Abstract
In the present investigation, an ecofriendly magnetic inorganic-protein hybrid system-based enzyme immobilization was developed using partially purified laccase from Trametes versicolor (TvLac), Fe3O4 nanoparticles, and manganese (Mn), and was successfully applied for synthetic dye decolorization in the presence of enzyme inhibitors. After the partial purification of crude TvLac, the specific enzyme activity reached 212 U∙mg total protein−1. The synthesized Fe3O4/Mn3(PO4)2-laccase (Fe3O4/Mn-TvLac) and Mn3(PO4)2-laccase (Mn-TvLac) nanoflowers (NFs) exhibited encapsulation yields of 85.5% and 90.3%, respectively, with relative activities of 245% and 260%, respectively, compared with those of free TvLac. One-pot synthesized Fe3O4/Mn-TvLac exhibited significant improvements in catalytic properties and stability compared to those of the free enzyme. Fe3O4/Mn-TvLac retained a significantly higher residual activity of 96.8% over that of Mn-TvLac (47.1%) after 10 reuse cycles. The NFs showed potential for the efficient decolorization of synthetic dyes in the presence of enzyme inhibitors. For up to five reuse cycles, Fe3O4/Mn-TvLac retained a decolorization potential of 81.1% and 86.3% for Coomassie Brilliant Blue R-250 and xylene cyanol, respectively. The synthesized Fe3O4/Mn-TvLac showed a lower acute toxicity towards Vibrio fischeri than pure Fe3O4 nanoparticles did. This is the first report of the one-pot synthesis of biofriendly magnetic protein-inorganic hybrids using partially purified TvLac and Mn.
1. Introduction
Enzymes, as natural catalysts, have been utilized by humans for centuries in various applications, such as in the production of cheese, brewing processes, baking, and the production of wines and alcohol [1,2]. They are essential for numerous biocatalytic industrial processes, including biofuel and food production, and pharmaceutical manufacturing [3,4,5]. However, the traditional use of enzymes in these processes, whether free or immobilized enzymes, has limitations [6,7]. For example, free enzymes are often unstable and can be deactivated or degraded easily under harsh reaction conditions. Additionally, free enzymes are difficult to recover and reuse, leading to increased production costs [8,9]. To address these limitations, researchers have explored the immobilization of enzymes, which involves immobilizing to a support. This approach offers several potential advantages, including improved enzyme stability, enhanced catalytic efficiency, and easier separation and recovery of enzymes [10,11]. Despite these advantages, there remains scientific gaps and limitations in enzyme immobilization that warrant further research. One limitation is the potential loss of enzyme activity during the immobilization process. This loss of activity can be caused by various factors, such as conformational changes, reduced accessibility to substrates, or limited diffusion of substrates and products within the immobilization support [11,12]. Other limitations include the potential for enzyme inactivation due to interactions with the immobilization support or other nearby enzymes, as well as difficulties in achieving optimal enzyme loading. Further research is required to better understand these limitations and develop strategies to overcome them [11,13,14].
Advancements in technology have led to the development of novel methods for enzyme immobilization, including the use of inorganic-protein hybrid systems that are known as nanoflowers (NFs) because their morphology resembles that of flowers [15,16]. In an established mechanism, NF synthesis occurs in phosphate-buffered saline (PBS) through three steps: (i) nucleation via interaction of metal ions with a nitrogen group available in the protein, (ii) followed by aggregation, and (iii) anisotropic growth [17]. Metals such as cobalt (Co), copper (Cu), manganese (Mn), and zinc (Zn) have been widely demonstrated for the immobilization of enzymes as NFs [11,16]. These systems combine the unique properties of inorganic materials with the catalytic activity of enzymes, resulting in enhanced enzyme stability and effectiveness. Moreover, these inorganic-protein hybrids have been utilized for enzyme immobilization, which allows enzymes to be securely attached to a solid support. Initially, the metal ions form complexes with the amide groups of the protein molecules and functional activated nanoparticles. Subsequently, the nanoparticles and metal/phosphate nuclei continue to grow as the enzyme integrates into the hybrid assemblies. Finally, larger nanoparticles and metal-based enzyme hybrid NFs form after longer incubation periods [11,17]. This immobilization technique improves the efficiency and reusability of enzymes in various applications, such as pharmaceutical production and green synthesis processes [16,18]. The combination of genetic engineering techniques and the utilization of inorganic-protein hybrid systems for enzyme immobilization offers promising solutions to overcome the drawbacks associated with enzyme instability and make enzymes more suitable for industrial applications [12,19,20]. One promising approach for enzyme immobilization is the use of magnetic inorganic-protein hybrids, which offer several advantages. These hybrids combine the magnetic properties of inorganic materials with the functional properties of proteins, such as enzymes [21,22]. This allows for the efficient and convenient recovery of the immobilized enzyme using magnetic separation techniques [11].
Laccase plays a crucial role in dye decolorization. It is an enzyme widely used in various industries, including textile and dyestuff manufacturing, because of its ability to oxidize aromatic amines and degrade azo dyes via a nonspecific, free radical-mediated mechanism [19,23]. Moreover, laccase can be produced from various sources, including fungi (Trametes versicolor), plants, and bacteria. Therefore, they provide diverse options for industrial applications [24,25]. The use of laccase for dye decolorization has several advantages. These include its effectiveness in removing dyes by forming relatively harmless byproducts [26,27]. Mechanistically, laccases act through direct phenolic substrate oxidation and indirect oxidation of non-phenolic substrates in the presence of a natural/synthetic mediator with a high redox potential, or through coupling reactions via reactive intermediate radicals created in a direct oxidation process [28,29]. Additionally, laccase is more stable and versatile than other enzymes because it can function under a wide range of temperature and pH conditions. Furthermore, laccase functions efficiently in both aerobic and anaerobic environments, making it suitable for various biological treatment systems [30,31,32]. Overall, laccase is a valuable enzyme for dye decolorization because of its effectiveness, versatility, and environmentally friendly nature. Therefore, the immobilization of laccase onto magnetic inorganic-protein hybrids as NFs enhances the stability and reusability of the enzyme and provides a larger surface area for substrate binding, resulting in increased catalytic activity [11,19]. Few reports have been published on magnetic inorganic-protein hybrid-based NFs [20,33]. Mechanistically, magnetic inorganic-protein hybrid NFs are synthesized via the interaction of 3-aminopropyltriethoxysilane (APTES)-functionalized magnetic (Fe3O4) nanoparticles with the enzyme, followed by precipitation in the presence of desirable metal ions [11,33]. In this study, partially purified laccase from T. versicolor (TvLac), functionally activated Fe3O4 nanoparticles, and Mn-based synthesis of the magnetic inorganic-protein hybrid NFs, Fe3O4/Mn3(PO4)2-laccase (Fe3O4/Mn-TvLac) and Mn3(PO4)2-laccase (Mn-TvLac) NFs, were evaluated to improve the properties of TvLac. After immobilization, significant improvements in the TvLac properties were observed, including its catalytic activity, pH and temperature profiles, stability, and reusability. Furthermore, these hybrid NFs were successfully applied in the decolorization of dyes in the presence of enzyme inhibitors.
2. Materials and Methods
2.1. Materials
Magnetic nanoparticles (Fe3O4) were bought from Nanostructured and Amorphous Materials, Inc. (Houston, TX, USA). Ultrapure-water and PBS were obtained from Life Technologies, Carlsbad, CA, USA. 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS), Cu sulfate (CuSO4), Mn sulfate (MnSO4), APTES, potato dextrose (PD) broth (PDB), dithiothreitol, fluorescein isothiocyanate (FITC), and l-cysteine were acquired from Sigma-Aldrich (St. Louis, MO, USA). Coomassie Brilliant Blue R-250 (CBBR-250) and xylene cyanol were obtained from BioShop (Canada Inc., Burlington, ON, Canada). Other analytic reagents were purchased from commercial suppliers.
2.2. Laccase Production and Partial Purification
For laccase production, mycelia of T. versicolor (~five disks with a 5 mm diameter) grown on a PD agar (five days) were inoculated into 100 mL PDB (500 mL conical flask) for incubation for five days (30 °C) with slight agitation of 180 rpm. Moreover, 10% (v/v) precultures were inoculated into 1 L of basal medium (pH 5.0) containing CuSO4 (0.1–0.5 mM) as a laccase inducer [2]. The TvLac production profile was analyzed by measuring enzyme activity for up to eight days of incubation. The supernatant was retrieved by centrifugation (6000 rpm, 30 min, and at 4 °C) and filtered by a membrane (0.22 µm, Millipore, Bedford, MA, USA). Further, it was purified via ultrafiltration using a 30 kDa cut-off membrane (Viva-flow; Vivascience, Hannover, Germany). Total protein content was measured using the Bradford method (4:1 ratio of supernatant to Bradford reagent) at 595 nm with bovine serum albumin as the protein standard [34]. For further use, partially purified laccase was kept at 4 °C [2].
2.3. Magnetic Nanoparticles and Laccase-Based Protein-Inorganic Hybrid Nanoflowers Synthesis
Initially, Fe3O4 was activated using APTES, as previously described [23]. The synthesis of magnetic nanoparticle-based protein-inorganic hybrids as Fe3O4/Mn-TvLac NFs was accomplished in 50 mL of 100 mM PBS (pH 6.0), including APTES-activated Fe3O4 (5 mg), partially purified laccase (0.25 mg∙mL−1), and 0.5 mL MnSO4 (200 mM) at 4 °C for incubation up to 24 h under mild shaking (60 rpm). Similarly, the synthesis of Mn-TvLac was achieved without Fe3O4 nanoparticles under similar conditions. The residual protein concentration in the supernatant was measured using the Bradford method after separation, as previously reported [34,35]. An encapsulation yield (EY) was determined by 100 × ratio of the immobilized amount of enzyme to their initially added amount. The RA of NFs was measured as follows (Equation (1)):
RA (%) = total specific activity of immobilized laccase/initial specific activity of free laccase × 100
2.4. Laccase Activity Measurements
Enzyme activity was assessed by spectrophotometer at 420 nm using 1 mM of ABTS (εmax = 3.6 × 104∙M−1∙cm−1) as a substrate [2]. The activity was defined in international units, which denotes the amount of enzyme required to form 1 mol of product per min using ABTS in buffer solution (pH, 3.5) at 25 °C.
2.5. Characterization of Immobilized Laccase as Nanoflowers
Using ABTS, the free TvLac, Mn-TvLac, and Fe3O4/Mn-TvLac NFs activities were evaluated at various pH (2.5–6.0). The influence of temperature on enzyme activity was assayed over the range of 25–85 °C at the optimum pH values under assay conditions. Nonlinear regression (GraphPad Prism 5.0; GraphPad Software, La Jolla, CA, USA) measurements were acquired to determine kinetic parameters using 0.005–2.0 mM of ABTS at 25 °C.
2.6. Stability, Reusability, and Leaching Measurement
Initially, room temperature (25 °C) stability of free TvLac, Mn-TvLac, and Fe3O4/Mn-TvLac NFs was investigated by assessing the residual activity at different intervals in sodium-citrate buffer (100 mM, at optimum pH values) up to 72 h of incubation. Similarly, storage stability (at 4 °C) was assessed for 30 days of incubation. Thermostability was determined between temperatures of 40–80 °C. The initial activity (residual) was considered 100%. The reusability of Mn-TvLac and Fe3O4/Mn-TvLac was determined under standard assay conditions for 10 reuse cycles. The immobilized laccase was recovered through centrifugation (1000 rpm, 10 min, and at 4 °C), pursued by buffer washing (two times), and subsequently employed in the next cycle. Leaching of the immobilized laccase was estimated by assessing the supernatant protein (total) to the initial immobilized enzyme ratio ×100 [2].
2.7. Acute Toxicity Analysis
The toxicity of the synthesized NFs was measured towards Vibrio fischeri using a Microtox, Model 500 Analyzer (Modern Water, London, UK). In brief, different dilutions of NFs (heat-inactivated, 2.0 mg∙mL−1) and pure Fe3O4 nanoparticles (1.0 mg∙mL−1) were prepared according to the manufacturer’s standard protocol, and a decline in the luminescence intensity of V. fischeri was monitored for 30 min. Effective concentration (EC) values of the synthesized NFs were denoted as EC50, where a 50% reduction in the luminescence of the bacteria was observed after 30 min of incubation.
2.8. Decolorization of Synthetic Dyes in the Presence of Laccase Inhibitors
The decolorization of the synthetic dyes, CBBR-250 (λmax = 585 nm) and xylene cyanol (λmax = 615 nm) was assessed in the presence of laccase inhibitors (0.1 mM), such as dithiothreitol, Fe(II), and l-cysteine. In brief, a 10 mL reaction mixture was added to a 50 mL conical flask containing dyes (120 µg∙mL−1), inhibitor, and free TvLac or NFs in buffer (50 mM) under optimum pH conditions at 25 °C for incubation up to 72 h with shaking (100 rpm). Repeated batch decolorization of the dyes was also measured for 12 h of incubation in the presence of Fe2+ (0.1 mM) for up to five reuse cycles. NFs were collected through centrifugation at 1000 rpm for 10 min (4 °C) and subsequently applied to the next cycle. The decolorization efficiency at zero cycles was considered 100%. The decolorization percentage was calculated as follows (Equation (2)) [36]:
Decolorization (%) = absorbance after incubation/absorbance at the initial point × 100
2.9. Instrumental Measurements and Statistical Analysis
The field-emission scanning electron microscopy (SEM) measurements of the synthesized NFs were achieved using a JSM-6700F, JEOL, Tokyo, Japan [35]. Diffraction patterns were assessed via X-ray diffraction (XRD, X’pert PRO MPD X-ray, Malvern Panalytical, Malvern, UK). All absorption analysis was achieved by UV–Vis spectrophotometer (Jenway Scientific 6705, Staffordshire, UK) [37]. FITC-labeled enzymes were examined by confocal laser scanning microscopy (CLSM, FV1000 confocal microscope, Olympus, Center Valley, PA, USA) [35]. The magnetic properties of the Fe3O4 nanoparticles and NFs were analyzed at 298 K using a SQUID/VSM magnetometer, MPMSR3, San Diego, CA, USA. Experimental data values were presented as mean ± standard deviations, and statistical significance was investigated via ANOVA (α = 0.05; GraphPad Prism 5).
3. Results and Discussion
3.1. TvLac Production and Purification
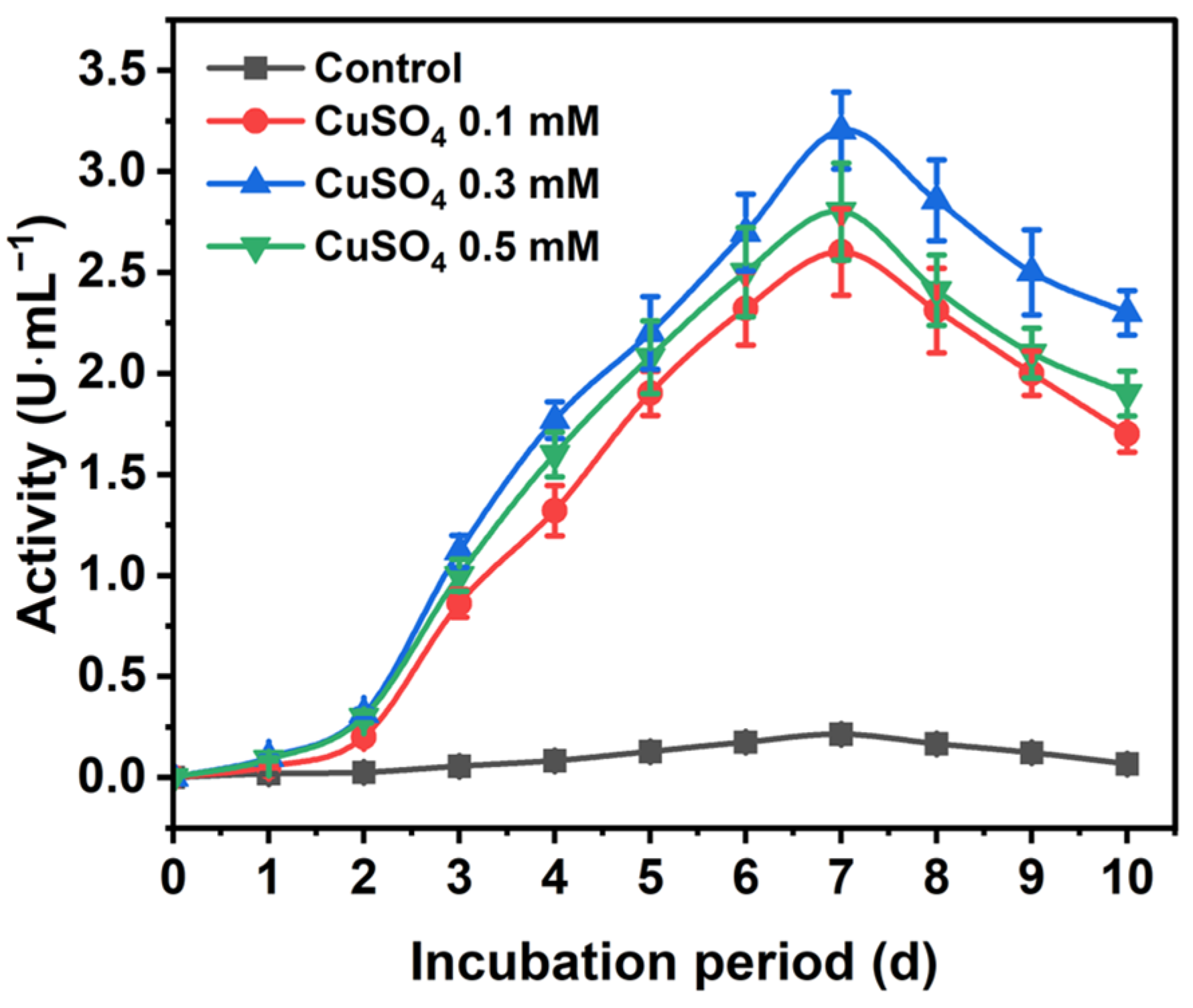
The TvLac production profile using various inducers is presented in Figure S1. Among different laccase mediators, Cu showed higher residual activity. Initially, laccase activity elevated with increasing incubation for up to seven days, with a crude activity reaching 3.2 U∙mL−1 (total activity of 3200 U∙L−1) (Figure 1 and Figure S2). Subsequently, the activity significantly declined to 2.3 U∙mL−1 after 10 days of incubation. Here, a 15-fold increase in TvLac production using CuSO4 (0.3 mM) was observed compared to the control. Furthermore, TvLac obtained from a seven-day culture was partially purified via ultrafiltration using a 30 kDa cut-off spin column (Corning, AZ, USA). The specific activity of the partially purified TvLac increased to 212 U∙mg total protein−1 with a fold purification and yield of 1.5 and 66.7%, respectively (Table S1). Previously, Coriolopsis gallica showed a low crude laccase production of 31 U∙mg protein−1 using inducers [38].
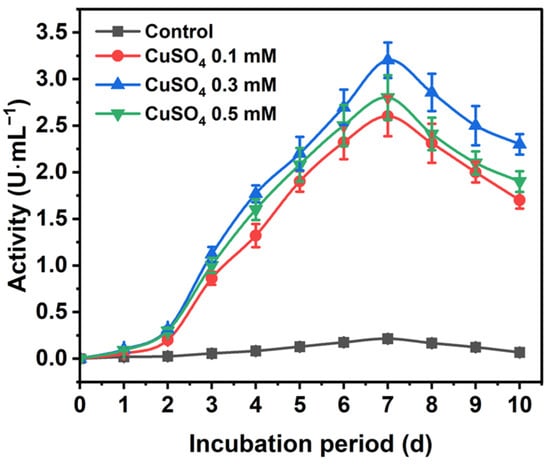
Figure 1.
Trametes versicolor laccase production profile in the presence of CuSO4 as an inducer.
3.2. Immobilization of TvLac Using Magnetic Nanoparticles and MnSO4 through Protein-Inorganic Hybrid Nanoflowers
The immobilization of partially purified TvLac as protein-inorganic hybrid NFs was performed using APTES-activated Fe3O4 nanoparticles and MnSO4 in PBS for 24 h of incubation at 4 °C. The synthesized Fe3O4/Mn-TvLac NFs exhibited EY and RA values of 85.5% and 245%, respectively (Table 1). A slightly higher EY of 90.3% and RA of 260% were observed for NFs synthesized without Fe3O4 nanoparticles (Mn-TvLac). Under assay conditions, free TvLac showed an RA of 127% in the presence of Mn ions (2 mM) (Table S2). Previously, purified TvLac immobilized using Co-based NFs, such as laccase@Co3(PO4)2·H NFs, showed a significantly reduced RA of 60.9% under a similar incubation period of 24 h [39]. In contrast, at a longer incubation time of 72 h, laccase NFs synthesized using Ni as laccase@Ni3(PO4)2·H NFs exhibited a low RA of 75% [40]. These results suggest that the immobilization of TvLac as NFs using magnetic nanoparticles and Mn is more effective for retaining a higher RA.

Table 1.
Immobilization of TvLac as inorganic-protein hybrid nanobiocatalysts as NFs.
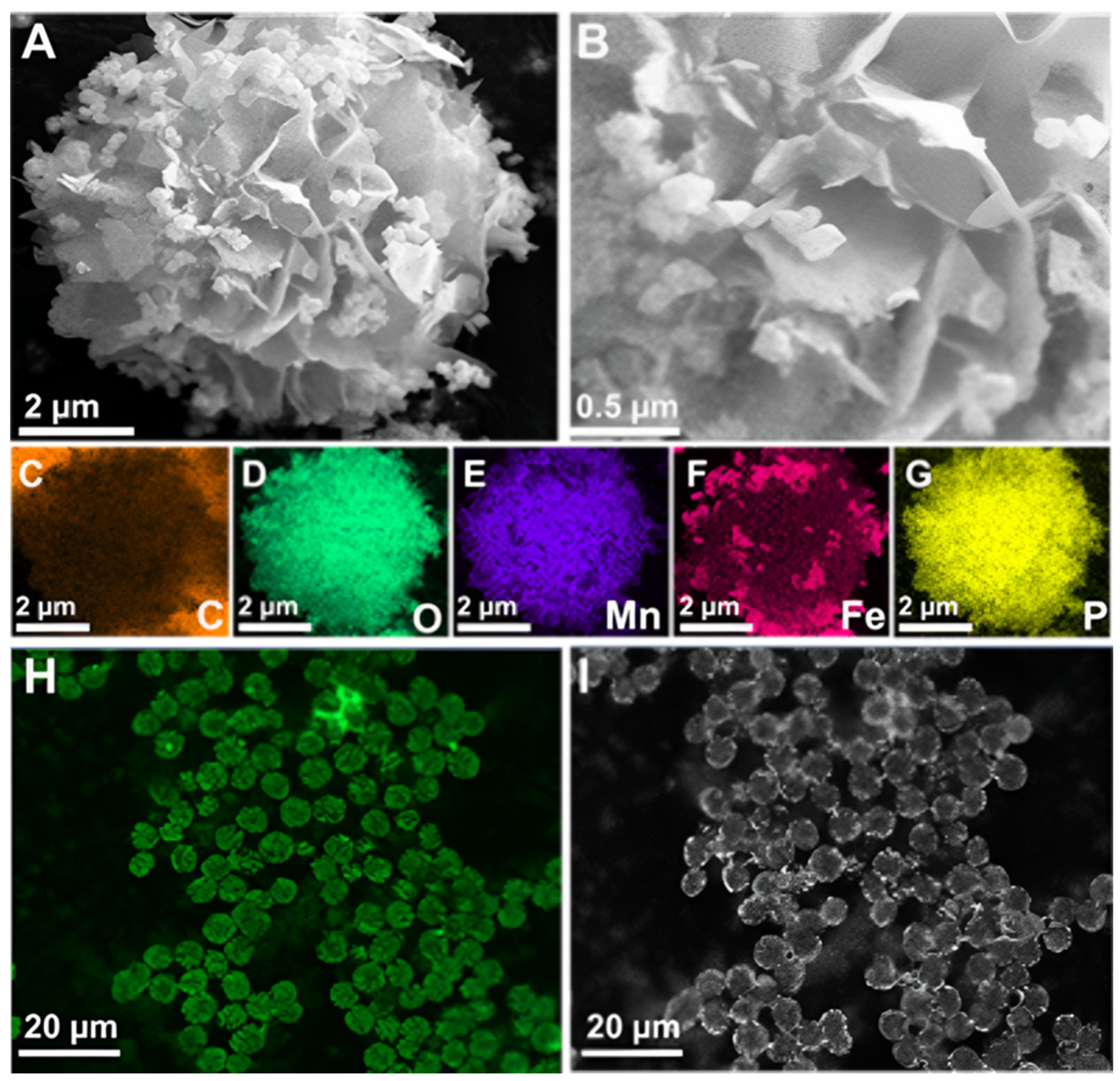
Synthesis incubation conditions and laccase origin highly influence the size and structural morphology of synthesized inorganic-protein hybrid NFs [39,40]. SEM was used to analyze the morphological structures of the synthesized inorganic-protein hybrids. The size of NFs increased from 0.7 µm at 2 h to 7 µm at 24 h of incubation at 4 °C (Figure S3). Under these conditions, the EY rose from 46.1% to 85.5%, with an optimum RA of 245% at 24 h (Table S3). The synthesized Fe3O4/Mn-TvLac and Mn-TvLac NFs showed a flower-like morphology with an average size of 7–9 µm (Figure 2A,B and Figure S4). Under a similar incubation conditions (24 h at 4 °C), the flower-like morphology of Cu-based laccase NFs exhibited higher sizes of ~50 µm [41]. NFs based on Co and laccase showed a size of nearly 2 µm with architecturally altered flower-resembling aggregates [39]. In another study, Ni-based laccase NFs exhibited hierarchically structured hybrids with sizes up to 10 µm [40]. In contrast, Zn-based TvLac NFs showed a larger size of 25 µm with a flake-like morphology [42]. Elemental mapping was performed to confirm the composition of the synthesized Fe3O4/Mn-TvLac NFs. The presence of carbon, Fe, Mn, and phosphorus (P) suggests the successful formation of magnetic inorganic-protein hybrid NFs (Figure 2C–G). Similarly, the presence of Mn, nitrogen, and P confirms Mn-TvLac NF formation (Figure S4). To validate TvLac immobilization, FITC-labeled TvLac was used to synthesize NFs under similar immobilization conditions (Figure 2H,I). In the CLSM analysis, the visualization of green fluorescence in the synthesized magnetic NFs confirmed TvLac immobilization. The synthesized hybrid NFs XRD patterns are shown in Figure S5. The relative peak locations of Fe3O4/Mn-TvLac and Mn-TvLac NFs were identical to Mn3(PO4)2·3H2O (JCPDS No. 03–0426) XRD patterns, Fe3O4 (JCPDS No. 75–0449), and protein-Mn3(PO4)2·3H2O hybrids, validating the synthesis of Mn-based magnetic inorganic-protein hybrids [43,44]. The Fe3O4 nanoparticles exhibited 24.2 electromagnetic unit∙g−1 (emu∙g−1) of magnetization saturation, while Fe3O4/Mn-TvLac showed 15.0 emu∙g−1 (Figure S6A). This finding confirms that the synthesized hybrids retained their magnetic properties, which can be quickly recovered by applying an external magnetic field (Figure S6B,C) [11,21].
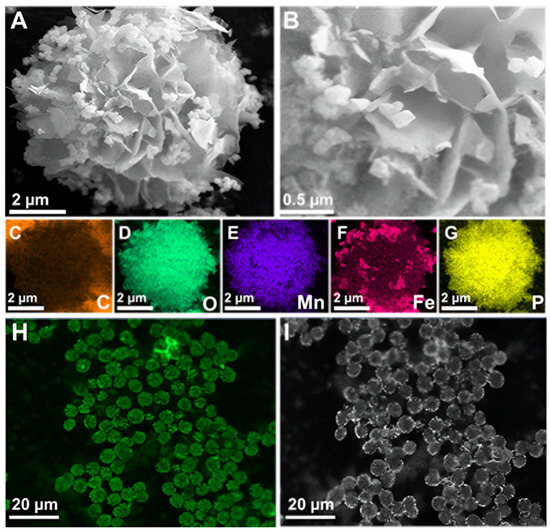
Figure 2.
Field-emission scanning electron microscopy examination of (A,B) magnetic inorganic-protein hybrids as Fe3O4/Mn-TvLac, (C–G) elemental mapping analysis for carbon (C), oxygen (D), manganese (E), iron (F), and phosphorus (G), and (H,I) confocal laser scanning microscope images of the synthesized TvLac hybrid NFs labeled with FITC under green channel (H) and bright-field (I).
3.3. Characterization of Free and Immobilized TvLac as Nanoflowers
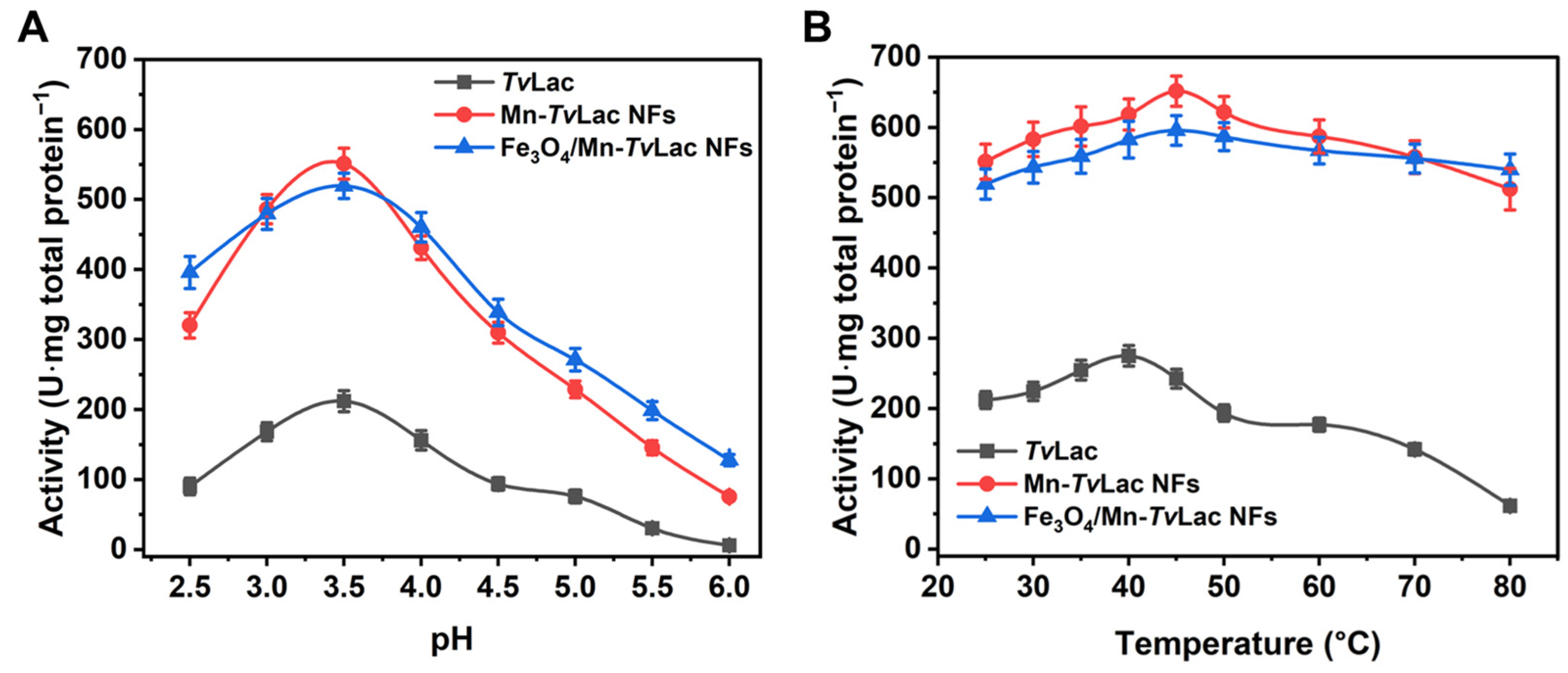
The free and immobilized TvLac pH profiles were assessed over a range of 2.5–6.0 in a buffer solution. Free TvLac had an optimum pH of 3.5 (Figure 3A). After being immobilized as magnetic NFs, a shift in the optimum pH from 3.5 to 4.0 was observed for immobilized TvLac as Fe3O4/Mn-TvLac and Mn-TvLac NFs. In contrast, no change in the optimal pH was recorded for free laccase and its NFs based on Co and Cu [39,41]. The Fe3O4/Mn-TvLac and Mn-TvLac NFs showed better activity profiles than those of free TvLac at lower and higher pH values of 2.5 and 6.0, respectively, under optimal conditions. Under these pH conditions, Fe3O4/Mn-TvLac and Mn-TvLac NFs showed up to 1.9- and 14.2-fold higher residual activity than the TvLac with values of 42.5% at pH 2.5 and 2.8% at pH 6.0, respectively. Previously, similar pH profiles were reported for free and Cu-NFs-based laccase [41]. A similar shift in the temperature-activity profiles of the Fe3O4/Mn-TvLac and Mn-TvLac NFs was observed over that of free TvLac (Figure 3B). Optimal temperature values of 40 °C for free TvLac and 45 °C to immobilized TvLac as Fe3O4/Mn-TvLac and Mn-TvLac NFs were measured. In contrast, a similar optimum temperature value was noted for free and immobilized laccase NFs synthesized using Cu and Co ions [41,45]. Another study on laccase immobilization using metal-organic frameworks showed analogous optimal temperature values for free and encapsulated enzymes [46]. Further, an increase in temperature above the optimum values resulted in the continual decline of the residual activity of TvLac up to 80 °C. At 80 °C, the TvLac, Fe3O4/Mn-TvLac, and Mn-TvLac NFs retained residual activities of 22.4%, 78.6%, and 90.6%, respectively. The Mn-based NFs displayed a wide pH and temperature activity profiles compared to the corresponding free TvLac. Above results imply that encapsulating partially purified TvLac through magnetic nanoparticles and Mn-based inorganic-protein hybrids could be more advantageous for shifting pH and temperature optimal values than when immobilizing purified-laccase using Co- or Cu-based NFs [39,41,45].
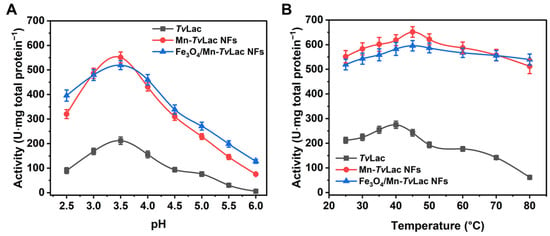
Figure 3.
Activity profiles of free and immobilized TvLac at different (A) pH values and (B) temperatures.
Among various substrates, TvLac showed high substrate specificity towards ABTS (Table S4). Using ABTS as the substrate, the kinetic parameter values of partially purified TvLac were observed, with a Km of 38.1 µM and Vmax of 219 µmol∙min−1∙mg protein−1 (Table 2). Fe3O4/Mn-TvLac and Mn-TvLac NFs exhibited adequate affinity against ABTS compared to the free TvLac, indicating a decline in Km with corresponding values of 17.6 and 17.1 µM, respectively. Previously, the comparable substrate affinity (Km of ~3.0 µM) towards free and immobilized Co-based NFs was described [40], whereas a 1.5-fold decline in substrate affinity was reported for TvLac and Zn-based NFs for an over-free laccase value of 6.3 µM [39]. After immobilization, the Vmax was enhanced to 542 µmol∙min−1∙mg protein−1 for Fe3O4/Mn-TvLac, and 570 µmol∙min−1∙mg protein−1 for Mn-TvLac. In contrast, no considerable Km and Vmax variations were reported on TvLac towards ABTS after immobilization as Co-based NFs [42].

Table 2.
Kinetic parameters of free and immobilized partially purified TvLac as inorganic-protein hybrid NFs.
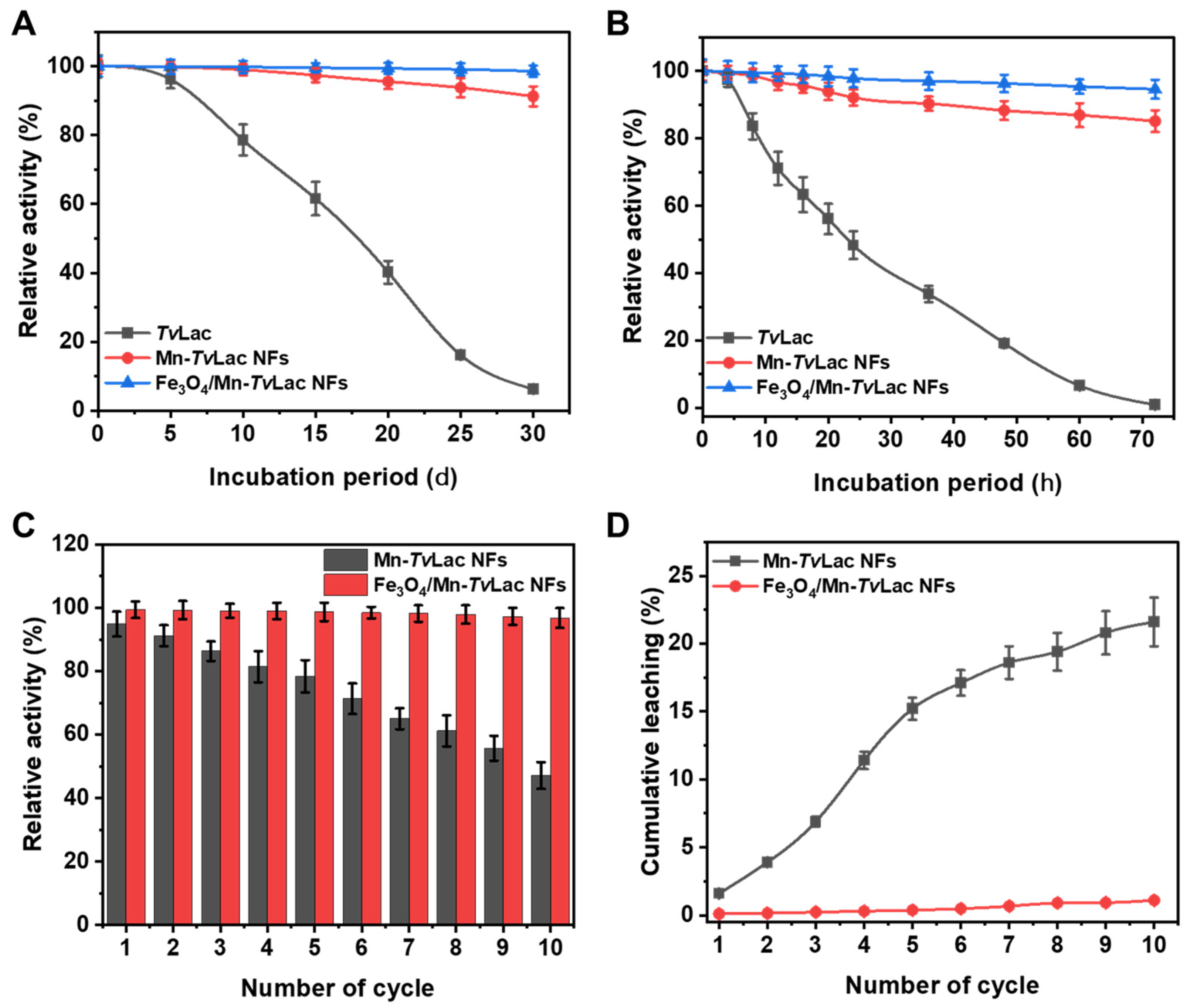
3.4. Storage, Thermal Stability, Reusability, and Leaching
Enzyme properties after immobilization, especially positive alternation in activity for better stability, are essential for defining the success of immobilization methods [39,47,48]. Initially, the room temperature storage stability of free TvLac, Fe3O4/Mn-TvLac, and Mn-TvLac NFs were compared after incubation up to 72 h under optimum pH conditions at 25 °C (Figure 4A). A successive decline in the activity of free TvLac was observed during the increasing incubation period, with ~99% loss of residual activity at 72 h. Under similar incubation conditions, Mn-TvLac and Fe3O4/Mn-TvLac NFs retained significantly higher residual activities of 85.1% and 94.6%, respectively. Here, the beneficial influence of immobilization was observed via improvements in TvLac’s storage stability by up to 135-fold. Previously, Cu-based immobilized laccase NFs showed a minor 4-fold improvement over that of free enzymes upon storage at room temperature [48]. Following one month of storage at 4 °C, the residual activity of free TvLac declined to 6.3% (Figure 4B). At similar incubation periods, Mn-TvLac and Fe3O4/Mn-TvLac NFs lost only marginal residual activities of 8.7% and 1.4%, respectively. Previously, Co-based laccase NFs demonstrated an insignificant 1.4-fold improvement, corresponding to free enzymes, after 40 days of incubation [39]. Meanwhile, Cu-derived laccase NFs at 4 °C storage for 10 days exhibited analogous residual activity [48]. Furthermore, the thermal stability of free TvLac, immobilized Fe3O4/Mn-TvLac, and Mn-TvLac NFs were investigated under optimum pH conditions at various temperatures to validate successful immobilization (Table 3). Thermostability measured for free TvLac at different temperatures showed a t1/2 value of 12.2 h (40 °C), 9.12 h (45 °C), 6.21 h (50 °C), 4.03 h (60 °C), and 0.90 h (70 °C). Whereas, Mn-TvLac exhibited a t1/2 of 25.6, 18.3, 14.1, 11.9, and 3.80 h at 40, 45, 50, 60, and 70 °C under similar conditions, respectively. In contrast, Fe3O4/Mn-TvLac showed higher stability, with up to 2.5-, 2.9-, 3.4-, 4.3-, and 8.6-fold higher t1/2 values at the corresponding temperatures than those of free TvLac. Previously, at 45 °C, laccase immobilized as Co-based NFs exhibited reduced residual activity compared to the free enzyme [39].
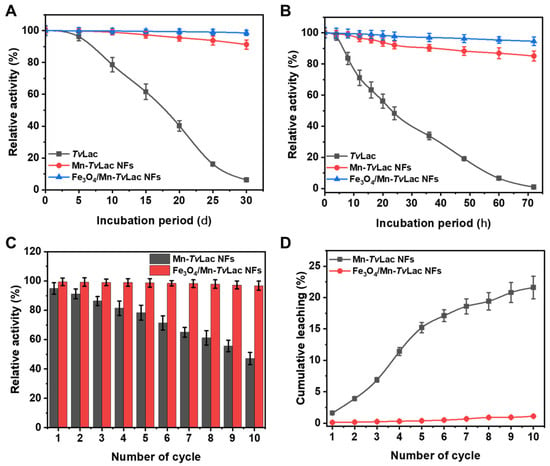
Figure 4.
Storage stability of free and immobilized TvLac at (A) room temperature, (B) 4 °C, (C) reusability, and (D) leaching.

Table 3.
The thermal deactivation constant (kd) and t1/2 of free and immobilized TvLac as inorganic-protein hybrid NFs.
After successive recycling, the activities of Fe3O4/Mn-TvLac and Mn-TvLac NFs were reduced (Figure 4C). For the Mn-TvLac NFs, a decline in residual activity to 78.4% and 47.1% was observed after five and 10 reuse cycles, respectively. Under similar recycling conditions, Fe3O4/Mn-TvLac retained much better residual activities of 98.7% and 96.8%, respectively. Under various recycling conditions, an immobilized system based on purified laccase exhibited residual activities of (i) ~50% after 10 cycles for Co-based, and dendrimer-grafted silica-coated hercynite-copper phosphate magnetic NFs [39,47], (ii) 25–40% after five cycles for ferrite microparticles based on Ni-Zn and Ni-Zn-Co [49], (iii) 30% after 10 cycles for Cu-based NFs [45], and (iv) 40% after 12 cycles for Zn-based NFs [41]. During the reusability test, successive decreases in the immobilized laccase residual activity of Mn-TvLac can be linked to the leaching of bound enzymes or their inactivation due to the deformation of the NF matrix [18,40]. Further, higher cumulative leaching is confirmed by higher structural deformation in Mn-TvLac than Fe3O4/Mn-TvLac (Figure S7). The high reuse stability of Fe3O4/Mn-TvLac was validated by its remarkably low cumulative leaching of 1.1% compared to the 21.6% of Mn-TvLac (Figure 4D).
3.5. Acute Toxicity Analysis of Nanoflowers
Nanomaterials, especially nanoparticles, are toxic to aquatic biodiversity [50]. The Fe3O4/Mn-TvLac exhibited an EC50 of 980 µg∙mL−1 compared to the 325 µg∙mL−1 of pure Fe3O4 nanoparticles for V. fischeri (Table 4). Whereas, synthesized NFs without Fe3O4 have considerably lower toxicity than Fe3O4/Mn-TvLac with an EC50 of 695 µg∙mL−1. Here, the larger size of Fe3O4/Mn-TvLac (7–9 μm) can be associated with a lower toxicity than Fe3O4 nanoparticles (~50 nm) that can quickly internalize within the cell via cell membrane pores (75–90 nm) and inhibit V. fischeri growth [51,52]. This finding implies that the synthesized magnetic NFs are more eco-friendly than pure Fe3O4 nanoparticles since they require ~3.0-fold higher concentrations to achieve a 50% viability reduction in V. fischeri. Previously, dust samples containing Fe and Mn showed high toxicity (90%) toward V. fischeri [51]. With a lower incubation period of 5 min, an EC50 of 240 µg∙mL−1 was reported for Fe3O4 nanoparticles against V. fischeri [52].

Table 4.
The acute toxicity level of Fe3O4 nanoparticles and synthesized inorganic-protein hybrid NFs.
3.6. Decolorization of Synthetic Dyes in the Presence of Inhibitors via Immobilized TvLac
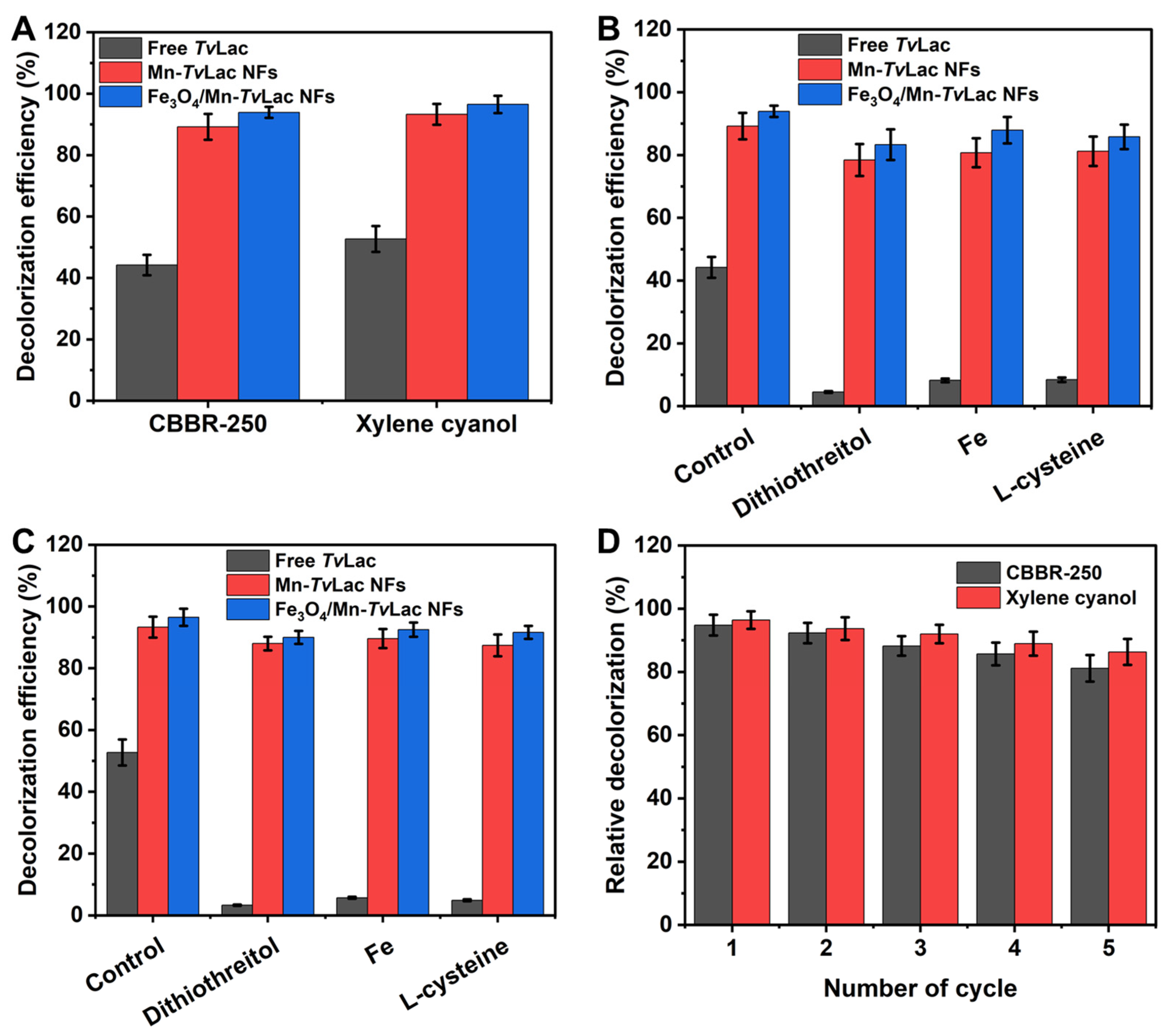
To investigate the prospective application of free and immobilized TvLac as Mn-TvLac and Fe3O4/Mn-TvLac NFs, the decolorization of CBBR-250 and xylene cyanol was evaluated at 25 °C during a 48 h incubation period and under agitation (100 rpm). The decolorization of dyes was enhanced with an increase in the incubation period up to 48 h (Table S5). Subsequently, the decolorization efficiency stabilized after 72 h of incubation. Incomplete decolorization may be associated with enzyme inactivation or substrate inhibition. In contrast, Fe3O4 nanoparticles and heat-inactivated Fe3O4/Mn-TvLac, used as controls, showed less than 1% dye decolorization, which could be associated with their inherent properties for dye adsorption. Free TvLac showed decolorization efficiencies of 44.2% and 52.7% for CBBR-250 and xylene cyanol, respectively (Figure 5A). Under similar incubation conditions, the Mn-TvLac NFs exhibited higher decolorization efficiencies (89.2% for CBBR-250 and 93.3% for xylene cyanol), whereas a maximum decolorization of 93.9% and 96.5% for CBBR-250 and xylene cyanol was observed by Fe3O4/Mn-TvLac NFs, respectively. Previously, lower decolorization efficiencies were reported in the range of 5–40% for Coomassie Brilliant Blue by Aspergillus oryzae-, Paraconiothyrium varia-bile-, and T. versicolor-based laccases [53]. In contrast, a low 36% decolorization of Coomassie Brilliant Blue by immobilized laccase from Myceliophthora thermophila on epoxy-functionalized silica was observed over free laccase with a value of 53% under similar reaction conditions [54]. Xylene cyanol decolorization of up to 35% was demonstrated using Phanerochaete chrysosporium-based lignin peroxidase combined with glucose oxidase in the presence of external hydrogen peroxide [55]. Similar dyes such as Brilliant Blue X-BR and Remazol Brilliant Blue R have been used for free and immobilized TvLac on chitosan beads [56]. Dithiothreitol, Fe(II) ions, and l-cysteine are well-known potent inhibitors of laccase that can potentially inhibit laccase bioremediation during waste treatment [36,57]. Therefore, decolorization of these dyes was assessed in the presence of these inhibitors. A remarkable decline in the decolorization efficiency from 44.2% to 4.5% for CBBR-250 and from 52.7% to 3.3% for xylene cyanol by TvLac was observed in the presence of these potent laccase inhibitors (Figure 5B,C). In contrast, Mn-TvLac and Fe3O4/Mn-TvLac retained excellent residual decolorization efficiencies of up to 87.9% for CBBR-250 and up to 92.5% for xylene cyanol. Furthermore, repeated batch decolorization of these dyes occurred when using Mn-TvLac and Fe3O4/Mn-TvLac NFs in the presence of 0.1 mM Fe(II). After five reuse cycles, Mn-TvLac and Fe3O4/Mn-TvLac retained residual decolorization efficiencies of up to 81.1% and 86.3% for CBBR-250 and xylene cyanol, respectively (Figure 5D). Previously, a low Brilliant Blue decolorization of 63.2% was reported for TvLac immobilized on lectin-modified cryogels [58]. These findings suggest that the immobilization of TvLac using magnetic nanoparticles and Mn metals as inorganic protein hybrid NFs is beneficial for retaining a high decolorization efficiency in the presence of enzyme inhibitors.
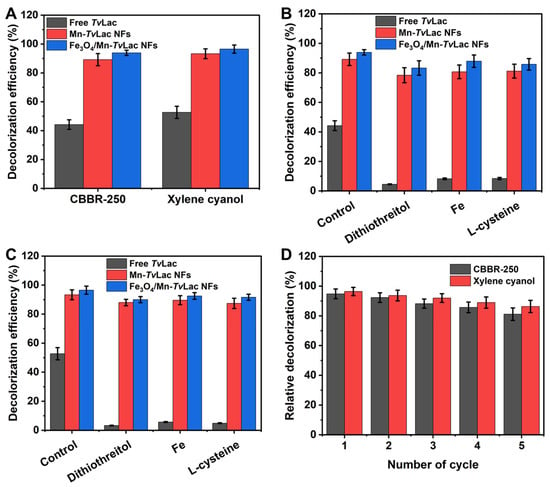
Figure 5.
Decolorization of dyes by free and immobilized TvLac (A), decolorization in the presence of laccase inhibitors for CBBR-250 (B), and xylene cyanol (C), and reusability in the presence of 0.1 mM of Fe(II) (D).
4. Conclusions
Enzyme immobilization plays a crucial role in various biotechnological applications, including in biocatalysis and wastewater treatment. In this study, a green synthesis approach was successfully employed to synthesize magnetic inorganic-protein hybrid NFs using partially purified TvLac, Fe3O4 nanoparticles, and Mn for potential application in the decolorization of dyes in the presence of inhibitors. The synthesized Fe3O4/Mn-TvLac and Mn-TvLac NFs showed broad pH and temperature profiles, improved catalytic properties, and high storage and thermostability compared to those of free TvLac. Incorporating Fe3O4 nanoparticles was found to be more suitable for achieving higher reusability and decolorization efficiency of dyes in the presence of known potent laccase inhibitors than when free enzymes are used. The synthesized NFs exhibited superior biocompatibility towards V. fischeri when compared to the Fe3O4 nanoparticles. This is the first report demonstrating the TvLac- and Mn-based one-pot synthesis of eco-friendly magnetic protein-inorganic hybrids that can be utilized for broad biotechnological applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma17081790/s1, Table S1. Details of the partial purification of Trametes versicolor laccase; Table S2. Activity of partially purified Trametes versicolor laccase in the presence of manganese ions; Table S3: Immobilization of TvLac as magnetic inorganic-protein hybrid nanobiocatalysts as NFs at different incubation; Table S4: The activity measurements of TvLac using various substrates; Table S5: The decolorization of dyes by free and immobilized laccase as NFs; Figure S1. Trametes versicolor laccase production profile in the presence of different inducers (0.5 mM). Figure S2. Activity measurements by the spectrophotometric procedure for produced laccase; Figure S3: The field-emission scanning electron microscopy images of magnetic nanoflowers synthesized after 3 h (A), 12 h (B), and 24 h (C) incubation at 4°C; Figure S4: Field-emission scanning electron microscopy and elemental mapping analysis of Mn3(PO4)2-laccase (Mn-TvLac) nanoflowers. (A) field-emission scanning electron micrograph of the inorganic-protein hybrids as Mn-TvLac nanoflowers, and (B-E) elemental mapping analysis for nitrogen (B), oxygen (C), manganese (D), and phosphorus (E); Figure S5: X-ray diffraction patterns of inorganic-protein hybrid nanoflowers (NFs). Mn-TvLac = Mn3(PO4)2-laccase; Figure S6: Magnetic property measurements of Fe3O4 nanoparticles and Fe3O4/Mn-TvLac hybrids (A), recovery of magnetic NFs by applying an external magnetic field (B), and under recycling conditions (C); Figure S7: The field-emission scanning electron microscopy images of Fe3O4/Mn-TvLac (A and B) and Mn-TvLac NFs (C and D): before recycling (A and C) and after ten cycles of reusability (B and D).
Author Contributions
Conceptualization, S.K.S.P. and J.-K.L.; methodology, S.K.S.P. and R.K.G.; software, J.-K.L.; validation, S.K.S.P., R.K.G. and J.-K.L.; formal analysis, S.K.S.P., K.K.K., D.K.P., S.R. and P.P.; investigation, S.K.S.P. and R.K.G.; resources and data curation, S.K.S.P., R.K.G., K.K.K., D.K.P., S.R., P.P. and J.-K.L.; writing—original draft preparation, S.K.S.P. and R.K.G.; writing—review and editing, S.K.S.P. and J.-K.L.; visualization, S.K.S.P. and J.-K.L.; supervision and project administration, J.-K.L.; funding acquisition, J.-K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) and funded by the Ministry of Science, ICT & Future Planning (grant numbers NRF-2022M3A9I3082366, RS-2023-00222078). This paper was supported by the Konkuk University Researcher Fund in 2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sheldon, R.A.; Woodley, J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Gupta, R.K.; Kim, I.-W.; Lee, J.-K. Coriolus versicolor laccase-based inorganic protein hybrid synthesis for application in biomass saccharification to enhance biological production of hydrogen and ethanol. Enzym. Microb. Technol. 2023, 170, 110301. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-Y.; Li, F.-L.; Zhang, Y.-W.; Gupta, R.K.; Patel, S.K.S.; Lee, J.-K. Recent strategies for the immobilization of therapeutic enzymes. Polymers 2022, 14, 1409. [Google Scholar] [CrossRef] [PubMed]
- Nezhad, N.G.; Rahman, R.N.Z.R.A.; Normi, Y.M.; Oslan, S.N.; Shariff, F.M.; Leow, T.C. Recent advances in the simultaneous thermostability-activity improvement of industrial enzymes through structure modification. Int. J. Biol. Macromol. 2023, 232, 123440. [Google Scholar] [CrossRef]
- Patel, H.; Upadhyay, R.V.; Parekh, K.; Reis, D.; Oliveira, C.L.P.; Neto, A.M.F. Optimized Mn0.5Zn0.5Fe2O4 nanoflowers based magnetic fluids for potential biomedical applications. J. Magn. Magn. Mater. 2024, 590, 171656. [Google Scholar] [CrossRef]
- Anwar, A.; Imran, M.; Iqbal, H.M.N. Smart chemistry and applied perceptions of enzyme-coupled nano-engineered assemblies to meet future biocatalytic challenges. Coord. Chem. Rev. 2023, 493, 215329. [Google Scholar] [CrossRef]
- Mohidem, N.A.; Mohamad, M.; Rashid, M.U.; Norizan, M.N.; Hamzah, F.; Mat, H.B. Recent advances in enzyme immobilisation strategies: An overview of techniques and composite carriers. J. Compos. Sci. 2023, 7, 488. [Google Scholar] [CrossRef]
- Lee, I.; Cheon, H.J.; Adhikari, M.D.; Tran, T.D.; Yeon, K.-M.; Kim, M.I.; Kim, J. Glucose oxidase-copper hybrid nanoflowers embedded with magnetic nanoparticles as an effective antibacterial agent. Int. J. Biol. Macromol. 2020, 155, 1520–1531. [Google Scholar] [CrossRef] [PubMed]
- Jafari-Nodoushan, H.; Mojtabavi, S.; Faramarzi; Samadi, N. Organic-inorganic hybrid nanoflowers: The known, the unknown, and the future. Adv. Colloid Interface Sci. 2022, 309, 102780. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Chun, M.; Kim, M.I.; Ha, S.H. Preparation of glutaraldehyde-treated lipaseinorganic hybrid nanoflowers and their catalytic performance as immobilized enzymes. Enzym. Microb. Technol. 2017, 105, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.D.; Kelkar, R.K.; Patil, N.P.; Pise, P.V.; Patil, S.P.; Patil, A.S.; Kulkarni, N.S.; Tiwari, M.S.; Phirke, A.N.; Nadar, S.S. Magnetic nanoflowers: A hybrid platform for enzyme immobilization. Crit. Rev. Biotechnol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Asgher, M.; Shah, S.Z.H.; Iqbal, H.M.N. Engineering enzyme-coupled hybrid nanoflowers: The quest for optimum performance to meet biocatalytic challenges and opportunities. Int. J. Biol. Macromol. 2019, 135, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; Azzazy, H.M.E. Enzyme immobilization technologies and industrial applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Chen, R.; Hui, Y.; Chen, D.; Zhao, C.-X. Boosting enzyme activity in enzyme metal–organic framework composites. Chem. Bio. Eng. 2024, 1, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, H.; Su, Z. Enzyme-based hybrid nanoflowers with high performances for biocatalytic, biomedical, and environmental applications. Coord. Chem. Rev. 2020, 416, 213342. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Yang, C.; Ma, C.; Tang, J. Enzyme-inorganic hybrid nanoflowers: Classification, synthesis, functionalization and potential applications. Chem. Eng. J. 2021, 415, 129075. [Google Scholar] [CrossRef]
- Ge, J.; Lei, J.; Zare, R.N. Protein-inorganic hybrid nanoflowers. Nat. Nanotechnol. 2012, 7, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, F.P.; Cipolatti, E.P.; Junior, A.F.; Henriques, R.O. Nanoflowers: A new approach of enzyme immobilization. Chem. Rec. 2022, 22, e202100293. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Xing, J.; Ge, Z. Preparation of laccase-loaded magnetic nanoflowers and their recycling for efficient degradation of bisphenol A. Sci. Total Environ. 2019, 651, 2857–2865. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, M.; Ahmadpoor, F.; Shojaosadati, S.A. Mussel-inspired magnetic nanoflowers as an effective nanozyme and antimicrobial agent for biosensing and catalytic reduction of organic dyes. ACS Omega 2020, 5, 18766–18777. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Luo, P.; Wang, L.; Li, C.; Mao, Y.; Wang, Y. Construction of magnetic nanoflower biocatalytic system with enhanced enzymatic performance by biomineralization and its application for bisphenol A removal. J. Hazard. Mater. 2019, 380, 120901. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Jiao, X.; Hu, H.; Shen, X.; Zhao, J.; Feng, Y.; Li, C.; Du, Y.; Cui, J.; Jia, S. Activated magnetic lipase-inorganic hybrid nanoflowers: A highly active and recyclable nanobiocatalyst for biodiesel production. Renew. Energy 2021, 171, 825–832. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Anwar, M.Z.; Kumar, A.K.; Otari, S.V.; Pagolu, R.T.; Kim, S.-Y.; Kim, I.-W.; Lee, J.-K. Fe2O3 yolk-shell particle-based laccase biosensor for efficient detection of 2,6-dimethoxyphenol. Biochem. Eng. J. 2018, 132, 1–8. [Google Scholar] [CrossRef]
- Shraddha, X.; Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: Microbial sources, production, purification, and potential biotechnological applications. Enzym. Res. 2011, 2011, 217861. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Varma, A.; Jha, S.; Patel, S.K.S.; Porwal, S. Production, purification, and characterization of recombinant Bhargavaea beijingensis laccase for potential lignin degradation and dyes decolorization. Catal. Lett. 2024, 154, 1537–1546. [Google Scholar] [CrossRef]
- Chen, Z.; Oh, W.-D.; Yap, P.-S. Recent advances in the utilization of immobilized laccase for the degradation of phenolic compounds in aqueous solutions: A review. Chemosphere 2022, 307, 135824. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, B.B.; Saibi, S.; Haroune, L.; Rios, N.S.; Gonçalves, L.R.B.; Cabana, H. Genipin and glutaraldehyde based laccase two-layers immobilization with improved properties: New biocatalysts with high potential for enzymatic removal of trace organic contaminants. Enzym. Microb. Technol. 2023, 169, 110261. [Google Scholar] [CrossRef] [PubMed]
- Morsy, S.A.G.Z.; Tajudin, A.A.; Ali, M.S.M.; Shariff, F.M. Current development in decolorization of synthetic dyes by immobilized laccases. Front. Microbiol. 2020, 11, 572309. [Google Scholar] [CrossRef] [PubMed]
- Peñaranda, P.A.; Noguera, M.J.; Florez, S.L.; Husserl, J.; Ornelas-Soto, N.; Cruz, J.C.; Osma, J.F. Treatment of wastewater, phenols and dyes using novel magnetic torus microreactors and laccase immobilized on magnetite nanoparticles. Nanomaterials 2022, 12, 1688. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hou, J.; Duan, L.; Ji, C.; Zhang, Y.; Chen, V. Graphene oxide-enzyme hybrid nanoflowers for efficient water soluble dye removal. J. Hazard. Mater. 2017, 445, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.K.; Malhotra, M.; Kurmi, A.A.; Narani, A.; Bhaskar, T.; Ghosh, S.; Jain, S.L. Jute sticks biomass delignification through laccase-mediator system for enhanced saccharification and sustainable release of fermentable sugar. Chemosphere 2022, 286, 131687. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Lu, C.; Li, X.; Zhou, J.; Wang, J. Lanthanum: A novel inducer for enhancement of fungal laccase production by Shiraia bambusicola. J. Rare Earth 2022, 40, 508–516. [Google Scholar] [CrossRef]
- Cheon, H.J.; Adhikari, M.D.; Chung, M.; Tran, T.D.; Kim, J.; Kim, M.I. Magnetic nanoparticles-embedded enzyme-inorganic hybrid nanoflowers with enhanced peroxidase-like activity and substrate channeling for glucose biosensing. Adv. Healthc. Mater. 2019, 8, 1801507. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Otari, S.V.; Kang, Y.C.; Lee, J.-K. Protein–inorganic hybrid system for efficient histagged enzymes immobilization and its application in L-xylulose production. RSC Adv. 2017, 7, 3488–3494. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Otari, S.V.; Li, J.; Kim, D.R.; Kim, S.C.; Cho, B.K.; Kalia, V.C.; Kang, Y.C.; Lee, J.-K. Synthesis of cross-linked protein-metal hybrid nanoflowers and its application in repeated batch decolorization of synthetic dyes. J. Hazard. Mater. 2018, 347, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Kalia, V.V.; Lee, J.-K. Laccase immobilization on copper-magnetic nanoparticles for efficient bisphenol degradation. J. Microbiol. Biotechnol. 2023, 33, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Cen, Q.; Wu, X.; Cao, L.; Lu, Y.; Lu, X.; Chen, J.; Fu, G.; Liu, Y.; Ruan, R. Green production of a yellow laccase by Coriolopsis gallica for phenolic pollutants removal. AMB Express 2022, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Vojdanitalab, K.; Jafari-Nodoushan, H.; Mojtabavi, S.; Shokri, M.; Jahandar, H.; Faramarzi, M. Instantaneous synthesis and full characterization of organic–inorganic laccase-cobalt phosphate hybrid nanoflowers. Sci. Rep. 2022, 12, 9297. [Google Scholar] [CrossRef] [PubMed]
- Jafari-Nodoushan, H.; Fazeli, M.R.; Faramarzi, M.A.; Samadi, N. Hierarchically-structured laccase@Ni3(PO4)2 hybrid nanoflowers for antibiotic degradation: Application in real wastewater effluent and toxicity evaluation. Int. J. Biol. Macromol. 2023, 234, 123574. [Google Scholar] [CrossRef]
- Al-Maqdi, K.A.; Elmerhi, N.; Alzamly, A.; Shah, I.; Ashraf, S.S. Laccase–copper phosphate hybrid nanoflower as potent thiazole remediation agent. J. Water Process Eng. 2023, 51, 103438. [Google Scholar] [CrossRef]
- Kiani, M.; Mojtabavi, S.; Jafari-Nodoushan, H.; Tabib, S.R.; Hassannejad, N.; Faramarzi, M.A. Fast anisotropic growth of the biomineralized zinc phosphate nanocrystals for a facile and instant construction of laccase@Zn3(PO4)2 hybrid nanoflowers. Int. J. Biol. Macromol. 2022, 204, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Y.; Song, R.; Wang, M.; Yan, F.; He, L.; Feng, X.; Fang, S.; Zhao, J.; Zhang, H. Manganese(II) phosphate nanoflowers as electrochemical biosensors for the high-sensitivity detection of ractopamine. Sens. Actuators B Chem. 2015, 211, 310–317. [Google Scholar] [CrossRef]
- Rai, S.K.; Narnoliya, L.K.; Sangwan, R.S.; Yadav, S.K. Self-assembled hybrid nanoflowers of manganese phosphate and L-arabinose isomerase: A stable and recyclable nanobiocatalyst for equilibrium level conversion of D-galactose to D-tagatose. ACS Sustain. Chem. Eng. 2018, 6, 6296–6304. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Yang, C.; Ma, C.; Zhang, Y.; Tang, J. Facile synthesis of recyclable laccase-mineral hybrid complexes with enhanced activity and stability for biodegradation of Evans Blue dye. Int. J. Biol. Macromol. 2021, 188, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yuan, F.; Jia, S.; Zhang, X.; Xing, W. Laccase encapsulation immobilized in mesoporous ZIF-8 for enhancement bisphenol A degradation. J. Hazard. Mater. 2023, 445, 130460. [Google Scholar] [CrossRef] [PubMed]
- Rezayaraghi, F.; Jafari-Nodoushan, H.; Mojtabavi, S.; Golshani, S.; Jahandar, H.; Faramarzi, M.A. Hybridization of laccase with dendrimer-grafted silica-coated hercynite-copper phosphate magnetic hybrid nanoflowers and its application in bioremoval of Gemifloxacin. Environ. Sci. Pollut. Res. 2022, 29, 89255–89272. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Wang, H.; Zheng, J.; Wang, S.; Yu, S.; Lu, L. Ultrafast synthesis of laccase copper phosphate hybrid nanoflowers for efficient degradation of tetracycline antibiotics. Environ. Res. 2023, 216, 114690. [Google Scholar] [CrossRef] [PubMed]
- Bîtcan, I.; Petrovici, A.; Pellis, A.; Klebertc, S.; Karolyc, Z.; Bereczkic, L.; Peter, F.; Todea, A. Enzymatic route for selective glycerol oxidation using covalently immobilized laccases. Enzym. Microb. Technol. 2023, 163, 110168. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wu, L.; Xiao, X.; Rong, L.; Li, M.; Zou, X. Mixture toxicity of zinc oxide nanoparticle and chemicals with different mode of action upon Vibrio fischeri. Environ. Sci. Eur. 2020, 32, 41. [Google Scholar] [CrossRef]
- Ledda, C.; Rapisarda, V.; Bracci, M.; Proietti, L.; Zuccarello, M.; Fallico, R.; Fiore, M.; Ferrante, M. Professional exposure to basaltic rock dust: Assessment by the Vibrio fischeri ecotoxicological test. J. Occup. Med. Toxicol. 2013, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Espinosa, R.; Delgado, L.; Casals, E.; Gonzalez, E.; Puntes, V.; Barata, C.; Font, X.; Sanchez, A. Acute toxicity of cerium oxide, titanium oxide and iron oxide nanoparticles using standardized tests. Desalination 2011, 269, 136–141. [Google Scholar] [CrossRef]
- Forootanfar, H.; Moezzi, A.; Aghaie-Khozani, M.; Mahmoudjanlou, Y.; Ameri, A.; Niknejad, F.; Faramarzi, M.A. Synthetic dye decolorization by three sources of fungal laccase. Iran. J. Environ. Health Sci. Eng. 2012, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Salami, F.; Habibi, Z.; Yousefi, M.; Mohammadi, M. Covalent immobilization of laccase by one pot three component reaction and its application in the decolorization of textile dyes. Int. J. Biol. Macromol. 2018, 120, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Huang, X.; Hu, M.; Li, Y.; Qu, Y.; Gao, P.; Wu, D. High efficient degradation of dyes with lignin peroxidase coupled with glucose oxidase. J. Biotechnol. 2006, 123, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Cui, B.-K.; Wu, X.-J.; Meng, G.; Liu, H.-X.; Si, J. Immobilization of laccase onto chitosan beads to enhance its capability to degrade synthetic dyes. Int. Biodeterior. Biodegrad. 2016, 110, 69–78. [Google Scholar] [CrossRef]
- Li, S.; Liu, Q.; Liu, J.; Sun, K.; Yang, W.; Si, Y.; Li, Y.; Gao, Y. Inhibition mechanisms of Fe2+/Fe3+ and Mn2+ on fungal laccase-enabled bisphenol a polyreaction. Chemosphere 2022, 307, 135685. [Google Scholar] [CrossRef] [PubMed]
- Bayraktaroğlu, M.; Husein, I.; Uygun, A.D.; Uygun, M. Lectin-modified cryogels for laccase immobilization: A decolorization study. Water Air Soil Pollut. 2020, 231, 31. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).