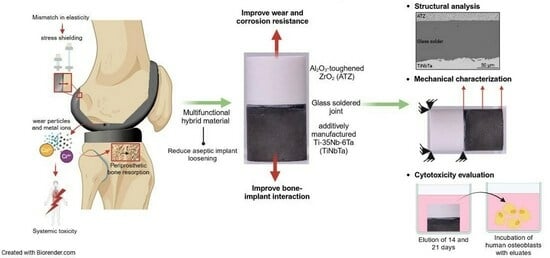
Multifunctional Hybrid Material for Endoprosthetic Implants Based on Alumina-Toughened Zirconia Ceramics and Additively Manufactured TiNbTa Alloys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Manufacturing of the Hybrid Material
2.2. Shear Testing, Artificial Aging, and Fracture Analysis
2.3. Biological Characterization
2.4. Biomechanical Characterization of Functional Demonstrators
2.4.1. Demonstrator Manufacturing
2.4.2. Biomechanical Characterization
2.5. Statistical Analysis
3. Results
3.1. Structural, Chemical, and Mechanical Characterization
3.2. Biological Characterization
3.3. Biomechanical Characterization of the Demonstrator
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B

References
- Lewis, P.L.; Robertsson, O.; Graves, S.E.; Paxton, E.W.; Prentice, H.A.; W-Dahl, A. Variation and trends in reasons for knee replacement revision: A multi-registry study of revision burden. Acta Orthop. 2021, 92, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.B.; Gallo, J. Periprosthetic Osteolysis: Mechanisms, Prevention and Treatment. J. Clin. Med. 2019, 8, 2091. [Google Scholar] [CrossRef]
- Järvenpää, J.; Soininvaara, T.; Kettunen, J.; Miettinen, H.; Kröger, H. Changes in bone mineral density of the distal femur after total knee arthroplasty: A 7-year DEXA follow-up comparing results between obese and nonobese patients. Knee 2014, 21, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Jonitz-Heincke, A.; Sellin, M.-L.; Seyfarth, A.; Peters, K.; Mueller-Hilke, B.; Fiedler, T.; Bader, R.; Klinder, A. Analysis of Cellular Activity Short-Term Exposure to Cobalt and Chromium Ions in Mature Human Osteoblasts. Materials 2019, 12, 2771. [Google Scholar] [CrossRef] [PubMed]
- Sansone, V.; Pagani, D.; Melato, M. The effects on bone cells of metal ions released from orthopaedic implants. A review. Clin. Cases Miner. Bone Metab. 2013, 10, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Scharf, B.; Clement, C.C.; Zolla, V.; Perino, G.; Yan, B.; Elci, S.G.; Purdue, E.; Goldring, S.; Macaluso, F.; Cobelli, N.; et al. Molecular analysis of chromium and cobalt-related toxicity. Sci. Rep. 2014, 4, 5729. [Google Scholar] [CrossRef] [PubMed]
- Crutsen, J.R.W.; Koper, M.C.; Jelsma, J.; Heymans, M.; Heyligers, I.C.; Grimm, B.; Mathijssen, N.M.C.; Schotanus, M.G.M. Prosthetic hip-associated cobalt toxicity: A systematic review of case series and case reports. EFORT Open Rev. 2022, 7, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Glaß, H.; Jonitz-Heincke, A.; Petters, J.; Lukas, J.; Bader, R.; Hermann, A. Corrosion Products from Metallic Implants Induce ROS and Cell Death in Human Motoneurons In Vitro. J. Funct. Biomater. 2023, 14, 392. [Google Scholar] [CrossRef]
- Costa, B.C.; Tokuhara, C.K.; Rocha, L.A.; Oliveira, R.C.; Lisboa-Filho, P.N.; Costa Pessoa, J. Vanadium ionic species from degradation of Ti-6Al-4V metallic implants: In vitro cytotoxicity and speciation evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 730–739. [Google Scholar] [CrossRef]
- Crapper, D.R.; Krishnan, S.S.; Dalton, A.J. Brain aluminum distribution in Alzheimer’s disease and experimental neurofibrillary degeneration. Science 1973, 180, 511–513. [Google Scholar] [CrossRef]
- Gomes, C.C.; Moreira, L.M.; Santos, V.J.S.V.; Ramos, A.S.; Lyon, J.P.; Soares, C.P.; Santos, F.V. Assessment of the genetic risks of a metallic alloy used in medical implants. Genet. Mol. Biol. 2011, 34, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Du Ro, H.; Han, H.-S.; Lee, M.C. Titanium Alloy Knee Implant Is Associated with Higher Bone Density over Cobalt Chromium: A Prospective Matched-Pair Case-Control Study. Clin. Orthop. Surg. 2023, 15, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Bahraminasab, M.; Arab, S.; Doostmohammadi, N. Cytotoxicity and Ion Release of Functionally Graded Al2O3- Ti Orthopedic Biomaterial. J. Biomim. Biomater. Biomed. Eng. 2022, 54, 103–118. [Google Scholar] [CrossRef]
- Bahraminasab, M.; Arab, S.; Ghaffari, S. Osteoblastic cell response to Al2O3-Ti composites as bone implant materials. Bioimpacts 2022, 12, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Bahraminasab, M.; Arab, S.; Safari, M.; Talebi, A.; Kavakebian, F.; Doostmohammadi, N. In vivo performance of Al2O3-Ti bone implants in the rat femur. J. Orthop. Surg. Res. 2021, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Bahraminasab, M.; Bozorg, M.; Ghaffari, S.; Kavakebian, F. Electrochemical corrosion of Ti-Al2O3 biocomposites in Ringer’s solution. J. Alloys Compd. 2019, 777, 34–43. [Google Scholar] [CrossRef]
- Bahraminasab, M.; Ghaffari, S.; Eslami-Shahed, H. Al2O3-Ti functionally graded material prepared by spark plasma sintering for orthopaedic applications. J. Mech. Behav. Biomed. Mater. 2017, 72, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bandyopadhyay, A. Direct fabrication of compositionally graded Ti-Al2O3 multi-material structures using Laser Engineered Net Shaping. Addit. Manuf. 2018, 21, 104–111. [Google Scholar] [CrossRef]
- Moayedee, Y.; Nikzad, L.; Majidian, H. Exploration into the microstructural, mechanical, and biological characteristics of the functionally graded 3Y-TZP/Ti6Al4V system as a potential material for dental implants. J. Mech. Behav. Biomed. Mater. 2024, 151, 106380. [Google Scholar] [CrossRef]
- Sun, Q.; Yang, L.; Yang, W.; Ji, H.; Li, M.; Li, Y. Microstructure evolution and bonding mechanism of ZrO2 ceramic and Ti-6Al-4V alloy joints brazed by Bi2O3-B2O3-ZnO glass paste. J. Eur. Ceram. Soc. 2022, 42, 5953–5963. [Google Scholar] [CrossRef]
- Mick, E.; Tinschert, J.; Mitrovic, A.; Bader, R. A Novel Technique for the Connection of Ceramic and Titanium Implant Components Using Glass Solder Bonding. Materials 2015, 8, 4287–4298. [Google Scholar] [CrossRef] [PubMed]
- Bahraminasab, M.; Sahari, B.B.; Edwards, K.L.; Farahmand, F.; Hong, T.S.; Naghibi, H. Material tailoring of the femoral component in a total knee replacement to reduce the problem of aseptic loosening. Mater. Des. 2013, 52, 441–451. [Google Scholar] [CrossRef]
- Mitrovic, M.; Zothner, A. Dentalimplantat. Patent DE102011015299A1, 27 September 2012. [Google Scholar]
- Sass, J.-O.; Burmeister, U.; Ganz, C.; Mitrovic, A.; Lang, H.; Bader, R.; Vogel, D. Fracture strength of monolithic and glass-soldered ceramic subcomponents of 5-unit fixed dental prosthesis. J. Prosthodont. 2023, 32, e71–e80. [Google Scholar] [CrossRef]
- van Vu, T.; Oh, G.-J.; Lim, H.-P.; Yun, K.-D.; Ryu, S.-K.; Yim, E.-K.; Fisher, J.G.; Ban, J.-S.; Park, S.-W. Shear Bond Strength of Zirconia to Titanium Implant Using Glass Bonding. J. Nanosci. Nanotechnol. 2019, 19, 967–969. [Google Scholar] [CrossRef]
- Markhoff, J.; Mick, E.; Mitrovic, A.; Pasold, J.; Wegner, K.; Bader, R. Surface modifications of dental ceramic implants with different glass solder matrices: In vitro analyses with human primary osteoblasts and epithelial cells. Biomed. Res. Int. 2014, 2014, 742180. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Boehlert, C.J. Titanium Alloys for Biomedical Applications. In Advances in Metallic Biomaterials; Niinomi, M., Narushima, T., Nakai, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 179–213. ISBN 978-3-662-46835-7. [Google Scholar]
- Rack, H.J.; Qazi, J.I. Titanium alloys for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1269–1277. [Google Scholar] [CrossRef]
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Grimberg, A.; Luetzner, J.; Melsheimer, O.; Morlock, M.; Steinbrueck, A. The German Arthroplasty Registry—Annual Report. 2023. Available online: https://www.eprd.de/fileadmin/user_upload/Dateien/Publikationen/Berichte/AnnualReport2023-Web_2024-03-26_F.pdf (accessed on 9 April 2024).
- Rho, J.Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Choi, K.; Kuhn, J.L.; Ciarelli, M.J.; Goldstein, S.A. The elastic moduli of human subchondral, trabecular, and cortical bone tissue and the size-dependency of cortical bone modulus. J. Biomech. 1990, 23, 1103–1113. [Google Scholar] [CrossRef]
- Matusiewicz, H. Potential release of in vivo trace metals from metallic medical implants in the human body: From ions to nanoparticles—A systematic analytical review. Acta Biomater. 2014, 10, 2379–2403. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Miyazaki, S. Martensitic Transformation and Superelastic Properties of Ti-Nb Base Alloys. Mater. Trans. 2015, 56, 625–634. [Google Scholar] [CrossRef]
- Kuroda, P.A.B.; Da Silva, L.M.; Sousa, K.D.S.J.; Donato, T.A.G.; Grandini, C.R. Preparation, structural, microstructural, mechanical, and cytotoxic characterization of Ti-15Nb alloy for biomedical applications. Artif. Organs 2020, 44, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Schulze, C.; Weinmann, M.; Schweigel, C.; Keßler, O.; Bader, R. Mechanical Properties of a Newly Additive Manufactured Implant Material Based on Ti-42Nb. Materials 2018, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Gustmann, T.; Günther, F.; Zimmermann, M.; Kühn, U.; Gebert, A. Controlling the Young’s modulus of a ß-type Ti-Nb alloy via strong texturing by LPBF. Mater. Des. 2022, 216, 110516. [Google Scholar] [CrossRef]
- Huang, S.; Sing, S.L.; de Looze, G.; Wilson, R.; Yeong, W.Y. Laser powder bed fusion of titanium-tantalum alloys: Compositions and designs for biomedical applications. J. Mech. Behav. Biomed. Mater. 2020, 108, 103775. [Google Scholar] [CrossRef]
- Soro, N.; Brodie, E.G.; Abdal-hay, A.; Alali, A.Q.; Kent, D.; Dargusch, M.S. Additive manufacturing of biomimetic Titanium-Tantalum lattices for biomedical implant applications. Mater. Des. 2022, 218, 110688. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Niinomi, M.; Akahori, T. Effects of Ta content on Young’s modulus and tensile properties of binary Ti–Ta alloys for biomedical applications. Mater. Sci. Eng. A 2004, 371, 283–290. [Google Scholar] [CrossRef]
- Bertrand, E.; Gloriant, T.; Gordin, D.M.; Vasilescu, E.; Drob, P.; Vasilescu, C.; Drob, S.I. Synthesis and characterisation of a new superelastic Ti-25Ta-25Nb biomedical alloy. J. Mech. Behav. Biomed. Mater. 2010, 3, 559–564. [Google Scholar] [CrossRef]
- Dubinskiy, S.; Prokoshkin, S.; Brailovski, V.; Inaekyan, K.; Korotitskiy, A. In situ X-ray diffraction strain-controlled study of Ti–Nb–Zr and Ti–Nb–Ta shape memory alloys: Crystal lattice and transformation features. Mater. Charact. 2014, 88, 127–142. [Google Scholar] [CrossRef]
- Hussein, A.H.; Gepreel, M.A.-H.; Gouda, M.K.; Hefnawy, A.M.; Kandil, S.H. Biocompatibility of new Ti-Nb-Ta base alloys. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 574–578. [Google Scholar] [CrossRef]
- Johannsen, J.; Lauhoff, C.; Stenzel, M.; Schnitter, C.; Niendorf, T.; Weinmann, M. Laser beam powder bed fusion of novel biomedical titanium/niobium/tantalum alloys: Powder synthesis, microstructure evolution and mechanical properties. Mater. Des. 2023, 233, 112265. [Google Scholar] [CrossRef]
- Kim, H.Y.; Fu, J.; Tobe, H.; Kim, J.I.; Miyazaki, S. Crystal Structure, Transformation Strain, and Superelastic Property of Ti–Nb–Zr and Ti–Nb–Ta Alloys. Shap. Mem. Superelasticity 2015, 1, 107–116. [Google Scholar] [CrossRef]
- Sass, J.-O.; Sellin, M.-L.; Kauertz, E.; Johannsen, J.; Weinmann, M.; Stenzel, M.; Frank, M.; Vogel, D.; Bader, R.; Jonitz-Heincke, A. Advanced Ti–Nb–Ta Alloys for Bone Implants with Improved Functionality. J. Funct. Biomater. 2024, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Kuramoto, S.; Hwang, J.; Nishino, K.; Saito, T. Elastic Deformation Behavior of Multi-Functional Ti–Nb–Ta–Zr–O Alloys. Mater. Trans. 2005, 46, 3001–3007. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Simakin, A.V.; Konushkin, S.V.; Ivannikov, A.Y.; Nasakina, E.O.; Shatova, L.A.; Kolmakov, A.G.; Sevostyanov, M.A. Preparation, structural and microstructural characterization of Ti–30Nb–10Ta–5Zr alloy for biomedical applications. J. Mater. Res. Technol. 2020, 9, 16018–16028. [Google Scholar] [CrossRef]
- Saito, T.; Furuta, T.; Hwang, J.-H.; Kuramoto, S.; Nishino, K.; Suzuki, N.; Chen, R.; Yamada, A.; Ito, K.; Seno, Y.; et al. Multifunctional Alloys Obtained via a Dislocation-Free Plastic Deformation Mechanism. Science 2003, 300, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Sevostyanov, M.A.; Kolmakov, A.G.; Sergiyenko, K.V.; Kaplan, M.A.; Baikin, A.S.; Gudkov, S.V. Mechanical, physical–chemical and biological properties of the new Ti–30Nb–13Ta–5Zr alloy. J. Mater. Sci. 2020, 55, 14516–14529. [Google Scholar] [CrossRef]
- Prigent, H.; Pellen-Mussi, P.; Cathelineau, G.; Bonnaure-Mallet, M. Evaluation of the biocompatibility of titanium-tantalum alloy versus titanium. J. Biomed. Mater. Res. 1998, 39, 200–206. [Google Scholar] [CrossRef]
- Weng, W.; Biesiekierski, A.; Li, Y.; Wen, C. Effects of selected metallic and interstitial elements on the microstructure and mechanical properties of beta titanium alloys for orthopedic applications. Materialia 2019, 6, 100323. [Google Scholar] [CrossRef]
- Kolli, R.; Devaraj, A. A Review of Metastable Beta Titanium Alloys. Metals 2018, 8, 506. [Google Scholar] [CrossRef]
- Farrahnoor, A.; Zuhailawati, H. Review on the mechanical properties and biocompatibility of titanium implant: The role of niobium alloying element. Int. J. Mater. Res. 2021, 112, 505–513. [Google Scholar] [CrossRef]
- Niinomi, M. Recent metallic materials for biomedical applications. Metall. Mater. Trans. A 2002, 33, 477–486. [Google Scholar] [CrossRef]
- Fu, J.; Yamamoto, A.; Kim, H.Y.; Hosoda, H.; Miyazaki, S. Novel Ti-base superelastic alloys with large recovery strain and excellent biocompatibility. Acta Biomater. 2015, 17, 56–67. [Google Scholar] [CrossRef]
- Hao, Y.L.; Li, S.J.; Sun, S.Y.; Zheng, C.Y.; Hu, Q.M.; Yang, R. Super-elastic titanium alloy with unstable plastic deformation. Appl. Phys. Lett. 2005, 87, 091906. [Google Scholar] [CrossRef]
- F04 Committee. Test Method for Shear and Bending Fatigue Testing of Calcium Phosphate and Metallic Medical and Composite Calcium Phosphate/Metallic Coatings; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- F04 Committee. Test Method for Shear Testing of Calcium Phosphate Coatings and Metallic Coatings; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ISO 14243-3:2014-11; Implants for Surgery, Wear of total Knee-Joint Prostheses, Part 3: Loading and Displacement Parameters for Wear-Testing Machines with Displacement Control and Corresponding Environmental Conditions for Test. Beuth Verlag GmbH: Berlin, Germany, 2014.
- F02 Committee. Guide for Accelerated Aging of Sterile Barrier Systems for Medical Devices; ASTM International: West Conshohocken, PA, USA, 2021. [Google Scholar]
- ISO 13179-1:2021; Implants for Surgery, Coatings on Metallic Surgical Implants, Part 1: Plasma-Sprayed Coatings Derived from Titanium or Titanium-6 Aluminum-4 Vanadium Alloy Powders. Beuth Verlag GmbH: Berlin, Germany, 2021.
- Lochner, K.; Fritsche, A.; Jonitz, A.; Hansmann, D.; Mueller, P.; Mueller-Hilke, B.; Bader, R. The potential role of human osteoblasts for periprosthetic osteolysis following exposure to wear particles. Int. J. Mol. Med. 2011, 28, 1055–1063. [Google Scholar] [CrossRef]
- Zietz, C.; Reinders, J.; Schwiesau, J.; Paulus, A.; Kretzer, J.P.; Grupp, T.; Utzschneider, S.; Bader, R. Experimental testing of total knee replacements with UHMW-PE inserts: Impact of severe wear test conditions. J. Mater. Sci. Mater. Med. 2015, 26, 134. [Google Scholar] [CrossRef]
- Suansuwan, N.; Swain, M.V. Adhesion of porcelain to titanium and a titanium alloy. J. Dent. 2003, 31, 509–518. [Google Scholar] [CrossRef]
- Vásquez, V.Z.C.; Ozcan, M.; Kimpara, E.T. Evaluation of interface characterization and adhesion of glass ceramics to commercially pure titanium and gold alloy after thermal- and mechanical-loading. Dent. Mater. 2009, 25, 221–231. [Google Scholar] [CrossRef]
- Vercaigne, S.; Wolke, J.G.; Naert, I.; Jansen, J.A. Histomorphometrical and mechanical evaluation of titanium plasma-spray-coated implants placed in the cortical bone of goats. J. Biomed. Mater. Res. 1998, 41, 41–48. [Google Scholar] [CrossRef]
- Ozeki, K.; Yuhta, T.; Aoki, H.; Nishimura, I.; Fukui, Y. Push-out strength of hydroxyapatite coated by sputtering technique in bone. Biomed. Mater. Eng. 2001, 11, 63–68. [Google Scholar]
- Müller, M.; Hennig, F.F.; Hothorn, T.; Stangl, R. Bone-implant interface shear modulus and ultimate stress in a transcortical rabbit model of open-pore Ti6Al4V implants. J. Biomech. 2006, 39, 2123–2132. [Google Scholar] [CrossRef]
- Li, J.; Liao, H.; Fartash, B.; Hermansson, L.; Johnsson, T. Surface-dimpled commercially pure titanium implant and bone ingrowth. Biomaterials 1997, 18, 691–696. [Google Scholar] [CrossRef]
- Chang, C.K.; Wu, J.S.; Mao, D.L.; Ding, C.X. Mechanical and histological evaluations of hydroxyapatite-coated and noncoated Ti6Al4V implants in tibia bone. J. Biomed. Mater. Res. 2001, 56, 17–23. [Google Scholar] [CrossRef]
- Zelle, J.; Janssen, D.; Peeters, S.; Brouwer, C.; Verdonschot, N. Mixed-mode failure strength of implant-cement interface specimens with varying surface roughness. J. Biomech. 2011, 44, 780–783. [Google Scholar] [CrossRef]
- Zhukova, Y.S.; Pustov, Y.A.; Konopatsky, A.S.; Filonov, M.R. Characterization of electrochemical behavior and surface oxide films on superelastic biomedical Ti–Nb–Ta alloy in simulated physiological solutions. J. Alloys Compd. 2014, 586, S535–S538. [Google Scholar] [CrossRef]
- Soni, R.; Pande, S.; Salunkhe, S.; Natu, H.; Abouel Nasr, E.; Shanmugam, R.; Hussein, H.M.A.M. In Vitro and Electrochemical Characterization of Laser-Cladded Ti-Nb-Ta Alloy for Biomedical Applications. Crystals 2022, 12, 954. [Google Scholar] [CrossRef]
- Hey, J.; Kasaliyska, M.; Kiesow, A.; Schweyen, R.; Arnold, C. Retentive Force of Glass-Ceramic Soldered Customized Zirconia Abutment Copings with Prefabricated Titanium Bases. Materials 2020, 13, 3193. [Google Scholar] [CrossRef]
- Travessa, D.; Ferrante, M. The Al2O3-titanium adhesion in the view of the diffusion bonding process. J. Mater. Sci. 2002, 37, 4385–4390. [Google Scholar] [CrossRef]
- Gibbesch, B.; Elssner, G.; Petzow, G. Microstructure of interface regions and mechanical properties of Ti/Al2O3 and Ti-alloy/Al2O3 joints for dental implants. Clin. Mater. 1990, 5, 177–189. [Google Scholar] [CrossRef]
- Gibbesch, B.; Elssner, G.; Petzow, G. Investigation of Ti/Al2O3 joints with intermediate tantalum and niobium layers. Biomaterials 1992, 13, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.N.; Huang, T.; Bal, B.S.; Li, Y. In vitro testing of Al2O3-Nb composite for femoral head applications in total hip arthroplasty. Acta Biomater. 2010, 6, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, G.; Bender, A.; Graichen, F.; Dymke, J.; Rohlmann, A.; Trepczynski, A.; Heller, M.O.; Kutzner, I. Standardized loads acting in knee implants. PLoS ONE 2014, 9, e86035. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, M.J.; Trepczynski, A.; Hosseini Nasab, S.H.; Kutzner, I.; Schütz, P.; Weisse, B.; Dymke, J.; Postolka, B.; Moewis, P.; Bergmann, G.; et al. European Society of Biomechanics S.M. Perren Award 2022: Standardized tibio-femoral implant loads and kinematics. J. Biomech. 2022, 141, 111171. [Google Scholar] [CrossRef]
- Eisenbarth, E.; Velten, D.; Müller, M.; Thull, R.; Breme, J. Biocompatibility of beta-stabilizing elements of titanium alloys. Biomaterials 2004, 25, 5705–5713. [Google Scholar] [CrossRef]
- Sass, J.-O.; Jakobi, A.; Mitrovic, A.; Ganz, C.; Wilken, J.; Burmeister, U.; Lang, H.; Bader, R.; Vogel, D. Bending strength of ceramic compounds bonded with silicate-based glass solder. Mater. Test. 2021, 63, 593–598. [Google Scholar] [CrossRef]








| Group | Material | Specifications | Cross-Section [mm2] |
|---|---|---|---|
| 1 | TiNbTa-ATZ | Static shear test | 279.4 ± 0.1 |
| 2 | Ti-ATZ | Static shear test | 280.0 ± 0.3 |
| 3 | TiNbTa-ATZ | Accelerated aging followed by static shear test | 281.0 ± 1.1 |
| 4 | Ti-ATZ | Accelerated aging followed by static shear test | 280.4 ± 0.4 |
| 5 | TiNbTa-ATZ | Fatigue shear test | 280.3 ± 0.8 |
| 6 | Ti-ATZ | Fatigue shear test | 281.9 ± 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sass, J.-O.; Henke, P.; Mitrovic, A.; Weinmann, M.; Kluess, D.; Johannsen, J.; Sellin, M.-L.; Lembke, U.; Reimer, D.; Lork, C.; et al. Multifunctional Hybrid Material for Endoprosthetic Implants Based on Alumina-Toughened Zirconia Ceramics and Additively Manufactured TiNbTa Alloys. Materials 2024, 17, 1838. https://doi.org/10.3390/ma17081838
Sass J-O, Henke P, Mitrovic A, Weinmann M, Kluess D, Johannsen J, Sellin M-L, Lembke U, Reimer D, Lork C, et al. Multifunctional Hybrid Material for Endoprosthetic Implants Based on Alumina-Toughened Zirconia Ceramics and Additively Manufactured TiNbTa Alloys. Materials. 2024; 17(8):1838. https://doi.org/10.3390/ma17081838
Chicago/Turabian StyleSass, Jan-Oliver, Paul Henke, Aurica Mitrovic, Markus Weinmann, Daniel Kluess, Jan Johannsen, Marie-Luise Sellin, Ulrich Lembke, Daniel Reimer, Cornelia Lork, and et al. 2024. "Multifunctional Hybrid Material for Endoprosthetic Implants Based on Alumina-Toughened Zirconia Ceramics and Additively Manufactured TiNbTa Alloys" Materials 17, no. 8: 1838. https://doi.org/10.3390/ma17081838