Comparing Electrochemical Passivation and Surface Film Chemistry of 654SMO Stainless Steel and C276 Alloy in Simulated Flue Gas Desulfurization Condensates
Abstract
1. Introduction
2. Experimental
2.1. Materials and Solutions
2.2. Electrochemical Measurements
2.3. Characterization of Passive Film
3. Results and Discussion
3.1. Electrochemical Behavior at Different Immersion Times
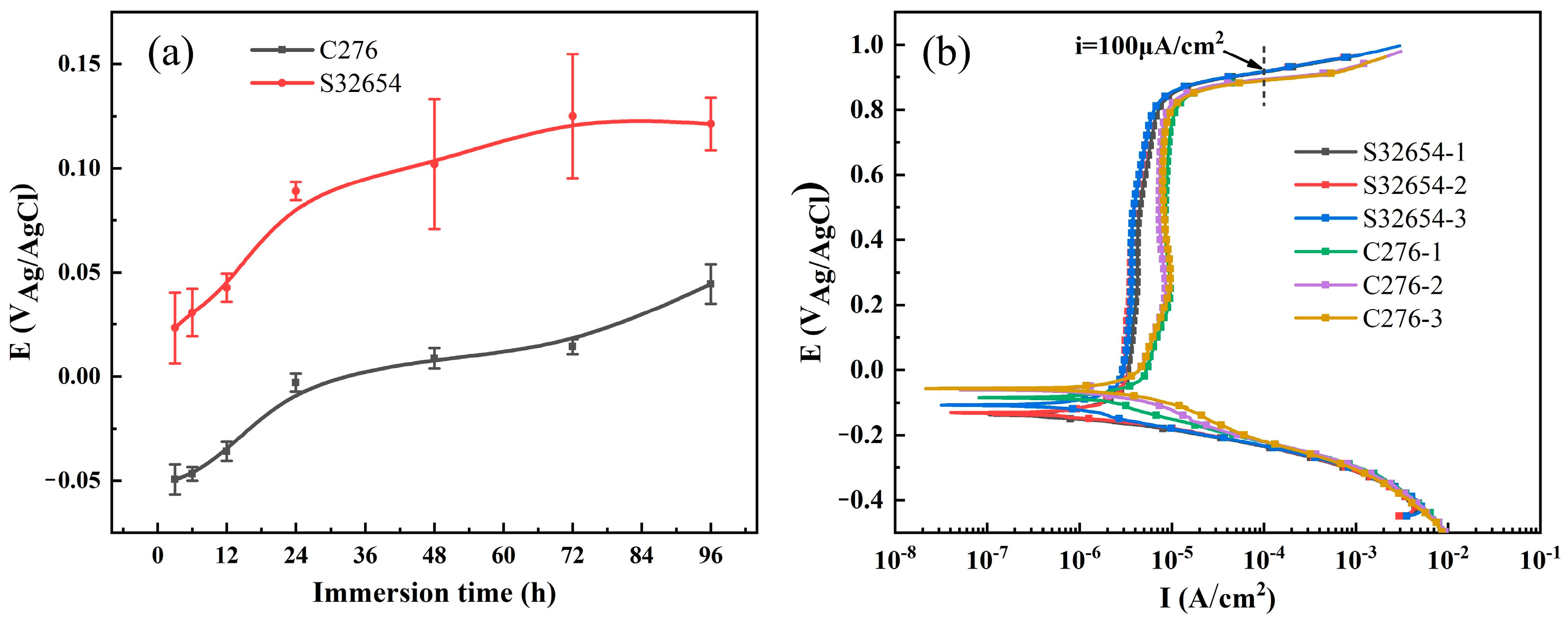
3.1.1. Open Circuit Potential and Potentiodynamic Polarization
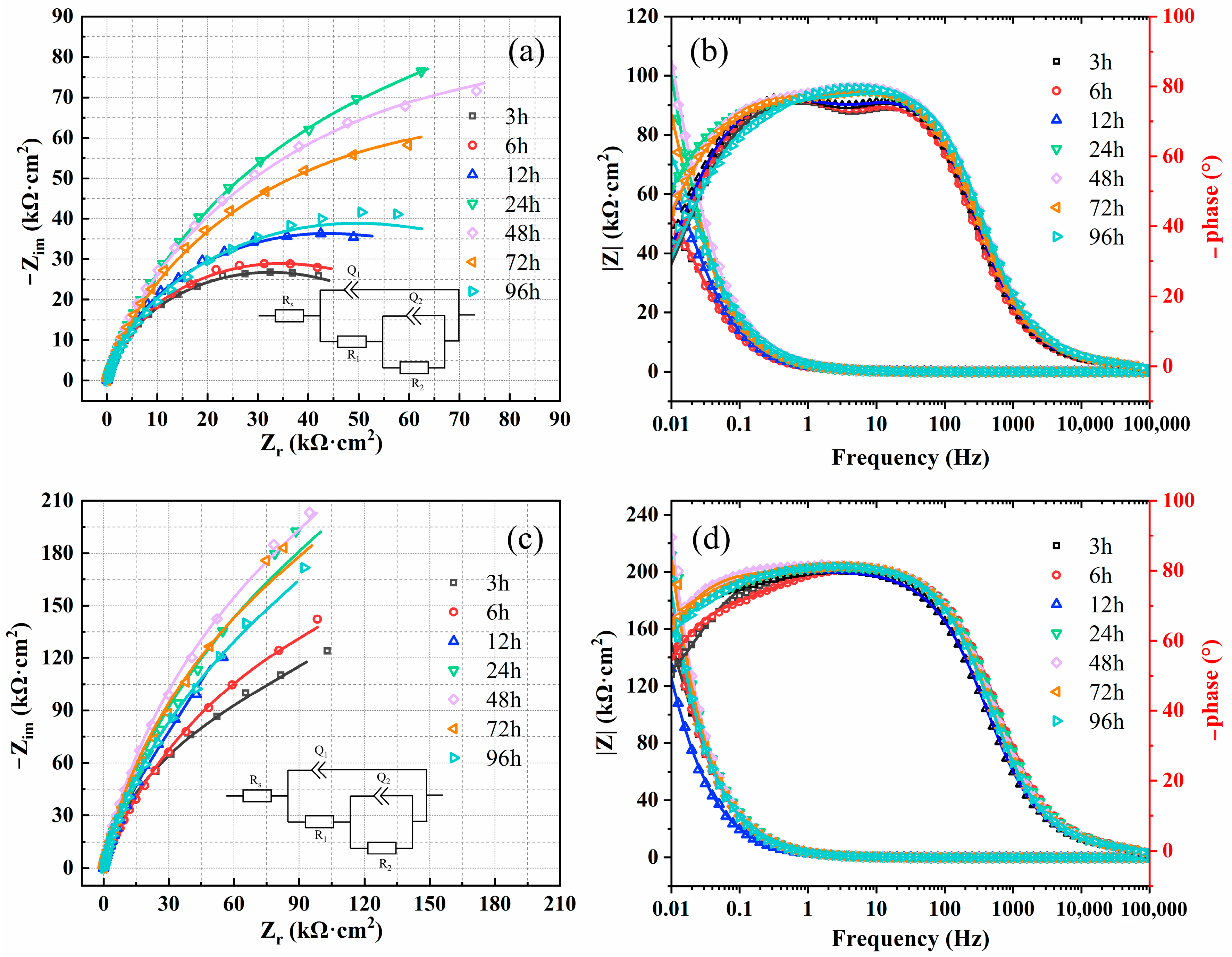
3.1.2. Electrochemical Impedance Spectroscopy
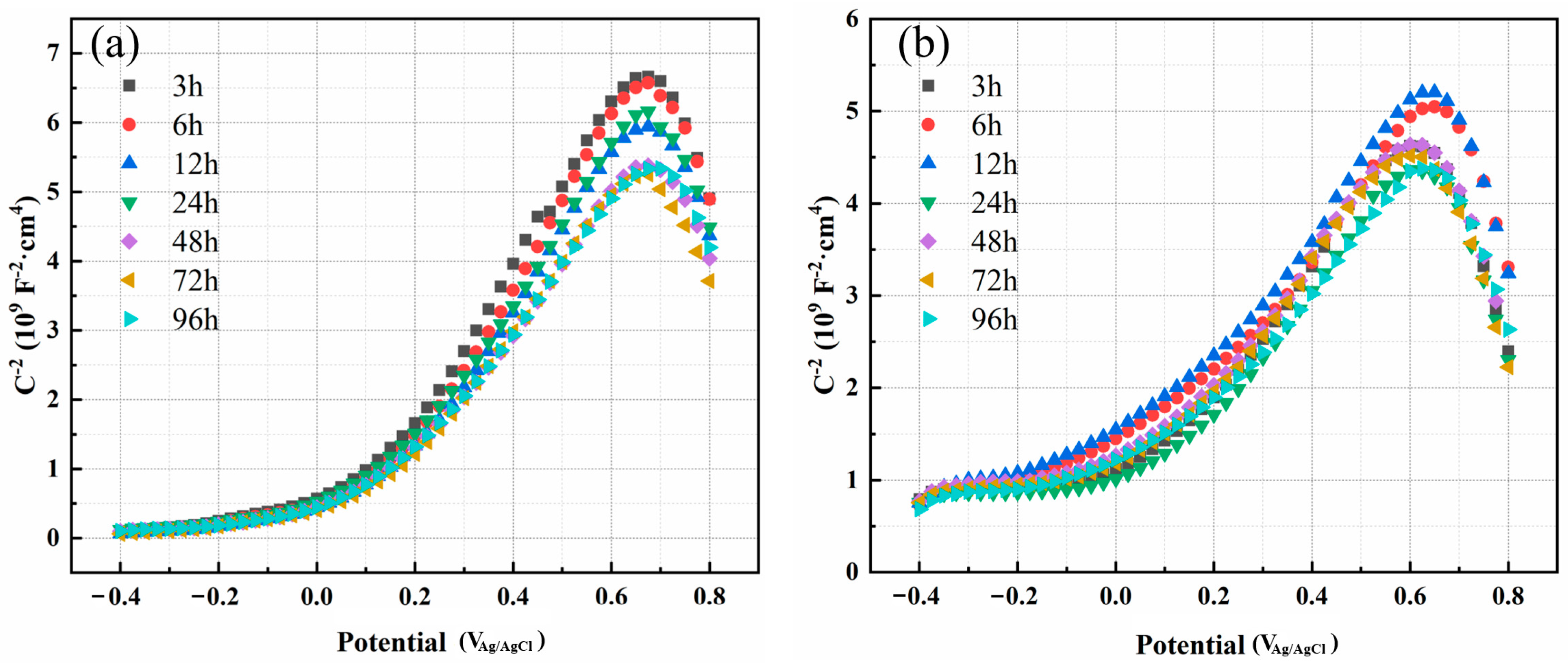
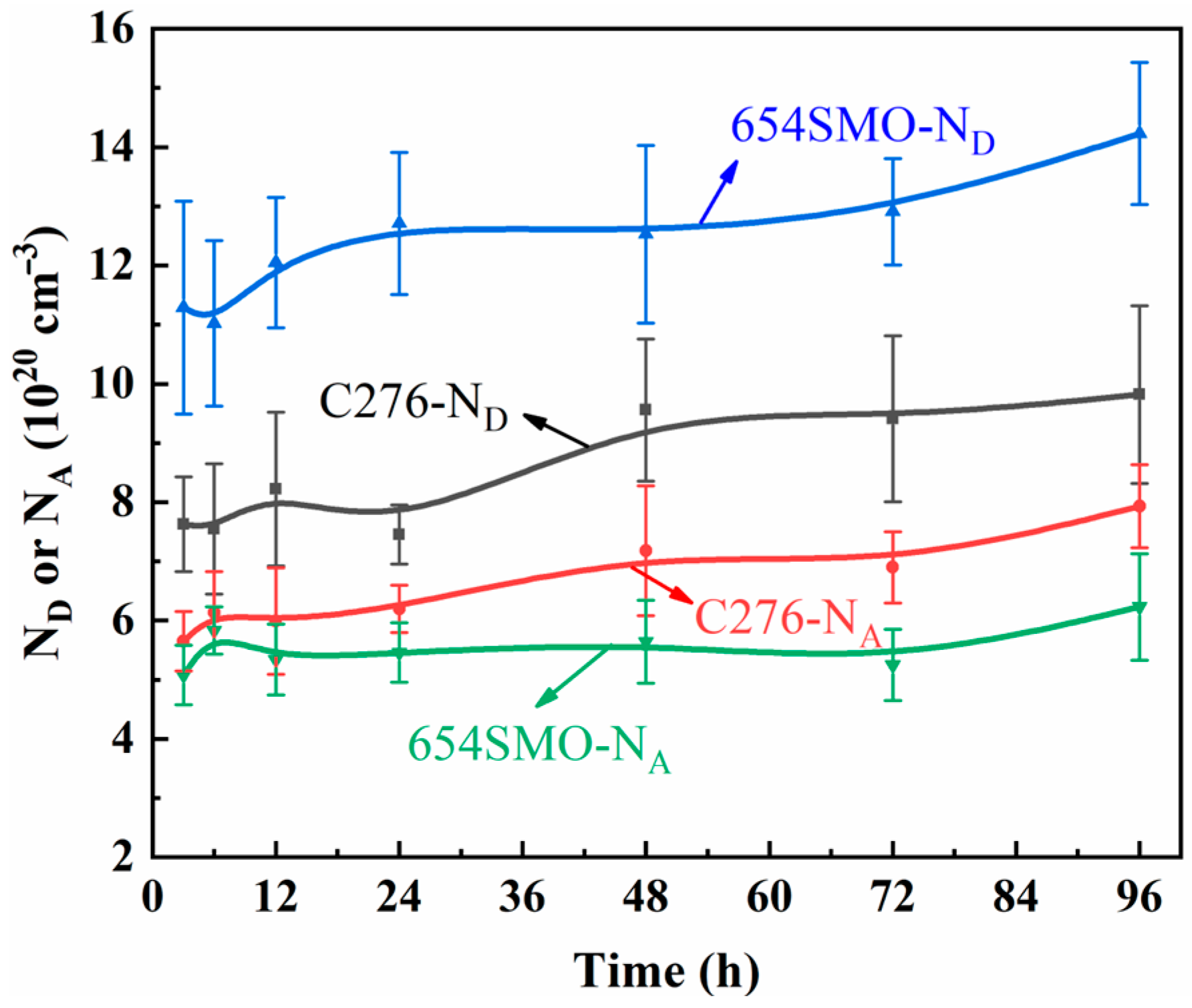
3.1.3. Mott–Schottky Analysis
3.2. Electrochemical Behavior of Anodic Polarization at Different Potentials
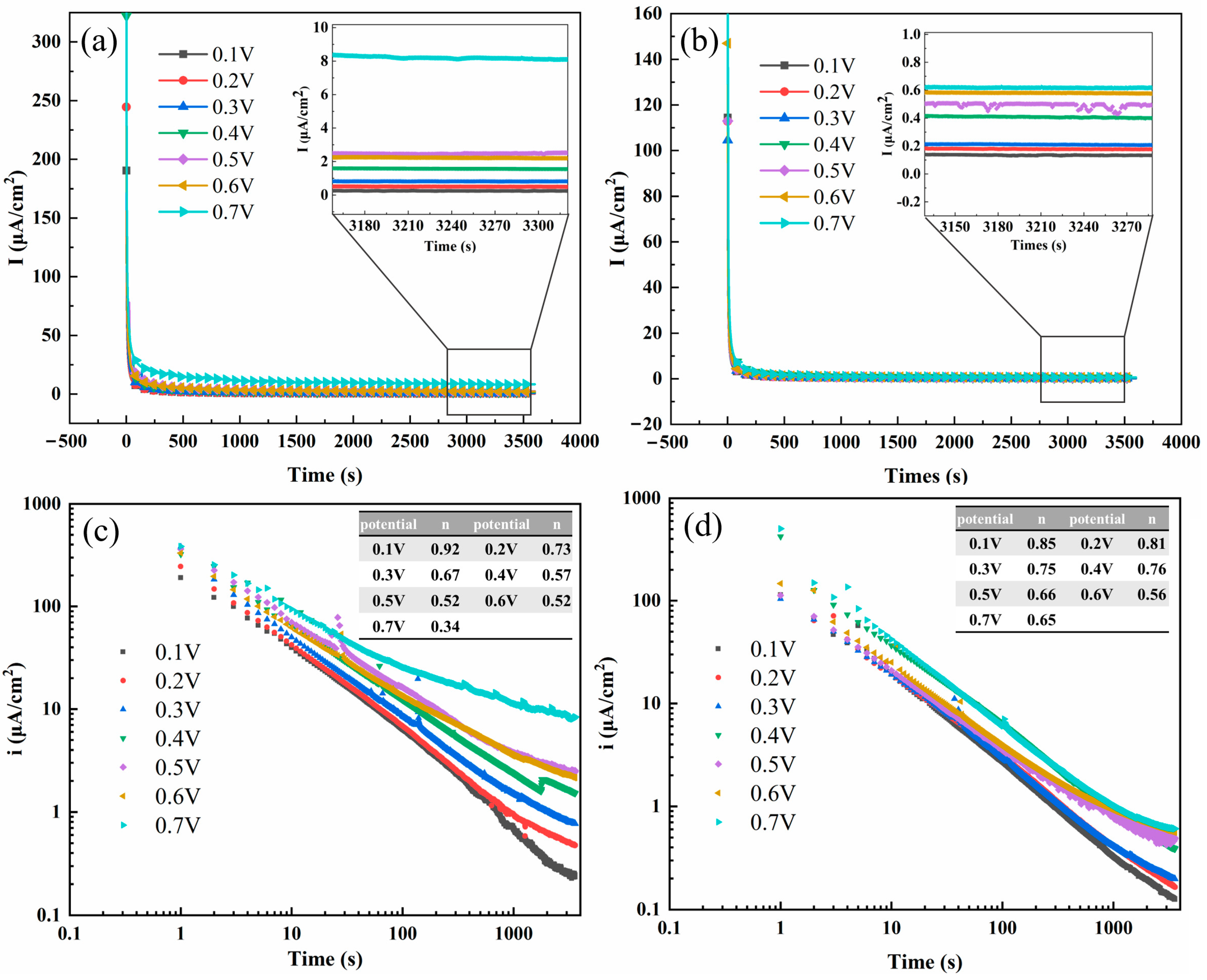
3.2.1. Potentiostatic Polarization Analysis
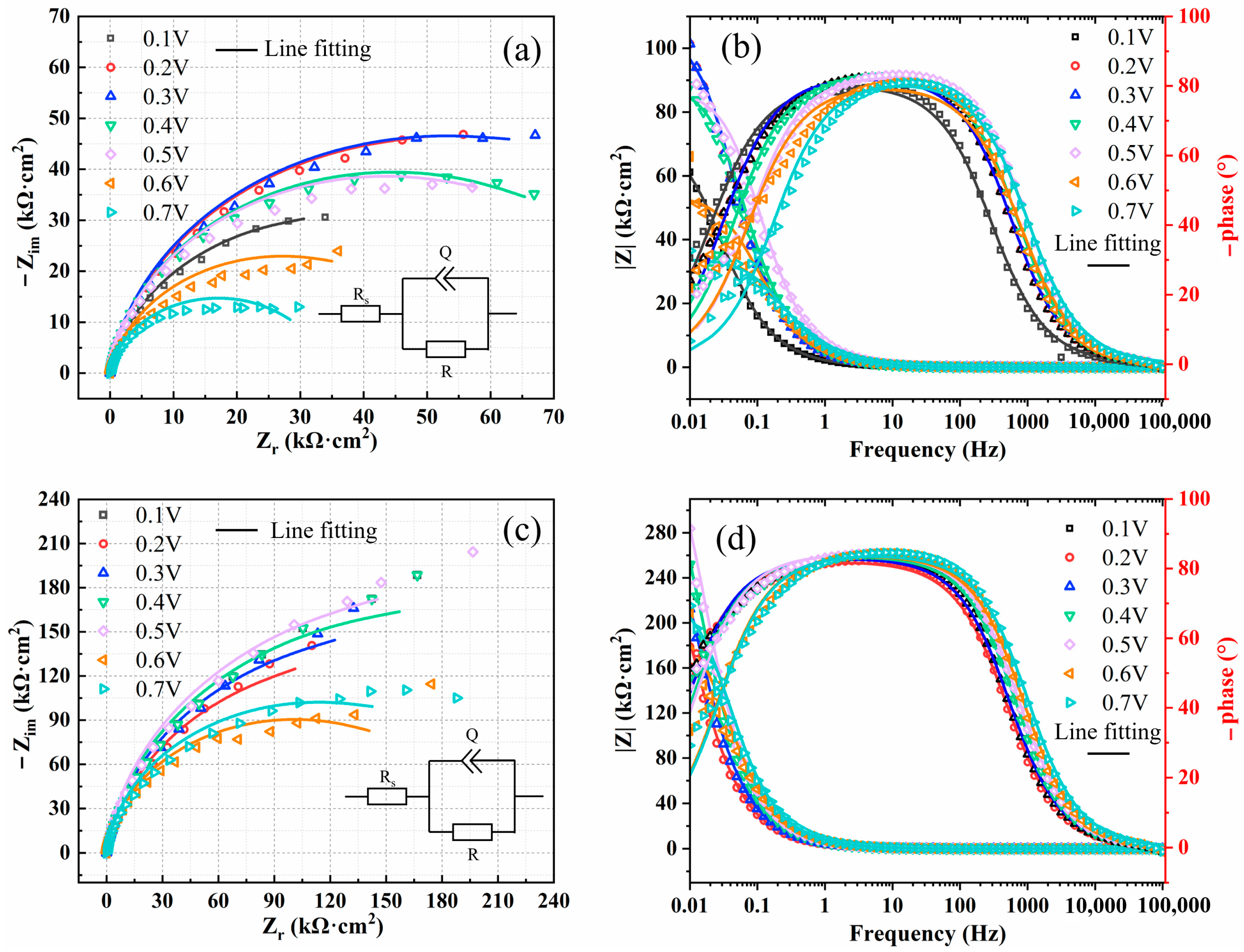
3.2.2. Electrochemical Impedance Spectroscopy
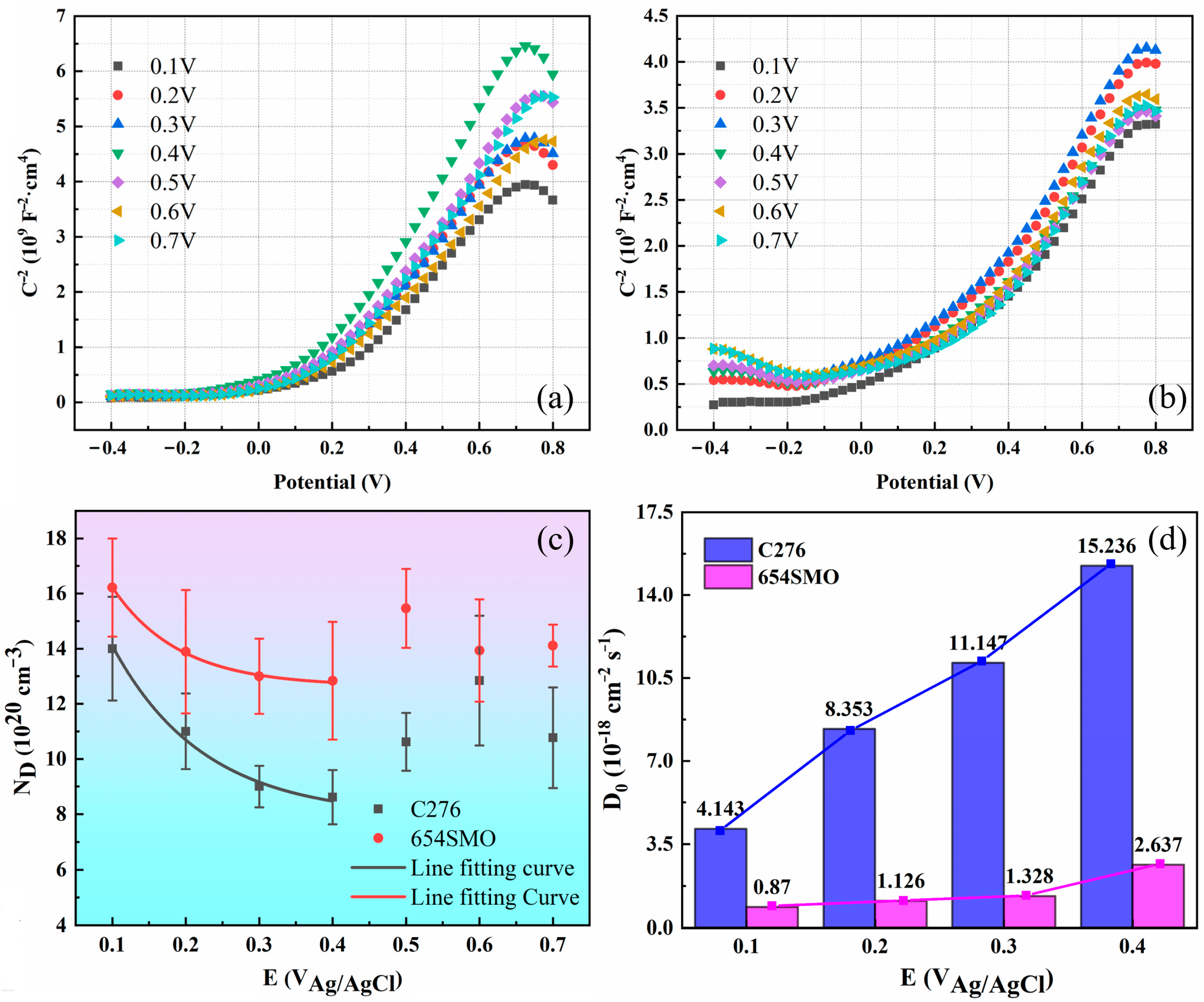
3.2.3. Mott–Schottky Analysis and Point Defect Diffusivity
3.3. Composition and Thickness Analysis of Passive Film
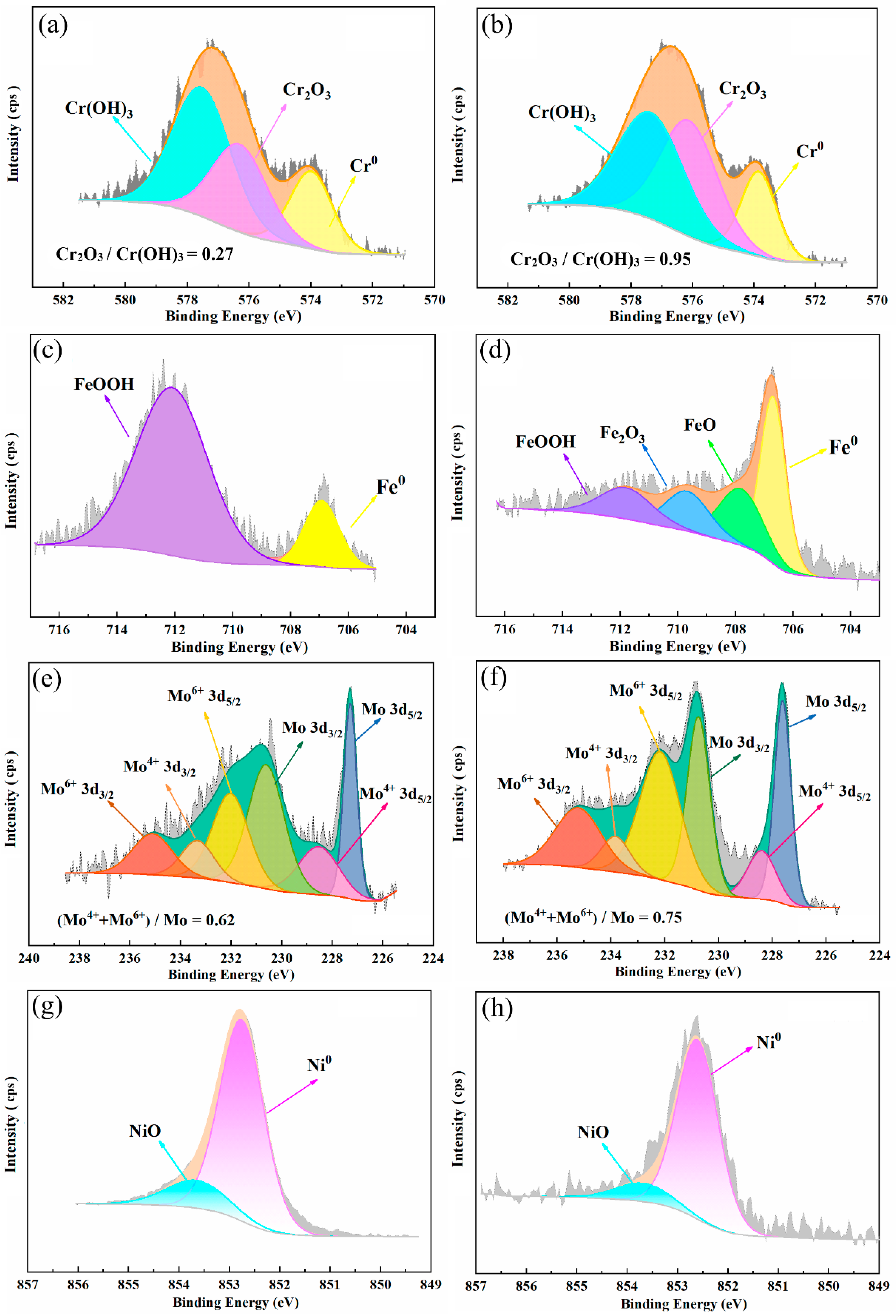
3.3.1. Surface Analysis of the Passive Film
3.3.2. Depth Profiles of the Passive Film
4. Conclusions
- (1)
- The open circuit potential of 654SMO is higher than that of C276. The results of potentiodynamic polarization curves show that the transpassive potential of 654SMO is higher than that of C276, and the passive current density of 654SMO is lower than that of C276.
- (2)
- With the prolongation of immersion time, the electrochemical impedance of the two materials increases first and then decreases. The impendence of 654SMO under different immersion time is greater than C276. The passive film exhibits n-type semiconductor characteristics at 0~0.6 V and p-type at 0.65~0.8 V. The donor density of 654SMO is higher than that of C276, while the acceptor density is lower than that of C276.
- (3)
- The steady current density of 654SMO and C276 increases with the increase in polarization potential from 0.1 V to 0.7 V. With the increase in polarization potential, the impedance of the two materials increases first and then decreases. 654SMO stainless steel has a significantly smaller diffusion coefficient of point defects than C276 alloy.
- (4)
- With increasing sputtering time, the atomic percentage of Cr, Fe, and Ni increases, and the atomic percentage of O decreases for both materials. The total oxide content of passive film formed in 654SMO is higher than that in C276 at different sputtering times.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jansen, P.; Hansen, V.; Jensen, T. Corrosion experience with carbon steel in spray absorption FGD plant. Mater. Corros. 1992, 43, 310–315. [Google Scholar] [CrossRef]
- Dahl, L. Corrosion in flue gas desulfurization plants and other low temperature equipment. Mater. Corros. 1992, 43, 298–304. [Google Scholar] [CrossRef]
- Le, D.P.; Ji, W.S.; Kim, J.G.; Jeong, K.J.; Lee, S.H. Effect of antimony on the corrosion behavior of low-alloy steel for flue gas desulfurization system. Corros. Sci. 2008, 50, 1195–1204. [Google Scholar] [CrossRef]
- Rajendran, N.; Latha, G.; Rajeswari, S. Localised corrosion behaviour of alloys 33 and 24 in simulated flue gas desulphurisation environment. Br. Corros. J. 2002, 37, 276–281. [Google Scholar] [CrossRef]
- Shuangchen, M.; Jin, C.; Kunling, J.; Lan, M.; Sijie, Z.; Kai, W. Environmental influence and countermeasures for high humidity flue gas discharging from power plants. Renew. Sustain. Energy Rev. 2017, 73, 225–235. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, Z.G. Corrosion evaluation of one wet desulfurization equipment flue gas desulfurization unit. Fuel Process. Technol. 2018, 181, 279–293. [Google Scholar] [CrossRef]
- Li, K.; Zeng, Y.; Luo, J.L. Corrosion of duplex stainless steel 2205 in hot flue gas environments produced at advanced oxy-fired pressurized fluidized bed combustion plants. Int. J. Greenh. Gas. Control 2020, 100, 103108. [Google Scholar] [CrossRef]
- Chen, J.R.; Tsai, W.T. In situ corrosion monitoring of Ti–6Al–4V alloy in H2SO4/HCl mixed solution using electrochemical AFM. Electrochim. Acta 2011, 56, 1746–1751. [Google Scholar] [CrossRef]
- Krakowiak, S.; Darowicki, K. Degradation of protective coatings in steel chimneys of flue gas desulfurization systems. Prog. Org. Coating 2018, 117, 141–145. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, H.; Zheng, Y.; Ke, W.; Qiao, Y. Comparison of the corrosion behavior of pure titanium and its alloys in fluoride-containing sulfuric acid. Corros. Sci. 2016, 103, 50–65. [Google Scholar] [CrossRef]
- Darowicki, K.; Krakowiak, S. Durability evaluation of Ni-Cr-Mo super alloys in a simulated scrubbed flue gas environment. Anti-Corros. Methods Mater. 1999, 46, 19–22. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, Z.D.; Chen, S.S.; Liu, C.C. Corrosion Behavior of a Ni-Cr-Mo Alloy Coating Fabricated by Laser Cladding in a Simulated Sulfuric Acid Dew Point Corrosion Environment. Coatings 2020, 10, 849. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, L.; Zhong, M.; Ge, F.; Gao, H.; Man, C.; Liu, C.; Wang, X. Electrochemical behavior and surface characteristics of pure titanium during corrosion in simulated desulfurized flue gas condensates. J. Electrochem. Soc. 2018, 165, 542–561. [Google Scholar] [CrossRef]
- Dou, Y.; Han, S.; Wang, L.; Wang, X.; Cui, Z. Characterization of the passive properties of 254SMO stainless steel in simulated desulfurized flue gas condensates by electrochemical analysis, XPS and ToF-SIMS. Corros. Sci. 2020, 165, 108405. [Google Scholar] [CrossRef]
- Ge, F.; Wang, L.; Dou, Y.; Wei, J.; Cheng, L.; Wang, X.; Cui, Z. Elucidating the passivation kinetics and surface film chemistry of 254SMO stainless steel for chimney construction in simulated desulfurized flue gas condensates. Constr. Build. Mater. 2021, 285, 122905. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, L.; Ni, H.; Hao, W.; Man, C.; Chen, S.; Wang, X.; Liu, Z.; Li, X. Influence of temperature on the electrochemical and passivation behavior of 2507 super duplex stainless steel in simulated desulfurized flue gas condensates. Corros. Sci. 2017, 118, 31–48. [Google Scholar] [CrossRef]
- Cui, Z.; Chen, S.; Dou, Y.; Han, S.; Wang, L. Passivation behavior and surface chemistry of 2507 super duplex stainless steel in artificial seawater: Influence of dissolved oxygen and pH. Corros. Sci. 2019, 150, 218–234. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, Z.; Liu, Q.; Li, Y.; Liu, C. Comparative investigation on corrosion behavior of laser cladding C22 coating, Hastelloy C22 alloy and Ti-6Al-4V alloy in simulated desulfurized flue gas condensates. J. Mater. Res. Technol. 2022, 18, 2194–2207. [Google Scholar] [CrossRef]
- Wang, M.; Wang, L.; Zhao, K.; Liu, Y.; Cui, Z. Understanding the passivation behavior and film chemistry of four corrosion-resistant alloys in the simulated flue gas condensates. Mater. Today Commun. 2022, 31, 103567. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, K.; Hughes, R.; Luo, J.L. Corrosion mechanisms and materials selection for the construction of flue gas component in advanced heat and power systems. Ind. Eng. Chem. Res. 2017, 56, 14141–14154. [Google Scholar] [CrossRef]
- Dansana, B.; Mahapatra, T.R.; Sahu, P.; Sahu, S.K. Tribological and electrochemical corrosion performanceanalysis of Hastelloy C276 under different loading conditions. Mater. Today Proc. 2020, 24, 1434–1441. [Google Scholar] [CrossRef]
- Salah, M.B.; Sabot, R.; Refait, P.; Liascukiene, I.; Méthivier, C.; Landoulsi, J.; Dhouibi, L.; Jeannin, M. Passivation behaviour of stainless steel (UNS N-08028) in industrial or simplified phosphoric acid solutions at different temperatures. Corros. Sci. 2015, 99, 320–332. [Google Scholar] [CrossRef]
- Luo, H.; Su, H.; Dong, C.; Li, X. Passivation and electrochemical behavior of 316L stainless steel in chlorinated simulated concrete pore solution. Appl. Surf. Sci. 2017, 400, 38–48. [Google Scholar] [CrossRef]
- Boissy, C.; Ter-Ovanessian, B.; Mary, N.; Normand, B. Correlation between predictive and descriptive models to characterize the passive film-Study of pure chromium by electrochemical impedance spectroscopy. Electrochim. Acta 2015, 274, 430–437. [Google Scholar] [CrossRef]
- Büchler, M.; Schmuki, P.; Böhni, H. A light reflectance technique for thickness measurements of passive films. Electrochim. Acta. 1998, 43, 635–637. [Google Scholar]
- Hamadou, L.; Kadri, A.; Benbrahim, N. Impedance investigation of thermally formed oxide films on AISI 304L stainless steel. Corros. Sci. 2010, 52, 859–864. [Google Scholar] [CrossRef]
- Freire, L.; Catarino, M.A.; Godinho, M.I.; Ferreira, M.J. Electrochemical and analytical investigation of passive films formed on stainless steels in alkaline media. Cem. Concr. Compos. 2012, 34, 1075–1081. [Google Scholar] [CrossRef]
- Feng, X.; Lu, X.; Zuo, Y.; Chen, D. The passive behaviour of 304 stainless steels insaturated calcium hydroxide solution under different deformation. Corros. Sci. 2014, 82, 347–355. [Google Scholar] [CrossRef]
- Huang, J.; Wu, X.; Han, E.H. Electrochemical properties and growth mechanism of passive films on Alloy 690 in high-temperature alkaline environments. Corros. Sci. 2010, 52, 3444–3452. [Google Scholar] [CrossRef]
- Macdonald, D.D. The history of the point defect model for the passive state: A brief review of film growth aspects. Electrochim. Acta 2011, 56, 1761–1772. [Google Scholar] [CrossRef]
- Fernández-Domene, R.M.; Blasco-Tamarit, E.; García-García, D.M.; GarcíaAntón, J. Repassivation of the damage generated by cavitation on UNS N08031 in a LiBr solution by means of electrochemical techniques and Confocal Laser Scanning Microscopy. Corros. Sci. 2010, 52, 3453–3464. [Google Scholar] [CrossRef]
- Lakatos-Varsányi, M.; Falkenberg, F.; Olefjord, I. The influence of phosphate on repassivation of 304 stainless steel in neutral chloride solution. Electrochim. Acta 1998, 43, 187–197. [Google Scholar] [CrossRef]
- Lee, J.B. Effects of alloying elements, Cr, Mo and N on repassivation characteristics of stainless steels using the abrading electrode technique. Mater. Chem. Phys. 2006, 99, 224–234. [Google Scholar] [CrossRef]
- Orazem, M.E.; Frateur, I.; Tribollet, B.; Vivier, V.; Marcelin, S.; Pébère, N.; Bunge, A.L.; White, E.A.; Riemer, D.P.; Musiani, M. Dielectric properties of materials showing constant-phase-element (CPE) impedance response. J. Electrochem. Soc. 2013, 160, 215–225. [Google Scholar] [CrossRef]
- Hsu, C.; Mansfeld, F. Technical note: Concerning the conversion of the constant phase element parameter Y0 into a capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Constant-phase-element behavior caused by resistivity distributions in films II. Applications. J. Electrochem. Soc. 2010, 157, 458–463. [Google Scholar] [CrossRef]
- Bojinov, M. The ability of a surface charge approach to describe barrier film growth on tungsten in acidic solutions. Electrochim. Acta 1997, 42, 3489–3498. [Google Scholar] [CrossRef]
- Sikora, E.; Sikora, J.; Macdonald, D.D. A new method for estimating the diffusivities of vacancies in passive films. Electrochim. Acta 1996, 41, 783–789. [Google Scholar] [CrossRef]
- Meng, G.; Li, Y.; Shao, Y.; Zhang, T.; Wang, Y.; Wang, F. Effect of Cl− on the Properties of the Passive Films Formed on 316L Stainless Steel in Acidic Solution. J. Mater. Sci. Technol. 2014, 30, 253–258. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, B.; Ren, Y.; Yang, K. A self-healing stainless steel: Role of nitrogen in eliminating detrimental effect of cold working on pitting corrosion resistance. Corros. Sci. 2018, 145, 55–66. [Google Scholar] [CrossRef]
- Baba, H.; Katada, Y. Effects of nitrogen on crevice corrosion in austenitic stainless steel. Corros. Sci. 2006, 48, 2510–2524. [Google Scholar] [CrossRef]
- Baba, H.; Kodama, T.; Katada, Y. Role of nitrogen on the corrosion behavior of austenitic stainless steels. Corros. Sci. 2002, 44, 2393–2407. [Google Scholar] [CrossRef]
- BenSalah, M.; Sabot, R.; Triki, E.; Dhouibi, L.; Refait, P.; Jeanin, M. Passivity of Sanicro28 (UNS N-08028) stainless steel in polluted phosphoric acid at different temperatures studied by electrochemical impedance spectroscopy and Mott-Schottky analysis. Corros. Sci. 2014, 86, 61–70. [Google Scholar] [CrossRef]
- Iken, H.; Basseguy, R.; Guenbour, A.; Bachir, A. Classic and local analysis of corrosion behaviour of graphite and stainless steels in polluted phosphoric acid. Electrochim. Acta 2007, 52, 2580–2587. [Google Scholar] [CrossRef]
- Kathleen, F.; Stephen, J.; Daniel, K.; Angela, Y.; Keren, M. Passivation of a corrosion resistant high entropy alloy in non-oxidizing sulfate solutions. Acta Mater. 2019, 164, 362–376. [Google Scholar]

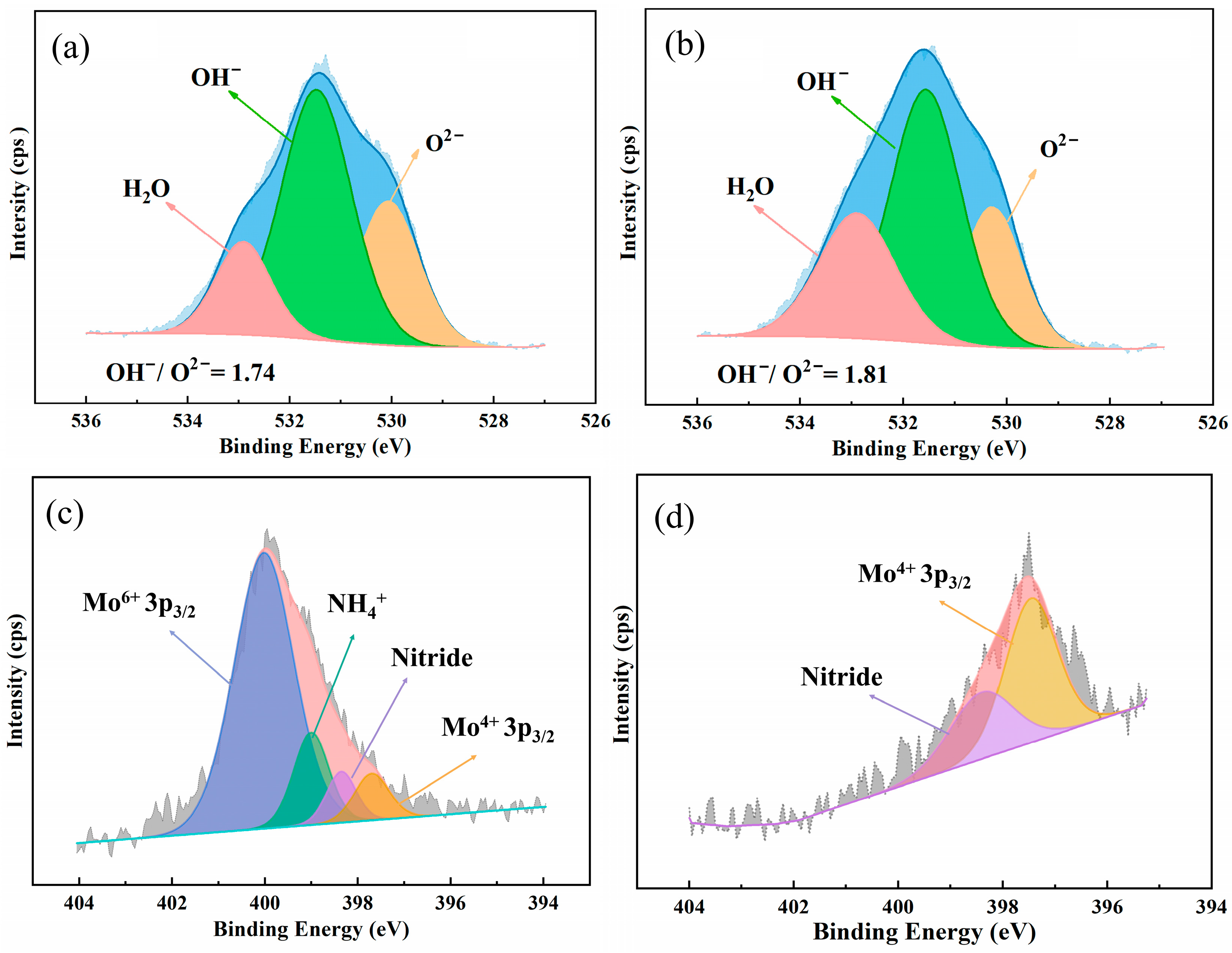
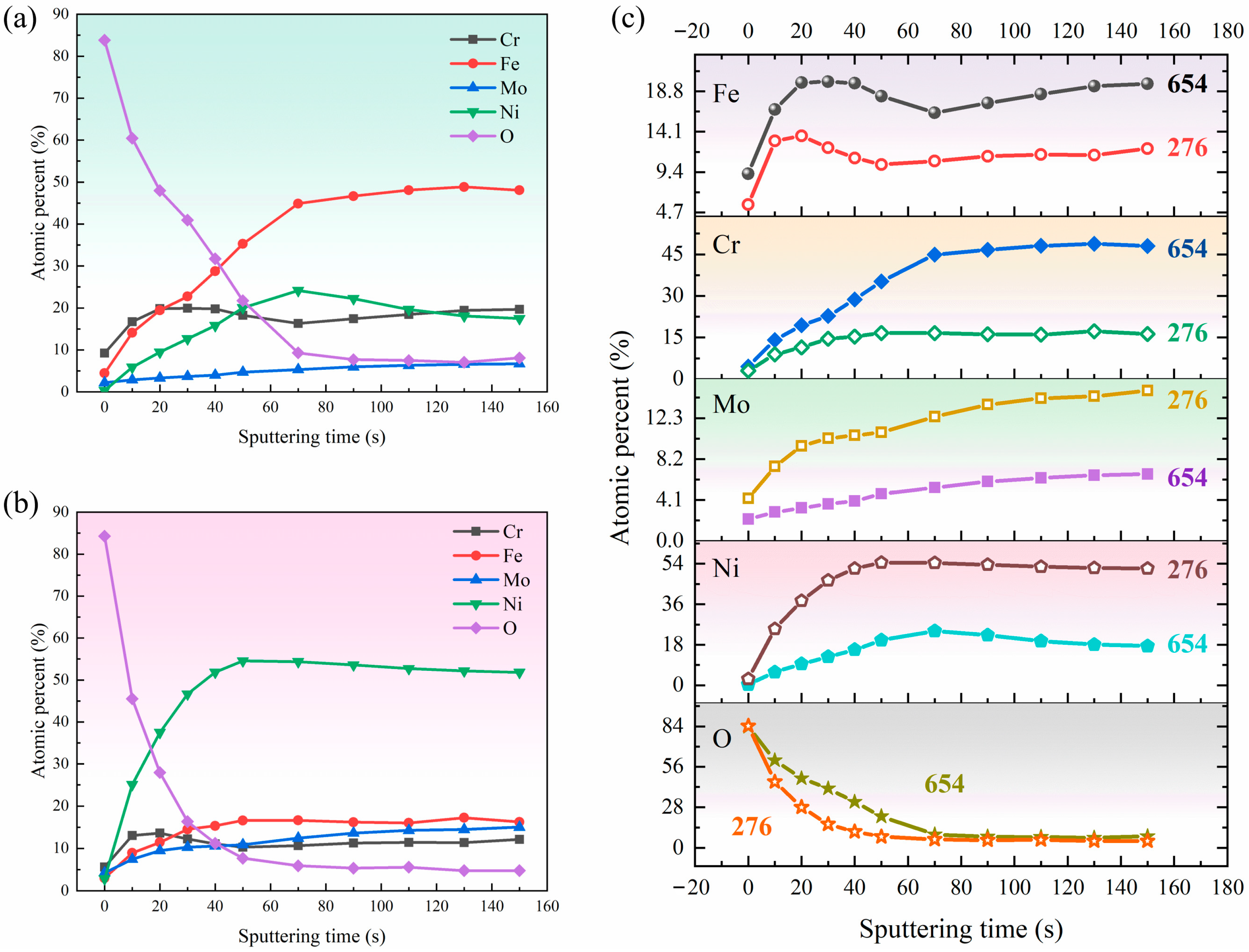

| Material | Fe | Ni | Cr | Mo | Cu | Mn | Co | Si | N | W | PREN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 654SMO | 42.25 | 22.03 | 24.56 | 7.02 | 0.33 | 3.19 | - | 0.071 | 0.509 | - | 56 |
| C276 | 5.61 | 57.57 | 15.76 | 15.57 | - | 0.53 | 1.53 | 0.04 | - | 3.38 | 73 |
| Agents | H2SO4 (mL) | HNO3 (mL) | HCl (mL) | Na2SO4 (g) | H2O (L) | pH |
|---|---|---|---|---|---|---|
| Content | 10 | 9 | 3 | 1.88 | 4 | 1.1 |
| Materials | Ecorr (VAg/AgCl) | ip (μA·cm−2) | Etr (VAg/AgCl) |
|---|---|---|---|
| 654SMO | −0.123 ± 0.015 | 4.12 ± 0.35 | 0.917 ± 0.002 |
| C276 | −0.067 ± 0.014 | 8.30 ± 0.58 | 0.891 ± 0.017 |
| Rs (Ω·cm2) | Q1 10−6 (Ω·cm−2·sn) | n1 | R1 (kΩ·cm2) | Q2 10−6 (Ω·cm−2·sn) | n2 | R2 (kΩ·cm2) | χ2 (10−4) | ||
|---|---|---|---|---|---|---|---|---|---|
| 276 | 3 h | 13.49 | 75.68 | 0.92 | 1.47 | 40.35 | 0.81 | 63.66 | 1.57 |
| 6 h | 13.01 | 85.77 | 0.91 | 1.79 | 36.84 | 0.83 | 67.58 | 1.48 | |
| 12 h | 12.83 | 75.64 | 0.92 | 2.28 | 31.65 | 0.82 | 85.51 | 2.52 | |
| 24 h | 13.13 | 72.78 | 0.90 | 2.03 | 17.76 | 0.70 | 205.4 | 0.77 | |
| 48 h | 12.74 | 65.28 | 0.91 | 2.11 | 14.35 | 0.77 | 174.6 | 0.99 | |
| 72 h | 13.40 | 72.95 | 0.91 | 8.52 | 19.08 | 0.68 | 152.3 | 2.24 | |
| 96 h | 13.27 | 55.66 | 0.93 | 3.03 | 24.03 | 0.71 | 87.14 | 9.33 | |
| 654 | 3 h | 12.67 | 43.94 | 0.91 | 65.31 | 16.73 | 0.75 | 3830 | 1.04 |
| 6 h | 12.84 | 50.31 | 0.91 | 228.7 | 19.14 | 0.81 | 4374 | 5.51 | |
| 12 h | 12.04 | 73.94 | 0.89 | 431.2 | 41.35 | 0.83 | 5135 | 3.80 | |
| 24 h | 12.98 | 42.98 | 0.92 | 102.4 | 10.65 | 0.68 | 6358 | 5.04 | |
| 48 h | 12.46 | 38.88 | 0.91 | 305.4 | 20.97 | 0.94 | 7784 | 1.59 | |
| 72 h | 13.17 | 47.06 | 0.91 | 245.7 | 18.05 | 0.81 | 6183 | 7.85 | |
| 96 h | 13.27 | 50.84 | 0.91 | 254.1 | 22.74 | 0.88 | 4753 | 1.39 |
| Time | C276 | 654SMO | ||
|---|---|---|---|---|
| ND (1020 cm−3) | NA (1020 cm−3) | ND (1020 cm−3) | NA (1020 cm−3) | |
| 3 h | 7.63 | 5.65 | 11.29 | 5.08 |
| 6 h | 7.55 | 6.13 | 11.02 | 5.83 |
| 12 h | 8.22 | 5.99 | 12.05 | 5.34 |
| 24 h | 7.45 | 6.20 | 12.71 | 5.46 |
| 48 h | 9.56 | 7.18 | 12.53 | 5.64 |
| 72 h | 9.41 | 6.90 | 12.91 | 5.25 |
| 96 h | 9.82 | 7.93 | 14.23 | 6.23 |
| 0.1 V | 0.2 V | 0.3 V | 0.4 V | 0.5 V | 0.6 V | 0.7 V | |
|---|---|---|---|---|---|---|---|
| C276 | 0.307 ± 0.112 | 0.619 ± 0.114 | 0.826 ± 0.048 | 1.129 ± 0.382 | 1.644 ± 0.784 | 1.588 ± 0.5316 | 5.490 ± 1.3440 |
| 654SMO | 0.129 ± 0.016 | 0.167 ± 0.011 | 0.197 ± 0.009 | 0.391 ± 0.028 | 0.495 ± 0.017 | 0.569 ± 0.151 | 0.641 ± 0.134 |
| Material | Potential (V) | Rs (Ω·cm2) | Q10−6 (Ω·cm−2·sn) | n | R (kΩ·cm2) | Ceff (μF·cm−2) | χ2 (10−3) |
|---|---|---|---|---|---|---|---|
| C276 | 0.1 | 13.21 | 87.22 | 0.8991 | 71.96 | 192.3933 | 1.55 |
| 0.2 | 13.52 | 54.54 | 0.9161 | 106.3 | 99.8503 | 1.63 | |
| 0.3 | 12.44 | 41.88 | 0.9224 | 105.9 | 70.8874 | 1.61 | |
| 0.4 | 11.83 | 35.22 | 0.9263 | 89.18 | 56.9153 | 1.66 | |
| 0.5 | 13.13 | 23.09 | 0.9269 | 88.87 | 36.2362 | 5.05 | |
| 0.6 | 13.46 | 36.76 | 0.8968 | 54.37 | 75.0651 | 2.58 | |
| 0.7 | 12.5 | 27.3 | 0.9097 | 35.91 | 48.7085 | 4.50 | |
| 654SMO | 0.1 | 12.25 | 68.69 | 0.8921 | 179.5 | 155.1235 | 1.74 |
| 0.2 | 13.01 | 49.71 | 0.9151 | 305.9 | 90.6178 | 1.34 | |
| 0.3 | 12.66 | 42.47 | 0.9258 | 339.8 | 70.2950 | 1.37 | |
| 0.4 | 12.51 | 33.97 | 0.9312 | 369.5 | 53.1235 | 1.80 | |
| 0.5 | 12.39 | 29.53 | 0.9365 | 396.3 | 44.0628 | 1.72 | |
| 0.6 | 12.04 | 26.68 | 0.9367 | 185.8 | 39.4085 | 3.37 | |
| 0.7 | 11.87 | 23.07 | 0.9357 | 227.6 | 33.9273 | 3.42 |
| Constituents | 654SMO | C276 | ||
|---|---|---|---|---|
| Binding Energy (eV) | FWHM (eV) | Binding Energy (eV) | FWHM (eV) | |
| Fe | 706.7 | 1.05 | 706.9 | 1.5 |
| FeO | 707.9 | 1.86 | - | - |
| Fe2O3 | 709.7 | 1.86 | - | - |
| FeOOH | 711.9 | 2.25 | 712.1 | 3.06 |
| Cr | 573.8 | 1.41 | 573.9 | 1.77 |
| Cr2O3 | 576.1 | 2.28 | 576.3 | 2.09 |
| Cr(OH)3 | 577.3 | 2.61 | 577.5 | 2.13 |
| Ni | 852.6 | 1 | 852.7 | 1.07 |
| NiO | 853.6 | 1.34 | 853.6 | 0.85 |
| Mo 3d5/2 | 227.3 | 0.63 | 227.6 | 0.75 |
| Mo4+ 3d5/2 | 228.5 | 1.96 | 228.4 | 1.3 |
| Mo 3d3/2 | 230.6 | 1.75 | 230.7 | 1.03 |
| Mo6+ 3d5/2 | 232.1 | 1.76 | 232.2 | 1.84 |
| Mo4+ 3d3/2 | 233.3 | 1.58 | 233.8 | 1.31 |
| Mo6+ 3d3/2 | 235.1 | 1.92 | 235.2 | 2.09 |
| O2− | 530.1 | 0.58 | 530.3 | 1.39 |
| OH− | 531.5 | 1 | 531.6 | 1.68 |
| H2O | 532.9 | 0.37 | 532.8 | 1.82 |
| Mo6+ 3p3/2 | 400.0 | 1.58 | ||
| Mo4+ 3p3/2 | 397.7 | 0.92 | ||
| Nitride | 398.4 | 0.78 | - | - |
| 399.0 | 0.93 | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, L.; Cheng, Y.; Zhang, H.; Yuan, X.; Li, F. Comparing Electrochemical Passivation and Surface Film Chemistry of 654SMO Stainless Steel and C276 Alloy in Simulated Flue Gas Desulfurization Condensates. Materials 2024, 17, 1827. https://doi.org/10.3390/ma17081827
Liao L, Cheng Y, Zhang H, Yuan X, Li F. Comparing Electrochemical Passivation and Surface Film Chemistry of 654SMO Stainless Steel and C276 Alloy in Simulated Flue Gas Desulfurization Condensates. Materials. 2024; 17(8):1827. https://doi.org/10.3390/ma17081827
Chicago/Turabian StyleLiao, Luhai, Yifan Cheng, He Zhang, Xuwen Yuan, and Fengguang Li. 2024. "Comparing Electrochemical Passivation and Surface Film Chemistry of 654SMO Stainless Steel and C276 Alloy in Simulated Flue Gas Desulfurization Condensates" Materials 17, no. 8: 1827. https://doi.org/10.3390/ma17081827
APA StyleLiao, L., Cheng, Y., Zhang, H., Yuan, X., & Li, F. (2024). Comparing Electrochemical Passivation and Surface Film Chemistry of 654SMO Stainless Steel and C276 Alloy in Simulated Flue Gas Desulfurization Condensates. Materials, 17(8), 1827. https://doi.org/10.3390/ma17081827






