |
|---|---|---|---|
| YY-1 | 15 | 1190 | 90 |
| YY-2 | 20 | 1190 | 90 |
| YY-3 | 25 | 1190 | 90 |
| YY-4 | 30 | 1190 | 90 |
| No. | Molding Pressure (MPa) | Temperature (°C) | Soaking Time (min) |
|---|---|---|---|
| YW-1 | 25 | 1185 | 90 |
| YW-2 | 25 | 1190 | 90 |
| YW-3 | 25 | 1195 | 90 |
| YW-4 | 25 | 1200 | 90 |
| No. | Molding Pressure (MPa) | Temperature (°C) | Soaking Time (min) |
|---|---|---|---|
| YB-1 | 25 | 1190 | 30 |
| YB-2 | 25 | 1190 | 60 |
| YB-4 | 25 | 1190 | 90 |
| No. | Modulus of Rupture (MPa) | Water Absorption (%) |
|---|---|---|
| Zj-1 | 31.60 | 10.81 |
| Zj-2 | 42.27 | 1.01 |
| Zj-3 | 42.12 | 0.33 |
| Zj-4 | 42.43 | 0.44 |
| Zj-5 | 41.63 | 4.58 |
| Zj-6 | 55.40 | 3.37 |
| Zj-7 | 61.84 | 0.19 |
| Zj-8 | 61.58 | 0.25 |
| Zj-9 | 30.95 | 12.12 |
| No. | Experimental Factor | ||
|---|---|---|---|
| SS (%) | WCB (%) | Temperature (°C) | |
| ki1 | 12.15 | 11.44 | 27.51 |
| ki2 | 8.39 | 5.84 | 4.57 |
| ki3 | 12.56 | 15.82 | 1.02 |
| ki1average | 4.05 | 3.81 | 9.17 |
| ki2average | 2.80 | 1.95 | 1.52 |
| ki3average | 4.12 | 5.27 | 0.34 |
| Ri | 1.39 | 3.33 | 7.65 |
| No. | Experimental Factor | ||
|---|---|---|---|
| SS (%) | WCB (%) | Temperature (°C) | |
| k1 | 115.99 | 135.87 | 104.18 |
| k2 | 139.46 | 145.48 | 159.51 |
| k3 | 154.37 | 128.47 | 146.13 |
| ki1average | 38.66 | 45.29 | 34.73 |
| ki2average | 46.49 | 48.49 | 53.17 |
| ki3average | 51.46 | 42.82 | 48.71 |
| Ri | 12.79 | 5.67 | 18.44 |
| No. | Modulus of Rupture (MPa) | Water Absorption (%) |
|---|---|---|
| Y-1 | 35.80 | 0.10 |
| Y-2 | 63.68 | 0.08 |
| Apparent Porosity (%) | Bulk Density (g/m3) | Linear Shrinkage (%) | |
|---|---|---|---|
| YY-1 | 0.171 | 2.56 | 12.9 |
| YY-2 | 0.130 | 2.61 | 13.5 |
| YY-3 | 0.086 | 2.78 | 14.6 |
| YY-4 | 0.170 | 2.56 | 12.8 |
| Crystal Size (nm) | Lattice Strain (%) | |
|---|---|---|
| YY-1 | 43.6 | 0.109 |
| YY-2 | 52.8 | 0.076 |
| YY-3 | 60.6 | 0.069 |
| YY-4 | 46.5 | 0.141 |
| Apparent Porosity (%) | Bulk Density (g/m3) | Linear Shrinkage (%) | |
|---|---|---|---|
| YW-1 | 0.155 | 2.62 | 13.5 |
| YW-2 | 0.086 | 2.78 | 14.6 |
| YW-3 | 0.114 | 2.68 | 13.8 |
| YW-4 | 0.129 | 2.64 | 13.6 |
| Crystal Size (nm) | Lattice Strain (%) | |
|---|---|---|
| YW-1 | 36.5 | 0.046 |
| YW-2 | 60.6 | 0.069 |
| YW-3 | 43.8 | 0.108 |
| YW-4 | 37.1 | 0.124 |
| Apparent Porosity (%) | Bulk Density (g/m3) | Linear Shrinkage (%) | |
|---|---|---|---|
| YB-1 | 0.101 | 2.69 | 13.8 |
| YB-2 | 0.058 | 2.82 | 14.9 |
| YB-3 | 0.086 | 2.78 | 14.6 |
| Crystal Size (nm) | Lattice Strain (%) | |
|---|---|---|
| YB-1 | 42.9 | 0.062 |
| YB-2 | 47.8 | 0.113 |
| YB-3 | 60.6 | 0.069 |
| ISO Standard | Experimental Sample | Commercial Sample 1 | Commercial Sample 2 | Commercial Sample 3 | |
|---|---|---|---|---|---|
| Water absorption (%) | E ≥ 0.5 | 0.04 | 0.33 | 0.05 | 0.02 |
| Modulus of rupture (MPa) | R ≥ 35 | 73.2 | 38 | 47 | 49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Li, E.; Zhu, G.; Wang, R.; Sha, Q. Preparation and Performance of Ceramic Tiles with Steel Slag and Waste Clay Bricks. Materials 2024, 17, 1755. https://doi.org/10.3390/ma17081755
Ji Y, Li E, Zhu G, Wang R, Sha Q. Preparation and Performance of Ceramic Tiles with Steel Slag and Waste Clay Bricks. Materials. 2024; 17(8):1755. https://doi.org/10.3390/ma17081755
Chicago/Turabian StyleJi, Ying, Enyao Li, Gang Zhu, Ruiqi Wang, and Qianqian Sha. 2024. "Preparation and Performance of Ceramic Tiles with Steel Slag and Waste Clay Bricks" Materials 17, no. 8: 1755. https://doi.org/10.3390/ma17081755





