Application of the Industrial Byproduct Gypsum in Building Materials: A Review
Abstract
1. Introduction
2. Overview of the Industrial Byproduct Gypsum
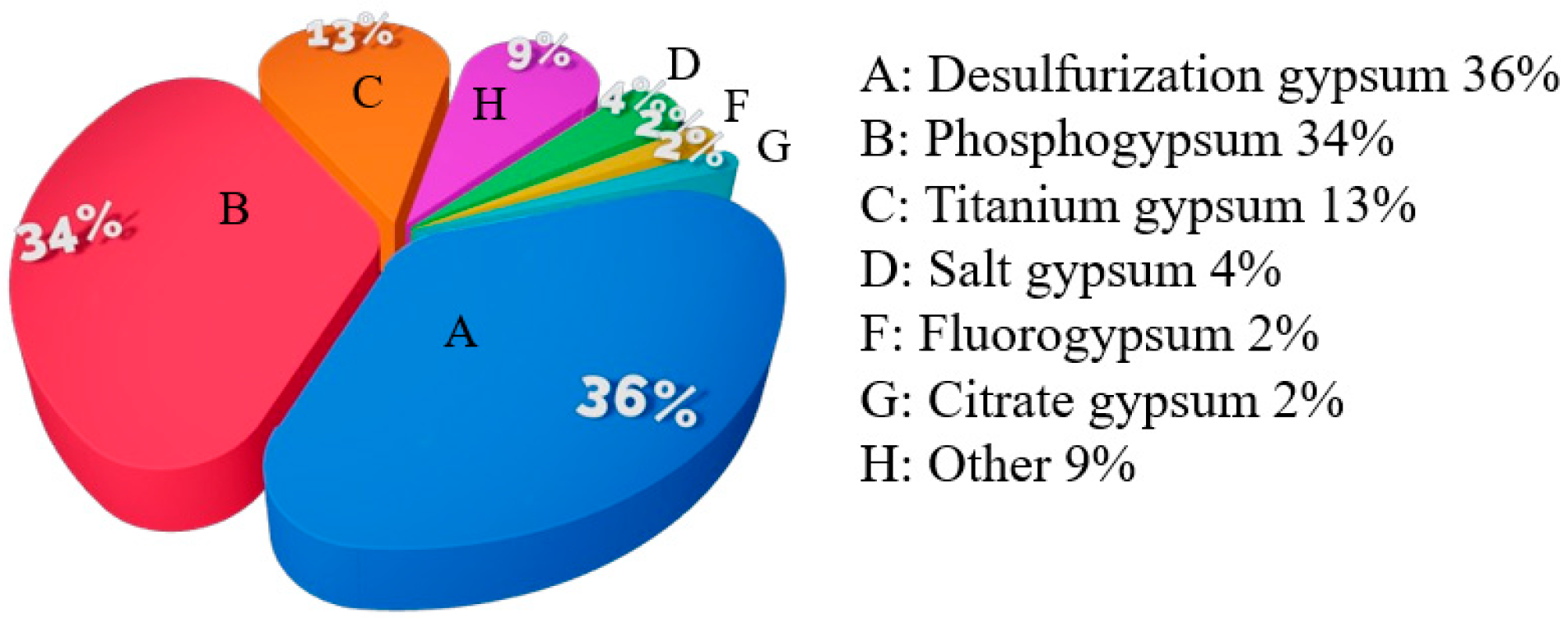
2.1. Source
2.2. Characteristics
| Species | Density (g/cm3) | Water Content (%) | Specific Surface Area (m2/kg) | Mean Particle Size (μm) | pH | Refs. |
|---|---|---|---|---|---|---|
| DG | 1.06–1.30 | 10–15 | 150–230 | 30–60 | 6–9 | [1,6,29] |
| PG | 1.05–1.20 | 25–35 | 450–520 | 5–150 | 1–4.5 | [30,32] |
| TG | 2.00–2.50 | 30–65 | 780–820 | 15–60 | 6–9 | [33,34] |
| FG | 1.30–1.50 | 15–20 | 300–500 | 10–80 | 2–4 | [31,35,36] |
3. Application in Building Materials
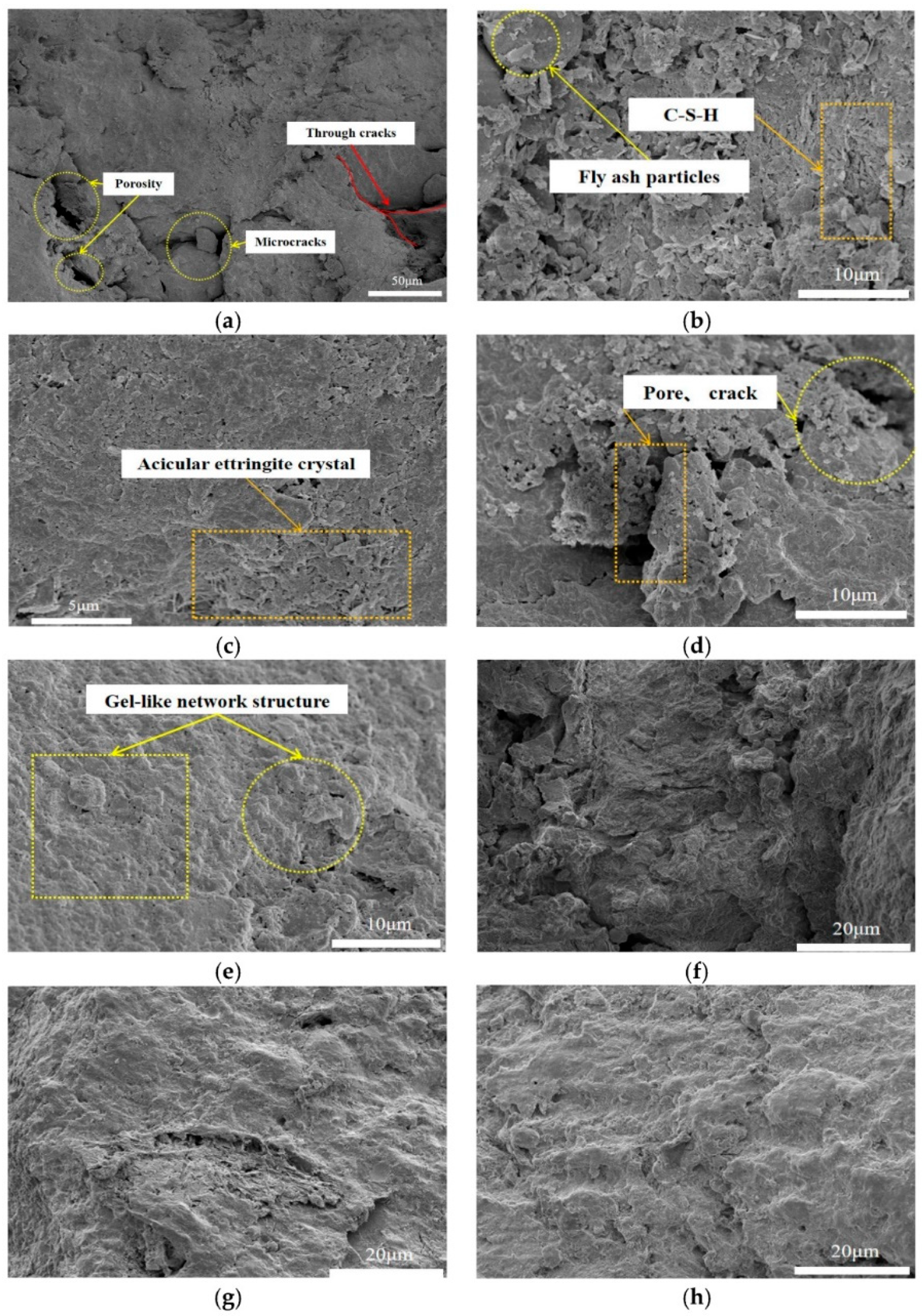
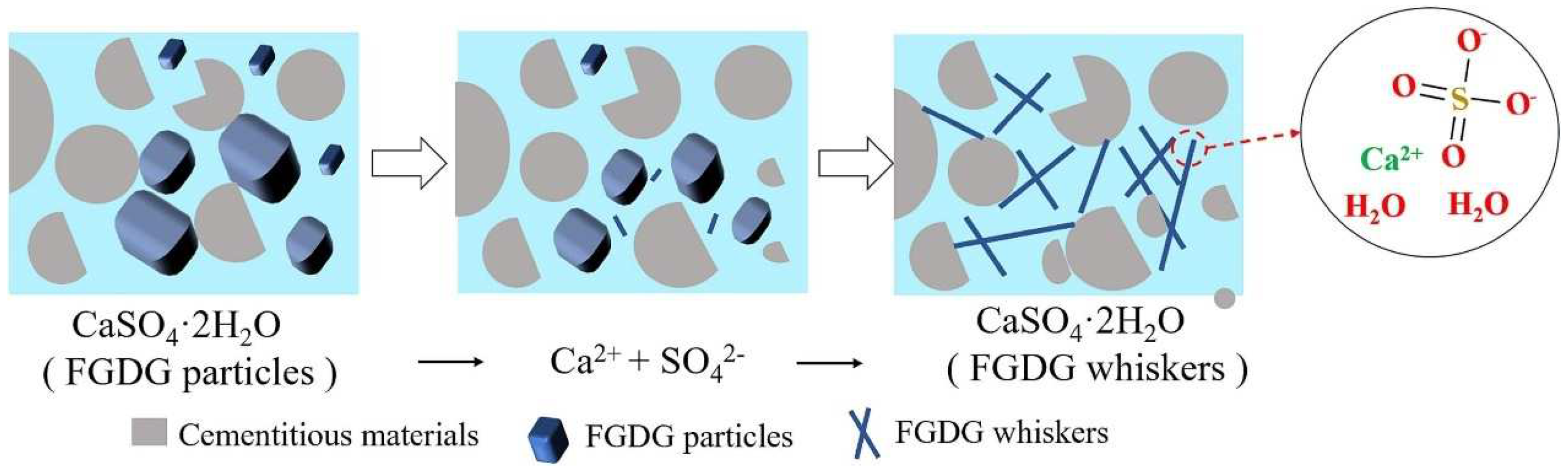
3.1. Cementitious Material
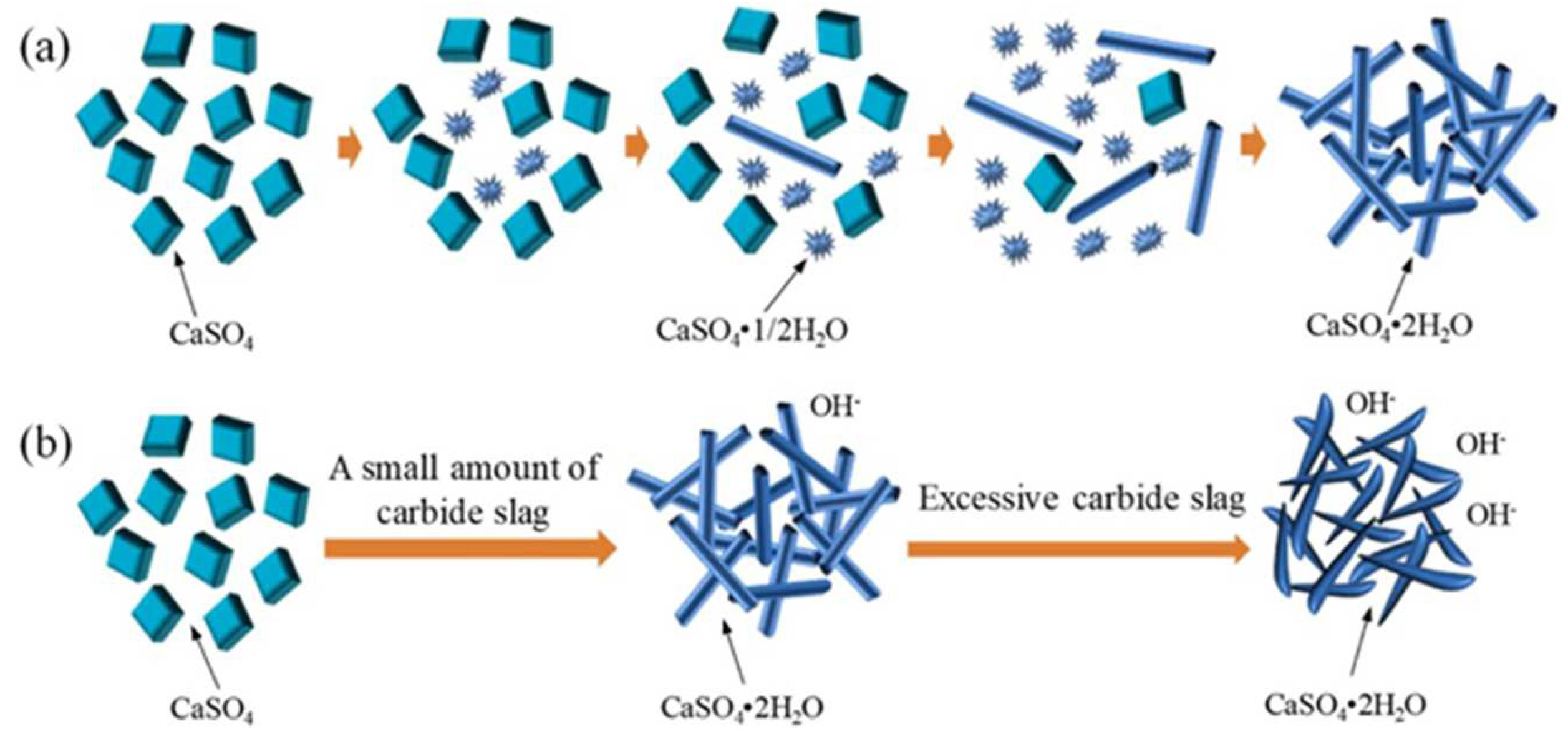
3.2. Cement Retarder
3.3. Road Base Material
3.4. Filling Material
3.5. Other Materials
4. Conclusions and Prospects
- (1)
- Research has shown that DG is equally suitable for all types of building blocks, with products designed in the appropriate proportions having good physical properties. However, the amount of DG was basically less than 10%. Excessive DG substantially affects the compressive strength and setting time of the slurry, and causes expansion cracking.
- (2)
- PG has also been shown to be a potentially viable construction material, although its low strength and presence of a number of impurities cause it to be underutilized. When PG is used in combination with other solid wastes (PG–red mud systems and PG–fly ash–steel slag), its mechanical properties can be improved. Moreover, compared with DG, PG can be blended up to approximately 30%, which is the main advantage of its industrial reuse.
- (3)
- Other gypsum byproducts have been less investigated, mainly because of the presence of various types of factors. For example, TG has a high Fe content, and impurities in FeSO4 lead to decreases in cement strength. The slow hydration rate and low early strength of FG make it difficult to develop and utilize directly, and prevent it from being incorporated into materials at too high a ratio. These impurities reduce the utilization of the byproduct gypsum.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sithole, T.; Mashifana, T.; Mahlangu, D.; Tchadjie, L. Physical, chemical and geotechnical characterization of wet flue gas desulfurization gypsum and its potential application as building materials. Buildings 2021, 11, 500. [Google Scholar] [CrossRef]
- Liu, L.; Zuo, H.; Xu, Z. Resource utilization approach of industrial gypsum and its prospect. Inorg. Chem. Ind. 2021, 53, 1–9. [Google Scholar]
- Chen, J.; Zhang, R. Report on Ecological Environment Technology Development of Industrial By-product Gypsum Resource Utilization. Guangzhou Chem. Ind. 2020, 48, 1–3. [Google Scholar]
- Li, L.; Li, G. Study on the preparation and properties of desulfurization gypsum-based self-leveling material. Appl. Mech. Mater. 2014, 541, 100–103. [Google Scholar] [CrossRef]
- Maichin, P.; Jitsangiam, P.; Nongnuang, T.; Boonserm, K.; Nusit, K.; Pra-Ai, S.; Binaree, T.; Aryupong, C. Stabilized high clay content lateritic soil using cement-FGD gypsum mixtures for road subbase applications. Materials 2021, 14, 1858. [Google Scholar] [CrossRef] [PubMed]
- Phutthimethakul, L.; Kumpueng, P.; Supakata, N. Use of flue gas desulfurization gypsum, construction and demolition waste, and oil palm waste trunks to produce concrete bricks. Crystals 2020, 10, 709. [Google Scholar] [CrossRef]
- Pliaka, M.; Gaidajis, G. Potential uses of phosphogypsum: A review. J. Environ. Sci. Health Part A 2022, 57, 746–763. [Google Scholar] [CrossRef]
- Xiao, Y.; Jin, H.; Wang, M.; Guo, Y. Collaborative utilization status of red mud and phosphogypsum: A review. J. Sustain. Metall. 2022, 8, 1422–1434. [Google Scholar] [CrossRef]
- Bouargane, B.; Laaboubi, K.; Biyoune, M.G.; Bakiz, B.; Atbir, A. Effective and innovative procedures to use phosphogypsum waste in different application domains: Review of the environmental, economic challenges and life cycle assessment. J. Mater. Cycles Waste Manag. 2023, 25, 1288–1308. [Google Scholar] [CrossRef]
- Men, J.; Li, Y.; Cheng, P.; Zhang, Z. Recycling phosphogypsum in road construction materials and associated environmental considerations: A review. Heliyon 2022, 8, 11581. [Google Scholar] [CrossRef]
- Gu, J.; Liu, X.; Zhang, Z. Road base materials prepared by multi-industrial solid wastes in China: A review. Constr. Build. Mater. 2023, 373, 130860. [Google Scholar] [CrossRef]
- Jiang, C.; Dong, F. Comprehensive Utilization and Research Progress of Industrial By-product Gypsum. Shandong Chem. Ind. 2016, 45, 42–45. [Google Scholar]
- Liu, T.; Wang, Z.; Li, G.; Shi, G.; Zhao, X. Water resistance of flue gas desulphurization gypsum-fly ash-steel slag composites. IOP Conf. Ser. Mater. Sci. Eng. 2019, 474, 012039. [Google Scholar] [CrossRef]
- Wu, M.; Hu, X.; Zhang, Q.; Cheng, W.; Hu, Z. Orthogonal experimental studies on preparation of mine-filling materials from carbide slag, granulated blast-furnace slag, fly ash, and flue-gas desulphurisation gypsum. Adv. Mater. Sci. Eng. 2018, 2018, 4173520. [Google Scholar] [CrossRef]
- Koralegedara, N.; Pinto, P.; Dionysiou, D.; Al-Abed, S. Recent advances in flue gas desulfurization gypsum processes and applications—A review. J. Environ. Manag. 2019, 251, 109572. [Google Scholar] [CrossRef] [PubMed]
- Mihara, N.; Kuchar, D.; Kojima, Y.; Matsuda, H. Reductive decomposition of waste gypsum with SiO2, Al2O3, and Fe2O3 additives. J. Mater. Cycles Waste Manag. 2007, 9, 21–26. [Google Scholar] [CrossRef]
- Engbrecht, D.; Hirschfeld, D. Thermal analysis of calcium sulfate dihydrate sources used to manufacture gypsum wallboard. Thermochim. Acta 2016, 639, 173–185. [Google Scholar] [CrossRef]
- Mesić, M.; Brezinščak, L.; Zgorelec, Ž.; Perčin, A.; Šestak, I.; Bilandžija, D.; Trdenić, M.; Lisac, H. The application of phosphogypsum in agriculture. Agric. Conspec. Sci. 2016, 81, 7–13. [Google Scholar]
- Tayibi, H.; Choura, M.; López, F.A.; Alguacil, F.J.; López-Delgado, A. Environmental impact and management of phosphogypsum. J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef]
- Al-Hwaiti, M.S. Influence of treated waste phosphogypsum materials on the properties of ordinary portland cement. Bangladesh J. Sci. Ind. Res. 2015, 50, 241–250. [Google Scholar] [CrossRef]
- Alaam, R. Potential use of phosphogypsum in alkali-activated fly ash under the effects of elevated temperatures and thermal shock cycles. J. Clean. Prod. 2015, 87, 717–725. [Google Scholar]
- Akfas, F.; Elghali, A.; Aboulaich, A.; Munoz, M.; Benzaazoua, M.; Bodinier, J.-L. Exploring the potential reuse of PG: A waste or a resource? Sci. Total Environ. 2023, 908, 168196. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Bai, H.; Gao, Y.; Xiu, X. Phosphogypsum comprehensive utilization present situation and development trend of “difference”. Inorg. Chem. Ind. 2022, 54, 1–4. [Google Scholar]
- Gázquez, M.J.; Contreras, M.; Pérez-Moreno, S.M.; Guerrero, J.L.; Casas-Ruiz, M.; Bolívar, J.P. A Review of the commercial uses of sulphate minerals from the titanium dioxide pigment industry: The case of Huelva (Spain). Minerals 2021, 11, 575. [Google Scholar] [CrossRef]
- Li, X.; Yang, J. Production, characterisation, and application of titanium gypsum: A review. Process Saf. Environ. Prot. 2024, 181, 64–74. [Google Scholar] [CrossRef]
- Li, R.; Li, L.; Cao, Z. Exploration and practice of developing building materials with fluoropgypsum waste slag. Environ. Eng. 2006, 2, 58–61. [Google Scholar]
- Feng, Q.; Wang, J.; Zhang, X. Study on comprehensive utilization of industrial waste fluoropgypsum. Non-Met. Mines 2010, 33, 36–38. [Google Scholar]
- Córdoba, P. Status of Flue Gas Desulphurisation (FGD) systems from coal-fired power plants: Overview of the physic-chemical control processes of wet limestone FGDs. Fuel 2015, 144, 274–286. [Google Scholar] [CrossRef]
- Mu, R.; Wang, L.; Tian, W.L.; Cao, W. Long-Term Bonding Strength between Flue Gas Desulfurization Gypsum and Polymer Cement Mortar. Adv. Mater. Res. 2012, 446, 1348–1351. [Google Scholar] [CrossRef]
- Gong, Y.; Dong, S.; Liu, L.; Wu, F. A sustainable composite cementitious material manufactured by phosphogypsum waste. Appl. Sci. 2022, 12, 12718. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Cheng, L.; Xiong, Z.; Zhan, S. Study on the proportion of paste filling materials based on fluorogypsum. PLoS ONE 2023, 18, e0286872. [Google Scholar]
- Vaičiukynienė, D.; Nizevičienė, D.; Kantautas, A.; Bocullo, V.; Kielė, A. Alkali activated paste and concrete based on of biomass bottom ash with phosphogypsum. Appl. Sci. 2020, 10, 5190. [Google Scholar] [CrossRef]
- Lin, Q.; Zhen, X.; Rong, Y.; Li, Y.; Zhang, H.; Zhang, Q.; Yao, Z.; Yao, K. Investigation on Mechanical and Microstructure Properties of Silt Improved by Titanium Gypsum-Based Stabilizer. Materials 2022, 16, 271. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Sun, H.; Peng, T.; Zhao, D.; Zhang, X. The Leaching Kinetics of Iron from Titanium Gypsum in a Citric Acid Medium and Obtain Materials by Leaching Liquid. Molecules 2023, 28, 952. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, M.; Zhao, X.; Tang, C. Mechanical properties and hydration mechanisms of high-strength fluorogypsum-blast furnace slag-based hydraulic cementitious binder. Constr. Build. Mater. 2016, 127, 137–143. [Google Scholar] [CrossRef]
- Kuzmin, M.; Larionov, L.; Kuzmina, M.; Kuzmina, A.; Ran, J.; Burdonov, A.; Zenkov, E. Production of Portland cement using fluorine gypsum–hydrofluoric acid waste. Mag. Civ. Eng. 2022, 111, 11113. [Google Scholar]
- Ding, Y.; Ren, Q.; Hu, P. Study of Inorganic Waterproof Agent on the Performance of Desulfurization Gypsum Products. Asian J. Chem. 2013, 25, 5678. [Google Scholar] [CrossRef]
- Lei, D.; Guo, L.; Sun, W.; Liu, J.; Miao, C. Study on properties of untreated FGD gypsum-based high-strength building materials. Constr. Build. Mater. 2017, 153, 765–773. [Google Scholar] [CrossRef]
- Wang, T.; Gao, X.; Jian, W. Preparation of foamed phosphogypsum lightweight materials by incorporating cementitious additives. Mater. Sci. 2019, 25, 340–347. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Zhu, X.; Zhu, W.; Yue, J. Synergistic effect of red mud, desulfurized gypsum and fly ash in cementitious materials: Mechanical performances and microstructure. Constr. Build. Mater. 2023, 404, 133302. [Google Scholar] [CrossRef]
- Zhu, J.; Yue, H.; Ma, L.; Li, Z.; Bai, R. The synergistic hydration mechanism and environmental safety of multiple solid wastes in red mud-based cementitious materials. Environ. Sci. Pollut. Res. 2023, 30, 79241–79257. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Liao, H.; Wei, F.; Wang, J.; Wu, J.; Cheng, F. Solid waste-based dry-mix mortar using fly ash, carbide slag, and flue gas desulfurization gypsum. J. Mater. Res. Technol. 2022, 21, 3636–3649. [Google Scholar] [CrossRef]
- Caillahua, M.; Moura, F. Technical feasibility for use of FGD gypsum as an additive setting time retarder for Portland cement. J. Mater. Res. Technol. 2018, 7, 190–197. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, Y.; Jing, J.; Wang, C.; Zhou, Y.; Zhang, K.; Zheng, Y.; Zhai, Y.; Liu, F. Properties and environmental impact of building foundation pit backfilling materials containing iron and steel solid waste. Front. Earth Sci. 2023, 11, 1181974. [Google Scholar] [CrossRef]
- Baran, E.; Hynowski, M.; Kotwica, Ł.; Rogowski, J. Effect of Portland Cement on the Selected Properties of Flue Gas Desulfurization Gypsum-Based Plasters. Materials 2023, 16, 5058. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Zhou, B.; Guo, R.; Yan, F.; Wang, R.; Tang, C. Preparation and Micromechanics of Red Sandstone–Phosphogypsum–Cement Composite Cementitious Materials. Materials 2023, 16, 4549. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Zhong, Y.; Li, X.; Peng, H.; Su, J.; Huang, Z. Experimental Study on the Road Performance of High Content of Phosphogypsum in the Lime–Fly Ash Mixture. Front. Mater. 2022, 9, 935113. [Google Scholar] [CrossRef]
- Pratap, B.; Mondal, S.; Rao, B.H. Development of geopolymer concrete using fly ash and phosphogypsum as a pavement composite material. Mater. Today Proc. 2023, 93, 35–40. [Google Scholar] [CrossRef]
- Feng, Z.; Yu, F.; Geng, J. Reseach on anti-carbonation performance of titanium gypsum-based composite cementitious material. Low Temp. Archit. Technol. 2021, 43, 1–4. [Google Scholar]
- Sotiriadis, K.; Kiyko, P.I.; Chernykh, T.N.; Kriushin, M.V. Self-cleaning ability of gypsum-cement-pozzolan binders based on thermally processed red gypsum waste of titanium oxide manufacture. J. Build. Eng. 2024, 87, 109009. [Google Scholar] [CrossRef]
- Tang, J. Application of titanium gypsum as retarding agent in cement production. Cement 2017, 2, 20–23. [Google Scholar]
- Li, L.; Li, G. The Preparation of Desulfurization Gypsum-Based Lightweight Insulation Board. Appl. Mech. Mater. 2015, 711, 185–188. [Google Scholar] [CrossRef]
- Thymotie, A.; Chang, T.-P.; Nguyen, H.-A. Improving properties of high-volume fly ash cement paste blended with β-hemihydrate from flue gas desulfurization gypsum. Constr. Build. Mater. 2020, 261, 120494. [Google Scholar] [CrossRef]
- Zhao, Q.; Fang, Z. Preparation of Desulfurization Gypsum for Ultra-sulfur Hydraulic Cementitions Material. J. Wuhan Univ. Technol. 2014, 5, 17–22+27. [Google Scholar]
- Wansom, S.; Chintasongkro, P.; Srijampan, W. Water resistant blended cements containing flue-gas desulfurization gypsum, Portland cement and fly ash for structural applications. Cem. Concr. Compos. 2019, 103, 134–148. [Google Scholar] [CrossRef]
- Bumanis, G.; Zorica, J.; Bajare, D. Properties of foamed lightweight high-performance phosphogypsum-based ternary system binder. Appl. Sci. 2020, 10, 6222. [Google Scholar] [CrossRef]
- Azifa, A.; Chouaybi, I.; Ennaciri, Y.; Zdah, I.; Cherrat, A.; Majid, F.; Bettach, M.; El Alaoui-Belghiti, H. Phosphogypsum valorization in cement: Experimental study of the PG effect and the water-to-cement ratio on the flexural strength of cement paste. Sustain. Chem. Pharm. 2024, 38, 101474. [Google Scholar] [CrossRef]
- GB 175-2007; Common Portland Cement. Standardization Administration: Beijing, China, 2007.
- Xu, C.; Ni, W.; Li, K.; Zhang, S.; Xu, D. Activation mechanisms of three types of industrial by-product gypsums on steel slag–granulated blast furnace slag-based binders. Constr. Build. Mater. 2021, 288, 123111. [Google Scholar] [CrossRef]
- Lv, X.; Xiang, L. The generation process, impurity removal and high-value utilization of phosphogypsum material. Nanomaterials 2022, 12, 3021. [Google Scholar] [CrossRef]
- Chuan, L.; Zheng, H.; Zhao, J.; Wang, A.; Sun, S. Phosphogypsum production and utilization in China. IOP Conf. Ser. Mater. Sci. Eng. 2018, 382, 022099. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Li, Z.; Ren, Y.; Wang, Y.; Zhang, W. Preparation, characterization and application of red mud, fly ash and desulfurized gypsum based eco-friendly road base materials. J. Clean. Prod. 2021, 284, 124777. [Google Scholar] [CrossRef]
- Meskini, S.; Mechnou, I.; Benmansour, M.; Remmal, T.; Samdi, A. Environmental investigation on the use of a phosphogypsum-based road material: Radiological and leaching assessment. J. Environ. Manag. 2023, 345, 118597. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ma, Y.; Wang, J.; Lin, Z.; Chen, T. Experimental Study on the Road Performance of Phosphogypsum-Modified Lime-Fly Ash Stabilized Red Clay. Appl. Sci. 2023, 13, 12689. [Google Scholar] [CrossRef]
- Mareya, M.; Tchadjie, L.; Sithole, T. Turning fly ash and waste gypsum into a resource for backfilling applications. Case Stud. Constr. Mater. 2024, 20, e02703. [Google Scholar] [CrossRef]
- Ruan, S.; Liu, L.; Zhu, M.; Shao, C.; Xie, L. Development and field application of a modified magnesium slag-based mine filling cementitious material. J. Clean. Prod. 2023, 419, 138269. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, Y.; Zhu, H.; Zhou, Q. Performance activation and strength evolution mechanism of carbide slag on anhydrous phosphogypsum backfill material. Constr. Build. Mater. 2024, 419, 135503. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, M.; Hou, H.; Zhu, X.; Rong, H. Properties and mechanism of mine tailings solidified and filled with fluorgypsum-based binder material. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2012, 27, 465–470. [Google Scholar] [CrossRef]
- Hou, L.; Li, J.; Lu, Z.; Niu, Y. Influence of foaming agent on cement and foam concrete. Constr. Build. Mater. 2021, 280, 122399. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Yang, H.-M.; Lee, H.-S. Preparation and Properties of Sustainable Concrete Using Activated Sludge of Industrial By-Products. Sustainability 2021, 13, 4671. [Google Scholar] [CrossRef]
- Dima, C.; Badanoiu, A.; Cirstea, S.; Nicoara, A.I.; Stoleriu, S. Lightweight gypsum materials with potential use for thermal insulations. Materials 2020, 13, 5454. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Calabrese, D.; Valenti, G.L.; Montagnaro, F. Flue gas desulfurization gypsum and coal fly ash as basic components of prefabricated building materials. Waste Manag. 2013, 33, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Song, H.; Liu, F.; Liu, Q.; Fan, Z.; Cheng, H.; Cheng, F. Study on the influence mechanism of flue gas desulphurisation gypsum on solid waste-based geopolymer grouting materials. Constr. Build. Mater. 2023, 403, 133137. [Google Scholar] [CrossRef]
- Tamošaitis, G.; Vaičiukynienė, D.; Jaskaudas, T.; Mockiene, J.; Pupeikis, D. Development of alkali-activated porous concrete composition from slag waste. Materials 2023, 16, 1360. [Google Scholar] [CrossRef] [PubMed]
- Guedri, A.; Abdallah, F.; Mefteh, N.; Hamdi, N.; Baeza-Urrea, O.; Wagner, J.-F.; Zagrarni, M.F. Addition of Phosphogypsum to Fire-Resistant Plaster Panels: A Physic–Mechanical Investigation. Inorganics 2023, 11, 35. [Google Scholar] [CrossRef]
- Dong, E.; Fu, S.; Wu, C.; Lv, W.; Zhang, L.; Feng, Y.; Shui, Z.; Yu, R. Value-added utilization of phosphogypsum industrial by-products in producing green Ultra-High performance Concrete: Detailed reaction kinetics and microstructure evolution mechanism. Constr. Build. Mater. 2023, 389, 131726. [Google Scholar] [CrossRef]
- Wang, G.; Zong, H.; Zhang, Z.; Sun, J.; Wang, F.; Feng, Y.; Huang, S.; Li, Q. Application of titanium gypsum as raw materials in cement-based self-leveling mortars. Case Stud. Constr. Mater. 2023, 19, e02536. [Google Scholar] [CrossRef]







| Species | CaO | Al2O3 | SO3 | SiO2 | Fe2O3 | TiO2 | Refs. |
|---|---|---|---|---|---|---|---|
| DG | 35.92–50.83 | 1.10–1.16 | 42.72–47.94 | 2.01–3.45 | 0.48–0.64 | - | [1,37,38] |
| PG | 32.43–44.92 | 0.13–0.47 | 43.05–53.54 | 2.32–4.13 | 0.03–0.18 | - | [30,32,39] |
| TG | 31.21–35.24 | 1.12–2.13 | 27.05–31.16 | 1.20–2.13 | 12.02–16.21 | 1.41–3.83 | [24,33,34] |
| FG | 33.62–36.34 | 0.61–0.72 | 44.64–53.26 | 1.17–1.93 | 0.43–0.82 | - | [31,35,36] |
| Types of Building Materials | Gypsum and Content | The Optimal Proportion | Performances | Refs. |
|---|---|---|---|---|
| Cementitious material | DG: 6% | 26.4% Red mud, 17.6% Fly ash, 50% cement | 28 d Compressive Strength: 50.6 MPa | [40] |
| Cementitious material | DG: 5% | 60% Red mud, 24.5% Fly ash, 10.5% Lime | 28 d Flexural strengths: 3.2 MPa | [41] |
| Cementitious material | DG: 9% | 64% Circulating fluidized bed fly ash, 27% Carbide slag | 28 d Compressive Strength: 6.35 MPa | [42] |
| Cement retarder | DG: 2.1% | 56.5% Clinker, 10% Limestone, 30%Slag, 1.4% Natural gypsum | Extended condensation time 1 h | [43] |
| Filling material | DG: 9.1% | 12.1% Carbide slag, 60.6% Fly ash, 18.2% Granulated blast furnace slag | 28 d Compressive Strength: 3.58 MPa | [14] |
| Filling material | DG: 10% | 58% Steel slag, 32% Granulated blast furnace slag | 28 d Compressive Strength: 6.22 MPa | [44] |
| Gypsum plasters | DG: 30% | 12% Portland cement | 28 d Compressive Strength: 7.21 MPa | [45] |
| Cementitious material | PG: 5% | 20% Red sandstone, 75% Cement | 28 d Compressive strength: 62.5 MPa | [46] |
| Cementitious material | PG: 30% | 70% Cement | 28 d Compressive strength: 52.1 MPa | [30] |
| Road base materials | PG: 15% | 76% Crushed stone, 12% Fly ash, 6% Lime | 28 d Unconfined compressive strength: 4.1 MPa | [47] |
| Geopolymer concrete | PG: 25% | 75% Fly ash, Partial additives | 28 d Compressive strength: 51.52 MPa | [48] |
| Foam concrete | PG: 49% | 25% Cement, 20% Fly ash, 6% Hydrated lime | Compressive strength: 1.7 MPa, Dry density: 521.7 kg/m3 | [39] |
| Fine-grained concretes | PG: 15% | 85% Biomass bottom ash | 28 d Compressive strength: 30 MPa | [32] |
| Cementitious material | TG: 35% | 10% Cement, 30% Granulated blast furnace slag, 5% Clinker, 20% Fly ash | 28 d Compressive strength: 37.8 MPa | [49] |
| Cementitious material | TG: 66.5% | 20% Cement, 13.5% Microsilica | 28 d Compressive strength: 9 MPa | [50] |
| Cement retarder | 650 °C roast TG: 6% | 74% Clinker, 5% Granulated blast furnace slag, 3% limestone, 12% Coal cinder | Extended coagulation time: 277 min | [51] |
| Filling material | FG: 18% | 61% Coal gangue, 18% Fly ash, 3% Lime | 28 d Compressive strength: 4–5 MPa | [31] |
| Cementitious material | FG: 40% | 55% Granulated blast furnace slag, 5% Cement | 28 d Compressive strength: 59.0 MPa | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Z.; Liu, X.; Zhang, Z.; Wei, C.; Gu, J. Application of the Industrial Byproduct Gypsum in Building Materials: A Review. Materials 2024, 17, 1837. https://doi.org/10.3390/ma17081837
Xie Z, Liu X, Zhang Z, Wei C, Gu J. Application of the Industrial Byproduct Gypsum in Building Materials: A Review. Materials. 2024; 17(8):1837. https://doi.org/10.3390/ma17081837
Chicago/Turabian StyleXie, Zhiqing, Xiaoming Liu, Zengqi Zhang, Chao Wei, and Jiarui Gu. 2024. "Application of the Industrial Byproduct Gypsum in Building Materials: A Review" Materials 17, no. 8: 1837. https://doi.org/10.3390/ma17081837
APA StyleXie, Z., Liu, X., Zhang, Z., Wei, C., & Gu, J. (2024). Application of the Industrial Byproduct Gypsum in Building Materials: A Review. Materials, 17(8), 1837. https://doi.org/10.3390/ma17081837