Structural Characterization of La0.6Sr0.4CoO3−δ Thin Films Grown on (100)-, (110)-, and (111)-Oriented La0.95Sr0.05Ga0.95Mg0.05O3−δ
Abstract
:1. Introduction
2. Experimental Procedure
3. Results And Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laguna-Bercero, M. Recent advances in high temperature electrolysis using solid oxide fuel cells: A review. J. Power Sources 2012, 203, 4–16. [Google Scholar] [CrossRef]
- Fergus, J.W. Perovskite oxides for semiconductor-based gas sensors. Sens. Actuators B 2007, 123, 1169–1179. [Google Scholar] [CrossRef]
- Yatoo, M.A.; Habib, F.; Malik, A.H.; Qazi, M.J.; Ahmad, S.; Ganayee, M.A.; Ahmad, Z. Solid-oxide fuel cells: A critical review of materials for cell components. MRS Commun. 2023, 13, 378–384. [Google Scholar] [CrossRef]
- Teraoka, Y.; Nobunaga, T.; Okamoto, K.; Miura, N.; Yamazoe, N. Influence of constituent metal cations in substituted LaCoO3 on mixed conductivity and oxygen permeability. Solid State Ionics 1991, 48, 207–212. [Google Scholar] [CrossRef]
- Petrov, A.N.; Kononchuk, O.F.; Andreev, A.V.; Cherepanov, V.A.; Kofstad, P. Crystal structure, electrical and magnetic properties of La1−xSrxCoO3−y. Solid State Ionics 1995, 80, 189–199. [Google Scholar] [CrossRef]
- Sehlin, S.R.; Anderson, H.U.; Sparlin, D.M. Electrical characterization of the (La,Ca) (Cr,Co) 03, system. Solid State Ionics 1995, 78, 235–243. [Google Scholar] [CrossRef]
- Januschewsky, J.; Ahrens, M.; Opitz, A.; Kubel, F.; Fleig, J. Optimized La0.6Sr0.4CoO3−δ Thin-Film Electrodes with Extremely Fast Oxygen-Reduction Kinetics. Adv. Funct. Mater. 2009, 19, 3151–3156. [Google Scholar] [CrossRef]
- Kawada, T.; Suzuki, J.; Sase, M.; Kaimai, A.; Yashiro, K.; Nigara, Y.; Mizusaki, J.; Kawamura, K.; Yugami, H. Determination of Oxygen Vacancy Concentration in a Thin Film of La0.6Sr0.4CoO3−δ by and Electrochemical Method. J. Electrochem. Soc. 2002, 149, E252–E259. [Google Scholar] [CrossRef]
- Siebenhofer, M.; Riedl, C.; Nenning, A.; Artner, W.; Rameshan, C.; Opitz, A.K.; Fleig, J.; Kubicek, M. Improving and degrading the oxygen exchange kinetics of La0.6Sr0.4CoO3−δ by Sr decoration. J. Mater. Chem. A 2023, 11, 12827. [Google Scholar] [CrossRef]
- Ražnjević, S.; Siebenhofer, M.; Bumberger, A.E.; Böhme, C.; Riedl, C.; Chen, Z.; Kubicek, M.; Zhang, Z. Electron beam-induced brownmillerite–perovskite phase transition in La0.6Sr0.4CoO3−δ. Appl. Phys. Lett. 2023, 122, 211903. [Google Scholar] [CrossRef]
- Kubicek, M.; Cai, Z.; Ma, W.; Yildiz, B.; Hutter, H.; Fleig, J. Tensile Lattice Strain Accelerates Oxygen Surface Exchange and Diffusion in La1−xSrxCoO3−δ Thin Films. J. Electrochem. Soc. 2013, 7, 3276–3286. [Google Scholar] [CrossRef]
- Reis, S.L.; Muccillo, E.N.S. Ionic Conductivity of Chemically Synthesized La0.9Sr0.1Ga0.8Mg0.2O3−δ Solid Electrolyte. Adv. Mater. Res. 2014, 975, 81–85. [Google Scholar] [CrossRef]
- Vasylechko, L.; Vashook, V.; Savytskii, D.; Senyshyn, A.; Niewa, R.; Knapp, M.; Ullmann, H.; Berkowski, M.; Matkovskii, A.; Bismayer, U. Crystal structure, thermal expansion and conductivity of anisotropic La1−xSrxGa1−2xMg2xO3−y (x=0.05, 0.1) single crystals. J. Solid State Chem. 2003, 172, 396–411. [Google Scholar] [CrossRef]
- Kharton, V.V.; Viskup, A.P.; Yaremchenko, A.A.; Baker, R.T.; Gharbage, B.; Mather, G.C.; Figueiredo, F.M.; Naumovich, E.N.; Marques, F.M.B. Ionic conductivity of La(Sr)Ga(Mg,M)O3−δ (M = Ti, Cr, Fe, Co, Ni): Effects of transition metal dopants. Solid State Ionics 2000, 132, 119–130. [Google Scholar] [CrossRef]
- Siebenhofer, M.; Riedl, C.; Nenning, A.; Ražnjević, S.; Fellner, F.; Artner, W.; Zhang, Z.; Rameshan, C.; Fleig, J.; Kubicek, M. Crystal-Orientation-Dependent Oxygen Exchange Kinetics on Mixed Conducting Thin-Film Surfaces Investigated by In Situ Studies. ACS Appl. Energy Mater. 2023, 6, 6712–6720. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, T.; Matsuda, H.; Takita, Y. Doped LaGaO3 Perovskite Type Oxide as a New Oxide Ionic Conductor. J. Am. Chem. Soc. 1994, 116, 3801–3803. [Google Scholar] [CrossRef]
- Ishihara, T.; Matsuda, H.; Takita, Y. Effects of rare earth cations doped for La site on the oxide ionic conductivity of LaGaO3,-based perovskite type oxide. Solid State Ionics 1995, 79, 147–151. [Google Scholar] [CrossRef]
- Huang, P.; Petric, A. Superior Oxygen Ion Conductiviiy of Lanthanum Gallate Doped with Strontium and Magnesium. J. Electrochem. Soc. 1996, 143, 1644–1648. [Google Scholar] [CrossRef]
- Vasylechko, L.; Senyshyn, A.; Pivak, Y.; Berkowski, M.; Vashook, V.; Ullmann, H.; Bähtz, C.; Bismayer, U. LSGM Single Crystals: Crystal Structure, Thermal Expansion, Phase Transitions and Conductivity. In Mixed Ionic Electronic Conducting Perovskites for Advanced Energy Systems; Springer: Dordrecht, The Netherlands, 2004; Volume 173, pp. 231–237. [Google Scholar] [CrossRef]
- Hubler, G.K. Pulsed Laser Deposition. MRS Bull. 1992, 17, 26–29. [Google Scholar] [CrossRef]
- Lunney, J.G. Pulsed laser deposition of metal and metal multilayer films. Appl. Surf. Sci. 1995, 86, 79–85. [Google Scholar] [CrossRef]
- Schneider, C.W.; Lippert, T. PLD plasma plume analysis: A summary of the PSI contribution. Appl. Phys. A 2023, 129, 138. [Google Scholar] [CrossRef] [PubMed]
- Shepelin, N.A.; Tehrani, Z.P.; Ohannessian, N.; Schneider, C.W.; Pergolesi, D.; Lippert, T. A practical guide to pulsed laser deposition. Chem. Soc. Rev. 2023, 52, 2294. [Google Scholar] [CrossRef] [PubMed]
- Scharf, T.; Krebs, H. Influence of inert gas pressure on deposition rate during pulsed laser deposition. Appl. Phys. A 2002, 75, 551–554. [Google Scholar] [CrossRef]
- Willmott, P.R. Deposition of complex multielemental thin films. Prog. Surf. Sci. 2004, 76, 6–8. [Google Scholar] [CrossRef]
- Rupp, G.M.; Glowacki, M.; Fleig, J. Electronic and Ionic Conductivity of La0.95Sr0.05Ga0.95Mg0.05O3−δ (LSGM) Single Crystals. J. Electrochem. Soc. 2016, 163, F1189–F1197. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Hÿtch, M.; Snoeck, E.; Kilaas, R. Quantitative measurement of displacement and strain fields from HREM micrographs. Ultramicroscopy 1998, 74, 131–146. [Google Scholar] [CrossRef]
- Pearson, D.H.; Fultz, B.; Ahn, C.C. Measurements of 3d state occupancy in transition metals using electron energy loss spectrometry. Appl. Phys. Lett. 1988, 53, 1405. [Google Scholar] [CrossRef]
- Pearson, D.H.; Ahn, C.C.; Fultz, B. White lines and d-electron occupancies for the 3d and 4d transition metals. Phys. Rev. B 1993, 47, 8471–8478. [Google Scholar] [CrossRef]
- Wang, Z.L.; Yin, J.S.; Jiang, Y.D. EELS analysis of cation valence states and oxygen vacancies in magnetic oxides. Micron 2000, 31, 571–580. [Google Scholar] [CrossRef]
- Riedl, T.; Gemming, T.; Wetzig, K. Extraction of EELS white-line intensities of manganese compounds: Methods, accuracy, and valence sensitivity. Ultramicroscopy 2006, 106, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal—Amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251. [Google Scholar] [CrossRef] [PubMed]
- Csonka, G.I.; Perdew, J.P.; Ruzsinszky, A.; Philipsen, P.H.T.; Lebègue, S.; Paier, J.; Vydrov, O.A.; Ángyán, J.G. Assessing the performance of recent density functionals for bulk solids. Phys. Rev. B 2009, 79, 155107. [Google Scholar] [CrossRef]
- Rupp, G.M.; Schmid, A.; Nenning, A.; Fleig, J. The Superior Properties of La0.6Ba0.4CoO3−δ Thin Film Electrodes for Oxygen Exchange in Comparison to La0.6Sr0.4CoO3−δ. J. Electrochem. Soc. 2016, 163, F564–F573. [Google Scholar] [CrossRef]
- Kim, H.; An, J.; Bae, H.B.; Chung, S. Atomic-scale observation of premelting at lattice defects inside oxide crystals. Nat. Commun. 2016, 14, 2255. [Google Scholar] [CrossRef]
- Stemmer, S.; Jacobson, A.J.; Chen, X.; Igantiev, A. Oxygen Vacancy Ordering in Epitaxial La0.5Sr0.5CoO3−δ. J. Appl. Phys. 2001, 90, 3319. [Google Scholar] [CrossRef]
- Gazquez, J.; Bose, S.; Sharma, M.; Torija, M.A.; Pennycook, S.J.; Leighton, C.; Varela, M. Lattice mismatch accommodation via oxygen vacancy ordering in epitaxial La0.5Sr0.5CoO3−δ thin films. APL Mater. 2013, 1, 012105. [Google Scholar] [CrossRef]
- Ivanov, Y.P.; Kubicek, M.; Siebenhofer, M.; Viernstein, A.; Hutter, H.; Fleig, J.; Chuvilin, A.; Zhang, Z. Strain-induced structure and oxygen transport interactions in epitaxial La0.6Sr0.4CoO3−δ. Commun. Mater. 2020, 1, 32. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, F.; Gunkel, F.; Wei, X.K.; Zhang, Y.; Wang, D.; Barthel, J.; Dunin-Borkowski, R.E.; Jia, C.L. Understanding Structural Incorporation of Oxygen Vacancies in Perovskite Cobaltite Films and Potential Consequences for Electrocatalysis. Chem. Mater. 2022, 34, 10373–10381. [Google Scholar] [CrossRef]
- Tan, H.; Verbeeck, J.; Abakumov, A.; Tendeloo, G.V. Oxidation state and chemical shift investigation in transition metal oxides by EELS. Ultramicroscopy 2012, 166, 24–33. [Google Scholar] [CrossRef]
- Haselmann, U.; Haberfehlner, G.; Pei, W.; Popov, M.N.; Romaner, L.; Knez, D.; Chen, J.; Ghasemi, A.; He, Y.; Kothleitner, G.; et al. Study on Ca segregation toward an epitaxial interface between bismuth ferrite and strontium titanate. ACS Appl. Mater. Interfaces 2020, 12, 12264–12274. [Google Scholar] [CrossRef] [PubMed]
- Stemmer, S.; Sane, A.; Browning, N.D.; Mazanec, T.J. Characterization of oxygen-deficient SrCoO3−δ by electron energy-loss spectroscopy and Z-contrast imaging. Solid State Ionics 2000, 130, 71–80. [Google Scholar] [CrossRef]
- Biškup, N.; Salafranca, J.; Mehta, V.; Oxley, M.P.; Suzuki, Y.; Pennycook, S.J.; Pantelides, S.T.; Varela, M. Insulating Ferromagnetic LaCoO3−δ Films: A Phase Induced by Ordering of Oxygen Vacancies. Phys. Rev. Lett. 2014, 112, 087202. [Google Scholar] [CrossRef]

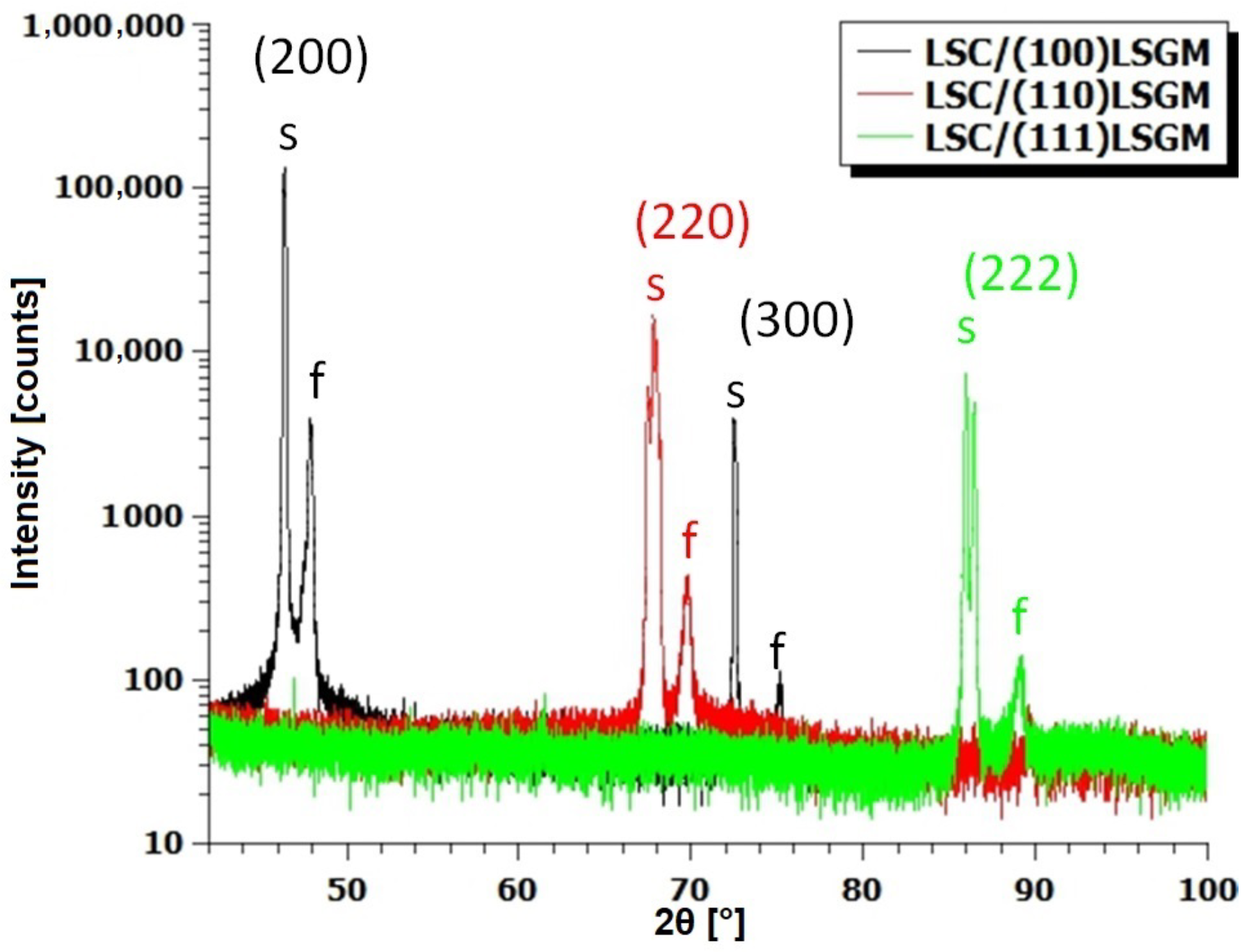


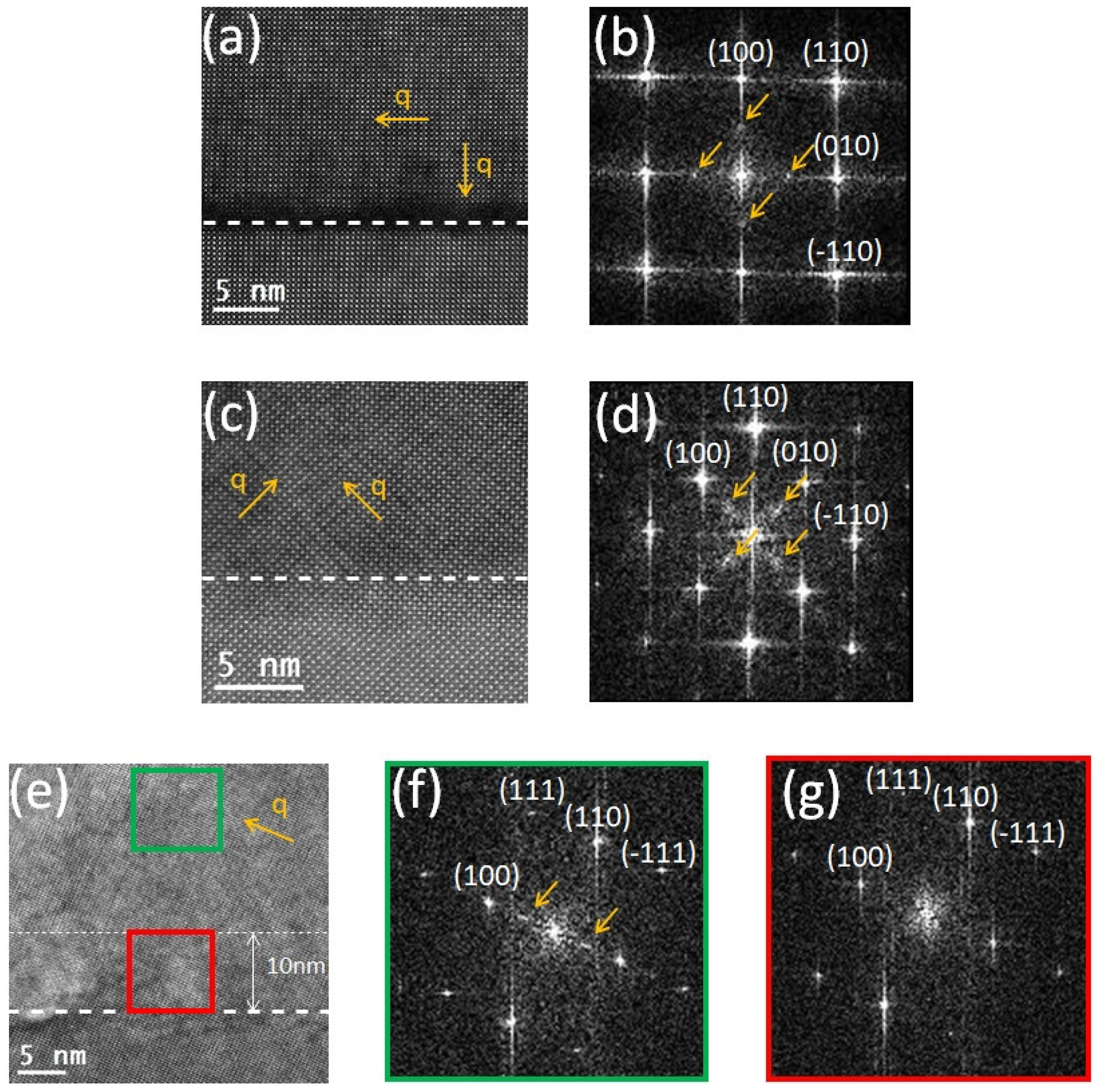
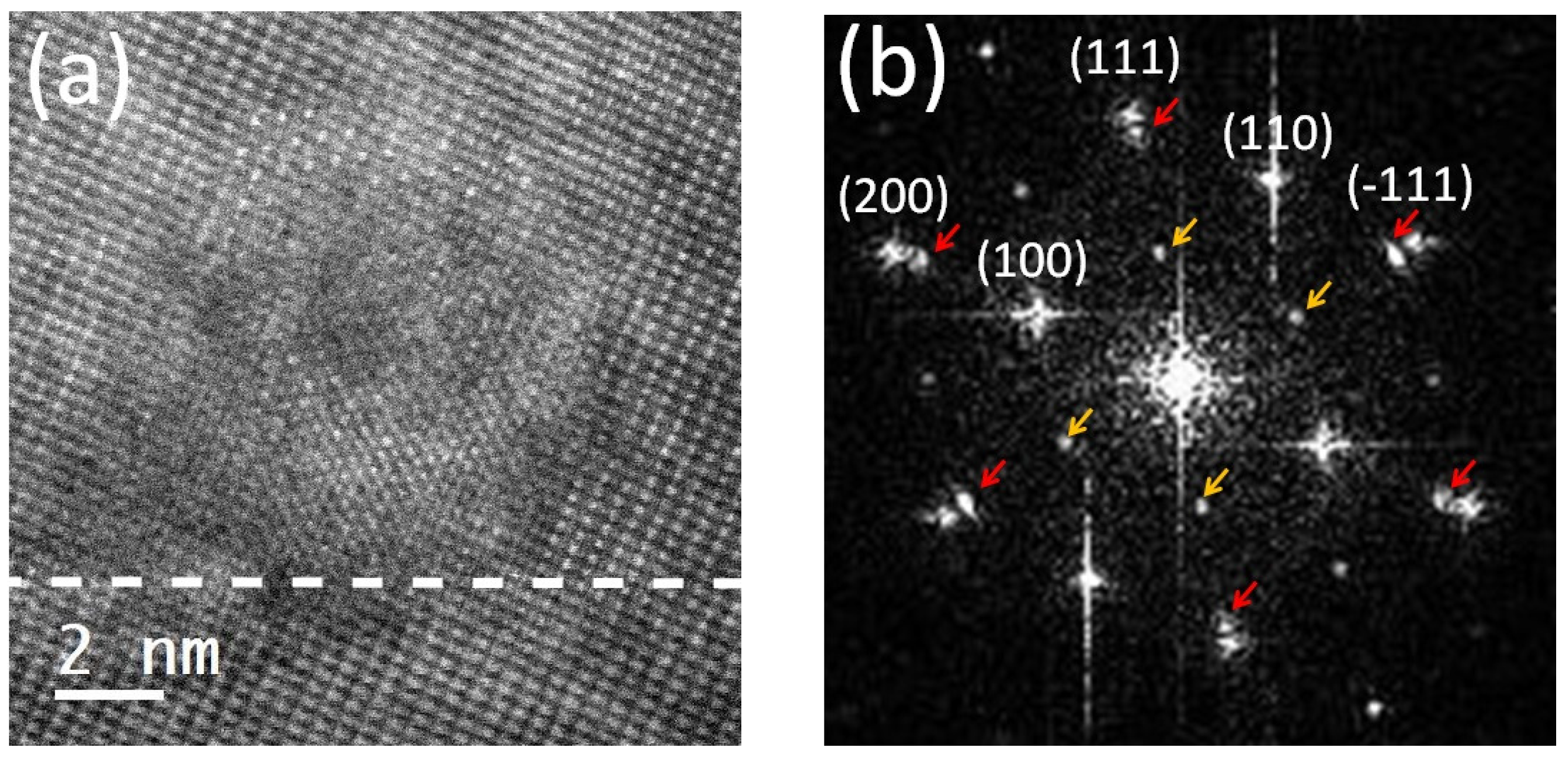

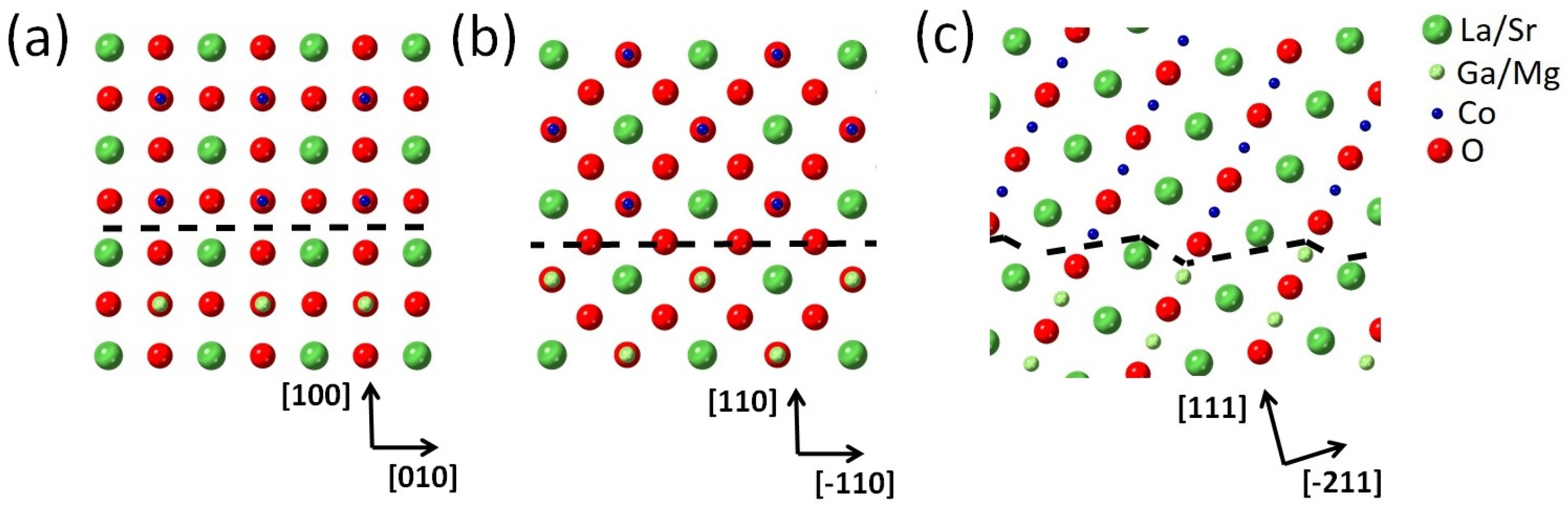
| Domain | / Å | / Å | / Å |
|---|---|---|---|
| A | 3.919 | 2.775 | 2.260 |
| B | 3.915 | 2.772 | 2.250 |
| C | 3.913 | 2.763 | |
| D | 3.911 | 2.761 | |
| E | 3.908 | 2.756 | |
| F | 3.906 | 2.754 | |
| G | 3.900 | 2.748 |
| Direction | (100) LSGM | (110) LSGM | (111) LSGM |
|---|---|---|---|
| d-spacing / Å | = 4.01 | = 2.78 | = 1.60 |
| = 3.90 | = 2.75 | = 2.23 | |
| Crystal system | Monoclinic | Tetragonal | Tetragonal |
| Orientation | / % | / % |
|---|---|---|
| (100) | 4.48 | 1.62 |
| (110) | 2.45 | 1.33 |
| (111) | 2.11 | 0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ražnjević, S.; Drev, S.; Bumberger, A.E.; Popov, M.N.; Siebenhofer, M.; Böhme, C.; Chen, Z.; Huang, Y.; Riedl, C.; Fleig, J.; et al. Structural Characterization of La0.6Sr0.4CoO3−δ Thin Films Grown on (100)-, (110)-, and (111)-Oriented La0.95Sr0.05Ga0.95Mg0.05O3−δ. Materials 2024, 17, 1802. https://doi.org/10.3390/ma17081802
Ražnjević S, Drev S, Bumberger AE, Popov MN, Siebenhofer M, Böhme C, Chen Z, Huang Y, Riedl C, Fleig J, et al. Structural Characterization of La0.6Sr0.4CoO3−δ Thin Films Grown on (100)-, (110)-, and (111)-Oriented La0.95Sr0.05Ga0.95Mg0.05O3−δ. Materials. 2024; 17(8):1802. https://doi.org/10.3390/ma17081802
Chicago/Turabian StyleRažnjević, Sergej, Sandra Drev, Andreas E. Bumberger, Maxim N. Popov, Matthäus Siebenhofer, Christin Böhme, Zhuo Chen, Yong Huang, Christoph Riedl, Jürgen Fleig, and et al. 2024. "Structural Characterization of La0.6Sr0.4CoO3−δ Thin Films Grown on (100)-, (110)-, and (111)-Oriented La0.95Sr0.05Ga0.95Mg0.05O3−δ" Materials 17, no. 8: 1802. https://doi.org/10.3390/ma17081802





