Valorization of Water Treatment Sludge for Applications in the Construction Industry: A Review
Abstract
1. Introduction
1.1. General Considerations
1.2. Scope of the Review Paper
2. Main Characteristics of Sludge Generated in Water Treatment Plants
| Chemical Composition (%) | Countries | |||||
|---|---|---|---|---|---|---|
| Iraq [26] | Egypt [27] | United Kingdom [28] | Brazil [29] | China [30] | Australia [31] | |
| SiO2 | 36.29 | 36.51 | 10.28 | 42.00 | 43.75 | 26.43 |
| Al2O3 | 27.92 | 22.21 | 44.24 | 35.00 | 36.57 | 28.27 |
| Fe2O3 | 5.33 | 5.65 | 2.51 | 18.00 | 6.00 | 6.66 |
| CaO | 3.77 | 2.66 | 2.50 | 0.41 | 1.00 | 5.36 |
| MgO | 1.12 | 1.34 | 0.34 | 1.13 | 0.60 | 1.11 |
| Na2O | 1.31 | 1.35 | 0.15 | 0.04 | - | - |
| K2O | 1.81 | 0.49 | 0.43 | 0.95 | 2.00 | 1.23 |
| SO3 | 0.55 | 0.08 | 1.24 | 0.86 | 2.04 | 0.48 |
| P2O5 | 0.43 | - | 0.44 | 0.47 | 0.62 | - |
Impacts of WTP Sludge on the Environment
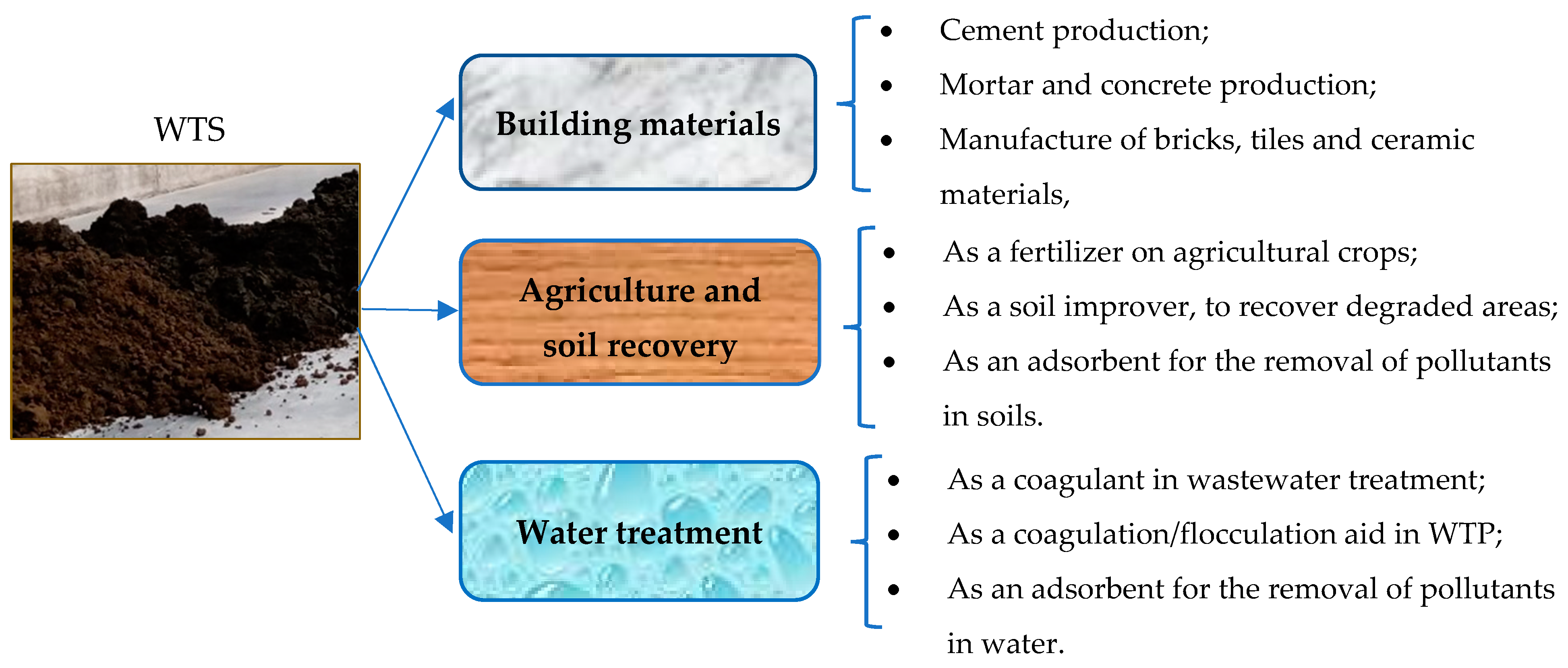
3. Emerging Applications for WTS
3.1. Applications in the Construction Industry
3.1.1. Cement Manufacturing
3.1.2. Mortar and Concrete Production
3.1.3. Manufacture of Bricks and Ceramic Products
3.1.4. Geotechnical Works
4. Conclusions
5. Perspectives
- Analysis of long-term durability indicators for applications such as SCMs in construction materials.
- Development of economic analyses, bearing in mind that the need for thermal treatment of sludge can increase costs.
- Development of life cycle analyses of materials with incorporated sludge, for better environmental assessment.
- Development of studies on the potential for valuing WTS for CO2 capture
- Development of full-scale studies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baillie, J.; Zhang, Y.P. Space for Nature. Science 2018, 361, 1051. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Ahmad, K.; Alam, M. Sludge Quantification at Water Treatment Plant and Its Management Scenario. Environ. Monit. Assess. 2017, 189, 453. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, K.G.; Possan, E.; Dezen, B.G.d.S.; Colombo, M. Potential Uses of Waste Sludge in Concrete Production. Manag. Environ. Qual. Int. J. 2017, 28, 821–838. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission: The European Green Deal; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Turner, L.K.; Collins, F.G. Carbon Dioxide Equivalent (CO2-e) Emissions: A Comparison between Geopolymer and OPC Cement Concrete. Constr. Build Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- He, Z.; Zhu, X.; Wang, J.; Mu, M.; Wang, Y. Comparison of CO2 Emissions from OPC and Recycled Cement Production. Constr. Build. Mater. 2019, 211, 965–973. [Google Scholar] [CrossRef]
- Carriço, A.; Real, S.; Bogas, J.A.; Costa Pereira, M.F. Mortars with Thermo Activated Recycled Cement: Fresh and Mechanical Characterisation. Constr. Build. Mater. 2020, 256, 119502. [Google Scholar] [CrossRef]
- Schneider, M.; Romer, M.; Tschudin, M.; Bolio, H. Sustainable Cement Production—Present and Future. Cem. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Hagemann, S.E.; Gastaldini, A.L.G.; Cocco, M.; Jahn, S.L.; Terra, L.M. Synergic Effects of the Substitution of Portland Cement for Water Treatment Plant Sludge Ash and Ground Limestone: Technical and Economic Evaluation. J. Clean. Prod. 2019, 214, 916–926. [Google Scholar] [CrossRef]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-Efficient Cements: Potential Economically Viable Solutions for a Low-CO2 Cement-Based Materials Industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Siddique, R. Recent Advances in Understanding the Role of Supplementary Cementitious Materials in Concrete. Cem. Concr. Res. 2015, 78, 71–80. [Google Scholar] [CrossRef]
- John, V.M.; Quattrone, M.; Abrão, P.C.R.A.; Cardoso, F.A. Rethinking Cement Standards: Opportunities for a Better Future. Cem. Concr. Res. 2019, 124, 105832. [Google Scholar] [CrossRef]
- Liu, Y.; Zhuge, Y.; Chow, C.W.K.; Keegan, A.; Pham, P.N.; Li, D.; Oh, J.-A.; Siddique, R. The Potential Use of Drinking Water Sludge Ash as Supplementary Cementitious Material in the Manufacture of Concrete Blocks. Resour. Conserv. Recycl. 2021, 168, 105291. [Google Scholar] [CrossRef]
- McCarthy, M.J.; Robl, T.; Csetenyi, L.J. 14-Recovery, Processing, and Usage of Wet-Stored Fly Ash. In Coal Combustion Products (CCP’s); Robl, T., Oberlink, A., Jones, R., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 343–367. [Google Scholar]
- Juenger, M.C.G.; Snellings, R.; Bernal, S.A. Supplementary Cementitious Materials: New Sources, Characterization, and Performance Insights. Cem. Concr. Res. 2019, 122, 257–273. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, W.; Peng, Y.; Tang, S.; Wang, L.; Shi, Y.; Li, Y.; Wang, Y.; Geng, Z.; Wu, K. Hydration and Fractal Analysis on Low-Heat Portland Cement Pastes Using Thermodynamics-Based Methods. Fractal Fract. 2023, 7, 606. [Google Scholar] [CrossRef]
- Dassanayake, K.B.; Jayasinghe, G.Y.; Surapaneni, A.; Hetherington, C. A Review on Alum Sludge Reuse with Special Reference to Agricultural Applications and Future Challenges. Waste Manag. 2015, 38, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Ahmad, K.; Alam, M. Characterization of Water Treatment Plant’s Sludge and Its Safe Disposal Options. Procedia Environ. Sci. 2016, 35, 950–955. [Google Scholar] [CrossRef]
- Ahmad, T.; Ahmad, K.; Ahad, A.; Alam, M. Characterization of Water Treatment Sludge and Its Reuse as Coagulant. J. Environ. Manag. 2016, 182, 606–611. [Google Scholar] [CrossRef]
- Tantawy, M.A. Characterization and Pozzolanic Properties of Calcined Alum Sludge. Mater. Res. Bull. 2015, 61, 415–421. [Google Scholar] [CrossRef]
- Bratby, J. Coagulation and Flocculation in Water and Wastewater Treatment; IWA Publishing: London, UK, 2016. [Google Scholar]
- Jeon, E.-K.; Ryu, S.; Park, S.-W.; Wang, L.; Tsang, D.C.W.; Baek, K. Enhanced Adsorption of Arsenic onto Alum Sludge Modified by Calcination. J. Clean. Prod. 2018, 176, 54–62. [Google Scholar] [CrossRef]
- Muisa, N.; Nhapi, I.; Ruziwa, W.; Manyuchi, M.M. Utilization of Alum Sludge as Adsorbent for Phosphorus Removal in Municipal Wastewater: A Review. J. Water Process Eng. 2020, 35, 101187. [Google Scholar] [CrossRef]
- Tavares, R.G. A Atenuação do Alumínio do Resíduo de Estações de Tratamento de Água por Vermicompostagem e Adsorção. Ph.D. Thesis, Universidade Federal de Pernambuco, Recife, Brazil, 2016. [Google Scholar]
- DI Bernardo, L.; Dantas, A.D.B.; Voltan, P.E.N. Métodos e Técnicas de Tratamento e Disposição Dos Resíduos Gerados Em Estações de Tratamento de Água, 1st ed.; LDiBe: São Carlos, Brazil, 2012. [Google Scholar]
- Ahmed, F.R.; Muhammad, M.A.; Ibrahim, R.K. Effect of Alum Sludge on Concrete Strength and Two Way Shear Capacity of Flat Slabs. Structures 2022, 40, 991–1001. [Google Scholar] [CrossRef]
- Abo-El-Enein, S.A.; Shebl, A.; Abo El-Dahab, S.A. Drinking Water Treatment Sludge as an Efficient Adsorbent for Heavy Metals Removal. Appl. Clay Sci. 2017, 146, 343–349. [Google Scholar] [CrossRef]
- Shamaki, M.; Adu-Amankwah, S.; Black, L. Reuse of UK Alum Water Treatment Sludge in Cement-Based Materials. Constr. Build. Mater. 2021, 275, 122047. [Google Scholar] [CrossRef]
- Altheman, D.; Andréia Gachet, L.; Siviero Pires, M.; Cristina Cecche Lintz, R. Water Treatment Waste as Supplementary Cementitious Material. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- He, Z.H.; Yang, Y.; Yuan, Q.; Shi, J.Y.; Liu, B.J.; Liang, C.F.; Du, S.G. Recycling Hazardous Water Treatment Sludge in Cement-Based Construction Materials: Mechanical Properties, Drying Shrinkage, and Nano-Scale Characteristics. J. Clean. Prod. 2021, 290, 125832. [Google Scholar] [CrossRef]
- Liu, Y.; Zhuge, Y.; Chow, C.W.K.; Keegan, A.; Li, D.; Pham, P.N.; Huang, J.; Siddique, R. Utilization of Drinking Water Treatment Sludge in Concrete Paving Blocks: Microstructural Analysis, Durability and Leaching Properties. J. Environ. Manag. 2020, 262, 110352. [Google Scholar] [CrossRef]
- Richter, C.A. Tratamento de Lodos de Estações de Tratamento de Água; Editora Blucher: Sao Paulo, Brazil, 2021. [Google Scholar]
- Mattoso, A.P.; Aguiar, J.L.B.d.; Duarte, A.A.L.S.D.; Lemos, H.M.O.; Cunha, S.R.L. Valorization of Water Treatment Sludge from a Circular Economy Perspective: The Case of the WTP of Areias de Vilar. In Proceedings of the 6th International Conference WASTES: Solutions, Treatments and Opportunities; Centro para a Valorização de Resíduos (CVR), Coimbra, Portugal, 6 September 2023; pp. 343–345. [Google Scholar]
- Azeddine, F.; Khadir, L.E.; Ali, I. Experimental Investigation of Solar Greenhouse Drying of Hydroxide Sludge under Summer and Winter Climate. Pol. J. Environ. Stud. 2022, 31, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- O’Kelly, B.C. Geotechnical Properties of a Municipal Water Treatment Sludge Incorporating a Coagulant. Can. Geotech. J. 2008, 45, 715–725. [Google Scholar] [CrossRef][Green Version]
- Keeley, J.; Jarvis, P.; Judd, S.J. Coagulant Recovery from Water Treatment Residuals: A Review of Applicable Technologies. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2675–2719. [Google Scholar] [CrossRef]
- Komlos, J.; Welker, A.; Punzi, V.; Traver, R. Feasibility Study of As-Received and Modified (Dried/Baked) Water Treatment Plant Residuals for Use in Storm-Water Control Measures. J. Environ. Eng. 2013, 139, 1237–1245. [Google Scholar] [CrossRef]
- O’kelly, B.C.; Quille, M.E. Compressibility and Consolidation of Water Treatment Residues. Proc. Inst. Civ. Eng. Waste Resour. Manag. 2009, 162, 85–97. [Google Scholar] [CrossRef]
- Wang, M.C.; Hull, J.Q.; Jao, M.; Dempsey, B.A.; Cornwell, D.A. Engineering Behavior of Water Treatment Sludge. J. Environ. Eng. 1992, 118, 848–864. [Google Scholar] [CrossRef]
- Lee, Y.-E.; Kim, I.-T.; Yoo, Y.-S. Stabilization of High-Organic-Content Water Treatment Sludge by Pyrolysis. Energies 2018, 11, 3292. [Google Scholar] [CrossRef]
- ABNT NBR 12653/2014; Materiais Pozolânicos: Requisitos. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2014.
- Gastaldini, A.L.G.; Hengen, M.F.; Gastaldini, M.C.C.; do Amaral, F.D.; Antolini, M.B.; Coletto, T. The Use of Water Treatment Plant Sludge Ash as a Mineral Addition. Constr. Build. Mater. 2015, 94, 513–520. [Google Scholar] [CrossRef]
- Owaid, H.M.; Hamid, R.; Taha, M.R. Influence of Thermally Activated Alum Sludge Ash on the Engineering Properties of Multiple-Blended Binders Concretes. Constr. Build. Mater. 2014, 61, 216–229. [Google Scholar] [CrossRef]
- Godoy, L.G.G.d.; Rohden, A.B.; Garcez, M.R.; da Costa, E.B.; Da Dalt, S.; Andrade, J.J. de O. Valorization of Water Treatment Sludge Waste by Application as Supplementary Cementitious Material. Constr. Build. Mater. 2019, 223, 939–950. [Google Scholar] [CrossRef]
- Bohórquez González, K.; Pacheco, E.; Guzmán, A.; Avila Pereira, Y.; Cano Cuadro, H.; Valencia, J.A.F. Use of Sludge Ash from Drinking Water Treatment Plant in Hydraulic Mortars. Mater. Today Commun. 2020, 23, 100930. [Google Scholar] [CrossRef]
- Agra, T.M.S.; Lima, V.M.E.; Basto, P.E.A.; Melo Neto, A.A. Characterizing and Processing a Kaolinite-Rich Water Treatment Sludge for Use as High-Reactivity Pozzolan in Cement Manufacturing. Appl. Clay Sci. 2023, 236, 106870. [Google Scholar] [CrossRef]
- Freddy, A.; Jos, V.; Ruben, S.; Özlem, C.; Liesbeth, H.; Céline, V.B.; Lucie, V.; Jonas, M.; Joris, D. BIND-AMOR: Flash-Calcined Dredging Sediments of the AMORAS Mechanical Dewatering Plant (*) as Cement Substitute; European Sediment Network: Genoa, Italy, 2017. [Google Scholar]
- Santos, L.A.R.; Michelan, D.C.d.G.; Jesus, T.M. Verificação da Produção de Lodo de ETA em Função da Quantidade e da Qualidade da Água Bruta. Rev. Bras. Eng. Biossistemas 2021, 15, 235–258. [Google Scholar]
- Katayama, V.T.; Montes, C.P.; Ferraz, T.H.; Morita, D.M. Quantificação da Produção de Lodo de Estações de Tratamento de Água de Ciclo Completo: Uma Análise Crítica. Eng. Sanit. E Ambient. 2015, 20, 559–569. [Google Scholar] [CrossRef][Green Version]
- American Water Works Association–AWWA. Water Quality and Treatment: A Handbook of Community Water Supplies, 5th ed.; Letterman, R.D., Ed.; McGraw-Hill: New York, NY, USA, 1999. [Google Scholar]
- Okuda, T.; Nishijima, W.; Sugimoto, M.; Saka, N.; Nakai, S.; Tanabe, K.; Ito, J.; Takenaka, K.; Okada, M. Removal of Coagulant Aluminum from Water Treatment Residuals by Acid. Water Res. 2014, 60, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Frias, M.; de la Villa, R.V.; García, R.; Sánchez de Rojas, M.I.; Baloa, T.A. Mineralogical Evolution of Kaolin-Based Drinking Water Treatment Waste for Use as Pozzolanic Material. The Effect of Activation Temperature. J. Am. Ceram. Soc. 2013, 96, 3188–3195. [Google Scholar] [CrossRef]
- AdP Relatório de Sustentabilidade 2022; Águas de Portugal: Lisbon, Portugal, 2022.
- Indústria e Ambiente. Available online: https://www.industriaeambiente.pt/noticias/lamas-epal-vao-ser-convertidas-em-tijolos/ (accessed on 1 April 2024).
- European Commission. Commission Decision of 18 December 2014 Amending Decision 2000/532/EC on the List of Waste Pursuant to Directive 2008/98/EC of the European Parliament and of the Council. 2014. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32014D0955 (accessed on 29 August 2023).
- ABNT NBR 10004/2004; Resíduos Sólidos: Classificação. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2004.
- Babatunde, A.O.; Zhao, Y.Q. Constructive Approaches Toward Water Treatment Works Sludge Management: An International Review of Beneficial Reuses. Crit. Rev. Environ. Sci. Technol. 2007, 37, 129–164. [Google Scholar] [CrossRef]
- Ahmad, T.; Ahmad, K.; Alam, M. Sustainable Management of Water Treatment Sludge through 3‘R’ Concept. J. Clean. Prod. 2016, 124, 1–13. [Google Scholar] [CrossRef]
- Sobrinho, M.A.d.M.; Tavares, R.G.; De Arruda, V.C.M.; Correa, M.M.; Pereira, L.J.R. Generation, Treatment and Final Disposal of the Waste of Water Treatment Stations in the State of Pernambuco, Brazil. Eng. Sanit. E Ambient. 2019, 24, 761–771. [Google Scholar]
- Achon, C.L.; Barroso, M.M.; Cordeiro, J.S. Resíduos de Estações de Tratamento de Água e a ISO 24512: Desafio Do Saneamento Brasileiro. Eng. Sanit. E Ambient. 2013, 18, 115–122. [Google Scholar] [CrossRef]
- George, D.B.; Berk, S.G.; Adams, V.D.; Ting, R.S.; Roberts, R.O.; Parks, L.H.; Lott, R.C. Toxicity of Alum Sludge Extracts to a Freshwater Alga, Protozoan, Fish, and Marine Bacterium. Arch. Environ. Contam. Toxicol. 1995, 29, 149–158. [Google Scholar] [CrossRef]
- Sotero-Santos, R.B.; Rocha, O.; Povinelli, J. Evaluation of Water Treatment Sludges Toxicity Using the Daphnia Bioassay. Water Res. 2005, 39, 3909–3917. [Google Scholar] [CrossRef]
- Soares, A.F.S.; Silva, L.F.M.; Araújo, B.J.R.S. Poluição Hídrica Ocasionada Pelo Lodo Gerado Em Estação de Tratamento de Água. In Proceedings of the Congresso Sul-Americano de Resíduos Sólidos e Sustentabilidade; IBEAS: Gramado, Brazil, 2018. [Google Scholar]
- Cordeiro, J.S. O Problema dos Lodos Gerados nos Decantadores em Estações de Tratamento de Água. Ph.D. Thesis, Universidade de São Paulo, São Carlos, Brazil, 1993. [Google Scholar]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60. [Google Scholar] [CrossRef]
- Silva, C.R. Avaliação do Efeito do Sulfato de Alumínio no Cérebro de Ratos Utilizando Modelos Neurofisiológicos, Comportamentais e Moleculares. Ph.D. Thesis, Universidade Federal de Pernambuco, Recife, Brazil, 2018. [Google Scholar]
- Cheng, D.; Wang, X.; Xi, Y.; Cao, J.; Jiang, W. Identification of the Al-Binding Proteins That Account for Aluminum Neurotoxicity and Transport “in Vivo”. Toxicol. Res. 2018, 7, 127–135. [Google Scholar] [CrossRef]
- Liu, Z.; Mayer, B.K.; Venkiteshwaran, K.; Seyedi, S.; Raju, A.S.K.; Zitomer, D.; McNamara, P.J. The State of Technologies and Research for Energy Recovery from Municipal Wastewater Sludge and Biosolids. Curr. Opin. Environ. Sci. Health 2020, 14, 31–36. [Google Scholar] [CrossRef]
- Cardoso, J.P.; Mantilla, V.; Lobo, G.; Martins, A. Estimativa do Potencial Técnico para Secagem de Lamas Provenientes de ETAR nas Regiões do Alentejo, Algarve e Andaluzia. In Proceedings of the CIES2020-XVII Congresso Ibérico e XIII Congresso Ibero-americano de Energia Solar, Online, 3–5 November 2020; pp. 1227–1235. [Google Scholar]
- Lombi, E.; Stevens, D.P.; McLaughlin, M.J. Effect of Water Treatment Residuals on Soil Phosphorus, Copper and Aluminium Availability and Toxicity. Environ. Pollut. 2010, 158, 2110–2116. [Google Scholar] [CrossRef] [PubMed]
- Balkaya, M. Evaluation of the Use of Alum Sludge as Hydraulic Barrier Layer and Daily Cover Material in Landfills: A Finite Element Analysis Study. Desalination Water Treat 2016, 57, 2400–2412. [Google Scholar] [CrossRef]
- Scalize, P.S.; Souza, L.M.D.; Albuquerque, A. Reuse of Alum Sludge for Reducing Flocculant Addition in Water Treatment Plants. Environ. Prot. Eng. 2019, 45, 57–70. [Google Scholar] [CrossRef]
- Hovsepyan, A.; Bonzongo, J.-C.J. Aluminum Drinking Water Treatment Residuals (Al-WTRs) as Sorbent for Mercury: Implications for Soil Remediation. J. Hazard. Mater. 2009, 164, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Siswoyo, E.; Qoniah, I.; Lestari, P.; Fajri, J.A.; Sani, R.A.; Sari, D.G.; Boving, T. Development of a Floating Adsorbent for Cadmium Derived from Modified Drinking Water Treatment Plant Sludge. Environ. Technol Innov. 2019, 14, 100312. [Google Scholar] [CrossRef]
- Castaldi, P.; Silvetti, M.; Garau, G.; Demurtas, D.; Deiana, S. Copper(II) and Lead(II) Removal from Aqueous Solution by Water Treatment Residues. J. Hazard. Mater. 2015, 283, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Godoy, L.G.G.d.; Rohden, A.B.; Garcez, M.R.; Da Dalt, S.; Bonan Gomes, L. Production of Supplementary Cementitious Material as a Sustainable Management Strategy for Water Treatment Sludge Waste. Case Stud. Constr. Mater. 2020, 12, e00329. [Google Scholar] [CrossRef]
- Ruviaro, A.S.; Silvestro, L.; Scolaro, T.P.; de Matos, P.R.; Pelisser, F. Use of Calcined Water Treatment Plant Sludge for Sustainable Cementitious Composites Production. J. Clean. Prod. 2021, 327, 129484. [Google Scholar] [CrossRef]
- Hemkemeier, T.A.; Almeida, F.C.R.; Sales, A.; Klemm, A.J. Repair Mortars with Water Treatment Plant Sludge (WTPS) and Sugarcane Bagasse Ash Sand (SBAS) for More Eco-Efficient and Durable Constructions. J. Clean. Prod. 2023, 386, 135750. [Google Scholar] [CrossRef]
- Tafarel, N.; Macioski, G.; Carvalho, K.; Nagalli, A.; Freitas, D.; Passig, F. Avaliação Das Propriedades Do Concreto Devido à Incorporação de Lodo de Estação de Tratamento de Água. Matéria 2016, 21, 974–986. [Google Scholar] [CrossRef]
- Cremades, L.V.; Cusidó, J.A.; Arteaga, F. Recycling of Sludge from Drinking Water Treatment as Ceramic Material for the Manufacture of Tiles. J. Clean. Prod. 2018, 201, 1071–1080. [Google Scholar] [CrossRef]
- Kizinievič, O.; Žurauskienė, R.; Kizinievič, V.; Žurauskas, R. Utilisation of Sludge Waste from Water Treatment for Ceramic Products. Constr. Build. Mater. 2013, 41, 464–473. [Google Scholar] [CrossRef]
- Teixeira, S.R.; Santos, G.T.A.; Souza, A.E.; Alessio, P.; Souza, S.A.; Souza, N.R. The Effect of Incorporation of a Brazilian Water Treatment Plant Sludge on the Properties of Ceramic Materials. Appl. Clay Sci. 2011, 53, 561–565. [Google Scholar] [CrossRef]
- Boaventura, R.A.R.; Duarte, A.A.L.S.; Almeida, M.F. Aluminum Recovery from Water Treatment Sludges. In Proceedings of the Internacional Conference on Water Supply and Water Quality; International Water Association Publishing (IWAP), IWA Publishing (CD-Rom): Kraków, Poland, September 2000. [Google Scholar]
- Hassan Basri, M.H.; Mohammad Don, N.N.; Kasmuri, N.; Hamzah, N.; Alias, S.; Azizan, F.A. Aluminium Recovery from Water Treatment Sludge under Different Dosage of Sulphuric Acid. J. Phys. Conf. Ser. 2019, 1349, 012005. [Google Scholar] [CrossRef]
- Moodley, M.; Johnston, M.A.; Hughes, J.C.; Titshall, L.W. Effects of a Water Treatment Residue, Lime, Gypsum, and Polyacrylamide on the Water Retention and Hydraulic Conductivity of Two Contrasting Soils under Field Conditions in KwaZulu-Natal, South Africa. Soil Res. 2004, 42, 273–282. [Google Scholar] [CrossRef]
- Moodley, M.; Hughes, J.C. The Effects of a Polyacrylamide-Derived Water Treatment Residue on the Hydraulic Conductivity, Water Retention and Evaporation of Four Contrasting South African Soils and Implications for Land Disposal. Water Sci. Technol. 2006, 54, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.; Sous, D.; Bernardo, M.; Matos, I.; Fonseca, I.; Vale Cardoso, V.; Carneiro, R.N.; Silva, S.; Fontes, P.; Daam, M.A.; et al. Study of the Potential of Water Treatment Sludges in the Removal of Emerging Pollutants. Molecules 2021, 26, 1010. [Google Scholar] [CrossRef] [PubMed]
- Mazari, L.; Abdessemed, D.; Szymczyk, A.; Trari, M. Assessment of Coagulation-ultrafiltration Performance for the Treatment of Primary Wastewater Using Alum Sludge. Water Environ. J. 2018, 32, 621–629. [Google Scholar] [CrossRef]
- Shrestha, S.; Kulandaivelu, J.; Sharma, K.; Jiang, G.; Yuan, Z. Effects of Dosing Iron- and Alum-Containing Waterworks Sludge on Sulfide and Phosphate Removal in a Pilot Sewer. Chem. Eng. J. 2020, 387, 124073. [Google Scholar] [CrossRef]
- Kang, C.; Zhao, Y.; Tang, C.; Addo-Bankas, O. Use of Aluminum-Based Water Treatment Sludge as Coagulant for Animal Farm Wastewater Treatment. J. Water Process Eng. 2022, 46, 102645. [Google Scholar] [CrossRef]
- Nair, A.T.; Ahammed, M.M. The Reuse of Water Treatment Sludge as a Coagulant for Post-Treatment of UASB Reactor Treating Urban Wastewater. J. Clean. Prod. 2015, 96, 272–281. [Google Scholar] [CrossRef]
- Taylor, M.; Elliott, H.A. Influence of Water Treatment Residuals on Dewaterability of Wastewater Biosolids. Water Sci. Technol. 2013, 67, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Lyczko, N.; Zhao, Y.; Nzihou, A. Integrating Alum Sludge with Waste-Activated Sludge in Co-Conditioning and Dewatering: A Case Study of a City in South France. Environ. Sci. Pollut. Res. 2020, 27, 14863–14871. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S. Processing, Effect and Reactivity Assessment of Artificial Pozzolans Obtained from Clays and Clay Wastes: A Review. Constr. Build Mater. 2017, 140, 10–19. [Google Scholar] [CrossRef]
- Tay, J.; Show, K. Properties of Cement Made from Sludge. J. Environ. Eng. 1991, 117, 236–246. [Google Scholar] [CrossRef]
- Pan, J.R.; Huang, C.; Lin, S. Reuse of Fresh Water Sludge in Cement Making. Water Sci. Technol. 2004, 50, 183–188. [Google Scholar] [CrossRef]
- Chen, H.; Ma, X.; Dai, H. Reuse of Water Purification Sludge as Raw Material in Cement Production. Cem. Concr. Compos. 2010, 32, 436–439. [Google Scholar] [CrossRef]
- Kaish, A.B.M.A.; Odimegwu, T.C.; Zakaria, I.; Abood, M.M.; Nahar, L. Properties of Concrete Incorporating Alum Sludge in Different Conditions as Partial Replacement of Fine Aggregate. Constr. Build. Mater. 2021, 284, 122669. [Google Scholar] [CrossRef]
- De Carvalho Gomes, S.; Zhou, J.L.; Zeng, X.; Long, G. Water Treatment Sludge Conversion to Biochar as Cementitious Material in Cement Composite. J. Environ. Manag. 2022, 306, 114463. [Google Scholar] [CrossRef]
- Arvaniti, E.C.; Juenger, M.C.G.; Bernal, S.A.; Duchesne, J.; Courard, L.; Leroy, S.; Provis, J.L.; Klemm, A.; De Belie, N. Physical Characterization Methods for Supplementary Cementitious Materials. Mater. Struct. 2015, 48, 3675–3686. [Google Scholar] [CrossRef]
- Juenger, M.; Provis, J.L.; Elsen, J.; Matthes, W.; Hooton, R.D.; Duchesne, J.; Courard, L.; He, H.; Michel, F.; Snellings, R.; et al. Supplementary Cementitious Materials for Concrete: Characterization Needs. MRS Proc. 2012, 1488, imrc12-1488-7b-026. [Google Scholar] [CrossRef]
- Benlalla, A.; Elmoussaouiti, M.; Dahhou, M.; Assafi, M. Utilization of Water Treatment Plant Sludge in Structural Ceramics Bricks. Appl. Clay Sci. 2015, 118, 171–177. [Google Scholar] [CrossRef]
- Wolff, E.; Schwabe, W.K.; Conceição, S.V. Utilization of Water Treatment Plant Sludge in Structural Ceramics. J. Clean. Prod. 2015, 96, 282–289. [Google Scholar] [CrossRef]
- Husillos Rodríguez, N.; Martínez-Ramírez, S.; Blanco-Varela, M.T.; Guillem, M.; Puig, J.; Larrotcha, E.; Flores, J. Evaluation of Spray-Dried Sludge from Drinking Water Treatment Plants as a Prime Material for Clinker Manufacture. Cem. Concr. Compos. 2011, 33, 267–275. [Google Scholar] [CrossRef]
- Orlov, A.; Belkanova, M.; Vatin, N. Structural Ceramics Modified by Water Treatment Plant Sludge. Materials 2020, 13, 5293. [Google Scholar] [CrossRef]
- Gonçalves, F.; Souza, C.H.U.d.; Tahira, F.S.; Fernandes, F.; Teixeira, R.S. Incremento de Lodo de ETA Em Barreiras Ipermeabilizantes de Aterro Sanitário. Rev. DAE 2017, 65, 5–14. [Google Scholar] [CrossRef]
- Caniani, D.; Masi, S.; Mancini, I.M.; Trulli, E. Innovative Reuse of Drinking Water Sludge in Geo-Environmental Applications. Waste Manag. 2013, 33, 1461–1468. [Google Scholar] [CrossRef]
- NEN 7345; Leaching Characteristics of Solid Earthy and Stony Building and Waste Materials—Leaching Tests—Determination of the Leaching of Inorganic Components from Buildings and Monolitic Waste Materials with the Diffusion Test. NNI: Delft, The Netherlands, 1995.
- ESA PSS-01-702; A Thermal Vacuum Test for the Screening of Space Materials. European Space Agency: Paris, France, 1994.
- ESA PSS-01-729; The Determination of Off-gassing Products from Materials and Assembled Articles to Be Used in a Manned Space Vehicle Crew Compartment. Issue 1. European Space Agency: Paris, France, 1991.
- EN 197-1; Cement—Part 1: Composition, Specifications, and Conformity Criteria for Common Cements. European Committee for Standardization: Brussels, Belgium, 2011.
- ABNT NBR 5752; Materiais Pozolânicos: Determinação do Índice de Desempenho com Cimento Portland aos 28 Dias. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2014.

| Characteristics | Cement | Dry Sludge (105 °C for 24 h) | Calcined Sludge (800 °C for 2 h) | Blast Furnace Slag | |
|---|---|---|---|---|---|
| Chemical composition (%) | SiO2 | 20.18 | 42.38 | 47.00 | 32.6 |
| Al2O3 | 5.23 | 35.03 | 41.94 | 12.57 | |
| Fe2O3 | 3.34 | 4.94 | 4.86 | 0.24 | |
| CaO | 64.40 | 0.13 | 0.41 | 41.0 | |
| MgO | 1.80 | 0.29 | 0.40 | 6.04 | |
| Na2O | 0.07 | 0.10 | 0.09 | 0.39 | |
| K2O | 0.44 | 1.87 | 0.99 | 0.35 | |
| SO3 | 2.98 | 0.14 | 0.10 | 1.31 | |
| P2O5 | - | 0.26 | 0.28 | - | |
| Loss on ignition | 2.17 | 11.4 | 2.64 | 1.48 | |
| Physical properties | Specific gravity | 3.12 | 2.34 | 2.53 | 2.83 |
| Specific surface (m2/kg) | 338 | 1110 | 1160 | 739 | |
| Average particle size (µm) (d50) | 16.9 | 11.0 | 10.1 | 16.8 | |
| Resistance activity index 7 days (%) | 100 | 78 | 84 | 84 | |
| Resistance activity index 28 days (%) | 100 | 86 | 93 | 101.4 | |
| Characteristics | Methodology | Observations |
|---|---|---|
| Chemical composition | X-ray Fluorescence Spectrometry (XRF) |
|
| Nuclear Magnetic Resonance Spectroscopy (NMR) |
| |
| Loss on Ignition—Volatiles | Thermogravimetric analysis (TGA) |
|
| Mineralogy/ Phase Composition | X-Ray Diffraction (XRD) |
|
| Particle Size Distribution | Laser Diffractometry (LD) |
|
| Scanning Electron Microscopy (SEM) |
| |
| Specific Surface Area | N2 sorption—BET (Brunauer, Emmett and Teller) |
|
| Air Permeability—Blaine |
| |
| Density | Helium (He) Gas Pycnometry |
|
| Study | Country | Application | Heat Treatment | Main Results |
|---|---|---|---|---|
| Chen et al. [97] | China, 2010. | Cement | Dry in an oven at 105 °C until constant weight. | Partially replacing the clay with WTS resulted in an “ecocement” with a higher compressive strength at 3 and 7 days than ordinary cement. By replacing the clay with 10% LETA, the strengths at 3 and 7 days were 13.0% and 5.6% higher, respectively. |
| Rodríguez et al. [104] | Spain, 2011. | Cement | Spray-drying | Atomized sludge was used as a raw material in the manufacture of clinker, replacing clay. The sludge showed high reactivity in the mixture. The clinker obtained had high proportions of allite (>70%), and its microstructure was like the reference clinker in terms of the size and composition of the allite and belite crystals. |
| Teixeira et al. [82] | Brazil, 2011. | Ceramic product | Oven-drying at 110 °C. | The use of WTS mixed with clay as a raw material resulted in bricks with characteristics within the standards defined for ceramics by Brazilian standards: flexural strength (bricks > 2.0 MPa, perforated bricks > 5.5 MPa), water absorption (perforated bricks < 25%), linear firing shrinkage (bricks < 6%) and apparent specific mass (>1.6 g/cm3). |
| Caniani et al. [107] | Italy, 2013. | Geotechnical work | - | Biosoil obtained from mixing WTS with the organic fraction of municipal solid waste proved to be suitable for use as daily cover and final cover in landfills. The mixture does not pose significant environmental risks, even at doses above 2000 tons ss/ha in single applications. |
| Kizinievič et al. [81] | Lithuania, 2013. | Ceramic product | Oven drying at 105 °C. | Incorporating 5% iron mud into the clay mixture, with the samples fired at 1000 °C or 1050 °C, resulted in an increase in the density and compressive strength of the ceramic body and in the reduction of water impregnation and effective porosity of the material produced. The incorporation of iron sludge resulted in a more intense tinting of the ceramic body, even in small proportions (5%). |
| Owaid et al. [43] | Malaysia, 2014. | Concrete | Drying in an oven at 105 °C for 24 h, followed by calcination at 800 °C for 2 h. | Binary mixtures containing 5%, 10% and 15% WTS as a partial cement substitute resulted in an increase in the concrete’s compressive strength at all ages compared to the control concrete: 3.4%, 8.4% and 9.3% at 7 days; 3.6%, 10% and 14.2% at 28 days; 5.4%, 9.3% and 12.5% at 56 days; and 3.1%, 6.3% and 9.8% at 90 days. |
| Benlalla et al. [102] | Morocco, 2015 | Ceramic product | Sun-dried for 72 h, followed by oven-dried at 105 °C for 48 h. | The use of the WTS–clay mixture resulted in bricks with properties within the standards defined for ceramic bricks. All the bricks produced met the criteria regarding the degree of shrinkage during firing. With the incorporation of 5 to 10% by weight of WTS, the bricks fell into the first-class category in terms of water absorption and compressive strength standards. |
| Gastaldini et al. [42] | Brazil, 2015. | Concrete | Drying in an oven at 110 °C for 24 h, followed by calcination at 600 °C for 1 h. | The use of WTS as a pozzolanic material to partially replace cement in concrete (up to 30% by mass) resulted in an increase in compressive strength of between 3% and 30% compared to the reference concrete, at both 7 and 28 days. With the replacement, cement consumption was reduced by between 37 and 200 kg/m3 of concrete. |
| Wolff et al. [103] | Brazil, 2015. | Ceramic product | Drying in an oven at 110 °C for 2 h. | WTS, waste from the recovery of chemical reagents (including lime sludge) and fine granite waste were mixed (8 different compositions) and used to make bricks, replacing clay. Some of these mixtures showed promising results, indicating that they could be used in the production of interior tiles or acoustic bricks. |
| Tafarel et al. [79] | Brazil, 2016. | Concrete | Wet sludge, without any heat treatment. | Replacing 5% of the fine aggregate with WTS resulted in concrete with satisfactory axial compressive strength conditions for non-structural use. |
| Gonçalves et al. [106] | Brazil, 2017. | Geotechnical work | Drying in drainage beds (layer of gravel No. 3, overlaid by geotextile blankets) for 30 days. | Mixing WTS with clay soil (proportions 1:0.5 and 1:1) and with sandy soil (proportion 1:0.25) resulted in materials with a coefficient of permeability between 10−10 and 10−9 m/s, suitable for use in landfill works. |
| Ramirez et al. [3] | Brazil, 2017. | Concrete | Wet sludge, without any heat treatment. | The study evaluated the effects of partially replacing sand with WTS on the mechanical properties and water absorption of concrete. Substitution of up to 5% proved suitable for non-structural concrete applications. |
| Cremades et al. [80] | Spain, 2018. | Ceramic product | Spray-drying: maximum hot air temperature 350 °C | Atomizing the sludge resulted in a powder with a low organic content and a high concentration of lime. The waste was used to partially replace clay in the manufacture of ceramic material and the product obtained passed the leaching test (NEN-7345 [108]) and the accelerated degassing tests (European Space Agency standards PSS-01-702 [109] and PSS-01-729 [110]). |
| Hagemann et al. [9] | Brazil, 2019. | Concrete | Drying in an oven at 110 °C for 24 h, followed by calcination at 700 °C for 1 h. | Mixture containing WTS, ground limestone and cement resulted in higher compressive strength than binary mixtures (ground limestone and cement) and simple cement. The ternary mixture made up of 15% WTS and 7.5% ground limestone reduced Portland cement consumption in concrete production by up to 38.4%. |
| Godoy et al. [76] | Brazil, 2020. | Cement | Drying in an oven at 110 °C for 24 h, followed by calcination at 600 °C for 1 h. | The use of 14% and 35% WTS resulted in SCM that met the compressive strength requirements to produce Portland cement mixtures equivalent to CEM II/A-M (25 MPa 32 MPa), according to EN 197-1 [111]. |
| Orlov et al. [105] | Russia, 2020. | Ceramic product | Freezing (−16 ± 2 °C) and thawing (20 ± 3 °C), followed by oven-drying at 105 ± 2 °C until constant weight was reached. | WTS pre-treated by the freeze–thaw method was used as an additive to partially replace clay in the production of ceramic bricks. The addition of WTS reduced the clay’s sensitivity to drying, decreased the ceramic’s density by 20% and increased its compressive strength from 7.0 to 10.2 MPa. |
| He et al. [30] | China, 2021. | Mortar | Drying in an oven at 105 °C for 24 h, followed by calcination at 900 °C for 2 h. | Mortar produced with 10% WTS as a partial substitute for the cement content showed higher compressive strength at 90 days than the reference sample |
| Kaish et al. [98] | Malaysia, 2021. | Concrete | Drying in an oven at 105 °C for 24 h. | Replacing the fine aggregate with WTS (at a rate of 10%) improved the density, mechanical properties and durability of the concrete. |
| Ruviaro et al. [77] | Brazil, 2021. | Cement paste | Drying in an oven at 105 °C for 24 h, followed by calcination at 700 °C for 1 h. | Replacing cement with up to 20% WTS resulted in pastes with comparable fresh-state properties and better mechanical strength than the reference paste. The CO2-eq emissions associated with the production of 1 m³ of paste decreased progressively, with a reduction of 42% for the highest level of WTS incorporation (45%) |
| De Carvalho et al. [99] | Australia, 2022 | Cement paste | Drying in an oven at 105 °C for 24 h, followed by pyrolysis at 700 °C for 2 h. | Mixtures containing 1%, 2% and 5% “biochar” (WTS bio-coal) in place of cement resulted in pastes with slightly higher compressive strength at 28 days compared to the reference material. With the use of 10% biochar, pastes with a compressive strength similar to the reference material were obtained. |
| Altheman et al. [29] | Brazil, 2023. | Mortar | Drying in an oven at 105 °C; followed by calcination at 725 °C for 3 h. | Mixtures of WTP sludge and cement and WTS, blast furnace slag and cement were tested for use as pozzolanic material. The WTS and cement mixture resulted in mortar with compressive strength close to the minimum standards required (ABNT NBR 5752 [112]), reaching 89.2% of that of the reference sample. The use of WTS mixed with blast furnace slag resulted in mortar with compressive strength within the required standards. |
| Hemkemeier et al. [78] | Brazil, 2023. | Mortar | Drying in an oven at 110 °C for 24 h. | WTS partially replaced the fine aggregate in the production of repair mortar (3% in mass), resulting in a material that performed similarly to the reference sample in terms of carbonation and chloride penetration tests. The probability of corrosion was delayed by 25% and the corrosion rate of the steel reinforcement was reduced by 70%. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattoso, A.P.; Cunha, S.; Aguiar, J.; Duarte, A.; Lemos, H. Valorization of Water Treatment Sludge for Applications in the Construction Industry: A Review. Materials 2024, 17, 1824. https://doi.org/10.3390/ma17081824
Mattoso AP, Cunha S, Aguiar J, Duarte A, Lemos H. Valorization of Water Treatment Sludge for Applications in the Construction Industry: A Review. Materials. 2024; 17(8):1824. https://doi.org/10.3390/ma17081824
Chicago/Turabian StyleMattoso, Ana Paula, Sandra Cunha, José Aguiar, António Duarte, and Helena Lemos. 2024. "Valorization of Water Treatment Sludge for Applications in the Construction Industry: A Review" Materials 17, no. 8: 1824. https://doi.org/10.3390/ma17081824
APA StyleMattoso, A. P., Cunha, S., Aguiar, J., Duarte, A., & Lemos, H. (2024). Valorization of Water Treatment Sludge for Applications in the Construction Industry: A Review. Materials, 17(8), 1824. https://doi.org/10.3390/ma17081824









