Medium- and High-Entropy Rare Earth Hexaborides with Enhanced Solar Energy Absorption and Infrared Emissivity
Abstract
1. Introduction
2. Experimental
2.1. Composition Design
2.2. Materials and Methods
2.3. Analysis and Characterization
3. Results and Discussion
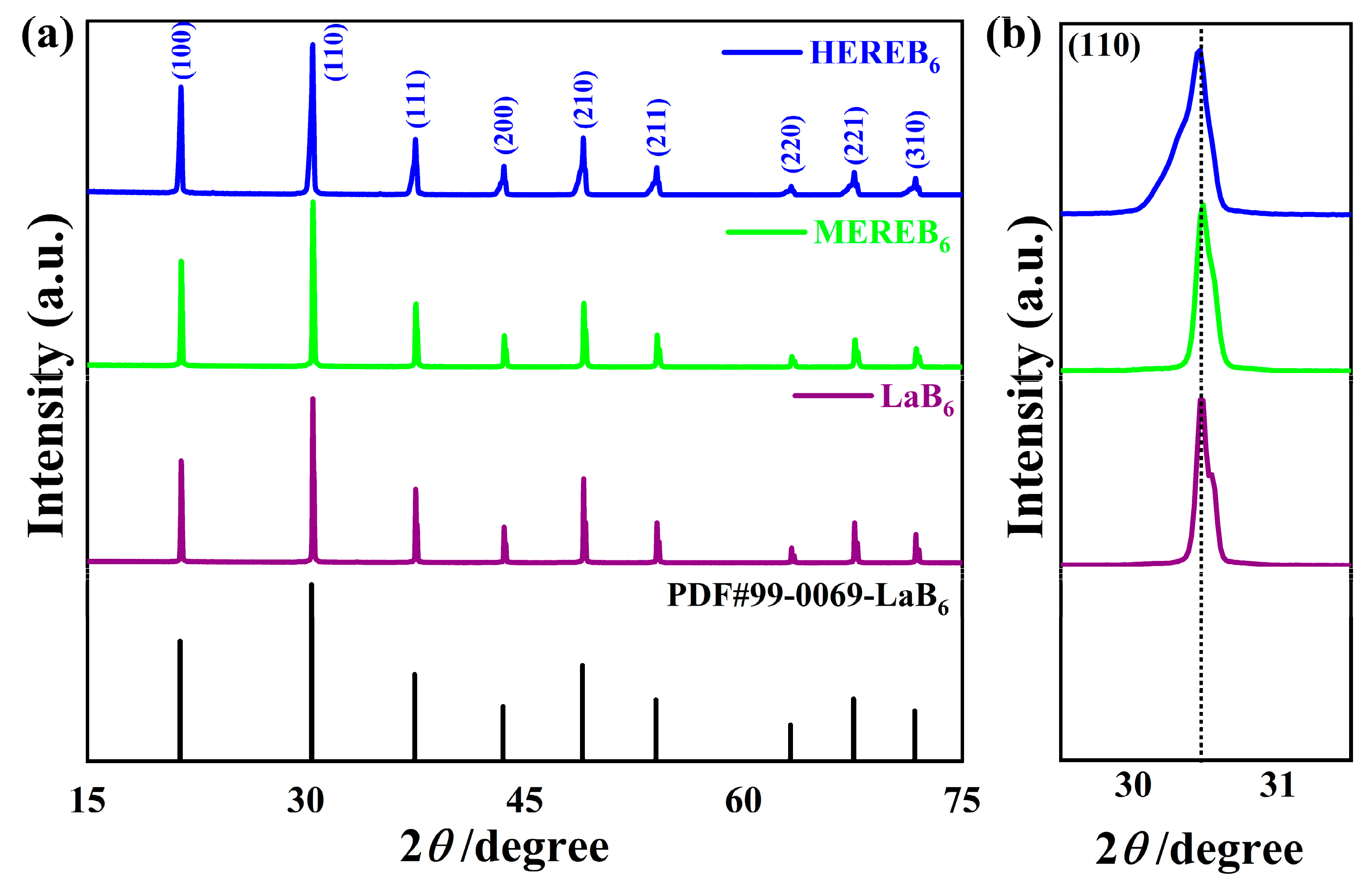
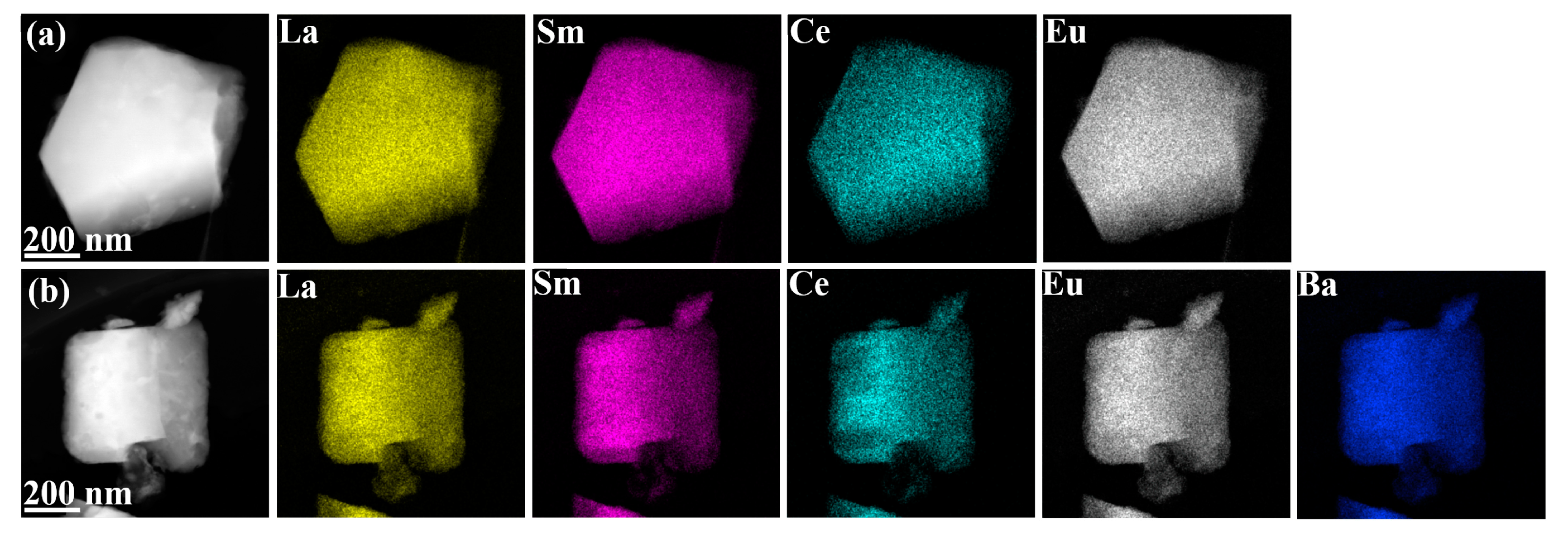
3.1. Phase Composition and Microstructure
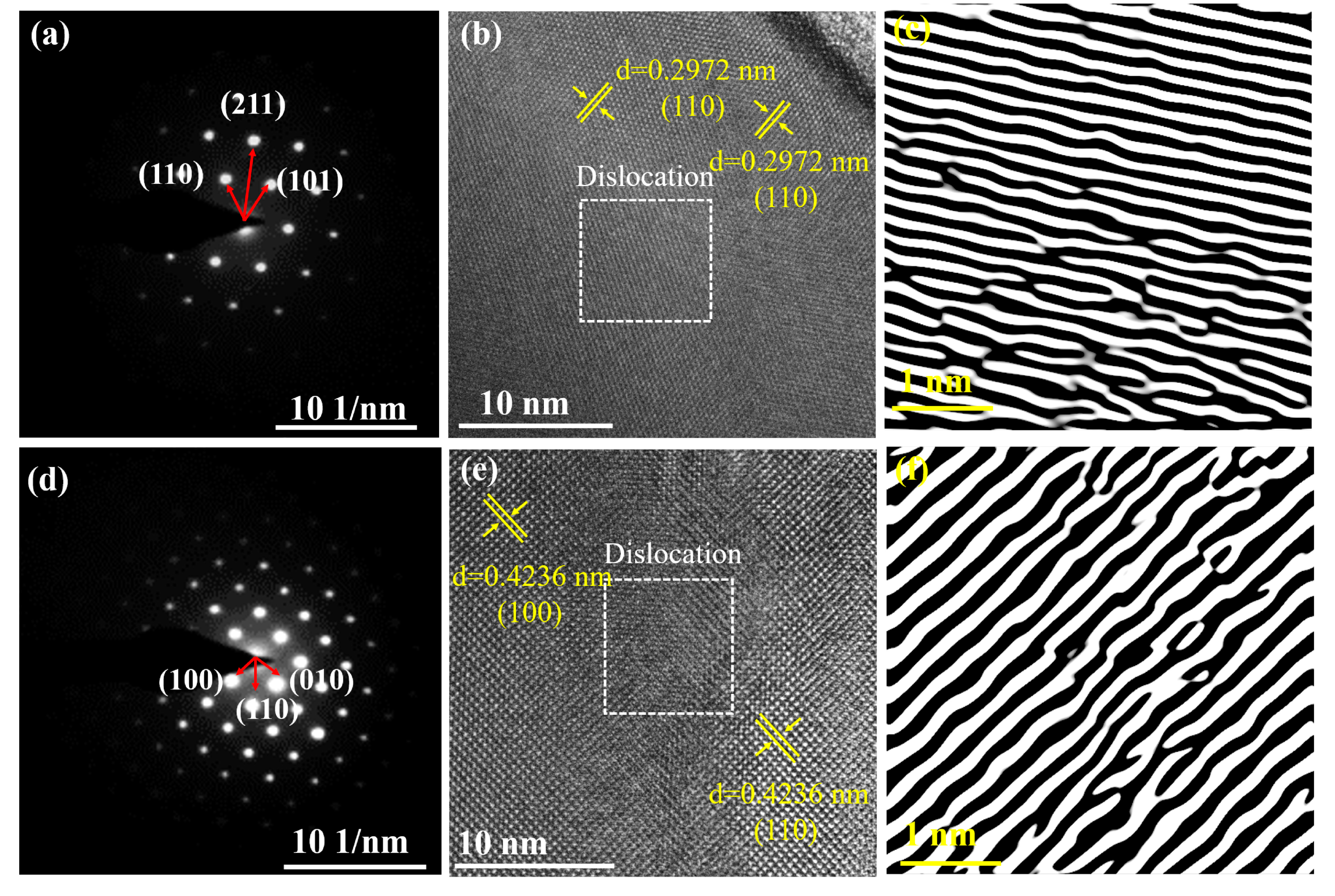
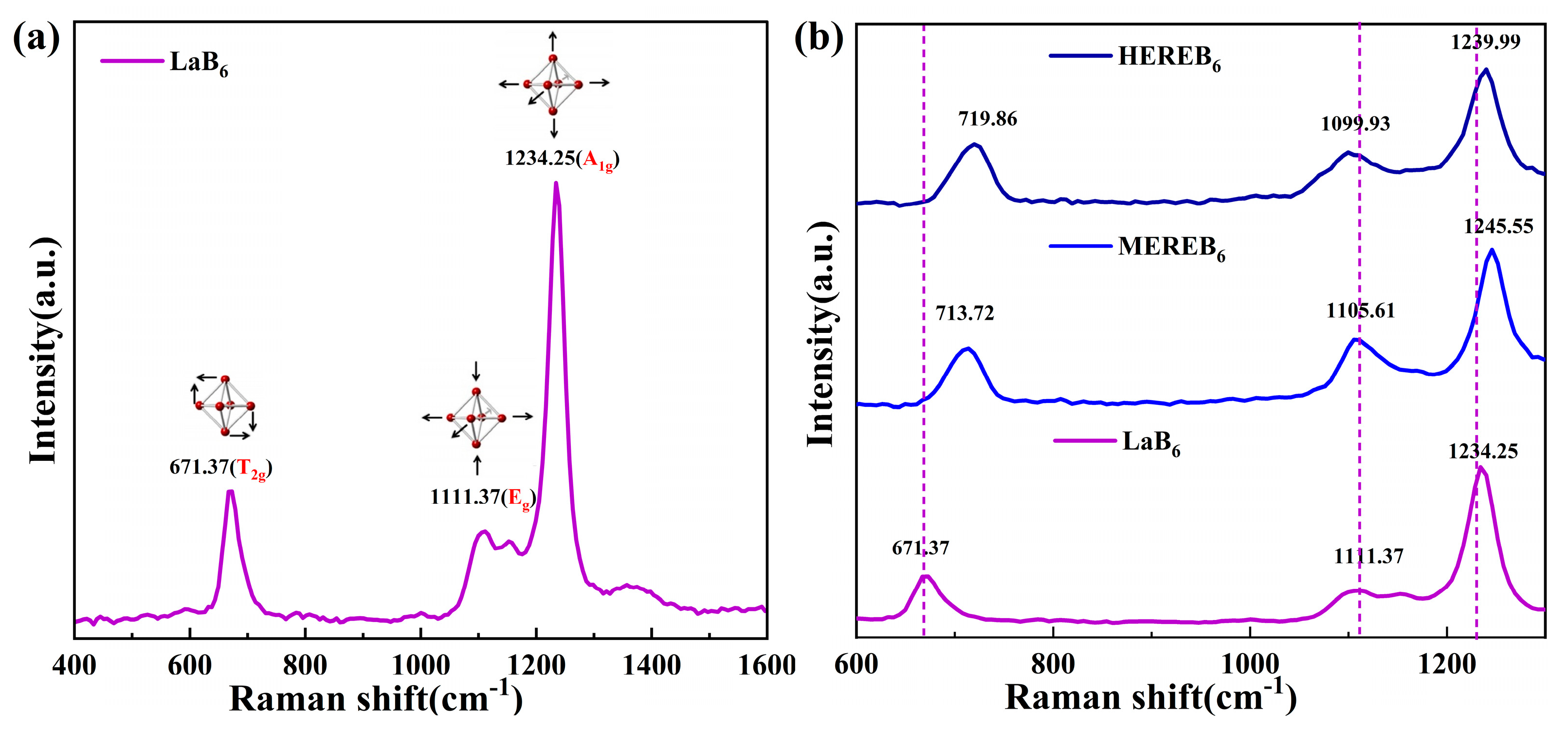
3.2. Lattice Distortion Analysis
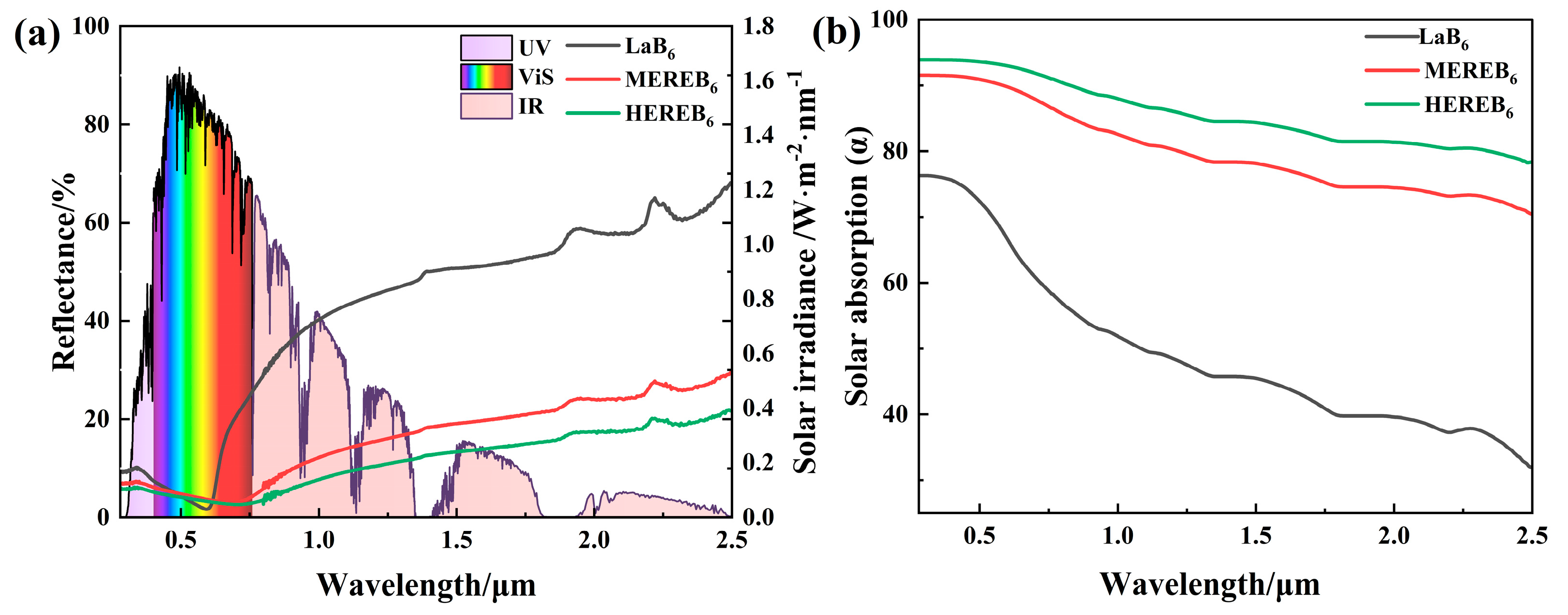
3.3. Solar Energy Absorption
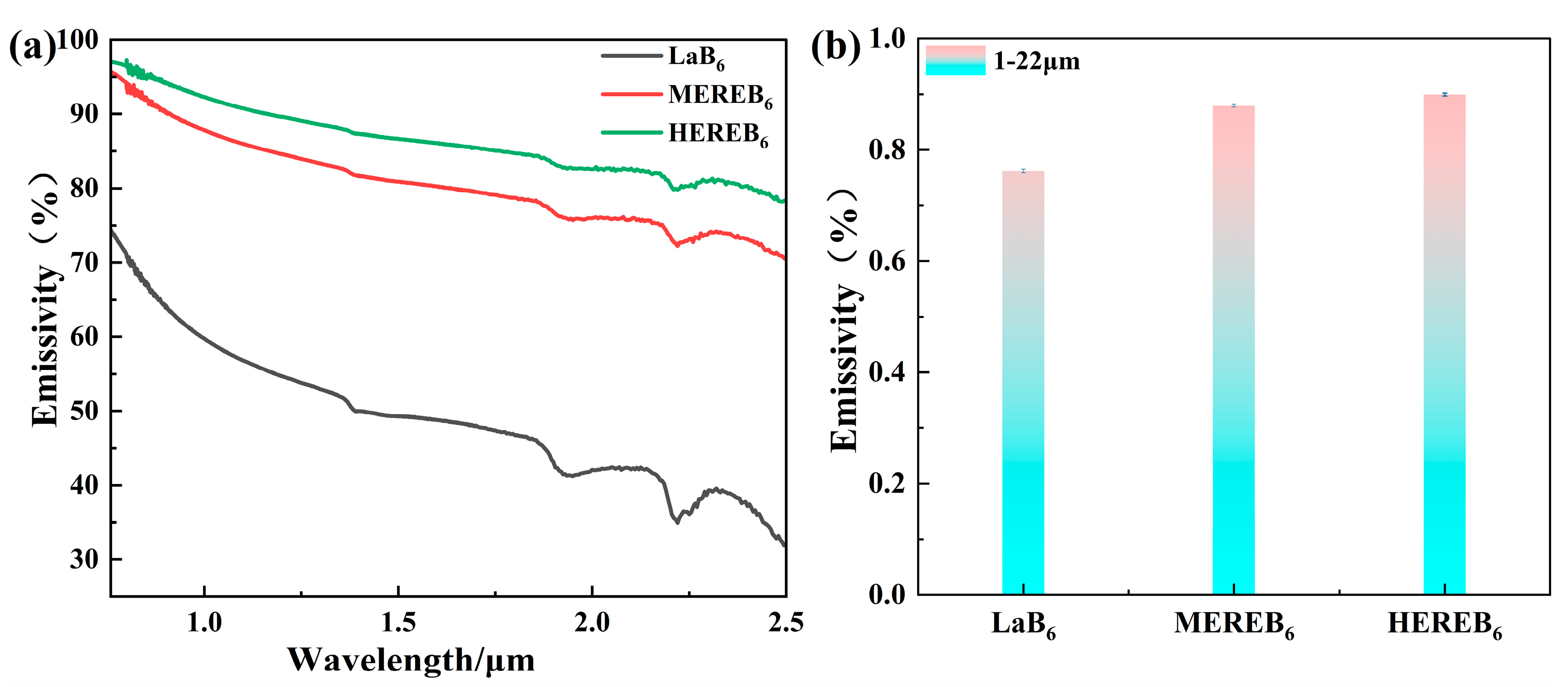
3.4. Infrared Emissivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, S.; Wei, Y.; Lu, Z.; Guo, S.; Chen, J.; Yu, Z. Review of Research Progress on Concentrated Solar Energy Utilization System. Renewables 2023, 1, 397–414. [Google Scholar] [CrossRef]
- Mehos, M.; Turchi, C.; Vidal, J.; Wagner, M.; Kruizenga, A.; Clifford, H.; William, K.; Charles, A.; Kruizenga, A. Concentrating Solar Power Gen3 Demonstration Roadmap; Office of Scientific and Technical Information (OSTI): Golden, CO, USA, 2017. [Google Scholar] [CrossRef]
- He, Y.-L.; Qiu, Y.; Wang, K.; Yuan, F.; Wang, W.-Q.; Li, M.-J.; Guo, J.-Q. Perspective of concentrating solar power. Energy 2020, 198, 117373. [Google Scholar] [CrossRef]
- Ho, C.K.; Christian, J.M.; Yellowhair, J.; Armijo, K.; Nguyen, C. Performance Evaluation of a High-Temperature Falling Particle Receiver. In Proceedings of the ASME 2016 14th International Conference on Fuel Cell Science, Engineering and Technology, Charlotte, NC, USA, 26–30 June 2016. [Google Scholar] [CrossRef]
- Le Gal, A.; Grange, B.; Casanova, M.; Perez, A.; Baltus, W.; Tessonneaud, M.; Flamant, G. Experimental results for a MW-scale fluidized particle-in-tube solar receiver in its first test campaign. Sol. Energy 2023, 262, 111907. [Google Scholar] [CrossRef]
- Nie, F.; Cui, Z.; Bai, F.; Wang, Z. Properties of solid particles as heat transfer fluid in a gravity driven moving bed solar receiver. Sol. Energy Mater. Sol. Cells 2019, 200, 110007. [Google Scholar] [CrossRef]
- Saeed, R.S.; Alswaiyd, A.; Saleh, N.S.; Alaqel, S.; Djajadiwinata, E.; El-Leathy, A.; Danish, S.N.; Al-Ansary, H.; Jeter, S.; Al-Suhaibani, Z.; et al. Characterization of Low-Cost Particulates Used as Energy Storage and Heat-Transfer Medium in Concentrated Solar Power Systems. Materials 2022, 15, 2946. [Google Scholar] [CrossRef]
- Chung, K.M.; Chen, R. Black coating of quartz sand towards low-cost solar-absorbing and thermal energy storage material for concentrating solar power. Sol. Energy 2023, 249, 98–106. [Google Scholar] [CrossRef]
- Baumann, T.; Zunft, S. Properties of granular materials as heat transfer and storage medium in CSP application. Sol. Energy Mater. Sol. Cells 2015, 143, 38–47. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Z.; Xu, X.; Ma, S.; Li, P.; Shi, X. Preparation and Thermal Shock Resistance of Mullite Ceramics for High Temperature Solar Thermal Storage. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2023, 38, 743–752. [Google Scholar] [CrossRef]
- Diago, M.; Iniesta, A.C.; Soum-Glaude, A.; Calvet, N. Characterization of desert sand to be used as a high-temperature thermal energy storage medium in particle solar receiver technology. Appl. Energy 2018, 216, 402–413. [Google Scholar] [CrossRef]
- Díaz-Heras, M.; Calderón, A.; Navarro, M.; Almendros-Ibáñez, J.A.; Fernández, A.I.; Barreneche, C. Characterization and testing of solid particles to be used in CSP plants: Aging and fluidization tests. Sol. Energy Mater. Sol. Cells 2021, 219, 110793–110811. [Google Scholar] [CrossRef]
- Radwan, O.A.; Humphrey, J.D.; Hakeem, A.S.; Zeama, M. Evaluating properties of Arabian desert sands for use in solar thermal technologies. Sol. Energy Mater. Sol. Cells 2021, 231, 111335–111345. [Google Scholar] [CrossRef]
- Siegel, N.P.; Gross, M.D.; Coury, R. The Development of Direct Absorption and Storage Media for Falling Particle Solar Central Receivers. J. Sol. Energy Eng. 2015, 137, 041003–041009. [Google Scholar] [CrossRef]
- Zhao, W.; Sun, Z.; Alwahabi, Z.T. Emissivity and absorption function measurements of Al2O3 and SiC particles at elevated temperature for the utilization in concentrated solar receivers. Sol. Energy 2020, 207, 183–191. [Google Scholar] [CrossRef]
- Kang, Q.; Flamant, G.; Dewil, R.; Baeyens, J.; Zhang, H.L.; Deng, Y.M. Particles in a circulation loop for solar energy capture and storage. Particuology 2019, 43, 149–156. [Google Scholar] [CrossRef]
- Jiang, K.; Du, X.; Kong, Y.; Xu, C.; Ju, X. A comprehensive review on solid particle receivers of concentrated solar power. Renew. Sustain. Energy Rev. 2019, 116, 109463. [Google Scholar] [CrossRef]
- Chao, L.; Bao, L.; Wei, W.; Tegus, O. A review of recent advances in synthesis, characterization and NIR shielding property of nanocrystalline rare-earth hexaborides and tungsten bronzes. Sol. Energy 2019, 190, 10–27. [Google Scholar] [CrossRef]
- Bao, L.; Qi, X.; Tana, T.; Chao, L.; Tegus, O. Synthesis, and magnetic and optical properties of nanocrystalline alkaline-earth hexaborides. CrystEngComm 2016, 18, 1223–1229. [Google Scholar] [CrossRef]
- Mattox, T.; Urban, J. Tuning the Surface Plasmon Resonance of Lanthanum Hexaboride to Absorb Solar Heat: A Review. Materials 2018, 11, 2473–2487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, B.; Ni, N.; Xiang, H.; Dai, F.-Z.; Wu, S.; Zhou, Y. High entropy rare earth hexaborides/tetraborides (HEREB6/HE REB4) composite powders with enhanced electromagnetic wave absorption performance. J. Mater. Sci. Technol. 2021, 87, 155–166. [Google Scholar] [CrossRef]
- Ji, X.H.; Zhang, Q.Y.; Xu, J.Q.; Zhao, Y.M. Rare-earth hexaborides nanostructures: Recent advances in materials, characterization and investigations of physical properties. Prog. Solid State Chem. 2011, 39, 51–69. [Google Scholar] [CrossRef]
- Sani, E.; Mercatelli, L.; Meucci, M.; Zoli, L.; Sciti, D. Lanthanum hexaboride for solar energy applications. Sci. Rep. 2017, 7, 718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-Z.; Reece, M.J. Review of high entropy ceramics: Design, synthesis, structure and properties. J. Mater. Chem. A 2019, 7, 22148–22162. [Google Scholar] [CrossRef]
- Chen, H.; Xiang, H.; Dai, F.-Z.; Liu, J.; Zhou, Y. Porous high entropy (Zr0.2Hf0.2Ti0.2Nb0.2Ta0.2)B2: A novel strategy towards making ultrahigh temperature ceramics thermal insulating. J. Mater. Sci. Technol. 2019, 35, 2404–2408. [Google Scholar] [CrossRef]
- Gild, J.; Zhang, Y.; Harrington, T.; Jiang, S.; Hu, T.; Quinn, M.C.; Mellor, W.M.; Zhou, N.; Vecchio, K.; Luo, J. High-Entropy Metal Diborides: A New Class of High-Entropy Materials and a New Type of Ultrahigh Temperature Ceramics. Sci. Rep. 2016, 6, 37946. [Google Scholar] [CrossRef]
- Chen, H.; Xiang, H.; Dai, F.-Z.; Liu, J.; Lei, Y.; Zhang, J.; Zhou, Y. High porosity and low thermal conductivity high entropy (Zr0.2Hf0.2Ti0.2Nb0.2Ta0.2)C. J. Mater. Sci. Technol. 2019, 35, 1700–1705. [Google Scholar] [CrossRef]
- Kovalev, D.Y.; Kochetov, N.A.; Chuev, I.I. Fabrication of high-entropy carbide (TiZrHfTaNb)C by high-energy ball milling. Ceram. Int. 2021, 47, 32626–32633. [Google Scholar] [CrossRef]
- He, C.-Y.; Zhao, P.; Gao, X.-H.; Liu, G.; La, P.-Q. High-entropy alloy nitride nanofilms via a co-sputtering method enable superior optical performance and thermal robustness. Mater. Lett. 2022, 329, 133198. [Google Scholar] [CrossRef]
- He, C.-Y.; Gao, X.-H.; Yu, D.-M.; Zhao, S.-S.; Guo, H.-X.; Liu, G. Toward high-temperature thermal tolerance in solar selective absorber coatings: Choosing high entropy ceramic HfNbTaTiZrN. J. Mater. Chem. A 2021, 9, 21270–21280. [Google Scholar] [CrossRef]
- McCormick, C.R.; Schaak, R.E. Simultaneous Multication Exchange Pathway to High-Entropy Metal Sulfide Nanoparticles. J. Am. Chem. Soc. 2021, 143, 1017–1023. [Google Scholar] [CrossRef]
- Guo, H.-X.; Wang, W.-M.; He, C.-Y.; Liu, B.-H.; Yu, D.-M.; Liu, G.; Gao, X.-H. Entropy-Assisted High-Entropy Oxide with a Spinel Structure toward High-Temperature Infrared Radiation Materials. ACS Appl. Mater. Interfaces 2021, 14, 1950–1960. [Google Scholar] [CrossRef]
- Xue, Y.; Zhao, X.; An, Y.; Wang, Y.; Gao, M.; Zhou, H.; Chen, J. High-entropy (La0.2Nd0.2Sm0.2Eu0.2Gd0.2)2Ce2O7: A potential thermal barrier material with improved thermo-physical properties. J. Adv. Ceram. 2022, 11, 615–628. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, L.; Xiang, H.; Dai, F.-Z.; Wang, X.; Ma, Z.; Liu, Y.; Zhou, Y. Improved thermal stability and infrared emissivity of high-entropy REMgAl11O19 and LaMAl11O19 (RE=La, Nd, Gd, Sm, Pr, Dy; M=Mg, Fe, Co, Ni, Zn). J. Mater. Sci. Technol. 2022, 104, 131–144. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Q.; Wang, G.; Zhang, Y.; Xia, M. High-entropy La(Fe0.2Co0.2Ni0.2Cr0.2Mn0.2)O3 ceramic exhibiting high emissivity and low thermal conductivity. Int. J. Appl. Ceram. Technol. 2022, 19, 2963–2966. [Google Scholar] [CrossRef]
- Song, J.; Cheng, Y.; Xiang, H.; Dai, F.-Z.; Dong, S.; Chen, G.; Hu, P.; Zhang, X.; Han, W.; Zhou, Y. Medium and high-entropy transition mental disilicides with improved infrared emissivity for thermal protection applications. J. Mater. Sci. Technol. 2023, 136, 149–158. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, B.; Xiang, H.; Dai, F.-Z.; Wu, S.; Zhou, Y. One-step synthesis and electromagnetic absorption properties of high entropy rare earth hexaborides (HEREB6) and high entropy rare earth hexaborides/borates (HEREB6/HEREBO3) composite powders. J. Adv. Ceram. 2020, 10, 62–77. [Google Scholar] [CrossRef]
- Min, G.; Zheng, S.; Zou, Z.; Yu, H.; Han, J. Reaction synthesis and formation mechanism of barium hexaboride. J. Ceram. 2002, 57, 1330–1333. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Zhou, Y.; Dai, F.; Xiang, H.; Liu, B.; Feng, Z. Shear anisotropy: Tuning high temperature metal hexaborides from soft to extremely hard. J. Mater. Sci. Technol. 2017, 33, 1371–1377. [Google Scholar] [CrossRef]
- Bai, L.; Ma, N.; Liu, F. Structure and chemical bond characteristics of LaB6. Phys. B Condens. Matter 2009, 404, 4086–4089. [Google Scholar] [CrossRef]
- Chao, L.; Bao, L.; Wei, W.; Tegus, O.; Zhang, Z. Effects of Nanoparticle Shape and Size on Optical Properties of LaB6. Plasmonics 2015, 11, 697–701. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, B.; Xiang, H.; Feng, Z.; Li, Z. YB6: A ‘Ductile’ and Soft Ceramic with Strong Heterogeneous Chemical Bonding for Ultrahigh-Temperature Applications. Mater. Res. Lett. 2015, 3, 210–215. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Duan, Y.; Liu, B.; Liang, J. Preparation and performance of Ce-doped far-infrared radiation ceramics by single iron ore tailings. Ceram. Int. 2022, 48, 11709–11717. [Google Scholar] [CrossRef]
- Johnson, R.W.; Daane, A.H. Electron Requirements of Bonds in Metal Borides. J. Chem. Phys. 1963, 38, 425–432. [Google Scholar] [CrossRef]
- Chazalviel, J.N.; Campagna, M.; Wertheim, G.K.; Schmidt, P.H. Study of valence mixing in SmB6 by X-ray photoelectron spectroscopy. Phys. Rev. B 1976, 14, 4586–4592. [Google Scholar] [CrossRef]
- Aita, O.; Watanabe, T.; Fujimoto, Y.; Tsutsum, K. Sm M4,5 Spectra of Metallic Samarium and Samarium Hexaboride. J. Phys. Soc. Jpn. 1982, 51, 483–489. [Google Scholar] [CrossRef]
- Pan, X.-J.; Bao, L.-H.; Ning, J.; Zhao, F.-Q.; Chao, L.-M.; Liu, Z.-Z. Synthesis and optical absorption properties of nanocrystalline rare earth hexaborides Nd1-xEuxB6 powders. Acta Phys. Sin. 2021, 70, 197–204. [Google Scholar] [CrossRef]
- Luo, X.; Luo, L.; Zhao, X.; Cai, H.; Duan, S.; Xu, C.; Huang, S.; Jin, H.; Hou, S. Single-phase rare-earth high-entropy zirconates with superior thermal and mechanical properties. J. Eur. Ceram. Soc. 2022, 42, 2391–2399. [Google Scholar] [CrossRef]
- Toby, B.H. R factors in Rietveld analysis: How good is good enough? Powder Diffr. 2012, 21, 67–70. [Google Scholar] [CrossRef]
- Lee, O.; Lee, M.; Choi, Y.; Sugio, K.; Matsugi, K.; Sasaki, G. Microstructure Observation of Preform for High Performance VGCF/Aluminum Composites. Mater. Trans. 2014, 55, 827–830. [Google Scholar] [CrossRef]
- Qiu, J.; Luo, T.; Yan, Y.; Xia, F.; Yao, L.; Tan, X.; Yang, D.; Tan, G.; Su, X.; Wu, J.; et al. Enhancing the Thermoelectric and Mechanical Properties of Bi0.5Sb1.5Te3 Modulated by the Texture and Dense Dislocation Networks. ACS Appl. Energy Mater. 2021, 13, 58974–58981. [Google Scholar] [CrossRef]
- Ye, Y.F.; Zhang, Y.H.; He, Q.F.; Zhuang, Y.; Wang, S.; Shi, S.Q.; Hu, A.; Fan, J.; Yang, Y. Atomic-scale distorted lattice in chemically disordered equimolar complex alloys. Acta Mater. 2018, 150, 182–194. [Google Scholar] [CrossRef]
- Dai, F.-Z.; Wen, B.; Sun, Y.; Xiang, H.; Zhou, Y. Theoretical prediction on thermal and mechanical properties of high entropy (Zr0.2Hf0.2Ti0.2Nb0.2Ta0.2)C by deep learning potential. J. Mater. Sci. Technol. 2020, 43, 168–174. [Google Scholar] [CrossRef]
- Aly, K.A.; Khalil, N.M.; Algamal, Y.; Saleem, Q.M.A. Lattice strain estimation for CoAl2O4 nano particles using Williamson-Hall analysis. J. Alloys Compd. 2016, 676, 606–612. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, P.; Huang, M.; Han, Y.; Xu, N.; Li, Y.; Zhang, Z.; Pan, W.; Wan, C. Effect of lattice distortion in high-entropy RE2Si2O7 and RE2SiO5 (RE=Ho, Er, Y, Yb, and Sc) on their thermal conductivity: Experimental and molecular dynamic simulation study. J. Eur. Ceram. Soc. 2023, 43, 6407–6415. [Google Scholar] [CrossRef]
- Teredesai, P.; Muthu, D.V.S.; Chandrabhas, N.; Meenakshi, S.; Vijayakumar, V.; Modak, P.; Rao, R.S.; Godwal, B.K.; Sikka, S.K.; Sood, A.K. High pressure phase transition in metallic LaB6: Raman and X-ray diffraction studies. Solid State Commun. 2004, 129, 791–796. [Google Scholar] [CrossRef]
- Mukherjee, A.; Gulnar, A.K.; Sahoo, D.K.; Krishnamurthy, N. Gas solid techniques for preparation of pure lanthanum hexaboride. Rare Met. 2012, 31, 285–289. [Google Scholar] [CrossRef]
- Mattox, T.M.; Chockkalingam, S.; Roh, I.; Urban, J.J. Evolution of Vibrational Properties in Lanthanum Hexaboride Nanocrystals. J. Phys. Chem. C 2016, 120, 5188–5195. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, S.; Li, W.; Chen, H.; Chen, Z. Synthesis of single-crystalline lanthanum hexaboride nanocubes by a low temperature molten salt method. Mater. Chem. Phys. 2018, 207, 325–329. [Google Scholar] [CrossRef]
- Massidda, S.; Monnier, R.; Stoll, E. Electronic structure of barium hexaboride. Eur. Phys. J. B 2000, 17, 645–649. [Google Scholar] [CrossRef]
- Singh, N.; Saini, S.M.; Nautiyal, T.; Auluck, S. Electronic structure and optical properties of rare earth hexaborides RB6(R = La, Ce, Pr, Nd, Sm, Eu, Gd). J. Phys. Condens. Matter 2007, 19, 346226–346235. [Google Scholar] [CrossRef]
- Kimura, S.; Nanba, T.; Kunii, S.; Suzuki, T.; Kasuya, T. Anomalous infrared absorption in rare-earth hexaborides. Solid State Commun. 1990, 75, 717–720. [Google Scholar] [CrossRef]
- ISO 9845-1:2022; Solar Energy—Reference Solar Spectral Irradiance at the Ground at Different Receiving Conditions—Part 1: Direct Normal and Hemispherical Solar Irradiance for Air Mass 1,5. International Standardization Organisation: Geneva, Switzerland, 2022.
- Dong, S.; Zhang, F.; Li, N.; Zeng, J.; Liang, P.; Zhang, H.; Liao, H.; Jiang, J.; Deng, L.; Cao, X. Thermal radiation and cycling properties of (Ca, Fe) or (Sr, Mn) co-doped La2Ce2O7 coatings. J. Eur. Ceram. Soc. 2020, 40, 2020–2029. [Google Scholar] [CrossRef]
- Gerald, D.; Mahan, K.R.S. Local Density Theory of Polarizability; Springer: New York, NY, USA, 1990; pp. 37–38. [Google Scholar]



| Composition Code | δr (%) | ΔSconf |
|---|---|---|
| MEREB6 | 2.58 | 1.3863 R |
| HEREB6 | 3.95 | 1.6094 R |
| Composition Code | Molar Ratio of Raw Materials |
|---|---|
| LaB6 | 1La2O3:3B4C |
| MEREB6 | 1La2O3:1Sm2O3: 2CeO2:1Eu2O3:12B4C |
| HEREB6 | 1La2O3:1Sm2O3:2CeO2:1Eu2O3:2BaCO3:15B4C |
| Samples | Lattice Parameters | Interatomic Distances (Å) | ||
|---|---|---|---|---|
| a (Å) = b (Å) = c (Å) | B–Binter Mean | B-Bintra Mean | M-B Mean | |
| LaB6 | 4.1576 | 1.647 (14) | 1.775 (10) | 3.0531 (18) |
| MEREB6 | 4.15546 | 1.672 (10) | 1.756 (7) | 3.0550 (13) |
| HEREB6 | 4.16005 | 1.677 (11) | 1.756 (8) | 3.0588 (15) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Pan, Y.; Zhang, J.; Wang, K.; Xue, L.; Huang, M.; Li, Y.; Yang, F.; Chen, H. Medium- and High-Entropy Rare Earth Hexaborides with Enhanced Solar Energy Absorption and Infrared Emissivity. Materials 2024, 17, 1789. https://doi.org/10.3390/ma17081789
Wang H, Pan Y, Zhang J, Wang K, Xue L, Huang M, Li Y, Yang F, Chen H. Medium- and High-Entropy Rare Earth Hexaborides with Enhanced Solar Energy Absorption and Infrared Emissivity. Materials. 2024; 17(8):1789. https://doi.org/10.3390/ma17081789
Chicago/Turabian StyleWang, Hongye, Yanyu Pan, Jincheng Zhang, Kaixian Wang, Liyan Xue, Minzhong Huang, Yazhu Li, Fan Yang, and Heng Chen. 2024. "Medium- and High-Entropy Rare Earth Hexaborides with Enhanced Solar Energy Absorption and Infrared Emissivity" Materials 17, no. 8: 1789. https://doi.org/10.3390/ma17081789
APA StyleWang, H., Pan, Y., Zhang, J., Wang, K., Xue, L., Huang, M., Li, Y., Yang, F., & Chen, H. (2024). Medium- and High-Entropy Rare Earth Hexaborides with Enhanced Solar Energy Absorption and Infrared Emissivity. Materials, 17(8), 1789. https://doi.org/10.3390/ma17081789







