Preparation of Ultrafine Co- and Ni-Coated (Ti,W,Mo,Ta)(C,N) Powders and Their Influence on the Microstructure of Ti(C,N)-Based Cermets
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Ultrafine Co- and Ni-Coated (Ti,W,Mo,Ta)(C,N) Powders
2.2. Characterization
3. Results and Discussion
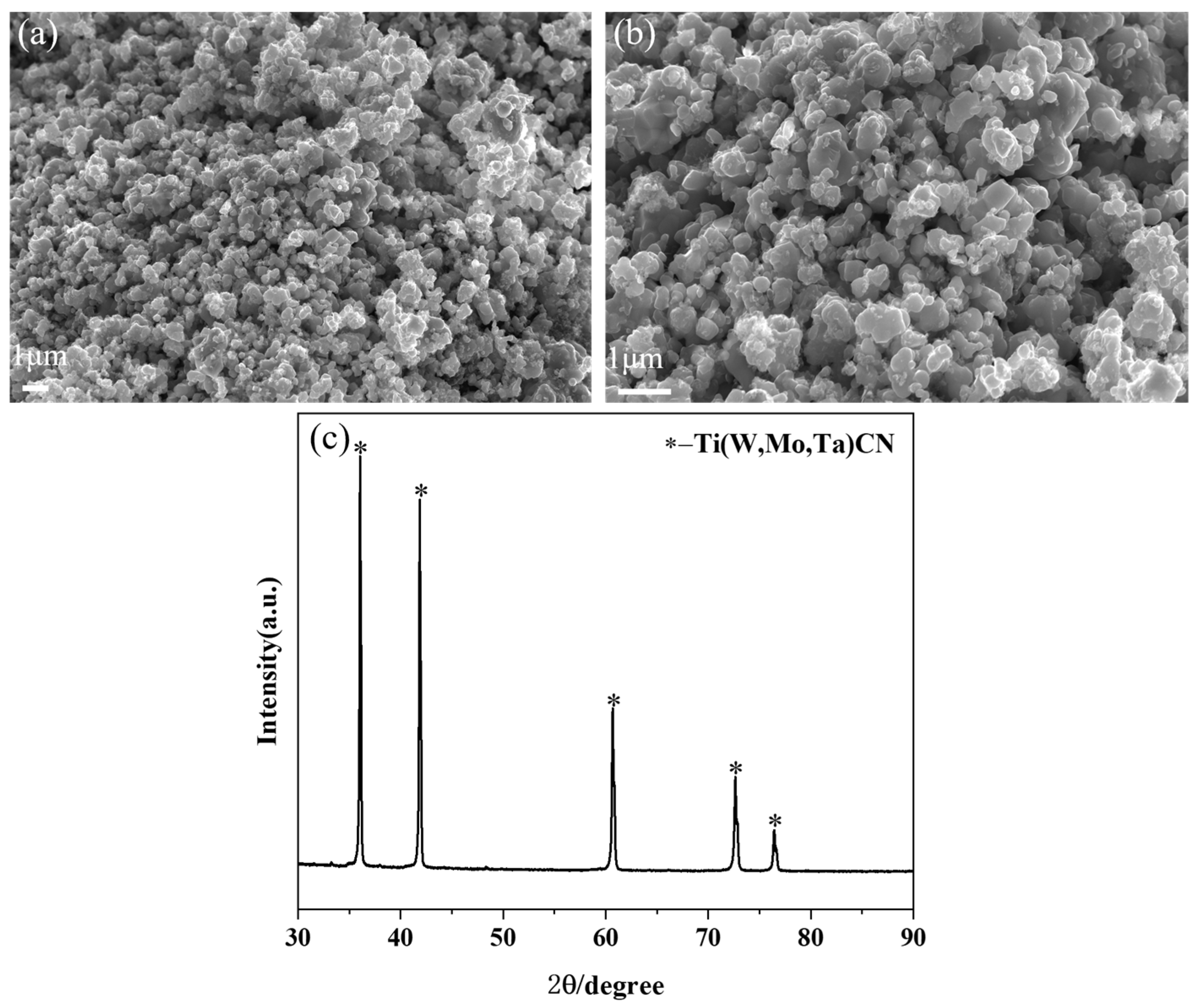
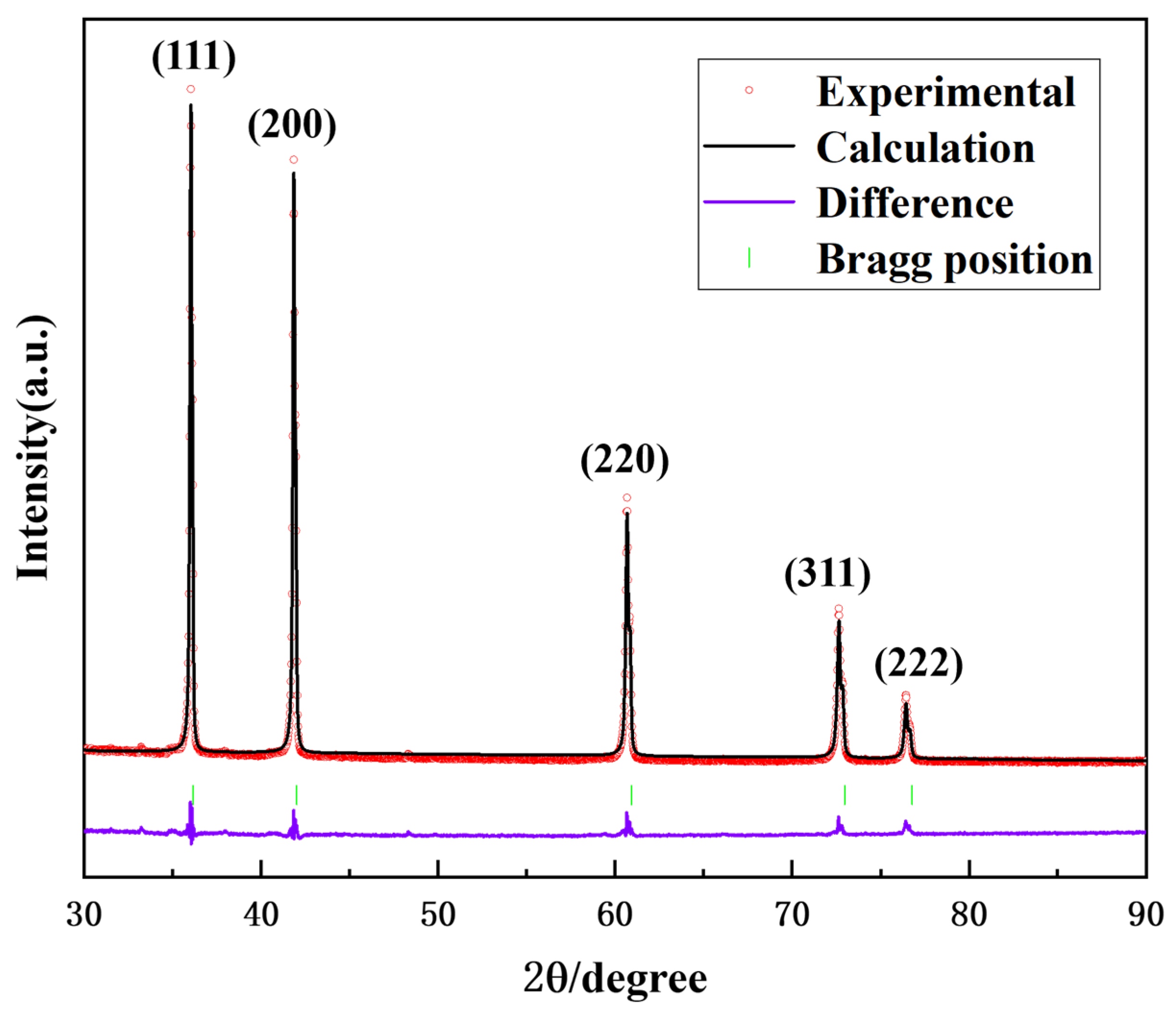
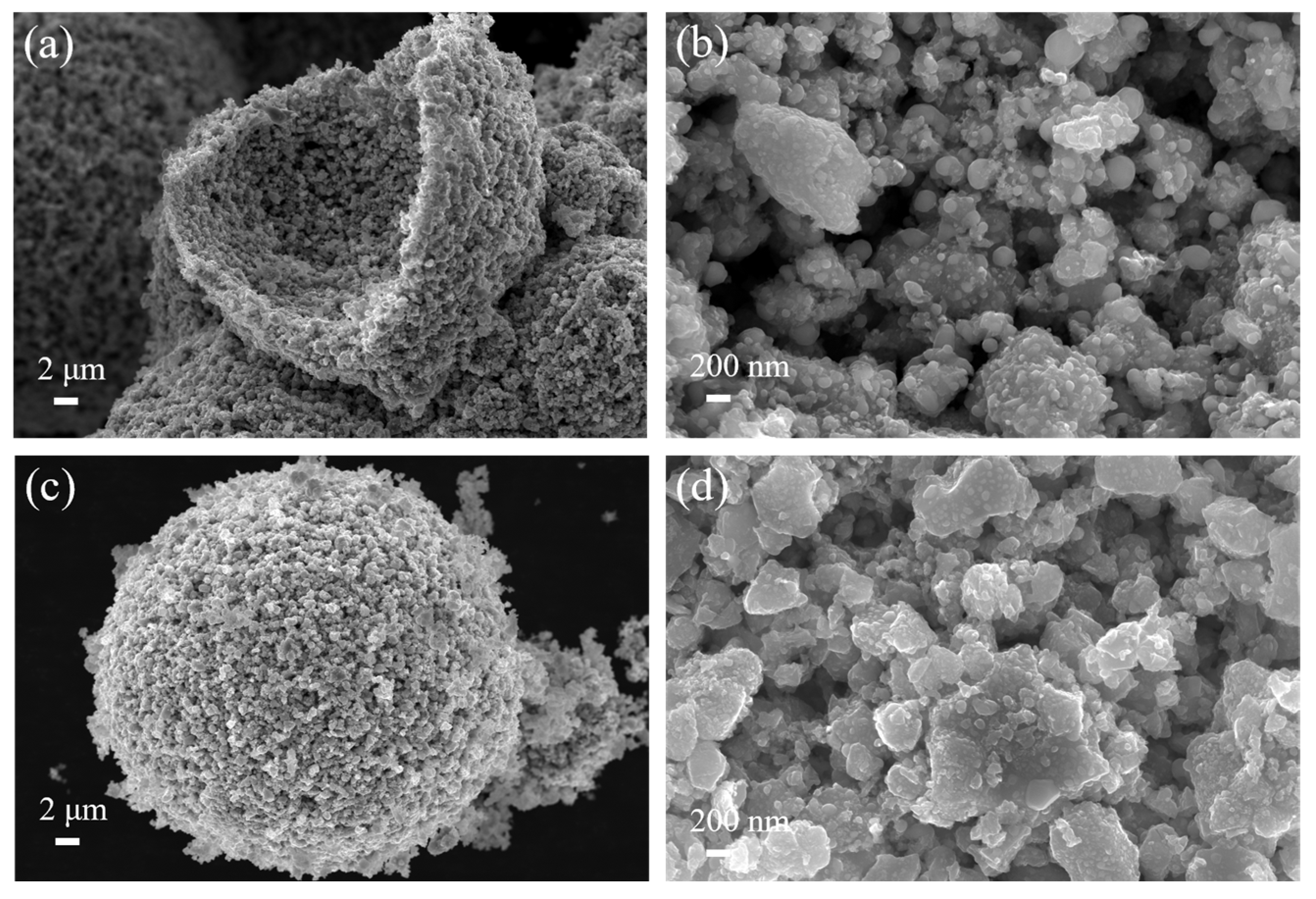
3.1. Ultrafine (Ti,W,Mo,Ta)(C,N) Powders
3.2. Precursor Powder
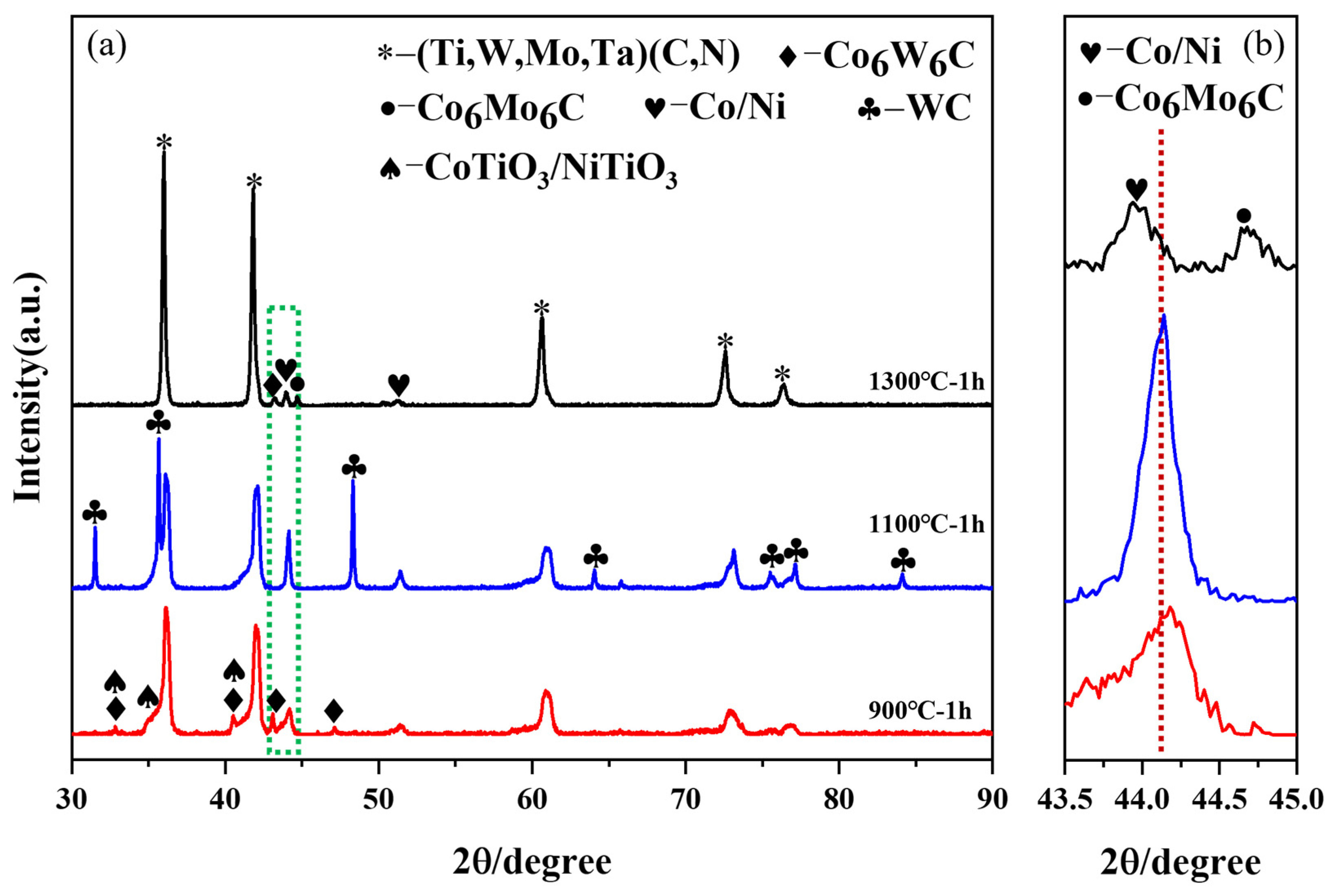
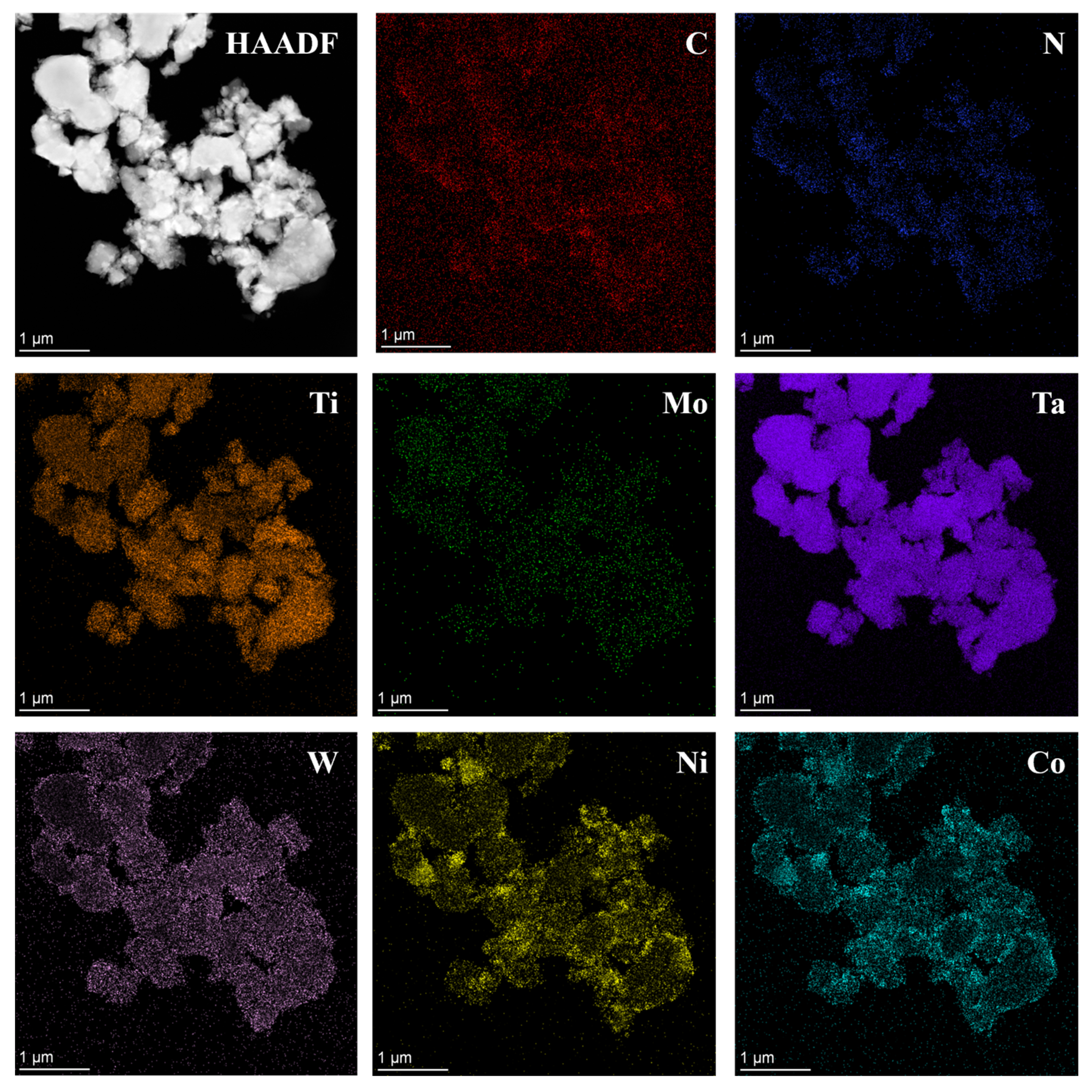
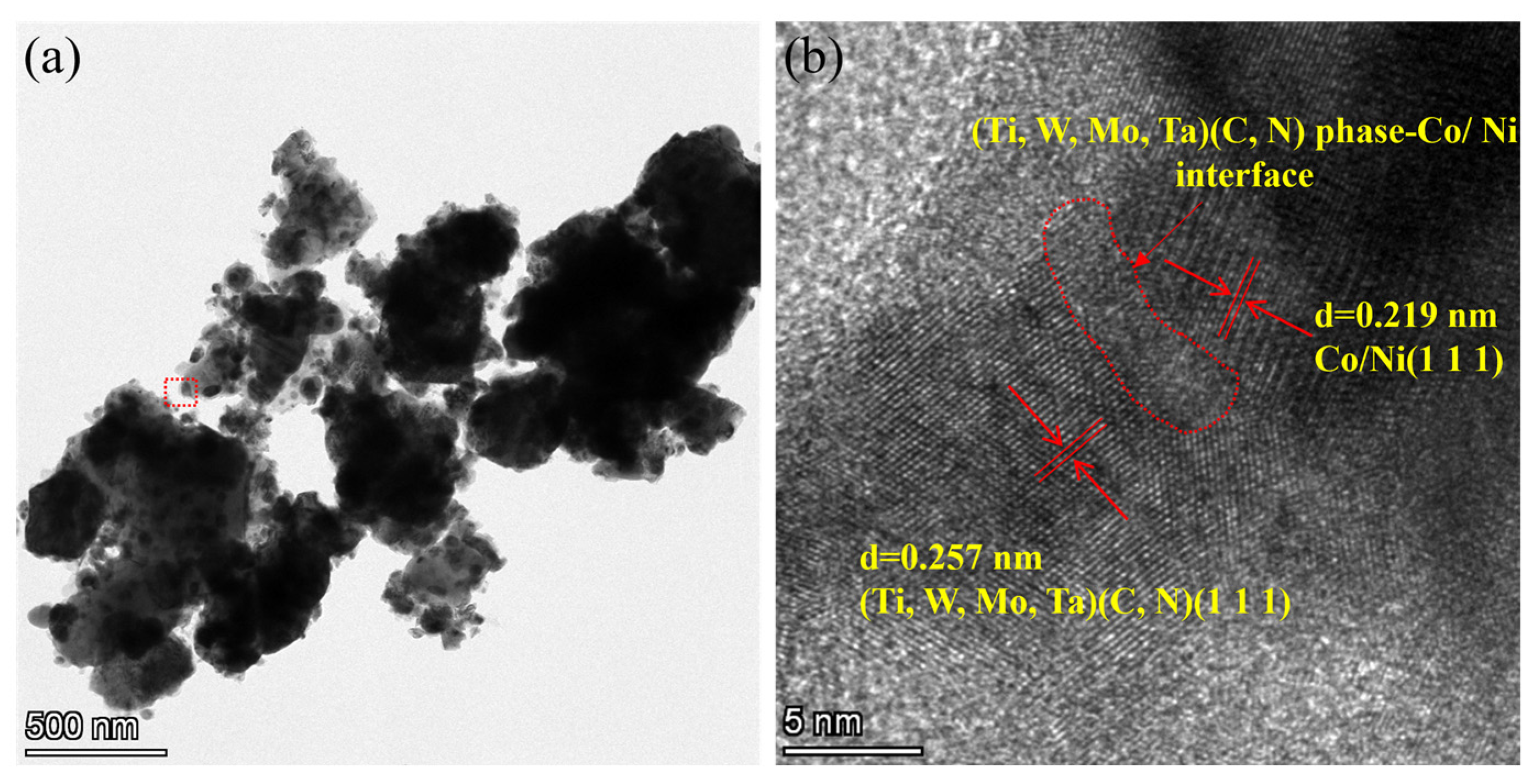
3.3. Phase Evolution and Microstructure of Coated Powder
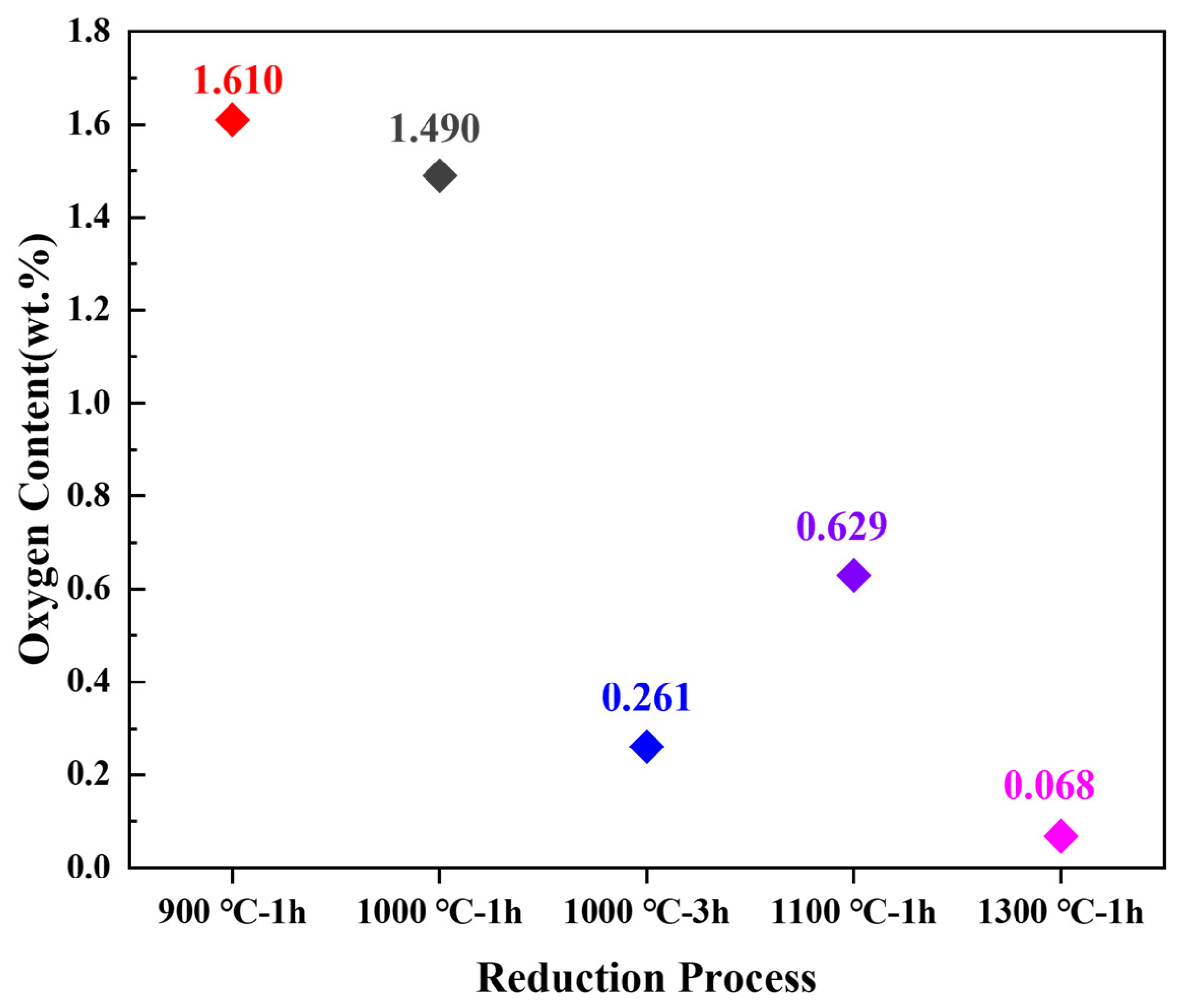
3.3.1. Influence of Reaction Temperature
3.3.2. Influence of Isothermal Time
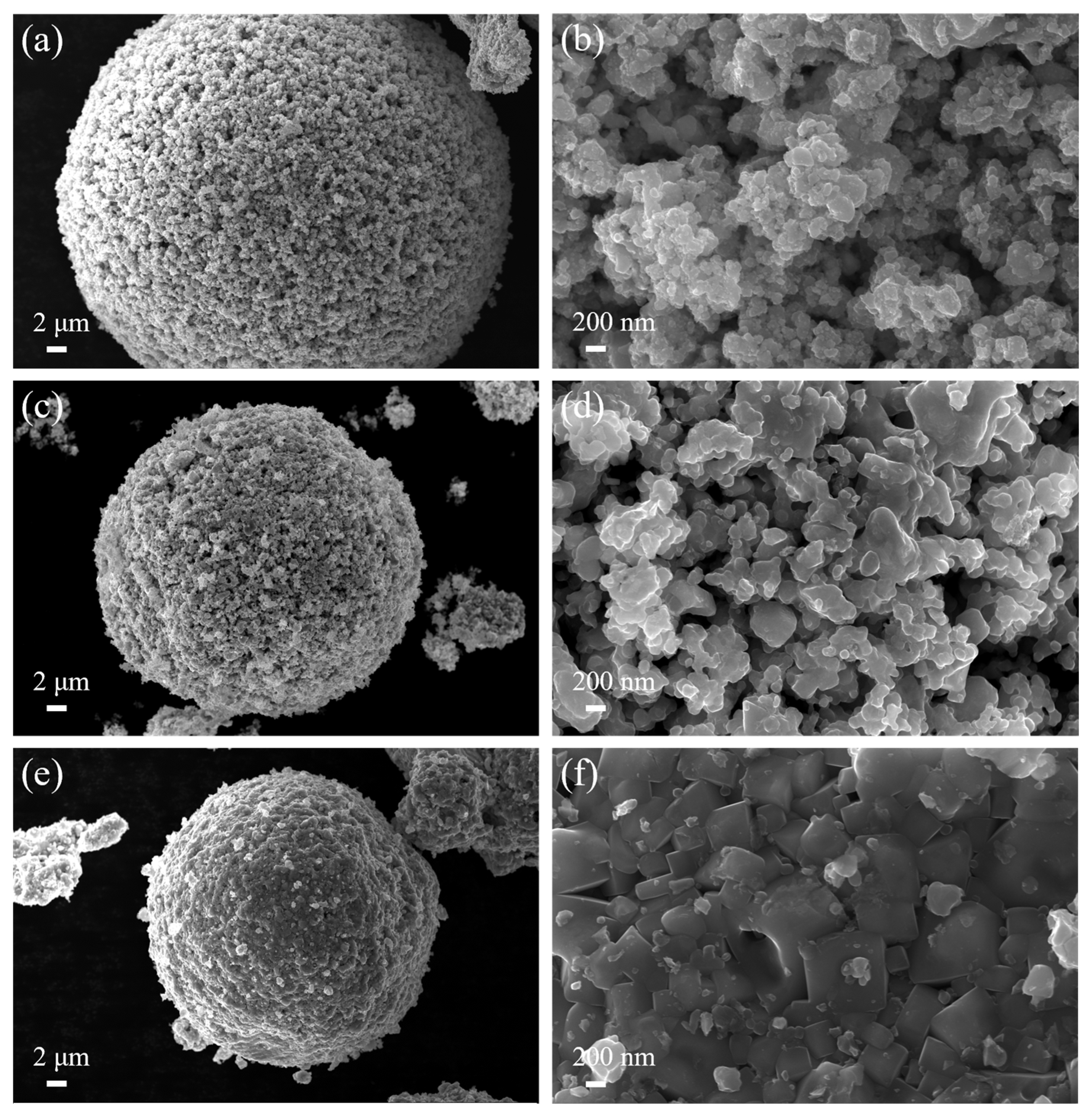
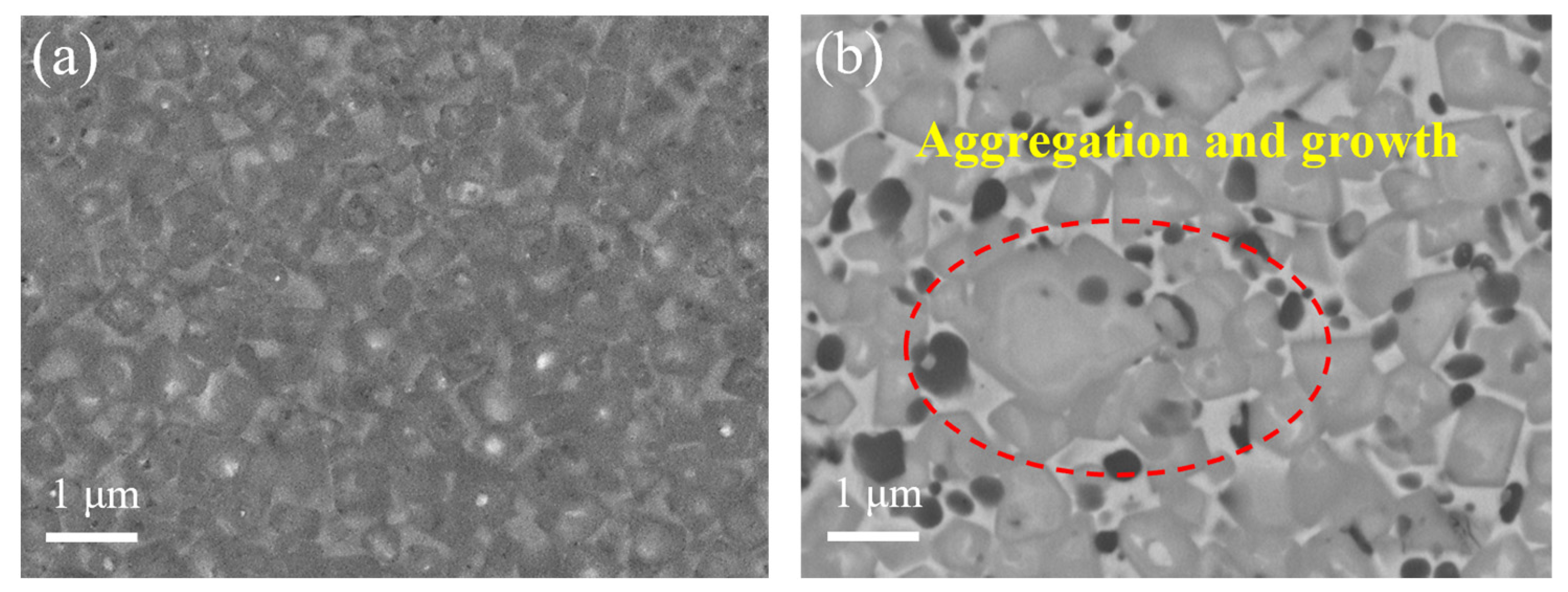
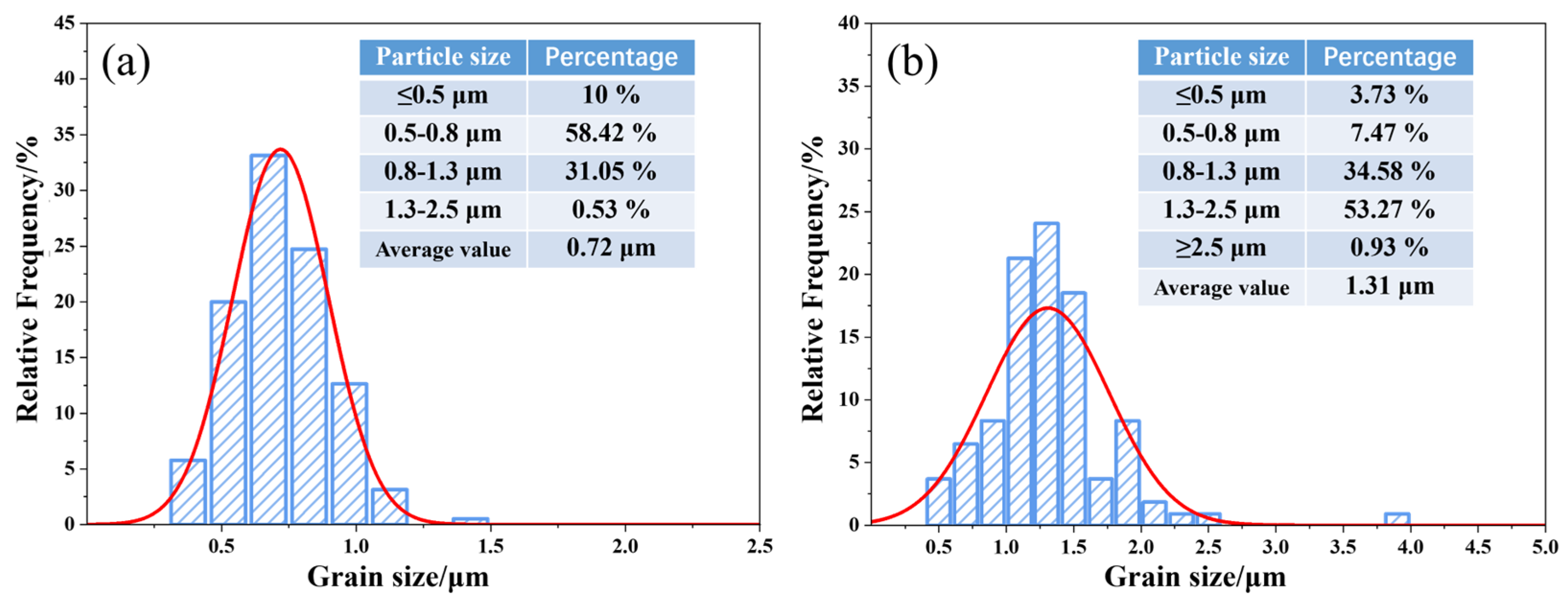
3.4. Microstructure of (Ti,W,Mo,Ta)(C,N)-Based Cermets
4. Conclusions
- (1).
- Ultrafine Co- and Ni-coated (Ti,W,Mo,Ta)(C,N) powders were successfully prepared via the spray-drying-in-situ carbothermal reduction method. Herein, the precursors of Co/Ni were (CH3COO)2Co·4H2O, and Ni(CH3COO)2·4H2O. Firstly, the (Ti,Me)(C,N) particles were physically coated by the precursors of Co/Ni through spray drying. Then, the acetates were completely reduced in situ into Co and Ni. TEM and other analytical methods proved that the prepared powders displayed a core ((Ti,W,Mo,Ta)(C,N))-shell (Co/Ni) structure.
- (2).
- Notably, the reduction reaction is facilitated by increasing the reduction temperature or extending the isothermal time. Moreover, the generation of intermediate phases can be avoided by rapid heating (10 °C/min) within a specific temperature range (600–1000 °C). It is feasible to achieve the complete reduction of Co and Ni metal salts pre-coated on the surface of (Ti,Me)(C,N) particles at a lower reduction temperature of 1000 °C with a long isothermal time of 3 h.
- (3).
- The cermets fabricated using the prepared ultrafine Co- and Ni-coated (Ti,W,Mo,Ta)(C,N) powders demonstrated a more uniform and finer microstructure compared with traditional cermets. They lacked the conventional core–rim structure. The weaker residual stresses at the core and rim interfaces and the finer particle size could theoretically enhance the toughness and hardness of Ti(C,N)-based cermets, simultaneously.
- (4).
- However, this article did not investigate the impact of ultrafine coated powders on the mechanical properties of Ti(C,N)-based cermets. According to the literature reports [10], the particle size of the raw materials directly influences the preparation process of cermets. Therefore, it is necessary to systematically study the preparation process of alloys in order to obtain optimal mechanical properties. The corresponding research will be systematically elaborated on in our subsequent studies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ji, W.B.; Zou, B.; Huang, C.Z.; Liu, Y.A.; Huang, C.M. Fabrication, microstructure and mechanical properties of self-diffusion gradient cermet composite tool materials. Mater. Sci. Eng. A 2017, 685, 332–341. [Google Scholar] [CrossRef]
- Chicardi, E.; Córdoba, J.M.; Gotor, F.J. High temperature oxidation resistance of (Ti,Ta)(C,N)-based cermets. Corros. Sci. 2016, 102, 125–136. [Google Scholar] [CrossRef]
- Jam, A.; Nikzad, L.; Razavi, M. TiC-based cermet prepared by high-energy ball-milling and reactive spark plasma sintering. Ceram. Int. 2017, 43, 2448–2455. [Google Scholar] [CrossRef]
- Seo, M.; Kim, J.; Kang, S. Effect of carbon content on the microstructure and properties of (Ti0.7W0.3)C-Ni cermet. Int. J. Refract. Met. Hard Mater. 2011, 29, 424–428. [Google Scholar] [CrossRef]
- Rajabi, A.; Ghazali, M.J.; Daud, A.R. Chemical composition, microstructure and sintering temperature modifications on mechanical properties of TiC-based cermet—A review. Mater. Des. 2015, 67, 95–106. [Google Scholar] [CrossRef]
- Maurya, H.; Juhani, K.; Viljus, M. Microstructural evolution and mechanical properties of Ti (C, N)–FeCrMo-based green cermets. Ceram. Int. 2024, 50, 8695–8705. [Google Scholar] [CrossRef]
- García, J.; Ciprés, V.C.; Blomqvist, A. Cemented carbide microstructures: A review. Int. J. Refract. Met. Hard Mater. 2019, 80, 40–68. [Google Scholar] [CrossRef]
- Ragothuman, A.; Natarajan, G.K.; Shanmugavel, B.P. Wear and thermal stability of TiCN-WC-Co-Cr3C2 cermets modified by TiN. Int. J. Refract. Met. Hard Mater. 2019, 84, 105020. [Google Scholar] [CrossRef]
- Lin, N.; Zheng, Z.P.; Zhao, L.B.; Ma, C.; Wang, Z.Y.; He, Y.H. Influences of ultrafine Ti(C,N) additions on microstructure and properties of micron Ti(C,N)-based cermets. Mater. Chem. Phys. 2019, 230, 197–206. [Google Scholar] [CrossRef]
- Ma, L.L.; Zhao, Z.Y.; Wu, Y.R.; Sun, J.J.; Gu, S.Y.; Zhang, H.A. Influences of Ultrafine Ti(C,N) on the Sintering Process and Mechanical Properties of Micron Ti(C,N)-Based Cermets. Materials 2023, 16, 3175. [Google Scholar] [CrossRef]
- Chicardi, E.; Gotor, J. Effects of boron addition on the microstructure and mechanical properties of (Ti, Ta)(C, N)-Co based cermets. Metals 2019, 9, 787. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Chen, C.H.; Xiong, J.; Guo, Z.X.; Liu, J.B.; You, Q.B. Effects of nano-Al2O3 addition on microstructure, mechanical properties, and wear resistance of Ti(C, N)-based cermets. Int. J. Refract. Met. Hard Mater. 2023, 115, 106290. [Google Scholar] [CrossRef]
- Xu, G.X.; Liu, W.J.; Yi, C.Y.; Zheng, K.; Yi, M.D. Formation of solid solution structures in (Ti, W, Ta, Mo)(C, N) cermet via spark plasma sintering. Int. J. Refract. Met. Hard Mater. 2023, 113, 106218. [Google Scholar] [CrossRef]
- Nie, R.X.; Zhang, L.; Liu, G.; Peng, Y.B. Effect of pre-solid solution treatment of secondary carbides on the microstructure and mechanical properties of Ti(C,N)-based cermets. J. Mater. Res. Technol. 2023, 27, 3621–3631. [Google Scholar] [CrossRef]
- Li, Y.; Katsui, H.; Goto, T. Phase decomposition of (Ti, Zr)(C, N) solid solutions prepared by spark plasma sintering. J. Eur. Ceram. Soc. 2019, 39, 4588–4594. [Google Scholar] [CrossRef]
- De, G.; Gotor, J.; Chicardi, E. Effect of the impact energy on the chemical homogeneity of a (Ti, Ta, Nb)(C, N) solid solution obtained via a mechanically induced self-sustaining reaction. J. Alloy Comp. 2017, 708, 1008–1017. [Google Scholar] [CrossRef]
- Moon, A.; Suh, C.Y.; Kwon, H. Microstructure and mechanical properties of ultrafine (Ti, Mo, W, Nb, Zr, Ta)(CN)–Ni composites prepared using the stabilized (Ti, Mo, W, Nb, Zr, Ta)(CN) phase. J. Alloy Comp. 2018, 732, 838–844. [Google Scholar] [CrossRef]
- Grosjean, A.; Rezrazi, M.; Bercot, P. Some morphological characteristics of the incorporation of silicon carbide (SiC) particles into electroless nickel deposits. Surf. Coat. Technol. 2000, 130, 252–256. [Google Scholar] [CrossRef]
- Juxian, Z.; Longqiao, G.; Fa, L. Progress in Research on Coated powders. Bull. Chin. Ceram. Soc. 2000, 19, 53–56. [Google Scholar] [CrossRef]
- Wang, H.L.; Zhang, R.; Wang, X.K.; Guo, J.K.; Sheng, S.H. Preparation of Cu/SiC composite particles by coating method. Chin. Ceram. Soc. 2004, 32, 398–401. [Google Scholar] [CrossRef]
- Zhou, S.; Luo, C.; Wu, X.; Zhu, L.; Tan, J. Preparation of Ni–Mo–C/Ti(C,N) coated powders and its influence on the microstructure and mechanical properties of Ti(C,N)-based cermets. Ceram. Int. 2015, 41, 9259–9264. [Google Scholar] [CrossRef]
- Dios, M.; Gonzalez, Z.; Gordo, B. Chemical precipitation of nickel nanoparticles on Ti(C,N) suspensions focused on cermet processing. Int. J. Refract. Met. Hard Mater. 2017, 63, 2–8. [Google Scholar] [CrossRef]
- Xu, X.Y.; Zheng, Y.; Zhang, G.T. Microstructure and mechanical properties of Ti(C,N)-based cermets fabricated using Ni-coated mixed powders. Ceram. Int. 2020, 46, 16944–16948. [Google Scholar] [CrossRef]
- Tang, Q.; Xie, Z.P.; Yang, J.L.; Huang, Y. Coating of silicon nitride and its colloidal behavior. J. Mater. Sci. Lett. 1998, 17, 1239–1241. [Google Scholar] [CrossRef]
- Chicinas, H.F.; Marton, L.E.; Jucan, O.D. Granulated WC-Co obtained by spray drying from aqueous suspensions. Int. J. Refract. Met. Hard Mater. 2023, 114, 106250. [Google Scholar] [CrossRef]
- Liu, W.B.; Song, X.Y.; Zhang, J.X.; Zhang, G.Z.; Liu, X.M. Thermodynamic analysis for in situ synthesis of WC-Co composite powder from metal oxides. Mater. Chem. Phys. 2008, 109, 235–240. [Google Scholar] [CrossRef]
- Jing, C.; Zhou, S.J.; Zhang, W.; Ding, Z.Y.; Liu, Z.G.; Wang, Y.J.; Ouyang, J.H. Low temperature synthesis and densification of (Ti,V,Nb,Ta,Mo)(C,N) high-entropy carbonitride ceramics. J. Alloy Compd. 2022, 927, 167095. [Google Scholar] [CrossRef]
- Wei, C.B.; Song, X.Y.; Zhao, S.X.; Zhang, L.; Liu, W.B. In-situ synthesis of WC-Co composite powder and densification by sinter-HIP. Int. J. Refract. Met. Hard Mater. 2010, 28, 567–571. [Google Scholar] [CrossRef]
- Zhou, S.J.; Ouyang, J.H.; Yu, Y.B.; Wang, Y.J. Grain growth behavior in TaC-modified ultrafine Ti (C, N)-based cermets. Int. J. Refract. Met. Hard Mater. 2024, 120, 106625. [Google Scholar] [CrossRef]
- Rong, P.C.; Liu, Y.; Ye, J.W.; Cao, Q.; Liu, A.R. Synthesis of (Ti, W, Mo, Ta, Cr)(C, N) solid solution powders by carbothermal reduction-nitridation in an open system. J. Alloy Compd. 2017, 718, 425–432. [Google Scholar] [CrossRef]
- Aramian, A.; Sadeghian, Z.; Narimani, M.; Razavi, N. A review on the microstructure and properties of TiC and Ti (C, N) based cermets. Int. J. Refract. Met. Hard Mater. 2023, 115, 106320. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Jia, P.; Zhang, Y.; Ma, L.; Sun, J.; Xu, Y.; Wu, Y. Preparation of Ultrafine Co- and Ni-Coated (Ti,W,Mo,Ta)(C,N) Powders and Their Influence on the Microstructure of Ti(C,N)-Based Cermets. Materials 2024, 17, 1807. https://doi.org/10.3390/ma17081807
Zhao Z, Jia P, Zhang Y, Ma L, Sun J, Xu Y, Wu Y. Preparation of Ultrafine Co- and Ni-Coated (Ti,W,Mo,Ta)(C,N) Powders and Their Influence on the Microstructure of Ti(C,N)-Based Cermets. Materials. 2024; 17(8):1807. https://doi.org/10.3390/ma17081807
Chicago/Turabian StyleZhao, Zaiyang, Pengmin Jia, Yuhui Zhang, Lili Ma, Jingjing Sun, Yiping Xu, and Yurong Wu. 2024. "Preparation of Ultrafine Co- and Ni-Coated (Ti,W,Mo,Ta)(C,N) Powders and Their Influence on the Microstructure of Ti(C,N)-Based Cermets" Materials 17, no. 8: 1807. https://doi.org/10.3390/ma17081807
APA StyleZhao, Z., Jia, P., Zhang, Y., Ma, L., Sun, J., Xu, Y., & Wu, Y. (2024). Preparation of Ultrafine Co- and Ni-Coated (Ti,W,Mo,Ta)(C,N) Powders and Their Influence on the Microstructure of Ti(C,N)-Based Cermets. Materials, 17(8), 1807. https://doi.org/10.3390/ma17081807




