Low-Temperature Synthesis of Bi2S3 Hierarchical Microstructures via Co-Precipitation and Digestive Process in Aqueous Medium
Abstract
:1. Introduction
2. Experimental Procedures
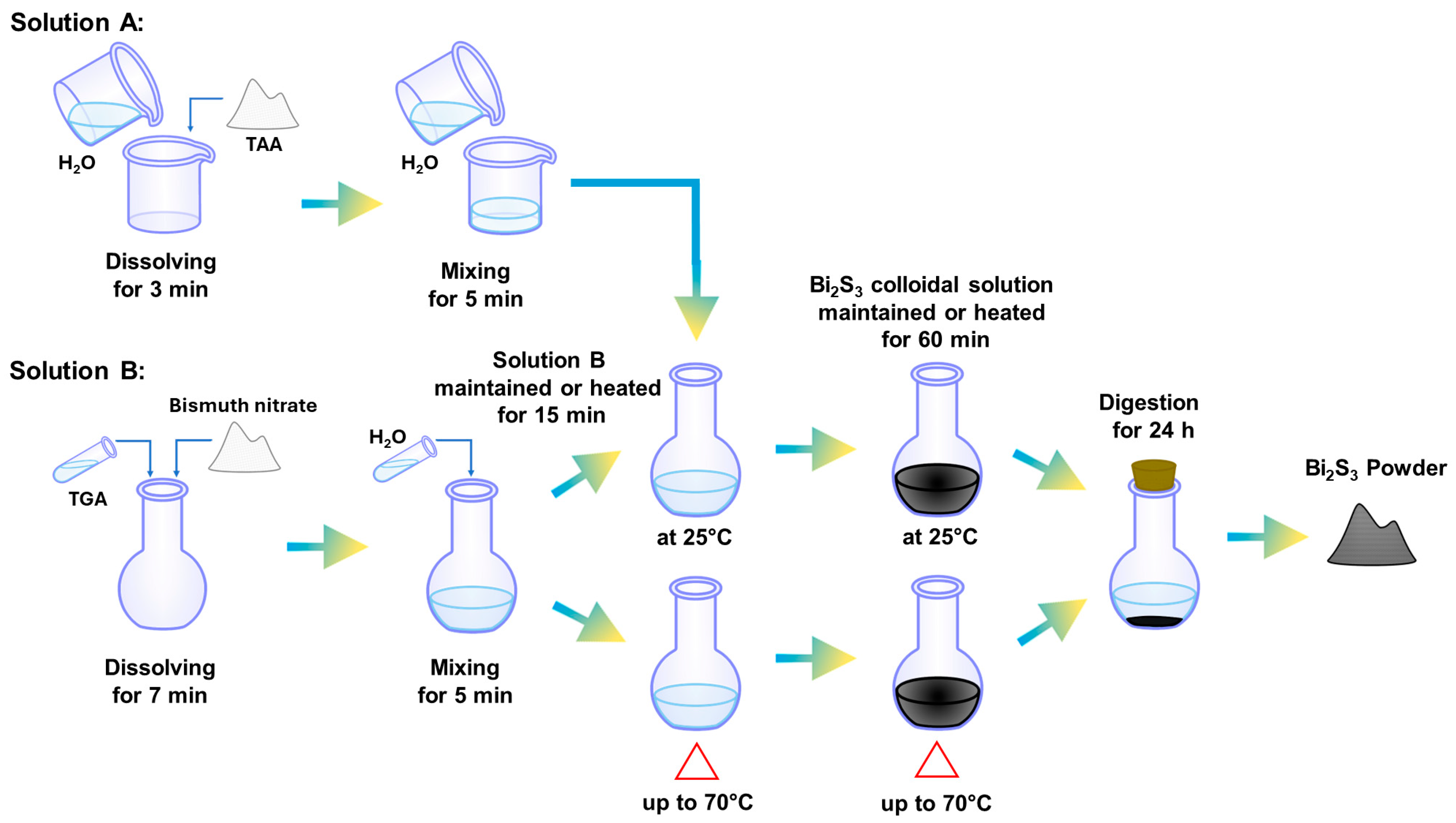
2.1. Reagents and Material Synthesis
2.2. Characterization Techniques
3. Results and Discussion
3.1. XRD Analysis
3.2. Morphological Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boles, M.A.; Engel, M.; Talapin, D.V. Self-assembly of colloidal nanocrystals: From intricate structures to functional materials. Chem. Rev. 2016, 116, 11220–11289. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.C.; Bernechea, M. Research Update: Bismuth based materials for photovoltaics. APL Mater. 2018, 6, 084503. [Google Scholar] [CrossRef]
- Aresti, M.; Saba, M.; Piras, R.; Marongiu, D.; Mula, G.; Quochi, F.; Mura, A.; Cannas, C.; Mureddu, M.; Ardu, A.; et al. Colloidal Bi2S3 nanocrystals: Quantum size effects and midgap states. Adv. Funct. Mater. 2014, 24, 3341–3350. [Google Scholar] [CrossRef]
- Ma, J.; Yang, J.; Jiao, L.; Wang, T.; Lian, J.; Duan, X.; Zheng, W. Bi2S3 nanomaterials: Morphology manipulation and related properties. Dalton Trans. 2011, 40, 10100–10109. [Google Scholar] [CrossRef] [PubMed]
- Bernechea, M.; Cao, Y.; Konstantatos, G. Size and bandgap tunability in Bi2S3 colloidal nanocrystals and its effect in solution processed solar cells. J. Mater. Chem. A 2015, 3, 20642–20648. [Google Scholar] [CrossRef]
- Calzia, V.; Malloci, G.; Bongiovanni, G.; Mattoni, A. Electronic Properties and Quantum Confinement in Bi2S3 Ribbon-like Nanostructures. J. Phys. Chem. C 2013, 117, 21923–21929. [Google Scholar] [CrossRef]
- Rincón, M.E.; Sánchez, M.; George, P.J.; Sánchez, A.; Nair, P.K. Comparison of the Properties of Bismuth Sulfide Thin Films Prepared by Thermal Evaporation and Chemical Bath Deposition. J. Solid State Chem. 1998, 136, 167–174. [Google Scholar] [CrossRef]
- Khan, Z.; Elahi, A.; Bukhari, D.A.; Rehman, A. Cadmium sources, toxicity, resistance and removal by microorganisms-A potential strategy for cadmium eradication. J. Saudi Chem. Soc. 2022, 26, 101569. [Google Scholar] [CrossRef]
- Min, X.; Xu, Q.; Ke, Y.; Xu, H.; Yao, L.; Wang, J.; Ren, H.; Li, T.; Lin, Z. Transformation behavior of the morphology, structure and toxicity of amorphous As2S3 during hydrothermal process. Hydrometallurgy 2021, 200, 105549. [Google Scholar] [CrossRef]
- Nádudvari, Á.; Cabała, J.; Marynowski, L.; Jabłońska, M.; Dziurowicz, M.; Malczewski, D.; Kozielska, B.; Siupka, P.; Piotrowska-Seget, Z.; Simoneit, B.R.T.; et al. High concentrations of HgS, MeHg and toxic gas emissions in thermally affected waste dumps from hard coal mining in Poland. J. Hazard Mater. 2022, 431, 128542. [Google Scholar] [CrossRef]
- Guo, J.; Lou, Q.; Qiu, Y.; Wang, Z.Y.; Ge, Z.H.; Feng, J.; He, J. Remarkably enhanced thermoelectric properties of Bi2S3 nanocomposites via modulation doping and grain boundary engineering. Appl. Surf. Sci. 2020, 520, 146341. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Onwudiwe, D.C. Bismuth sulfide based compounds: Properties, synthesis and applications. Results Chem. 2021, 3, 100151. [Google Scholar] [CrossRef]
- Dong, Y.; Hu, M.; Zhang, Z.; Zapien, J.A.; Wang, X.; Lee, J.M. Hierarchical self-assembled Bi2S3 hollow nanotubes coated with sulfur-doped amorphous carbon as advanced anode materials for lithium ion batteries. Nanoscale 2018, 10, 13343–13350. [Google Scholar] [CrossRef] [PubMed]
- Arabzadeh, A.; Salimi, A. Facile Synthesis of Ultra-wide Two Dimensional Bi2S3 Nanosheets: Characterizations, Properties and Applications in Hydrogen Peroxide Sensing and Hydrogen Storage. Electroanalysis 2017, 29, 2027–2035. [Google Scholar] [CrossRef]
- Sahu, M.; Park, C. A comprehensive review on bismuth-sulfide-based compounds. Mater. Today Sustain. 2023, 23, 10441. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, M. Synthesis, characterization, and hydrophobic properties of Bi2S3 hierarchical nanostructures. Mater. Res. Bull. 2011, 46, 555–562. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, P. Low-temperature urea-assisted hydrothermal synthesis of Bi2S3 nanostructures with different morphologies. Cryst. Res. Technol. 2009, 44, 713–720. [Google Scholar] [CrossRef]
- Miniach, E.; Zyna Gryglewicz, G. Solvent-controlled morphology of bismuth sulfide for supercapacitor applications. J. Mater. Sci. 2018, 53, 16511–16523. [Google Scholar] [CrossRef]
- Deshpande, M.P.; Sakariya, P.N.; Bhatt, S.V.; Garg, N.; Patel, K.; Chaki, S.H. Characterization of Bi2S3 nanorods prepared at room temperature. Mater. Sci. Semicond. Process. 2014, 21, 180–185. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Ghanbari, D.; Davar, F. Synthesis of different morphologies of bismuth sulfide nanostructures via hydrothermal process in the presence of thioglycolic acid. J. Alloys Compd. 2009, 488, 442–447. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Z.; Lian, J.; Duan, X.; Kim, T.; Peng, P.; Liu, X.; Chen, Q.; Yao, G.; Zheng, W. Ionic liquids-assisted synthesis and electrochemical properties of Bi2S3 nanostructures. CrystEngComm 2011, 13, 3072–3079. [Google Scholar] [CrossRef]
- Li, Y.; Wei, F.; Ma, Y.; Zhang, H.; Gao, Z.; Dai, L.; Qin, G. Selected-control hydrothermal synthesis and photoresponse properties of Bi2S3 micro/nanocrystals. CrystEngComm 2013, 15, 6611. [Google Scholar] [CrossRef]
- Carrillo-Castillo, A.; Rivas-Valles, B.G.; Castillo, S.J.; Ramirez, M.M.; Luque-Morales, P.A. New Formulation to Synthetize Semiconductor Bi2S3 Thin Films Using Chemical Bath Deposition for Optoelectronic Applications. Symmetry 2022, 14, 2487. [Google Scholar] [CrossRef]
- Han, P.; Mihi, A.; Ferre-borrull, J.; Pallarés, J.; Marsal, L.F. Interplay Between Morphology, Optical Properties, and Electronic Structure of Solution-Processed Bi2S3 Colloidal Nanocrystals. J. Phys. Chem. C 2015, 119, 10693–10699. [Google Scholar] [CrossRef]
- Song, H.; Zhan, X.; Li, D.; Zhou, Y.; Yang, B.; Zeng, K.; Zhong, J.; Miao, X.; Tang, J. Rapid thermal evaporation of Bi2S3 layer for thin film photovoltaics. Sol. Energy Mater. Sol. C 2016, 146, 1–7. [Google Scholar] [CrossRef]
- Wu, Y.; Lou, Q.; Qiu, Y.; Guo, J.; Mei, Z.Y.; Xu, X.; Feng, J.; He, J.; Ge, Z.H. Highly enhanced thermoelectric properties of nanostructured Bi2S3 bulk materials: Via carrier modification and multi-scale phonon scattering. Inorg. Chem. Front. 2019, 6, 1374–1381. [Google Scholar] [CrossRef]
- Liu, W.; Lukas, K.C.; McEnaney, K.; Lee, S.; Zhang, Q.; Opeil, C.P.; Chen, G.; Ren, Z. Studies on the Bi2Te3–Bi2Se3–Bi2S3 system for mid-temperature thermoelectric energy conversion. Energy Environ. Sci. 2013, 6, 552–560. [Google Scholar] [CrossRef]
- Saha, S.K.; Pal, A.J. Schottky diodes between Bi2S3 nanorods and metal nanoparticles in a polymer matrix as hybrid bulk-heterojunction solar cells. J. Appl. Phys. 2015, 118, 014503. [Google Scholar] [CrossRef]
- Jin, R.; Li, G.; Xu, Y.; Liu, J.; Chen, G. Uniform Bi2S3 nanorods-assembled hollow spheres with excellent electrochemical hydrogen storage abilities. Int. J. Hydrogen. Energy 2014, 39, 356–365. [Google Scholar] [CrossRef]
- Shkir, M. Improved opto-electronic properties of Bi2S3 thin films through trivalent atom (Al3+) doping for photodiode applications. Surf. Interfaces 2023, 40, 103025. [Google Scholar] [CrossRef]
- Chitara, B.; Kolli, B.S.C.; Yan, F. Near-Infrared photodetectors based on 2D Bi2S3. Chem. Phys. Lett. 2022, 804, 139876. [Google Scholar] [CrossRef]
- Kim, Y.; Jeong, E.; Joe, M.; Lee, C. Synthesis of 2D semiconducting single crystalline Bi2S3 for high performance electronics. Phys. Chem. Chem. Phys. 2021, 23, 26806–26812. [Google Scholar] [CrossRef] [PubMed]
- Uddin, I.; Abzal, S.M.; Kalyan, K.; Janga, S.; Rath, A.; Patel, R.; Gupta, D.K.; Ravindran, T.R.; Ateeq, H.; Khan, M.S.; et al. Starch-Assisted Synthesis of Bi2S3 Nanoparticles for Enhanced Dielectric and Antibacterial Applications. ACS Omega 2022, 7, 42438–42445. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qin, S.; Song, G.; Xiang, T.; Xin, F.; Yin, X. Shape-controlled solvothermal synthesis of Bi2S3 for photocatalytic reduction of CO2 to methyl formate in methanol. Dalton Trans. 2013, 42, 15133. [Google Scholar] [CrossRef] [PubMed]
- Sasikala, S.; Balakrishnan, M.; Kumar, M.; Chang, J.H.; Manivannan, M.; Thangabalu, S. Effect of sulfur source and temperature on the morphological characteristics and photocatalytic activity of Bi2S3 nanostructure synthesized by microwave irradiation technique. J. Mater. Sci. Mater. Electron. 2023, 34, 997. [Google Scholar] [CrossRef]
- Sang, Y.; Dai, G.; Wang, L.; Gao, X.; Fang, C. Hydrothermal Synthesis of Urchin-like Bi2S3 Nanostructures for Superior Visible-light-driven Cr(VI) Removal Capacity. ChemistrySelect 2018, 3, 7123–7128. [Google Scholar] [CrossRef]
- ten Haaf, S.; Sträter, H.; Brüggemann, R.; Bauer, G.H.; Felser, C.; Jakob, G. Physical vapor deposition of Bi2S3 as absorber material in thin film photovoltaics. Thin Solid Films 2013, 535, 394–397. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Q.; Dan, M.; Guo, Z.; Zhou, Y. A facile preparation route of Bi2S3 nanorod films for photocatalytic H2 production from H2S. Mater. Lett. 2017, 201, 118–121. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, C.; Lu, H.; Jia, M.; Lai, Y.; Li, J. Facile synthesis of dandelion-like Bi2S3 microspheres and their electrochemical properties for lithium-ion batteries. Mater. Lett. 2013, 91, 100–102. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrasco-González, J.A.; Ortega-Amaya, R.; Díaz-Torres, E.; Pérez-Guzmán, M.A.; Ortega-López, M. Low-Temperature Synthesis of Bi2S3 Hierarchical Microstructures via Co-Precipitation and Digestive Process in Aqueous Medium. Materials 2024, 17, 1818. https://doi.org/10.3390/ma17081818
Carrasco-González JA, Ortega-Amaya R, Díaz-Torres E, Pérez-Guzmán MA, Ortega-López M. Low-Temperature Synthesis of Bi2S3 Hierarchical Microstructures via Co-Precipitation and Digestive Process in Aqueous Medium. Materials. 2024; 17(8):1818. https://doi.org/10.3390/ma17081818
Chicago/Turabian StyleCarrasco-González, José Alfonso, Rebeca Ortega-Amaya, Esteban Díaz-Torres, Manuel A. Pérez-Guzmán, and Mauricio Ortega-López. 2024. "Low-Temperature Synthesis of Bi2S3 Hierarchical Microstructures via Co-Precipitation and Digestive Process in Aqueous Medium" Materials 17, no. 8: 1818. https://doi.org/10.3390/ma17081818





