Zirconium-Modified Medium-Entropy Alloy (TiVNb)85Cr15 for Hydrogen Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Design
- -
- All the alloys can be categorized as medium-entropy alloys since their mixing entropy values fall within the range of 1 R < ΔSmix < 1.5 R.
- -
- High values of the parameter Ω were observed in all the alloys, suggesting their potential for forming a single disordered solid solution.
- -
- Increasing Zr addition leads to a rise in the δ parameter (atomic size difference), causing the Zr7 alloy ((TiVNb)78Cr15Zr7) to fall outside the empirically determined region of solid solutions.
- -
- The value of the VEC parameter in all alloys is less than 6.87, indicating that the alloys should have a BCC structure [21].
2.2. Material Preparation
2.3. Material Characterization
2.4. Hydrogen Absorption and Desorption Experiments
- Approximately 0.5 g of powder alloy was placed into the reaction chamber of the magnetic suspension balance. The system was then sealed and evacuated to a rotary pump vacuum <0.02 bar (2 kPa).
- The alloy was activated by exposure to low hydrogen pressure of approximately 0.1 MPa at room temperature for 1 h to reduce oxides on the surfaces of the powder particles. To remove the absorbed hydrogen, the sample was then heated to 400 °C for 3 h in a vacuum.
- After activation, the reaction chamber was cooled to room temperature. Once reached, it was filled with hydrogen to a pressure of 2 MPa. The sample mass was monitored by the magnetic suspension balance. The chamber temperature (and thus that of the sample) was increased from room temperature to 250 °C in steps of 25 °C. At each step, the sample was held for 25 min. The aim of this isobaric measurement was to determine the temperature at which the sample starts significantly absorbing hydrogen.
- After this measurement, the sample was cooled down to room temperature under 2 MPa of H2. Measurement in hydrogen after cooling allowed us to determine the amount of total absorbed hydrogen by the sample in step 3.
- The chamber was then evacuated again and heated to 400 °C for 3 h to desorb hydrogen from the sample.
- The chamber was heated to the temperature at which the alloy absorbed hydrogen significantly, and hydrogen was again introduced to the chamber at a pressure of 2 MPa. During this second absorption measurement lasting 1 h, the sample weight was monitored again.
- Following hydrogenation, the sample was removed from the chamber for X-ray diffraction (XRD) and thermogravimetric analysis (TGA) using the Netsch Jupiter STA 449-F1 analyzer, Selb, Germany.
3. Results and Discussion
3.1. Chemical Composition and Density
3.2. Phase Composition
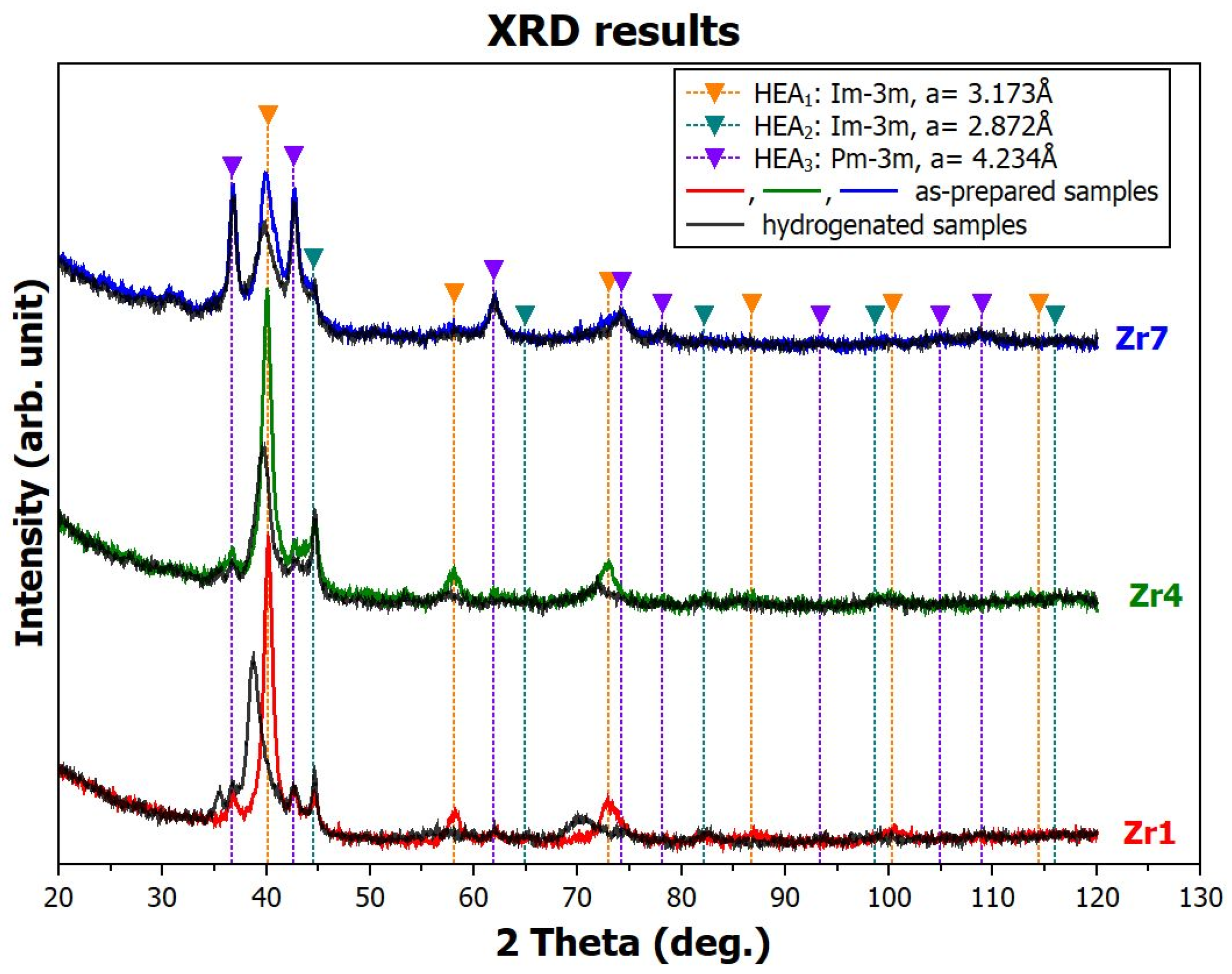
3.2.1. Analysis of the As-Prepared Samples
3.2.2. Analysis of the Hydrogenated Samples
- -
- Based on the analysis of the Bragg peak shift (lattice parameter changes), we believe that the HEA1 phase is the phase that significantly absorbs hydrogen in all samples. The HEA2 and HEA3 phases are practically unaffected by hydrogen, which means that they either do not absorb hydrogen or release it after removal from the reaction vessel. Unfortunately, this study did not allow us to perform in situ XRD experiments during hydrogenation of our alloys. Synchrotron sources would be the most suitable for this purpose. However, we are not aware of any beamlines that allow experiments at hydrogen pressure of 20 bar.
- -
- The increase in the lattice parameter of the Zr1 sample is a manifestation of the chemical bonding of absorbed hydrogen in the metal matrix. As will be shown later, samples Zr4 and Zr7 absorb hydrogen equally and even more, but they bind it with weaker bonds, which causes hydrogen to escape from the matrix at ambient conditions.
- -
- Since no permanent changes in the diffraction profiles were induced by hydrogen, it can be concluded that hydrogen is dissolved within interstitial positions in the absorbing phases and does not form hydrides with a completely different crystallography.
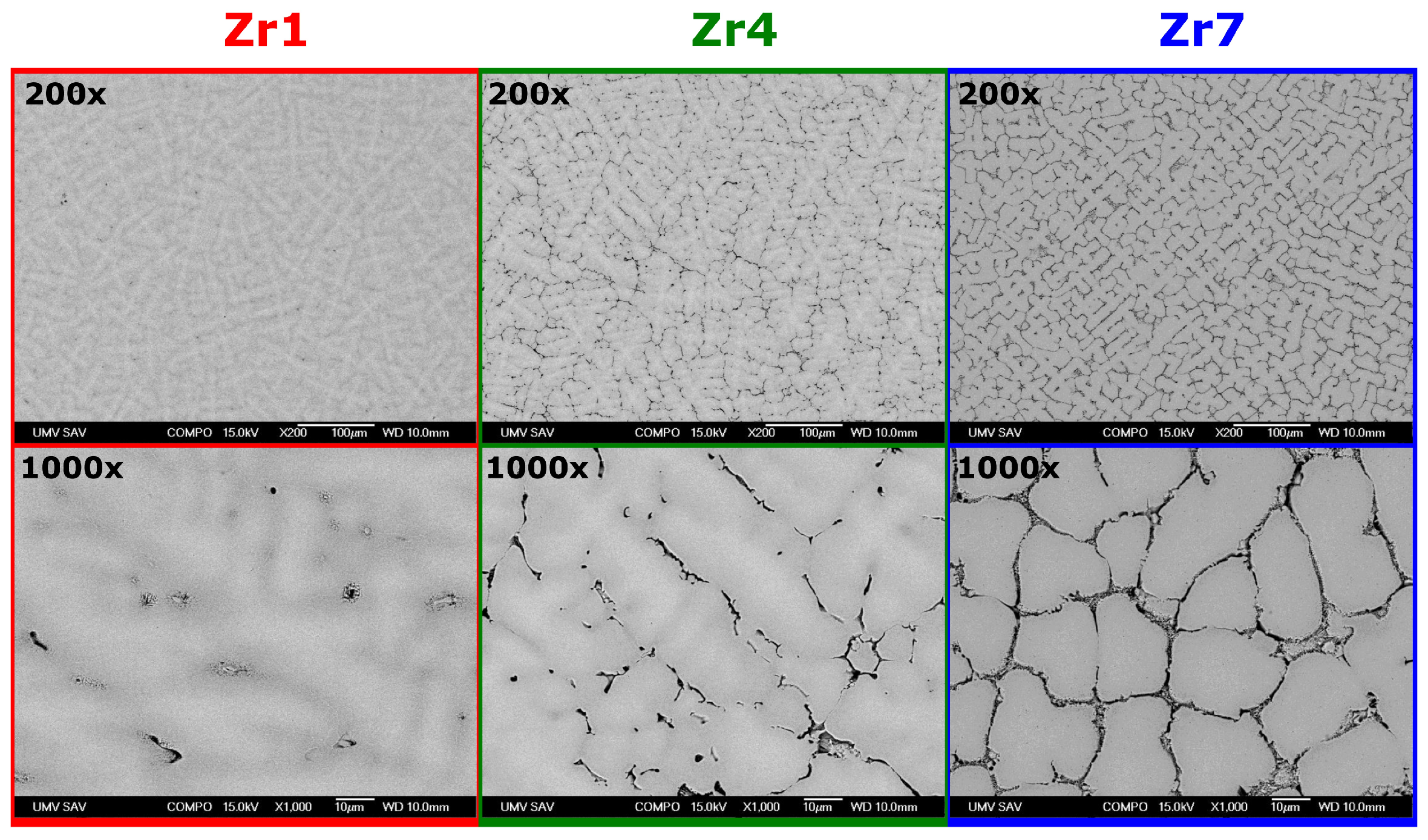
3.3. Microstructures
3.4. Hydrogen Absorption and Desorption
3.4.1. Absorption
- -
- The alloy with the lowest zirconium content, Zr1, begins to absorb hydrogen at temperatures above 150 °C. The maximum storage capacity of 0.77 wt.% (corresponding to H/M = 0.47) was reached at the highest measurement temperature of 250 °C.
- -
- The Zr4 alloy is activated already at room temperature. A significant increase in this alloy weight was observed already when the reaction chamber was filled with hydrogen. The maximum amount of hydrogen absorbed by this alloy is 0.92 wt.% (H/M = 0.57). As can be seen, from 150 °C, the alloy starts to desorb hydrogen, so it is very likely that if we increased the pressure of gaseous hydrogen in the chamber, its absorption capacity would be higher.
- -
- By adding additional 3 at.% Zr to the alloy (sample Zr7), this trend was significantly reverted. Activation again occurs only at high temperatures above 150 °C. The overall hydrogen absorption capacity is low, only 0.34 wt.% (H/M = 0.21), and only at the highest measured temperature.
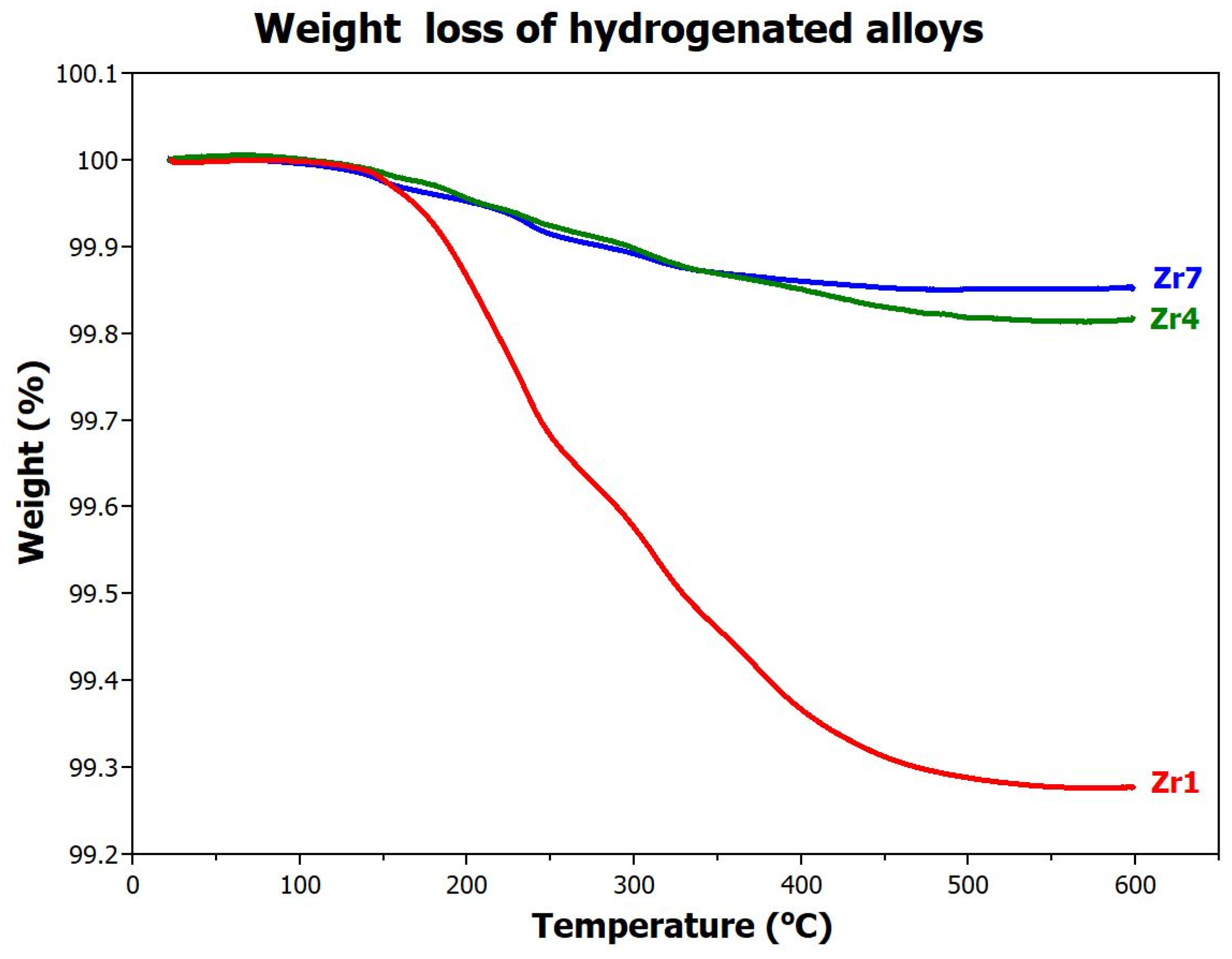
3.4.2. Desorption
- -
- The Zr1 sample contains a large amount of chemically bounded hydrogen, which significantly starts to desorb from the alloy at ~160 °C. Complete release of hydrogen from the metal lattice occurs only at 500 °C. These results approximately correspond to the desorption temperatures from reference [25]. The amount of hydrogen released in this way is 0.73 wt.%. For this alloy, only 0.04 wt.% of hydrogen is, therefore, available for reversible low-temperature (up to 160 °C) absorption/desorption.
- -
- The situation is completely different for the Zr4 alloy where the amount of hydrogen released at 600 °C is significantly lower, only 0.18 wt.%. This means that this alloy has up to 0.74 wt.% (H/M = 0.46) hydrogen available for reversible use.
- -
- The situation is similar for the Zr7 alloy, but since this alloy absorbs the least, the amount of reversibly available hydrogen is only 0.19 wt.%.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jena, P. Materials for Hydrogen Storage: Past, Present, and Future. J. Phys. Chem. Lett. 2011, 2, 206–211. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal Hydride Materials for Solid Hydrogen Storage: A Review. Int. J. Hydrog. Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Marques, F.; Balcerzak, M.; Winkelmann, F.; Zepon, G.; Felderhoff, M. Review and Outlook on High-Entropy Alloys for Hydrogen Storage. Energy Environ. Sci. 2021, 14, 5191. [Google Scholar] [CrossRef]
- Dantzer, P.; Millet, P.; Flanagan, T.B. Thermodynamic Characterization of Hydride Phase Growth in ZrNi-H 2. Metall. Mater. Trans. A 2001, 32, 29–38. [Google Scholar] [CrossRef]
- Moreno-Pirajan, J.C.; Moreno-Pirajan, J.C. Thermodynamics—Interaction Studies—Solids, Liquids and Gases; InTech Open Access Publishers: London, UK, 2011. [Google Scholar] [CrossRef]
- Yartys, V.A.; Lototskyy, M.V.; Akiba, E.; Albert, R.; Antonov, V.E.; Ares, J.R.; Baricco, M.; Bourgeois, N.; Buckley, C.E.; Bellosta von Colbe, J.M.; et al. Magnesium Based Materials for Hydrogen Based Energy Storage: Past, Present and Future. Int. J. Hydrog. Energy 2019, 44, 7809–7859. [Google Scholar] [CrossRef]
- Lin, H.-J.; Lu, Y.-S.; Zhang, L.-T.; Liu, H.-Z.; Edalati, K.; Révész, A. Recent Advances in Metastable Alloys for Hydrogen Storage: A Review. Rare Met. 2022, 41, 1797–1817. [Google Scholar] [CrossRef]
- Liu, J.; Yong, H.; Zhao, Y.; Wang, S.; Chen, Y.; Liu, B.; Hu, J.; Zhang, Y. Phase Evolution, Hydrogen Storage Thermodynamics, and Kinetics of Ternary Mg98Ho1.5Fe0.5 Alloy. J. Rare Earths, 2023, in press. [CrossRef]
- Miracle, D.B.; Senkov, O.N. A Critical Review of High Entropy Alloys and Related Concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Yeh, J.-W. Alloy Design Strategies and Future Trends in High-Entropy Alloys. JOM 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Gao, M.C.; Liaw, P.K.; Yeh, J.W.; Zhang, Y. High-Entropy Alloys: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–516. [Google Scholar] [CrossRef]
- Marques, F.; Pinto, H.C.; Figueroa, S.J.A.; Winkelmann, F.; Felderhoff, M.; Botta, W.J.; Zepon, G. Mg-Containing Multi-Principal Element Alloys for Hydrogen Storage: A Study of the MgTiNbCr0.5Mn0.5Ni0.5 and Mg0.68TiNbNi0.55 Compositions. Int. J. Hydrog. Energy 2020, 45, 19539–19552. [Google Scholar] [CrossRef]
- Sahlberg, M.; Karlsson, D.; Zlotea, C.; Jansson, U. Superior Hydrogen Storage in High Entropy Alloys OPEN. Sci. Rep. 2016, 6, 36770. [Google Scholar] [CrossRef]
- Ek, G.; Nygård, M.M.; Pavan, A.F.; Montero, J.; Henry, P.F.; Sørby, M.H.; Witman, M.; Stavila, V.; Zlotea, C.; Hauback, B.C.; et al. Elucidating the Effects of the Composition on Hydrogen Sorption in TiVZrNbHf-Based High-Entropy Alloys. Inorg. Chem. 2021, 60, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Montero, J.; Zlotea, C.; Ek, G.; Crivello, J.C.; Laversenne, L.; Sahlberg, M. TiVZrNb Multi-Principal-Element Alloy: Synthesis Optimization, Structural, and Hydrogen Sorption Properties. Molecules 2019, 24, 2799. [Google Scholar] [CrossRef] [PubMed]
- Nygård, M.M.; Fjellvåg, Ø.S.; Sørby, M.H.; Sakaki, K.; Ikeda, K.; Armstrong, J.; Vajeeston, P.; Sławiński, W.A.; Kim, H.; Machida, A.; et al. The Average and Local Structure of TiVCrNbDx (X=0,2.2,8) from Total Scattering and Neutron Spectroscopy. Acta Mater. 2021, 205, 116496. [Google Scholar] [CrossRef]
- Silva, B.H.; Zlotea, C.; Champion, Y.; Botta, W.J.; Zepon, G. Design of TiVNb-(Cr, Ni or Co) Multicomponent Alloys with the Same Valence Electron Concentration for Hydrogen Storage. J. Alloys Compd. 2021, 865, 158767. [Google Scholar] [CrossRef]
- Chanchetti, L.F.; Silva, B.H.; Montero, J.; Zlotea, C.; Champion, Y.; Jos E Botta, W.; Zepon, G. Structural Characterization and Hydrogen Storage Properties of the Ti 31 V 26 Nb 26 Zr 12 M 5 (M ¼ Fe, Co, or Ni) Multi-Phase Multicomponent Alloys. Int. J. Hydrog. Energy 2022, 48, 2247–2255. [Google Scholar] [CrossRef]
- Yang, S.; Yang, F.; Wu, C.; Chen, Y.; Mao, Y.; Luo, L. Hydrogen Storage and Cyclic Properties of (VFe) 60 (TiCrCo) 40-x Zr x (0 x 2) Alloys. J. Alloys Compd. 2015, 663, 460–465. [Google Scholar] [CrossRef]
- Hume-Rothery, W.; Powell, H.M. On the Theory of Super-Lattice Structures in Alloys. Z. Für Krist. -Cryst. Mater. 1935, 91, 23–47. [Google Scholar] [CrossRef]
- Guo, S. Phase Selection Rules for Cast High Entropy Alloys: An Overview. Mater. Sci. Technol. 2015, 31, 1223–1230. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Moellmer, J.; Moeller, A.; Dreisbach, F.; Glaeser, R.; Staudt, R. High Pressure Adsorption of Hydrogen, Nitrogen, Carbon Dioxide and Methane on the Metal–Organic Framework HKUST-1. Microporous Mesoporous Mater. 2011, 138, 140–148. [Google Scholar] [CrossRef]
- Strozi, R.B.; Leiva, D.R.; Zepon, G.; Botta, W.J.; Huot, J. Effects of the Chromium Content in (TiVNb)100−xCrx Body-Centered Cubic High Entropy Alloys Designed for Hydrogen Storage Applications. Energies 2021, 14, 3068. [Google Scholar] [CrossRef]
- Silva, B.H.; Zlotea, C.; Vaughan, G.; Champion, Y.; Botta, W.J.; Zepon, G. Hydrogen Absorption/Desorption Reactions of the (TiVNb)85Cr15 Multicomponent Alloy. J. Alloys Compd. 2022, 901, 163620. [Google Scholar] [CrossRef]
- Floriano, R.; Zepon, G.; Edalati, K.; Fontana, G.L.B.G.; Mohammadi, A.; Ma, Z.; Li, H.W.; Contieri, R.J. Hydrogen Storage in TiZrNbFeNi High Entropy Alloys, Designed by Thermodynamic Calculations. Int. J. Hydrogen Energy 2020, 45, 33759–33770. [Google Scholar] [CrossRef]
- China Bans Export of Rare Earths Processing Tech over National Security|Reuters. Available online: https://www.reuters.com/markets/commodities/china-bans-export-rare-earths-processing-technologies-2023-12-21/ (accessed on 27 February 2024).


| Alloy | δ [%] | ΔHmix [kJ·mol−1] | Ω | ΔSmix [J.K−1.mol−1] | Tm [K] | VEC |
|---|---|---|---|---|---|---|
| Precursor (TiVNb)85Cr15 | 5.76 | −3.04 | 8.37 | 1.36 R | 2257 | 4.87 |
| Zr1 (TiVNb)84Cr15Zr1 | 5.96 | −3.07 | 8.54 | 1.40 R | 2255 | 4.86 |
| Zr4 (TiVNb)81Cr15Zr4 | 6.48 | −3.17 | 8.70 | 1.47 R | 2251 | 4.84 |
| Zr7 (TiVNb)78Cr15Zr7 | 6.93 | −3.27 | 8.69 | 1.52 R | 2246 | 4.82 |
| Alloy EDX Composition [at.%] | Density [g.cm−3] | Hardness HV03 | Elastic Modulus [GPa] | Activation Temperature [°C] | Maximum H2 Capacity [wt.%] (H/M) | Residual H2 Content [wt.%] |
|---|---|---|---|---|---|---|
| Zr1 (TiVNb)84Cr15Zr1 Ti28V27Nb30Cr14Zr1 | 6.53 | 482 ± 8 | 130 ± 1 | >150 | 0.77 (0.47) | 0.73 |
| Zr4 (TiVNb)81Cr15Zr4 Ti27V26Nb29Cr14Zr4 | 6.58 | 463 ± 13 | 112 ± 4 | <RT | 0.92 (0.57) | 0.18 |
| Zr7 (TiVNb)78Cr15Zr7 Ti26V25Nb28Cr14Zr7 | 6.57 | 475 ± 17 | 136 ± 6 | >150 | 0.34 (0.21) | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saksl, K.; Matvija, M.; Fujda, M.; Ballóková, B.; Varcholová, D.; Kubaško, J.; Möllmer, J.; Lange, M.; Podobová, M. Zirconium-Modified Medium-Entropy Alloy (TiVNb)85Cr15 for Hydrogen Storage. Materials 2024, 17, 1732. https://doi.org/10.3390/ma17081732
Saksl K, Matvija M, Fujda M, Ballóková B, Varcholová D, Kubaško J, Möllmer J, Lange M, Podobová M. Zirconium-Modified Medium-Entropy Alloy (TiVNb)85Cr15 for Hydrogen Storage. Materials. 2024; 17(8):1732. https://doi.org/10.3390/ma17081732
Chicago/Turabian StyleSaksl, Karel, Miloš Matvija, Martin Fujda, Beáta Ballóková, Dagmara Varcholová, Jakub Kubaško, Jens Möllmer, Marcus Lange, and Mária Podobová. 2024. "Zirconium-Modified Medium-Entropy Alloy (TiVNb)85Cr15 for Hydrogen Storage" Materials 17, no. 8: 1732. https://doi.org/10.3390/ma17081732
APA StyleSaksl, K., Matvija, M., Fujda, M., Ballóková, B., Varcholová, D., Kubaško, J., Möllmer, J., Lange, M., & Podobová, M. (2024). Zirconium-Modified Medium-Entropy Alloy (TiVNb)85Cr15 for Hydrogen Storage. Materials, 17(8), 1732. https://doi.org/10.3390/ma17081732







