Co and Co3O4 in the Hydrolysis of Boron-Containing Hydrides: H2O Activation on the Metal and Oxide Active Centers
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalytic Materials under Study
2.2. Experimental Methods of Investigations
2.3. Catalytic Hydrolysis of Boron-Containing Hydrides
2.4. Density Functional Theory Calculations
3. Results
3.1. The Characteristics of Reactions When Co0 Is Used as a Catalyst
3.1.1. DFT Modeling of H2O and NH3BH3 Activation on Metallic Surfaces of Co, Ni, and Cu
3.1.2. Activity of Co Metal Catalyst in the Hydrolysis of NaBH4, NH3BH3, and (CH2NH2BH3)2
3.2. Features of Co3O4 Catalyst Use
3.2.1. Catalytic Activity of Co3O4 in the Hydrolysis of NaBH4, NH3BH3, and (CH2NH2BH3)2
3.2.2. Study of Co3O4 Activated in the Reaction Medium of NaBH4
3.2.3. Modeling of H2O Adsorption on the Surface of Co3O4 and Co3O4 with Oxygen Vacancies
3.2.4. Comparison of Kinetic Isotope Effect Results for Co3O4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baum, Z.J.; Diaz, L.L.; Konovalova, T.; Zhou, Q.A. Materials Research Directions Toward a Green Hydrogen Economy: A Review. ACS Omega 2022, 7, 32908–32935. [Google Scholar] [CrossRef] [PubMed]
- Vivanco-Martín, B.; Iranzo, A. Analysis of the European Strategy for Hydrogen: A Comprehensive Review. Energies 2023, 16, 3866. [Google Scholar] [CrossRef]
- Salman, M.S.; Pratthana, C.; Lai, Q.; Wang, T.; Rambhujun, N.; Srivastava, K.; Aguey-Zinsou, K.F. Catalysis in Solid Hydrogen Storage: Recent Advances, Challenges, and Perspectives. Energy Technol. 2022, 10, 2200433. [Google Scholar] [CrossRef]
- Luconi, L.; Tuci, G.; Giambastiani, G.; Rossin, A.; Peruzzini, M. H2 Production from Lightweight Inorganic Hydrides Catalyzed by 3d Transition Metals. Int. J. Hydrogen Energy 2019, 44, 25746–25776. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. A Review on Hydrogen Generation from the Hydrolysis of Sodium Borohydride. Int. J. Hydrogen Energy 2021, 46, 726–765. [Google Scholar] [CrossRef]
- Guan, S.; Liu, Y.; Zhang, H.; Shen, R.; Wen, H.; Kang, N.; Zhou, J.; Liu, B.; Fan, Y.; Jiang, J.; et al. Recent Advances and Perspectives on Supported Catalysts for Heterogeneous Hydrogen Production from Ammonia Borane. Adv. Sci. 2023, 10, 2300726. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Zhao, X.; Qin, G.; Zhang, X.; Lu, Z.; Yu, X.; Li, L.; Yang, X. Hydrogen Production from Ammonia Borane Hydrolysis Catalyzed by Metal Oxide-Based Materials. Russ. J. Phys. Chem. A 2023, 97, 854–877. [Google Scholar] [CrossRef]
- Engin, M.; Ozay, O. The First Catalytic Hydrolysis of Ethylenediamine Bisborane with Hydrogel-Supported Metallic Nanoparticles. Int. J. Hydrogen Energy 2018, 43, 15083–15094. [Google Scholar] [CrossRef]
- Coşkuner, Ö.; Kantürk Figen, A. Catalytic Semi-Continuous Operation Modes for Hydrogen Generation from Carbon Derivatives of Ammonia Boranes. Int. J. Hydrogen Energy 2022, 47, 40304–40316. [Google Scholar] [CrossRef]
- Yao, J.; Wu, Z.; Wang, H.; Yang, F.; Ren, J.; Zhang, Z. Application-Oriented Hydrolysis Reaction System of Solid-State Hydrogen Storage Materials for High Energy Density Target: A Review. J. Energy Chem. 2022, 74, 218–238. [Google Scholar] [CrossRef]
- Chen, W.; Ouyang, L.Z.; Liu, J.W.; Yao, X.D.; Wang, H.; Liu, Z.W.; Zhu, M. Hydrolysis and Regeneration of Sodium Borohydride (NaBH4)—A Combination of Hydrogen Production and Storage. J. Power Sources 2017, 359, 400–407. [Google Scholar] [CrossRef]
- Xu, Q.; Chandra, M. A Portable Hydrogen Generation System: Catalytic Hydrolysis of Ammonia-Borane. J. Alloys Compd. 2007, 446–447, 729–732. [Google Scholar] [CrossRef]
- Netskina, O.V.; Tayban, E.S.; Prosvirin, I.P.; Komova, O.V.; Simagina, V.I. Hydrogen Storage Systems Based on Solid-State NaBH4/Co Composite: Effect of Catalyst Precursor on Hydrogen Generation Rate. Renew. Energy 2020, 151, 278–285. [Google Scholar] [CrossRef]
- Keskin, E.; Coşkuner Filiz, B.; Kılıç Depren, S.; Kantürk Figen, A. Recommendations for Ammonia Borane Composite Pellets as a Hydrogen Storage Medium. Int. J. Hydrogen Energy 2018, 43, 20354–20371. [Google Scholar] [CrossRef]
- Komova, O.V.; Simagina, V.I.; Pochtar, A.A.; Bulavchenko, O.A.; Ishchenko, A.V.; Odegova, G.V.; Gorlova, A.M.; Ozerova, A.M.; Lipatnikova, I.L.; Tayban, E.S.; et al. Catalytic Behavior of Iron-Containing Cubic Spinel in the Hydrolysis and Hydrothermolysis of Ammonia Borane. Materials 2021, 14, 5422. [Google Scholar] [CrossRef]
- Coşkuner, Ö.; Kantürk Figen, A. Hydro-Catalytic Treatment of Organoamine Boranes for Efficient Thermal Dehydrogenation for Hydrogen Production. Int. J. Hydrogen Energy 2021, 46, 35641–35652. [Google Scholar] [CrossRef]
- Simagina, V.I.; Komova, O.V.; Ozerova, A.M.; Netskina, O.V.; Odegova, G.V.; Kellerman, D.G.; Bulavchenko, O.A.; Ishchenko, A.V. Cobalt Oxide Catalyst for Hydrolysis of Sodium Borohydride and Ammonia Borane. Appl. Catal. A Gen. 2011, 394, 86–92. [Google Scholar] [CrossRef]
- Krishnan, P.; Advani, S.G.; Prasad, A.K. Cobalt Oxides as Co2B Catalyst Precursors for the Hydrolysis of Sodium Borohydride Solutions to Generate Hydrogen for PEM Fuel Cells. Int. J. Hydrogen Energy 2008, 33, 7095–7102. [Google Scholar] [CrossRef]
- Tomboc, G.R.M.; Tamboli, A.H.; Kim, H. Synthesis of Co3O4 Macrocubes Catalyst Using Novel Chitosan/Urea Template for Hydrogen Generation from Sodium Borohydride. Energy 2017, 121, 238–245. [Google Scholar] [CrossRef]
- Pfeil, T.L.; Pourpoint, T.L.; Groven, L.J. Effects of Crystallinity and Morphology of Solution Combustion Synthesized Co3O4 as a Catalyst Precursor in Hydrolysis of Sodium Borohydride. Int. J. Hydrogen Energy 2014, 39, 2149–2159. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Xu, B.; Liu, X.; Zhang, K.; Fan, G.; Jiang, W. Efficient Hydrogen Generation from the NaBH4 Hydrolysis by Cobalt-Based Catalysts: Positive Roles of Sulfur-Containing Salts. ACS Appl. Mater. Interfaces 2020, 12, 9376–9386. [Google Scholar] [CrossRef] [PubMed]
- Ugale, A.D.; Ghodke, N.P.; Kang, G.S.; Nam, K.B.; Bhoraskar, S.V.; Mathe, V.L.; Yoo, J.B. Cost-Effective Synthesis of Carbon Loaded Co3O4 for Controlled Hydrogen Generation via NaBH4 Hydrolysis. Int. J. Hydrogen Energy 2022, 47, 16–29. [Google Scholar] [CrossRef]
- Simagina, V.I.; Ozerova, A.M.; Komova, O.V.; Netskina, O.V. Recent Advances in Applications of Co-B Catalysts in NaBH4-Based Portable Hydrogen Generators. Catalysts 2021, 11, 268. [Google Scholar] [CrossRef]
- Arzac, G.M.; Rojas, T.C.; Fernández, A. Boron Compounds as Stabilizers of a Complex Microstructure in a Co-B-Based Catalyst for NaBH4 Hydrolysis. ChemCatChem 2011, 3, 1305–1313. [Google Scholar] [CrossRef]
- Netskina, O.V.; Kochubey, D.I.; Prosvirin, I.P.; Malykhin, S.E.; Komova, O.V.; Kanazhevskiy, V.V.; Chukalkin, Y.G.; Bobrovskii, V.I.; Kellerman, D.G.; Ishchenko, A.V.; et al. Cobalt-Boron Catalyst for NaBH4 Hydrolysis: The State of the Active Component Forming from Cobalt Chloride in a Reaction Medium. Mol. Catal. 2017, 441, 100–108. [Google Scholar] [CrossRef]
- Chen, W.; Fu, W.; Qian, G.; Zhang, B.; Chen, D.; Duan, X.; Zhou, X. Synergistic Pt-WO3 Dual Active Sites to Boost Hydrogen Production from Ammonia Borane. iScience 2020, 23, 100922. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, S.; Shen, R.; Peng, Z.; Liu, B.; Li, J.; Zhang, Z.; Li, B. A Catalytic Copper/Cobalt Oxide Interface for Efficient Hydrogen Generation. Energy Environ. Mater. 2023, 6, e12279. [Google Scholar] [CrossRef]
- Mo, B.; Li, S.; Wen, H.; Zhang, H.; Zhang, H.; Wu, J.; Li, B.; Hou, H. Functional Group Regulated Ni/Ti3C2Tx (Tx = F, -OH) Holding Bimolecular Activation Tunnel for Enhanced Ammonia Borane Hydrolysis. ACS Appl. Mater. Interfaces 2022, 14, 16320–16329. [Google Scholar] [CrossRef]
- Yao, F.; Guan, S.; Bian, L.; Fan, Y.; Liu, X.; Zhang, H.; Li, B.; Liu, B. Ensemble-Exciting Effect in Pd/Alk-Ti3C2 on the Activity for Efficient Hydrogen Production. ACS Sustain. Chem. Eng. 2021, 9, 12332–12340. [Google Scholar] [CrossRef]
- Karakaya Akbaş, N.; Kutlu, B. Effect of Hydroxyl (OH) Radicals on the Progression of NaBH4 Hydrolysis Reaction on Fcc-Co Surfaces: A DFT Study. Phys. B Condens. Matter. 2022, 647, 414385. [Google Scholar] [CrossRef]
- Ji, J.; Deng, K.; Li, J.; Zhang, Z.; Duan, X.; Huang, H. In Situ Transformation of 3D Co3O4 Nanoparticles to 2D Nanosheets with Rich Surface Oxygen Vacancies to Boost Hydrogen Generation from NaBH4. Chem. Eng. J. 2021, 424, 130350. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, S.; Ma, Z.; Kundu, M.; Tang, B.; Li, J.; Wang, X. Oxygen Vacancies Engineered Self-Supported B Doped Co3O4 Nanowires as an Efficient Multifunctional Catalyst for Electrochemical Water Splitting and Hydrolysis of Sodium Borohydride. Chem. Eng. J. 2021, 404, 126474. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Mu, J.; Fan, S.; Chen, X.; Wang, L.; Yin, Z.; Tadé, M.; Liu, S. Oxygen Vacancy-Rich Porous Co3O4 Nanosheets toward Boosted NO Reduction by CO and CO Oxidation: Insights into the Structure-Activity Relationship and Performance Enhancement Mechanism. ACS Appl. Mater. Interfaces 2019, 11, 41988–41999. [Google Scholar] [CrossRef] [PubMed]
- Xiang, K.; Xu, Z.; Qu, T.; Tian, Z.; Zhang, Y.; Wang, Y.; Xie, M.; Guo, X.; Ding, W.; Guo, X. Two Dimensional Oxygen-Vacancy-Rich Co3O4 Nanosheets with Excellent Supercapacitor Performances. Chem. Commun. 2017, 53, 12410–12413. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, T.; Jiang, K.; Da, P.; Peng, Z.; Tang, J.; Kong, B.; Cai, W.B.; Yang, Z.; Zheng, G. Reduced Mesoporous Co3O4 Nanowires as Efficient Water Oxidation Electrocatalysts and Supercapacitor Electrodes. Adv. Energy Mater. 2014, 4, 1400696. [Google Scholar] [CrossRef]
- Guan, S.; An, L.; Ashraf, S.; Zhang, L.; Liu, B.; Fan, Y.; Li, B. Oxygen Vacancy Excites Co3O4 Nanocrystals Embedded into Carbon Nitride for Accelerated Hydrogen Generation. Appl. Catal. B Environ. 2020, 269, 118775. [Google Scholar] [CrossRef]
- Dong, G.; Hu, H.; Huang, X.; Zhang, Y.; Bi, Y. Rapid Activation of Co3O4 Cocatalysts with Oxygen Vacancies on TiO2 Photoanodes for Efficient Water Splitting. J. Mater. Chem. A 2018, 6, 21003–21009. [Google Scholar] [CrossRef]
- Lu, Y.; Li, C.; Zhang, Y.; Cao, X.; Xie, G.; Wang, M.; Peng, D.; Huang, K.; Zhang, B.; Wang, T.; et al. Engineering of Cation and Anion Vacancies in Co3O4 Thin Nanosheets by Laser Irradiation for More Advancement of Oxygen Evolution Reaction. Nano Energy 2021, 83, 105800. [Google Scholar] [CrossRef]
- Ozerova, A.M.; Skobelkina, A.A.; Simagina, V.I.; Komova, O.V.; Prosvirin, I.P.; Bulavchenko, O.A.; Lipatnikova, I.L.; Netskina, O.V. Magnetically Recovered Co and Co@Pt Catalysts Prepared by Galvanic Replacement on Aluminum Powder for Hydrolysis of Sodium Borohydride. Materials 2022, 15, 3010. [Google Scholar] [CrossRef]
- Komova, O.V.; Simagina, V.I.; Butenko, V.R.; Odegova, G.V.; Bulavchenko, O.A.; Nikolaeva, O.A.; Ozerova, A.M.; Lipatnikova, I.L.; Tayban, E.S.; Mukha, S.A.; et al. Dehydrogenation of Ammonia Borane Recrystallized by Different Techniques. Renew. Energy 2022, 184, 460–472. [Google Scholar] [CrossRef]
- Tang, W.; Sanville, E.; Henkelman, G. A Grid-Based Bader Analysis Algorithm without Lattice Bias. J. Phys. Condens. Matter 2009, 21, 084204. [Google Scholar] [CrossRef] [PubMed]
- Bligaard, T.; Nørskov, J.K.; Dahl, S.; Matthiesen, J.; Christensen, C.H.; Sehested, J. The Brønsted-Evans-Polanyi Relation and the Volcano Curve in Heterogeneous Catalysis. J. Catal. 2004, 224, 206–217. [Google Scholar] [CrossRef]
- Wang, C.; Tuninetti, J.; Wang, Z.; Zhang, C.; Ciganda, R.; Salmon, L.; Moya, S.; Ruiz, J.; Astruc, D. Hydrolysis of Ammonia-Borane over Ni/ZIF-8 Nanocatalyst: High Efficiency, Mechanism, and Controlled Hydrogen Release. J. Am. Chem. Soc. 2017, 139, 11610–11615. [Google Scholar] [CrossRef] [PubMed]
- Medford, A.J.; Vojvodic, A.; Hummelshøj, J.S.; Voss, J.; Abild-Pedersen, F.; Studt, F.; Bligaard, T.; Nilsson, A.; Nørskov, J.K. From the Sabatier Principle to a Predictive Theory of Transition-Metal Heterogeneous Catalysis. J. Catal. 2015, 328, 36–42. [Google Scholar] [CrossRef]
- Demirci, U.B.; Miele, P. Cobalt in NaBH4 Hydrolysis. Phys. Chem. Chem. Phys. 2010, 12, 14651–14665. [Google Scholar] [CrossRef]
- Li, Z.; He, T.; Liu, L.; Chen, W.; Zhang, M.; Wu, G.; Chen, P. Covalent Triazine Framework Supported Non-Noble Metal Nanoparticles with Superior Activity for Catalytic Hydrolysis of Ammonia Borane: From Mechanistic Study to Catalyst Design. Chem. Sci. 2016, 8, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Paterson, R.; Alharbi, A.A.; Wills, C.; Dixon, C.; Šiller, L.; Chamberlain, T.W.; Griffiths, A.; Collins, S.M.; Wu, K.; Simmons, M.D.; et al. Heteroatom Modified Polymer Immobilized Ionic Liquid Stabilized Ruthenium Nanoparticles: Efficient Catalysts for the Hydrolytic Evolution of Hydrogen from Sodium Borohydride. Mol. Catal. 2022, 528, 112476. [Google Scholar] [CrossRef]
- Feng, Y.; Liao, J.; Chen, X.; Wang, H.; Guo, B.; Li, H.; Zhou, L.; Huang, J.; Li, H. Synthesis of Rattle-Structured CuCo2O4 Nanospheres with Tunable Sizes Based on Heterogeneous Contraction and Their Ultrahigh Performance toward Ammonia Borane Hydrolysis. J. Alloys Compd. 2021, 863, 158089. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, S.; Liao, J.; Feng, K.; Zheng, Y.; Pollet, B.G.; Li, H. CuCo2O4 Nanoplate Film as a Low-Cost, Highly Active and Durable Catalyst towards the Hydrolytic Dehydrogenation of Ammonia Borane for Hydrogen Production. J. Power Sources 2017, 355, 191–198. [Google Scholar] [CrossRef]
- Lu, D.; Liao, J.; Zhong, S.; Leng, Y.; Ji, S.; Wang, H.; Wang, R.; Li, H. Cu0.6Ni0.4Co2O4 Nanowires, a Novel Noble-Metal-Free Catalyst with Ultrahigh Catalytic Activity towards the Hydrolysis of Ammonia Borane for Hydrogen Production. Int. J. Hydrogen Energy 2018, 43, 5541–5550. [Google Scholar] [CrossRef]
- Lu, D.; Li, J.; Lin, C.; Liao, J.; Feng, Y.; Ding, Z.; Li, Z.; Liu, Q.; Li, H. A Simple and Scalable Route to Synthesize CoxCu1−xCo2O4@CoyCu1−yCo2O4 Yolk–Shell Microspheres, A High-Performance Catalyst to Hydrolyze Ammonia Borane for Hydrogen Production. Small 2019, 15, 1805460. [Google Scholar] [CrossRef]
- Lu, D.; Liao, J.; Leng, Y.; Zhong, S.; He, J.; Wang, H.; Wang, R.; Li, H. Mo-Doped Cu0.5Ni0.5Co2O4 Nanowires, a Strong Substitute for Noble-Metal-Based Catalysts towards the Hydrolysis of Ammonia Borane for Hydrogen Production. Catal. Commun. 2018, 114, 89–92. [Google Scholar] [CrossRef]
- Gupta, S.K.; Desai, R.; Jha, P.K.; Sahoo, S.; Kirin, D. Titanium Dioxide Synthesized Using Titanium Chloride: Size Effect Study Using Raman Spectroscopy and Photoluminescence. J. Raman Spectrosc. 2010, 41, 350–355. [Google Scholar] [CrossRef]
- Gawali, S.R.; Gandhi, A.C.; Gaikwad, S.S.; Pant, J.; Chan, T.S.; Cheng, C.L.; Ma, Y.R.; Wu, S.Y. Role of Cobalt Cations in Short Range Antiferromagnetic Co3O4 Nanoparticles: A Thermal Treatment Approach to Affecting Phonon and Magnetic Properties. Sci. Rep. 2018, 8, 249. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.C.; Pan, L.; Shen, G.Q.; Mahmood, N.; Ma, Y.H.; Shi, Y.; Jia, W.; Wang, L.; Zhang, X.; et al. Engineering Cobalt Defects in Cobalt Oxide for Highly Efficient Electrocatalytic Oxygen Evolution. ACS Catal. 2018, 8, 3803–3811. [Google Scholar] [CrossRef]
- Lorite, I.; Del Campo, A.; Romero, J.J.; Fernández, J.F. Isolated Nanoparticle Raman Spectroscopy. J. Raman Spectrosc. 2012, 43, 889–894. [Google Scholar] [CrossRef]
- Pasquini, C.; D’Amario, L.; Zaharieva, I.; Dau, H. Operando Raman Spectroscopy Tracks Oxidation-State Changes in an Amorphous Co Oxide Material for Electrocatalysis of the Oxygen Evolution Reaction. J. Chem. Phys. 2020, 152, 194202. [Google Scholar] [CrossRef]
- Zhang, R.; Pan, L.; Guo, B.; Huang, Z.F.; Chen, Z.; Wang, L.; Zhang, X.; Guo, Z.; Xu, W.; Loh, K.P.; et al. Tracking the Role of Defect Types in Co3O4 Structural Evolution and Active Motifs during Oxygen Evolution Reaction. J. Am. Chem. Soc. 2023, 145, 2271–2281. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, J.; Zhang, Z.; Wei, T.; Wang, J.; Ma, J.; Ren, Y.; Zhang, H. Co3O4 Crystal Plane Regulation to Efficiently Activate Peroxymonosulfate in Water: The Role of Oxygen Vacancies. J. Colloid Interface Sci. 2022, 623, 520–531. [Google Scholar] [CrossRef]
- Mo, S.; Zhang, Q.; Li, S.; Ren, Q.; Zhang, M.; Xue, Y.; Peng, R.; Xiao, H.; Chen, Y.; Ye, D. Integrated Cobalt Oxide Based Nanoarray Catalysts with Hierarchical Architectures: In Situ Raman Spectroscopy Investigation on the Carbon Monoxide Reaction Mechanism. ChemCatChem 2018, 10, 3012–3026. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Zhang, L.; Jiang, D. Surface Oxygen Vacancies on Co3O4 Mediated Catalytic Formaldehyde Oxidation at Room Temperature. Catal. Sci. Technol. 2016, 6, 3845–3853. [Google Scholar] [CrossRef]
- Arora, A.K.; Rajalakshmi, M.; Ravindran, T.R.; Sivasubramanian, V. Raman spectroscopy of optical phonon confinement in nanostructured materials. J. Raman Spectrosc. 2007, 38, 604–617. [Google Scholar] [CrossRef]
- Hadjiev, V.G.; Iliev, M.N.; Vergilov, I.V. The Raman Spectra of Co3O4. J. Phys. C Solid State Phys. 1988, 21, L199–L201. [Google Scholar] [CrossRef]
- Wang, C.; Ren, Y.; Zhao, J.; Sun, S.; Du, X.; Wang, M.; Ma, G.; Yu, H.; Li, L.; Yu, X.; et al. Oxygen Vacancy-Attired Dual-Active-Sites Cu/Cu0.76Co2.24O4 Drives Electron Transfer for Efficient Ammonia Borane Dehydrogenation. Appl. Catal. B Environ. 2022, 314, 121494. [Google Scholar] [CrossRef]
- Patil, K.N.; Prasad, D.; Bhanushali, J.T.; Kim, H.; Atar, A.B.; Nagaraja, B.M.; Jadhav, A.H. Sustainable Hydrogen Generation by Catalytic Hydrolysis of NaBH4 Using Tailored Nanostructured Urchin-like CuCo2O4 Spinel Catalyst. Catal. Lett. 2020, 150, 586–604. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Y.; Hao, Y.; Yang, X.; Goddard, W.A.; Zhang, X.L.; Cao, B. Oxygen-Vacancy Abundant Ultrafine Co3O4/Graphene Composites for High-Rate Supercapacitor Electrodes. Adv. Sci. 2018, 5, 1700659. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, K.; Ashraf, S.; Fan, Y.; Guan, S.; Wu, X.; Liu, Y.; Liu, B.; Li, B. Polar O–Co–P Surface for Bimolecular Activation in Catalytic Hydrogen Generation. Energy Environ. Mater. 2023, 6, e12273. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, G.; Lei, T.; Lv, Z.; Xiong, M.; Wang, L.; Xing, S.; Ma, J.; Jiang, Z.; Qin, Y. Enhanced Hydrogen Generation by Reverse Spillover Effects over Bicomponent Catalysts. Nat. Commun. 2022, 13, 118. [Google Scholar] [CrossRef]
- Gorlova, A.M.; Kayl, N.L.; Komova, O.V.; Netskina, O.V.; Ozerova, A.M.; Odegova, G.V.; Bulavchenko, O.A.; Ishchenko, A.V.; Simagina, V.I. Fast Hydrogen Generation from Solid NH3BH3 under Moderate Heating and Supplying a Limited Quantity of CoCl2 or NiCl2 Solution. Renew. Energy 2018, 121, 722–729. [Google Scholar] [CrossRef]
- Rueda, M.; Sanz-Moral, L.M.; Segovia, J.J.; Martín, Á. Enhancement of Hydrogen Release Kinetics from Ethane 1,2 Diamineborane (EDAB) by Micronization Using Supercritical Antisolvent (SAS) Precipitation. Chem. Eng. J. 2016, 306, 164–173. [Google Scholar] [CrossRef]
- Leardini, F.; Valero-Pedraza, M.J.; Perez-Mayoral, E.; Cantelli, R.; Bañares, M.A. Thermolytic Decomposition of Ethane 1,2-Diamineborane Investigated by Thermoanalytical Methods and in Situ Vibrational Spectroscopy. J. Phys. Chem. C 2014, 118, 17221–17230. [Google Scholar] [CrossRef]
- Li, H.; He, W.; Xu, L.; Pan, Y.; Xu, R.; Sun, Z.; Wei, S. Synergistic Interface between Metal Cu Nanoparticles and CoO for Highly Efficient Hydrogen Production from Ammonia Borane. RSC Adv. 2023, 13, 11569–11576. [Google Scholar] [CrossRef]
- Fu, F.; Wang, C.; Wang, Q.; Martinez-Villacorta, A.M.; Escobar, A.; Chong, H.; Wang, X.; Moya, S.; Salmon, L.; Fouquet, E.; et al. Highly Selective and Sharp Volcano-Type Synergistic Ni2Pt@ZIF-8-Catalyzed Hydrogen Evolution from Ammonia Borane Hydrolysis. J. Am. Chem. Soc. 2018, 140, 10034–10042. [Google Scholar] [CrossRef]
- Wang, Q.; Fu, F.; Escobar, A.; Moya, S.; Ruiz, J.; Astruc, D. “Click” Dendrimer-Stabilized Nanocatalysts for Efficient Hydrogen Release upon Ammonia-Borane Hydrolysis. ChemCatChem 2018, 10, 2673–2680. [Google Scholar] [CrossRef]
- Zhao, Q.; Espuche, B.; Kang, N.; Moya, S.; Astruc, D. Cobalt Sandwich-Stabilized Rhodium Nanocatalysts for Ammonia Borane and Tetrahydroxydiboron Hydrolysis. Inorg. Chem. Front. 2022, 9, 4651–4660. [Google Scholar] [CrossRef]
- Kang, N.; Wang, Q.; Djeda, R.; Wang, W.; Fu, F.; Moro, M.M.; Ramirez, M.D.L.A.; Moya, S.; Coy, E.; Salmon, L.; et al. Visible-Light Acceleration of H2 Evolution from Aqueous Solutions of Inorganic Hydrides Catalyzed by Gold-Transition-Metal Nanoalloys. ACS Appl. Mater. Interfaces 2020, 12, 53816–53826. [Google Scholar] [CrossRef]
- Meng, Y.; Sun, Q.; Zhang, T.; Zhang, J.; Dong, Z.; Ma, Y.; Wu, Z.; Wang, H.; Bao, X.; Sun, Q.; et al. Cobalt-Promoted Noble-Metal Catalysts for Efficient Hydrogen Generation from Ammonia Borane Hydrolysis. J. Am. Chem. Soc. 2023, 145, 5486–5495. [Google Scholar] [CrossRef]
- Song, J.; Gu, X.; Zhang, H. Electrons and Hydroxyl Radicals Synergistically Boost the Catalytic Hydrogen Evolution from Ammonia Borane over Single Nickel Phosphides under Visible Light Irradiation. ChemistryOpen 2020, 9, 366–373. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Z.; Qu, B.; Tao, Y.; Liu, Y. Highly Efficient Dehydrogenation of Ammonia Borane over Reduced Graphene Oxide-Supported Pd@NiP Nanoparticles at Room Temperature. Int. J. Energy Res. 2023, 2023, 9889312. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, J.; Tu, Y.; Wang, Q.; Pan, H.; Wu, L.; Zheng, X.; Zhu, J. Oxygen Modified CoP2 Supported Palladium Nanoparticles as Highly Efficient Catalyst for Hydrolysis of Ammonia Borane. Nano Res. 2022, 15, 3034–3041. [Google Scholar] [CrossRef]
- Chen, W.; Li, D.; Wang, Z.; Qian, G.; Sui, Z.; Duan, X.; Zhou, X.; Yeboah, I.; Chen, D. Reaction Mechanism and Kinetics for Hydrolytic Dehydrogenation of Ammonia Borane on a Pt/CNT Catalyst. AIChE J. 2017, 63, 60–65. [Google Scholar] [CrossRef]
- Wang, Q.; Fu, F.; Yang, S.; Martinez Moro, M.; Ramirez, M.D.L.A.; Moya, S.; Salmon, L.; Ruiz, J.; Astruc, D. Dramatic Synergy in CoPt Nanocatalysts Stabilized by “Click” Dendrimers for Evolution of Hydrogen from Hydrolysis of Ammonia Borane. ACS Catal. 2019, 9, 1110–1119. [Google Scholar] [CrossRef]
- Fu, W.; Wang, Q.; Chen, W.; Qian, G.; Zhang, J.; Chen, D.; Yuan, W.-K.; Zhou, X.; Duan, X.; Yuan, W. Fabrication and Engineering of Ru Local Structures toward Enhanced Kinetics of Hydrogen Generation. Authorea Prepr. 2020, 2. [Google Scholar] [CrossRef]
- Mohsenzadeh, A.; Bolton, K.; Richards, T. DFT Study of the Adsorption and Dissociation of Water on Ni(111), Ni(110) and Ni(100) Surfaces. Surf. Sci. 2014, 627, 1–10. [Google Scholar] [CrossRef]
- Cui, C.; Liu, Y.; Mehdi, S.; Wen, H.; Zhou, B.; Li, J.; Li, B. Enhancing Effect of Fe-Doping on the Activity of Nano Ni Catalyst towards Hydrogen Evolution from NH3BH3. Appl. Catal. B Environ. 2020, 265, 118612. [Google Scholar] [CrossRef]
- Liu, R. Adsorption and Dissociation of H2O on Au(1 1 1) Surface: A DFT Study. Comput. Theor. Chem. 2013, 1019, 141–145. [Google Scholar] [CrossRef]
- Petersen, T.; Klüner, T. Water Adsorption on Ideal Anatase-TiO2(101)—An Embedded Cluster Model for Accurate Adsorption Energetics and Excited State Properties. Z. Phys. Chem. 2020, 234, 813–834. [Google Scholar] [CrossRef]
- Zhao, W.; Bajdich, M.; Carey, S.; Vojvodic, A.; Nørskov, J.K.; Campbell, C.T. Water Dissociative Adsorption on NiO(111): Energetics and Structure of the Hydroxylated Surface. ACS Catal. 2016, 6, 7377–7384. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, W.; Cao, J.; Fu, W.; Qian, G.; Chen, D.; Zhou, X.; Duan, X. Atomic Insights into Robust Pt-PdO Interfacial Site-Boosted Hydrogen Generation. ACS Catal. 2020, 10, 11417–11429. [Google Scholar] [CrossRef]
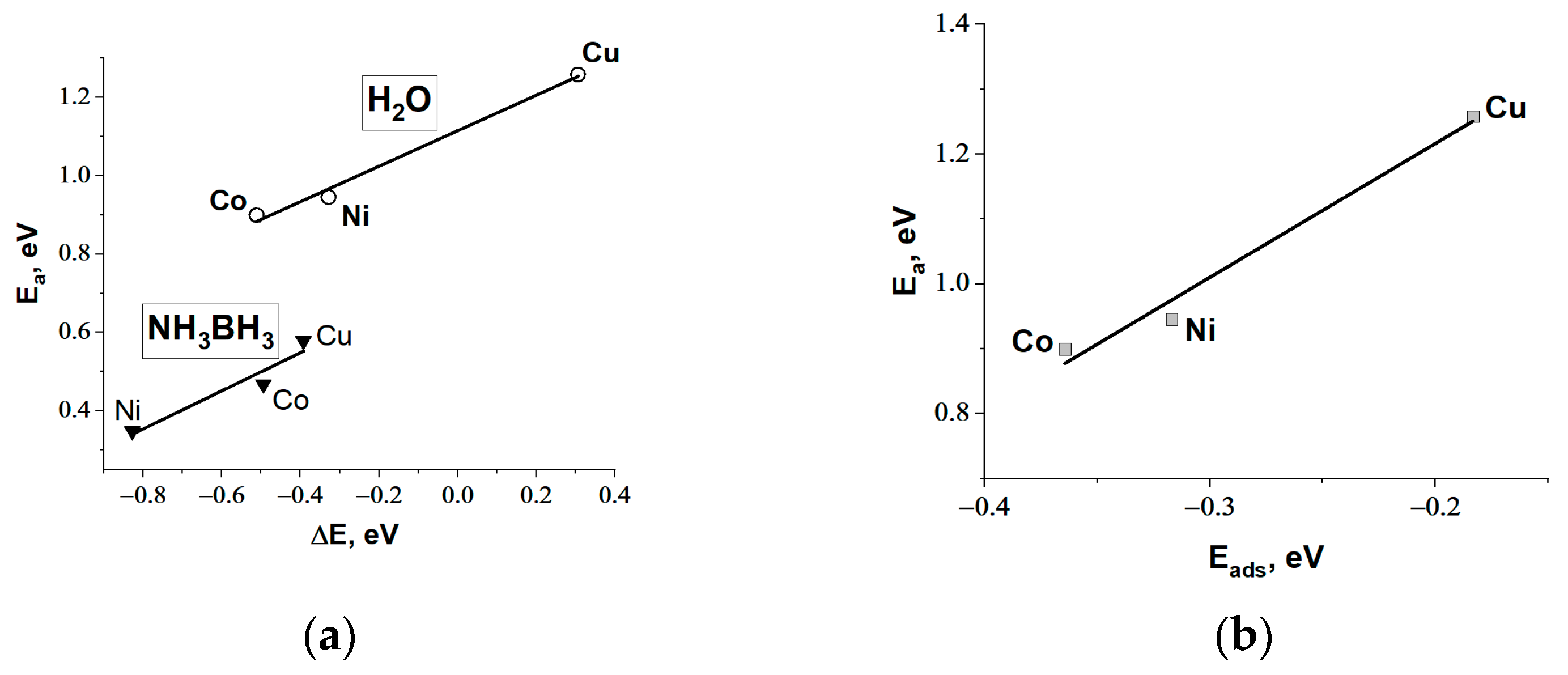

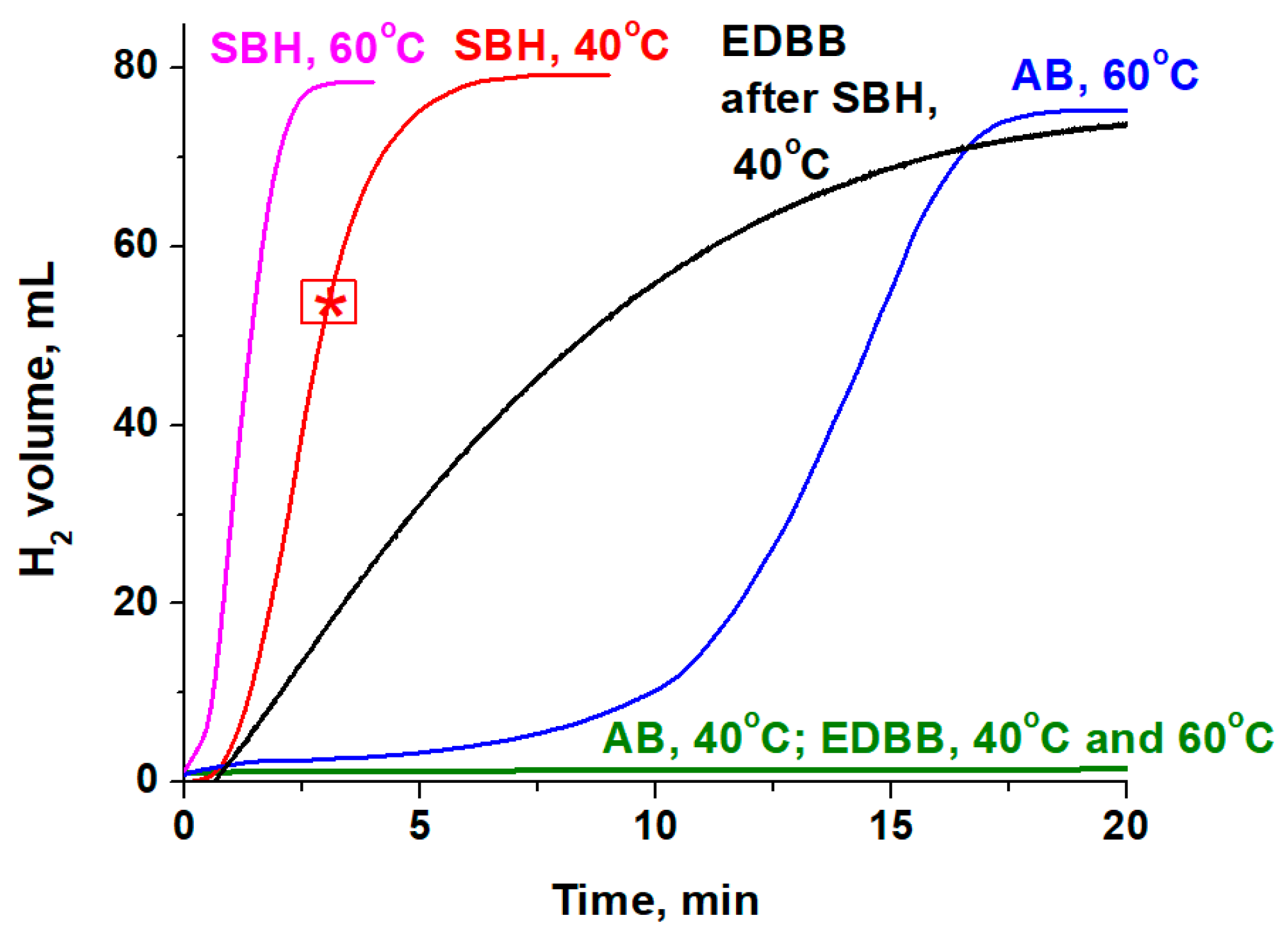
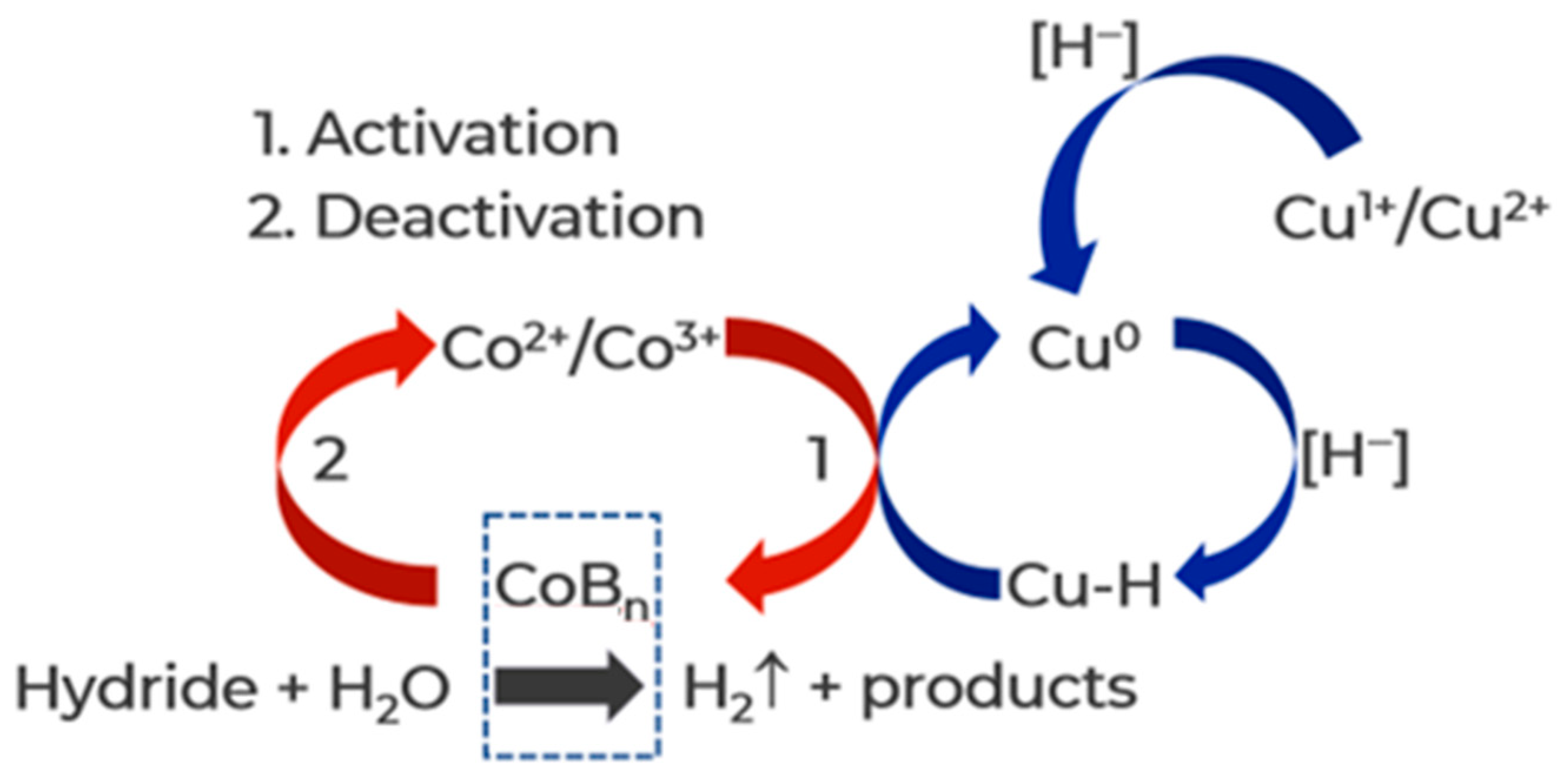




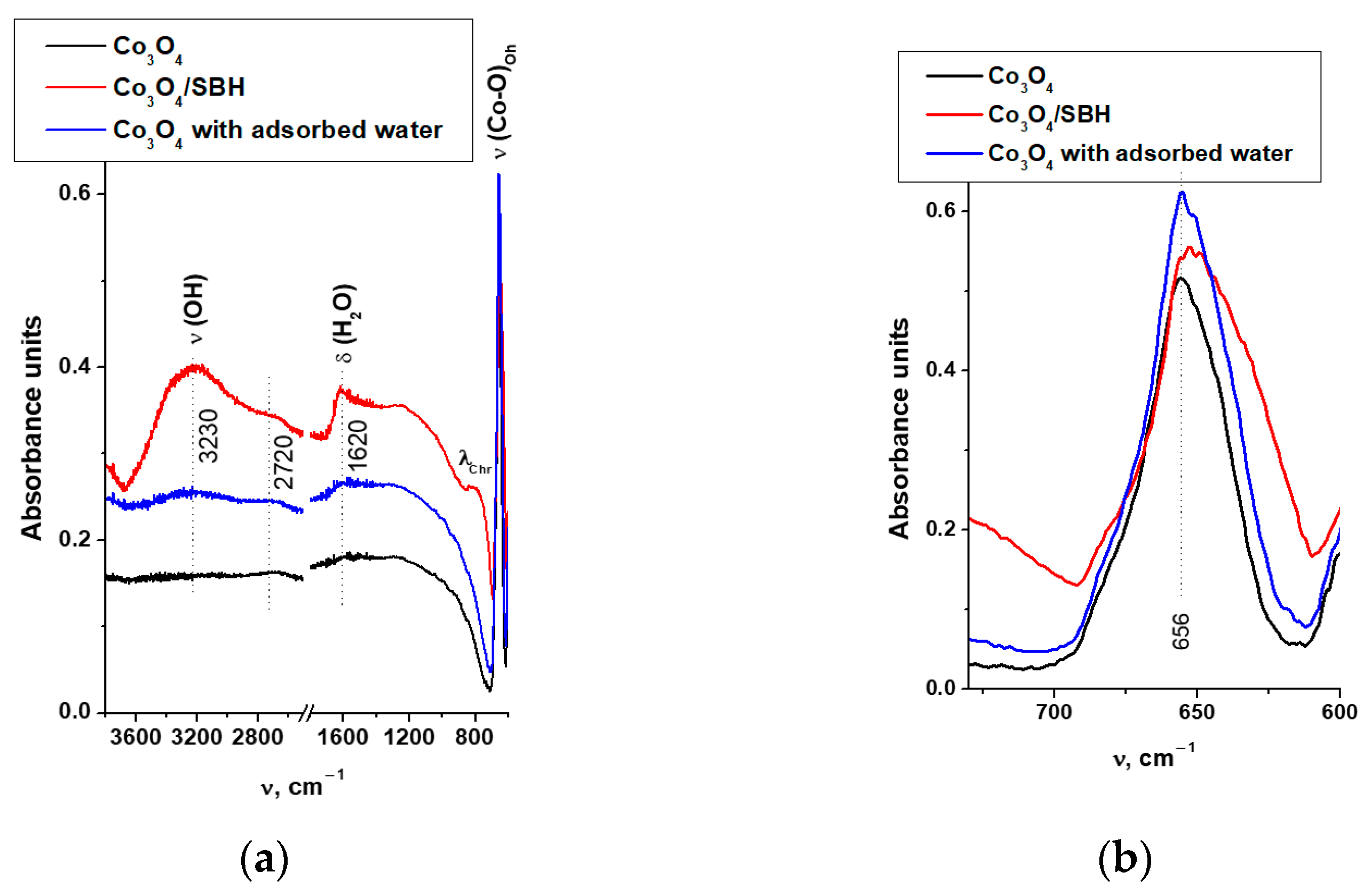
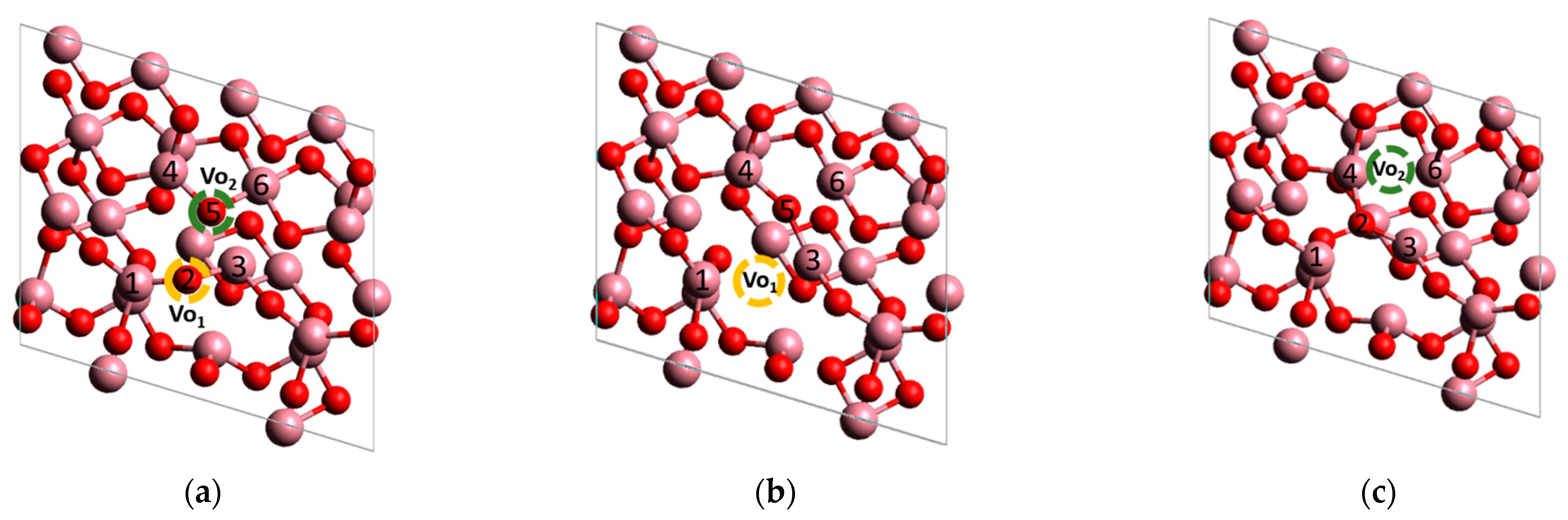
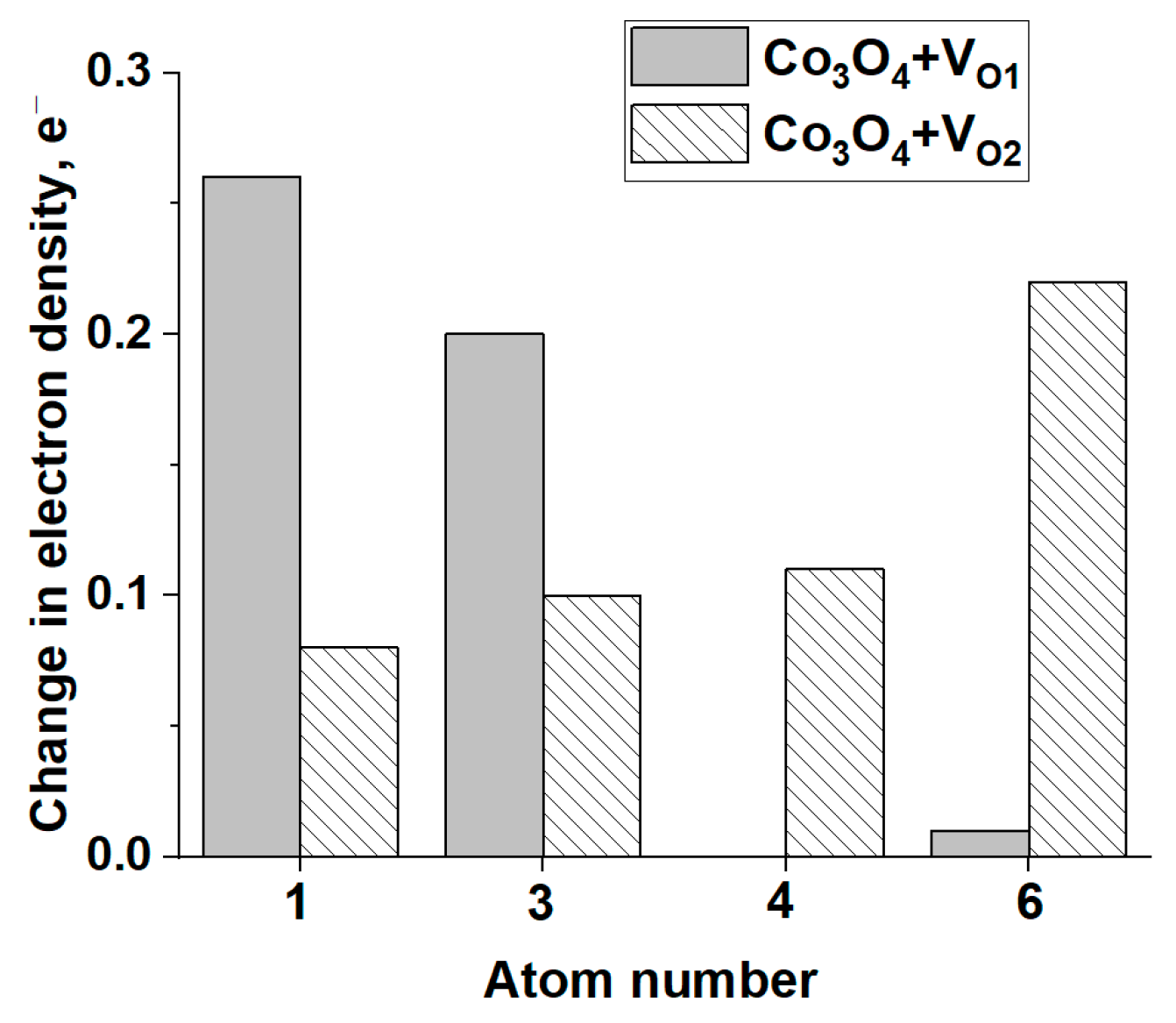


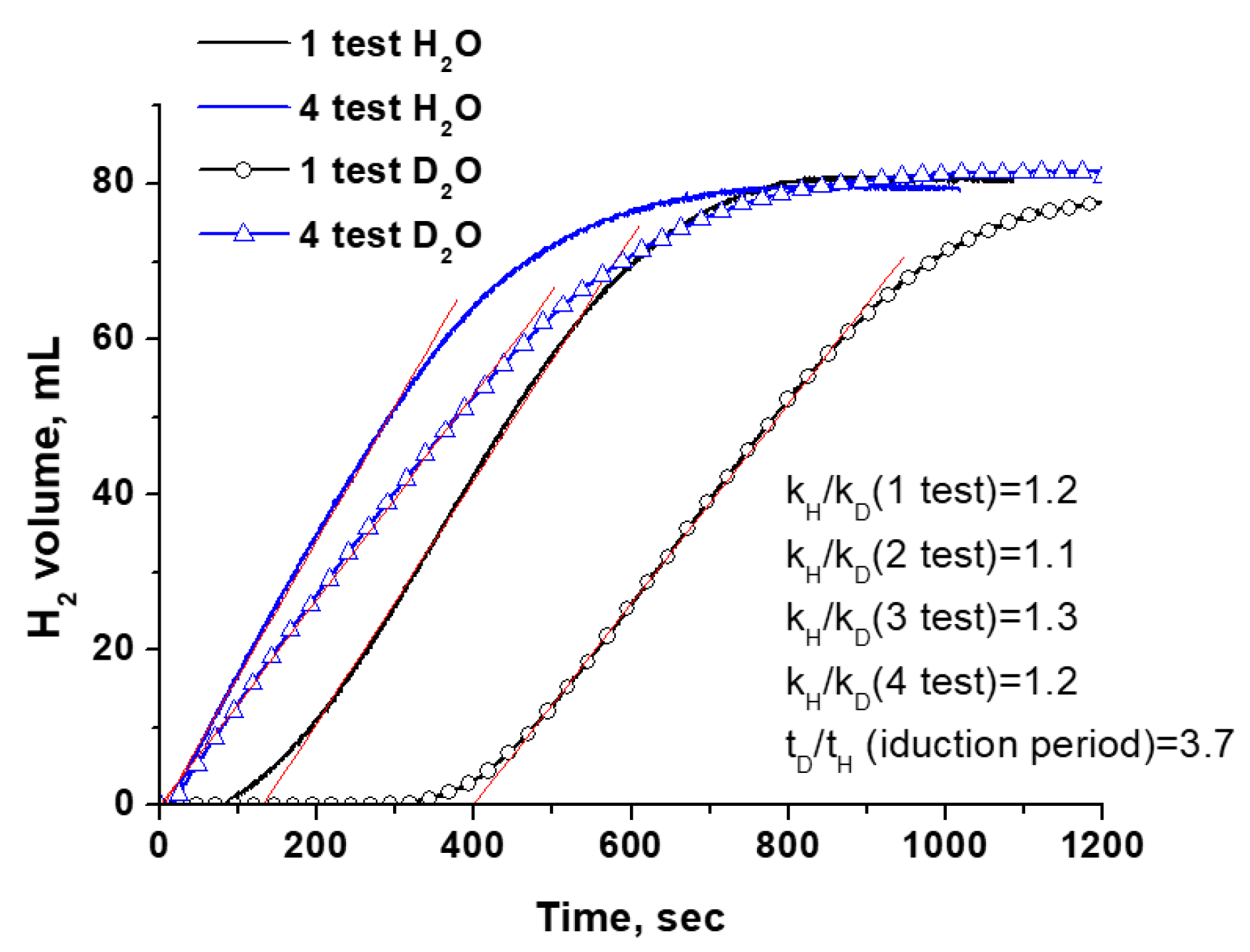
| Metal | Eads H2O, eV | M-O, Å | ∠H-O-H, ° | Eads AB, eV | M-H, Å | ∠H-B-H, ° |
|---|---|---|---|---|---|---|
| Co | −0.364 | 2.200 | 105.7 | −2.451 | 1.162 | 142.7 |
| Ni | −0.317 | 2.167 | 105.6 | −1.864 | 1.594 | 137.2 |
| Cu | −0.184 | 2.349 | 105.0 | −1.233 | 1.814 | 115.6 |
| H2O/NH3BH3 | 104.4 | 113.4 |
| Sample | Phase Composition, wt% | SCR 1, nm | a(Co3O4) 2, Å |
|---|---|---|---|
| Co3O4 | 100% Co3O4 | 41 | 8.084 (±0.001) |
| Co3O4/SBH | 95% Co3O4 | 40 | 8.085 (±0.001) |
| 5% CoO 3 | 17 |
| Sample | NaBH4 | NH3BH3 | (CH2NH2BH3)2 |
|---|---|---|---|
| Co0 | 2.4 | 2.5 | 2.3 |
| Co3O4 1 | 1.2 | - | - |
| 10% CuO-90% Co3O4 | 1.7 | 1.6 | 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butenko, V.R.; Komova, O.V.; Simagina, V.I.; Lipatnikova, I.L.; Ozerova, A.M.; Danilova, N.A.; Rogov, V.A.; Odegova, G.V.; Bulavchenko, O.A.; Chesalov, Y.A.; et al. Co and Co3O4 in the Hydrolysis of Boron-Containing Hydrides: H2O Activation on the Metal and Oxide Active Centers. Materials 2024, 17, 1794. https://doi.org/10.3390/ma17081794
Butenko VR, Komova OV, Simagina VI, Lipatnikova IL, Ozerova AM, Danilova NA, Rogov VA, Odegova GV, Bulavchenko OA, Chesalov YA, et al. Co and Co3O4 in the Hydrolysis of Boron-Containing Hydrides: H2O Activation on the Metal and Oxide Active Centers. Materials. 2024; 17(8):1794. https://doi.org/10.3390/ma17081794
Chicago/Turabian StyleButenko, Vladislav R., Oksana V. Komova, Valentina I. Simagina, Inna L. Lipatnikova, Anna M. Ozerova, Natalya A. Danilova, Vladimir A. Rogov, Galina V. Odegova, Olga A. Bulavchenko, Yuriy A. Chesalov, and et al. 2024. "Co and Co3O4 in the Hydrolysis of Boron-Containing Hydrides: H2O Activation on the Metal and Oxide Active Centers" Materials 17, no. 8: 1794. https://doi.org/10.3390/ma17081794
APA StyleButenko, V. R., Komova, O. V., Simagina, V. I., Lipatnikova, I. L., Ozerova, A. M., Danilova, N. A., Rogov, V. A., Odegova, G. V., Bulavchenko, O. A., Chesalov, Y. A., & Netskina, O. V. (2024). Co and Co3O4 in the Hydrolysis of Boron-Containing Hydrides: H2O Activation on the Metal and Oxide Active Centers. Materials, 17(8), 1794. https://doi.org/10.3390/ma17081794






