Characterization of CuAg Alloys with Low Ag Concentrations
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Description and Growth Process
2.2. Methods
3. Results and Discussion
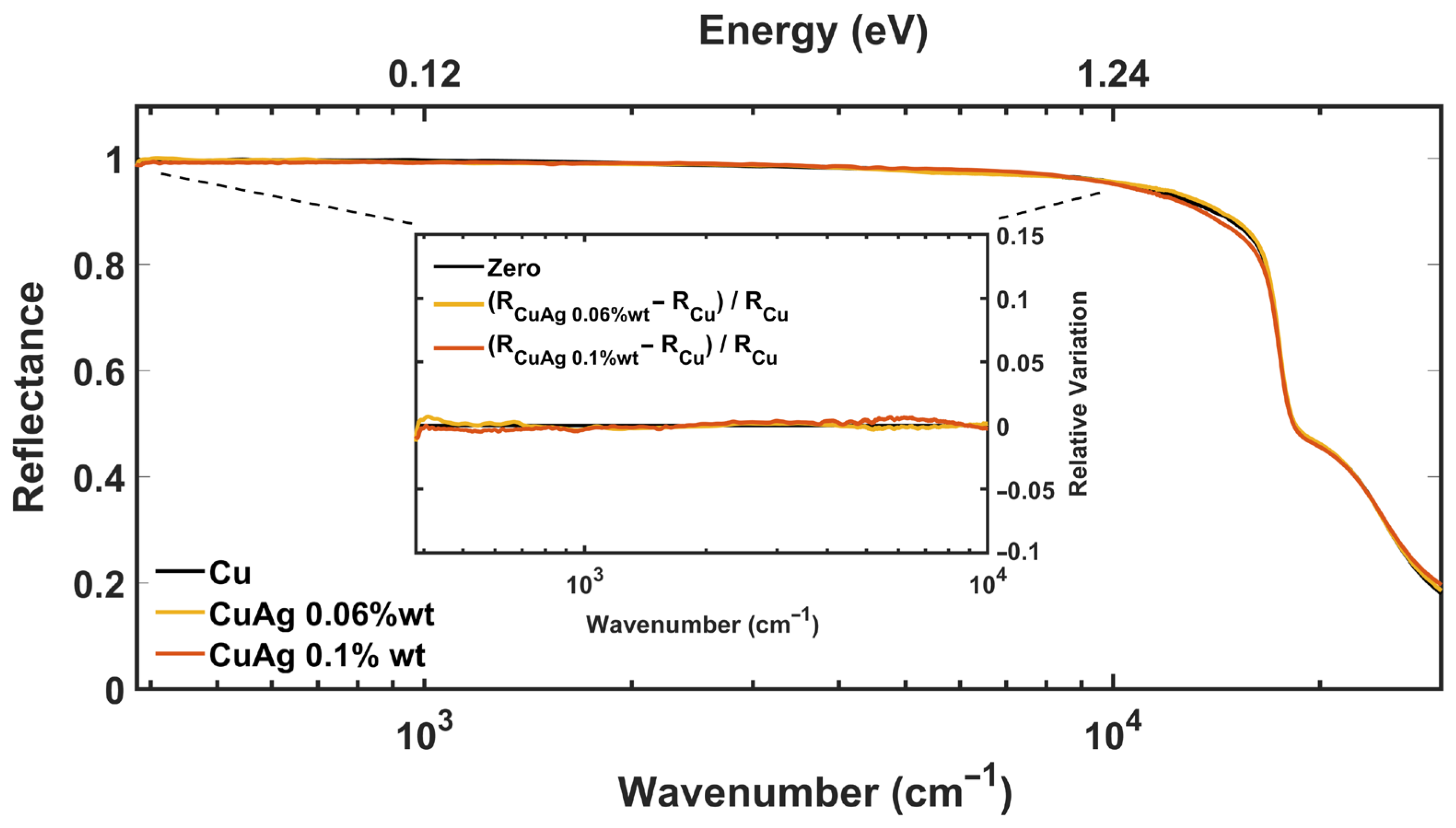
3.1. Optical Spectroscopy Results
3.2. SEM Microscopy Results
3.3. Raman Spectroscopy Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Callister, W.D., Jr.; Rethwisch, D.G. Callister’s Materials Science and Engineering; John Wiley & Sons: Hoboken, NJ, USA, 2020; ISBN 978-1-119-45391-8. [Google Scholar]
- Campbell, F.C. Phase Diagrams: Understanding the Basics; ASM International: Almere, The Netherlands, 2012; ISBN 978-1-61503-986-9. [Google Scholar]
- Liu, J.B.; Zhang, L.; Meng, L. Relationships between Mechanical Strength and Electrical Conductivity for Cu–Ag Filamentary Microcomposites. Appl. Phys. A 2007, 86, 529–532. [Google Scholar] [CrossRef]
- Sakai, Y.; Schneider-Muntau, H.-J. Ultra-High Strength, High Conductivity Cu-Ag Alloy Wires. Acta Mater. 1997, 45, 1017–1023. [Google Scholar] [CrossRef]
- Meng, L.; Liu, J.B. Progress and Current Status in Research on Nanostructured Cu-Ag Microcomposites for Conductor Wires. Mater. Sci. Forum 2007, 539–543, 2798–2803. [Google Scholar] [CrossRef]
- Liu, J.B.; Meng, L.; Zeng, Y.W. Microstructure Evolution and Properties of Cu–Ag Microcomposites with Different Ag Content. Mater. Sci. Eng. A 2006, 435–436, 237–244. [Google Scholar] [CrossRef]
- Shu-sen, W.; Yuan-wang, Z.; Da-wei, Y. Micro-Structure and Properties of Cu-0.5 wt%Ag Alloy Fine Wires with Severe Cold Plastic Deformation Treatment. Mater. Res. Express 2018, 5, 046532. [Google Scholar] [CrossRef]
- Mamala, A.; Knych, T.; Kwaśniewski, P.; Pacewicz, A.; Kawecki, A.; Kiesiewicz, G.; Smyrak, B.; Sieja-Smaga, E. Fabrication, Properties and Microstructures of High Strength and High Conductivity Copper-Silver Wires. Arch. Met. Mater. 2012, 57, 1261–1270. [Google Scholar]
- Choi, E.-A.; Lee, S.J.; Ahn, J.H.; Choe, S.; Lee, K.H.; Lim, S.H.; Choi, Y.S.; Han, S.Z. Enhancement of Strength and Electrical Conductivity for Hypo-Eutectic Cu-12Ag Alloy. J. Alloys Compd. 2023, 931, 167506. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, H.; He, X.; Wang, C.; Jiang, L.; Chen, L.-Q.; Xie, J. Dramatically Enhanced Combination of Ultimate Tensile Strength and Electric Conductivity of Alloys via Machine Learning Screening. Acta Mater. 2020, 200, 803–810. [Google Scholar] [CrossRef]
- Benghalem, A.; Morris, D.G. Microstructure and Strength of Wire-Drawn Cu-Ag Filamentary Composites. Acta Mater. 1997, 45, 397–406. [Google Scholar] [CrossRef]
- Chu, C.; Kexing, S.; Xujun, M.; Baoan, W.; Zhu, X.; Haofeng, X.; Yanjun, Z.; Xiuhua, G.; Haitao, L.; Dingbiao, C.; et al. Microstructural Evolution and Properties of Cu–20 wt% Ag Alloy Wire by Multi-Pass Continuous Drawing. Nanotechnol. Rev. 2020, 9, 1359–1367. [Google Scholar] [CrossRef]
- Sakai, Y.; Inoue, K.; Asano, T.; Maeda, H. Development of a High Strength, High Conductivity Copper-Silver Alloy for Pulsed Magnets. IEEE Trans. Magn. 1992, 28, 888–891. [Google Scholar] [CrossRef]
- Simakov, E.I.; Dolgashev, V.A.; Tantawi, S.G. Advances in High Gradient Normal Conducting Accelerator Structures. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2018, 907, 221–230. [Google Scholar] [CrossRef]
- Dolgashev, V.A. Progress on High-Gradient Structures. AIP Conf. Proc. 2012, 1507, 76–84. [Google Scholar] [CrossRef]
- Cahill, A.D.; Rosenzweig, J.B.; Dolgashev, V.A.; Tantawi, S.G.; Weathersby, S. High Gradient Experiments with X-Band Cryogenic Copper Accelerating Cavities. Phys. Rev. Accel. Beams 2018, 21, 102002. [Google Scholar] [CrossRef]
- Wang, G.; Simakov, E.I.; Perez, D. Ab Initio Cu Alloy Design for High-Gradient Accelerating Structures. Appl. Phys. Lett. 2022, 120, 134101. [Google Scholar] [CrossRef]
- Korsbäck, A.; Djurabekova, F.; Morales, L.M.; Profatilova, I.; Castro, E.R.; Wuensch, W.; Calatroni, S.; Ahlgren, T. Vacuum Electrical Breakdown Conditioning Study in a Parallel Plate Electrode Pulsed Dc System. Phys. Rev. Accel. Beams 2020, 23, 033102. [Google Scholar] [CrossRef]
- Dolgashev, V.; Tantawi, S.G.; Higashi, Y. Study of Basic RF Breakdown Phenomena in High Gradient Vacuum Structures. In Proceedings of the Linear Accelerator Conference LINAC, Tsukuba, Japan, 12–17 September 2010. [Google Scholar]
- Antoine, C.Z.; Peauger, F.; Le Pimpec, F. Electromigration Occurences and Its Effects on Metallic Surfaces Submitted to High Electromagnetic Field: A Novel Approach to Breakdown in Accelerators. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers, Detect. Assoc. Equip. 2011, 665, 54–69. [Google Scholar] [CrossRef]
- Dressel, M.; Grüner, G. Electrodynamics of Solids: Optical Properties of Electrons in Matter; Cambridge University Press: Cambridge, UK, 2002; ISBN 978-0-521-59253-6. [Google Scholar]
- Kuzmenko, A.B. RefFit Manual. Available online: https://reffit.ch/manual/ (accessed on 11 April 2024).
- Querry, M. Optical Constants of Minerals and Other Materials from the Millimeter to the Ultraviolet; Contractor Report; US Army Armament Munitions Chemical Command: Picatinny Arsenal, NJ, USA, 1987. [Google Scholar]
- Zeman, E.J.; Schatz, G.C. An Accurate Electromagnetic Theory Study of Surface Enhancement Factors for Silver, Gold, Copper, Lithium, Sodium, Aluminum, Gallium, Indium, Zinc, and Cadmium. J. Phys. Chem. 1987, 91, 634–643. [Google Scholar] [CrossRef]
- Gharbi, T.; Barchiesi, D.; Kessentini, S.; Maalej, R. Fitting Optical Properties of Metals by Drude-Lorentz and Partial-Fraction Models in the [0.5;6] eV Range. Opt. Mater. Express OME 2020, 10, 1129–1162. [Google Scholar] [CrossRef]
- Beaglehole, D.; Erlbach, E. Electronic Structure of Noble-Metal-Noble-Metal Alloys. Phys. Rev. B 1972, 6, 1209–1225. [Google Scholar] [CrossRef]
- Nilsson, P.; Forssell, G. Optical Properties of Metastable One Phase Cu-Ag Alloys. J. Phys. Colloq. 1974, 35, C4. [Google Scholar] [CrossRef][Green Version]
- Song, J.; Li, H.; Li, J.; Wang, S.; Zhou, S. Fabrication and Optical Properties of Metastable Cu–Ag Alloys. Appl. Opt. 2002, 41, 5413–5416. [Google Scholar] [CrossRef]
- Davies, J.R. Copper and Copper Alloys. In ASM Speciality Handbook; ASM International: Almere, The Netherlands, 2009. [Google Scholar]
- Macis, S.; Tomarchio, L.; Tofani, S.; Rezvani, S.J.; Faillace, L.; Lupi, S.; Irizawa, A.; Marcelli, A. Angular Dependence of Copper Surface Damage Induced by an Intense Coherent THz Radiation Beam. Condens. Matter 2020, 5, 16. [Google Scholar] [CrossRef]
- Deng, Y.; Handoko, A.D.; Du, Y.; Xi, S.; Yeo, B.S. In Situ Raman Spectroscopy of Copper and Copper Oxide Surfaces during Electrochemical Oxygen Evolution Reaction: Identification of CuIII Oxides as Catalytically Active Species. ACS Catal. 2016, 6, 2473–2481. [Google Scholar] [CrossRef]
- Yu, W.; Han, M.; Jiang, K.; Duan, Z.; Li, Y.; Hu, Z.; Chu, J. Enhanced Fröhlich Interaction of Semiconductor Cuprous Oxide Films Determined by Temperature-Dependent Raman Scattering and Spectral Transmittance. J. Raman Spectrosc. 2013, 44, 142–146. [Google Scholar] [CrossRef]
- Toparli, C.; Sarfraz, A.; Erbe, A. A New Look at Oxide Formation at the Copper/Electrolyte Interface by in Situ Spectroscopies. Phys. Chem. Chem. Phys. 2015, 17, 31670–31679. [Google Scholar] [CrossRef]
- Meyer, B.K.; Polity, A.; Reppin, D.; Becker, M.; Hering, P.; Klar, P.J.; Sander, T.; Reindl, C.; Benz, J.; Eickhoff, M.; et al. Binary Copper Oxide Semiconductors: From Materials towards Devices. Phys. Status Solidi 2012, 249, 1487–1509. [Google Scholar] [CrossRef]
- Debbichi, L.; Marco de Lucas, M.C.; Pierson, J.F.; Krüger, P. Vibrational Properties of CuO and Cu4O3 from First-Principles Calculations, and Raman and Infrared Spectroscopy. J. Phys. Chem. C 2012, 116, 10232–10237. [Google Scholar] [CrossRef]
- Paparoni, F.; Mijit, E.; Kazim, S.; Minicucci, M.; Pinto, N.; D’Elia, A.; Macis, S.; Kim, C.; Huh, S.; Gunnella, R.; et al. Metallic Interface Induced Ionic Redistribution within Amorphous MoO3 Films. Adv. Mater. Interfaces 2022, 9, 2200453. [Google Scholar] [CrossRef]
- Balerna, A.; Bartocci, S.; Batignani, G.; Cianchi, A.; Chiadroni, E.; Coreno, M.; Cricenti, A.; Dabagov, S.; Di Cicco, A.; Faiferri, M.; et al. The Potential of EuPRAXIA@SPARC_LAB for Radiation Based Techniques. Condens. Matter 2019, 4, 30. [Google Scholar] [CrossRef]



| Operation | T (in °C) | Power (kW) | Pressure (mbar) | Notes |
|---|---|---|---|---|
| Power on | 32 | Power on in vacuum to avoid oxidation | ||
| Melting | Above liquidus line | 7 | ||
| Casting | 1140 | Off | 100 | Top pouring into ceramic shells (investment casting) in argon atmosphere |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosesso, L.; Macis, S.; D’Arco, A.; Marcelli, A.; Notargiacomo, A.; Pea, M.; Spataro, B.; Stagno, V.; Lupi, S. Characterization of CuAg Alloys with Low Ag Concentrations. Materials 2024, 17, 1823. https://doi.org/10.3390/ma17081823
Mosesso L, Macis S, D’Arco A, Marcelli A, Notargiacomo A, Pea M, Spataro B, Stagno V, Lupi S. Characterization of CuAg Alloys with Low Ag Concentrations. Materials. 2024; 17(8):1823. https://doi.org/10.3390/ma17081823
Chicago/Turabian StyleMosesso, Lorenzo, Salvatore Macis, Annalisa D’Arco, Augusto Marcelli, Andrea Notargiacomo, Marialilia Pea, Bruno Spataro, Vincenzo Stagno, and Stefano Lupi. 2024. "Characterization of CuAg Alloys with Low Ag Concentrations" Materials 17, no. 8: 1823. https://doi.org/10.3390/ma17081823
APA StyleMosesso, L., Macis, S., D’Arco, A., Marcelli, A., Notargiacomo, A., Pea, M., Spataro, B., Stagno, V., & Lupi, S. (2024). Characterization of CuAg Alloys with Low Ag Concentrations. Materials, 17(8), 1823. https://doi.org/10.3390/ma17081823











