Effects and Mechanism of Hyperbranched Phosphate Polycarboxylate Superplasticizers on Reducing Viscosity of Cement Paste
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Synthesis of PCEs
- (1)
- Synthesis of hyperbranched PCEs
- (2)
- Synthesis of comb-like PCE
2.3. Characterization of PCEs
2.4. Dispersion of PCEs
2.5. Adsorption Layer Thickness
2.6. Apparent Adsorption Amount
2.7. Viscosity of Aqueous Solution and Simulated Pore Solution
3. Results
3.1. Structure Characterization of PCEs
3.2. Surface Tension
3.3. Fluidity
3.4. Marsh Time of Cement Paste
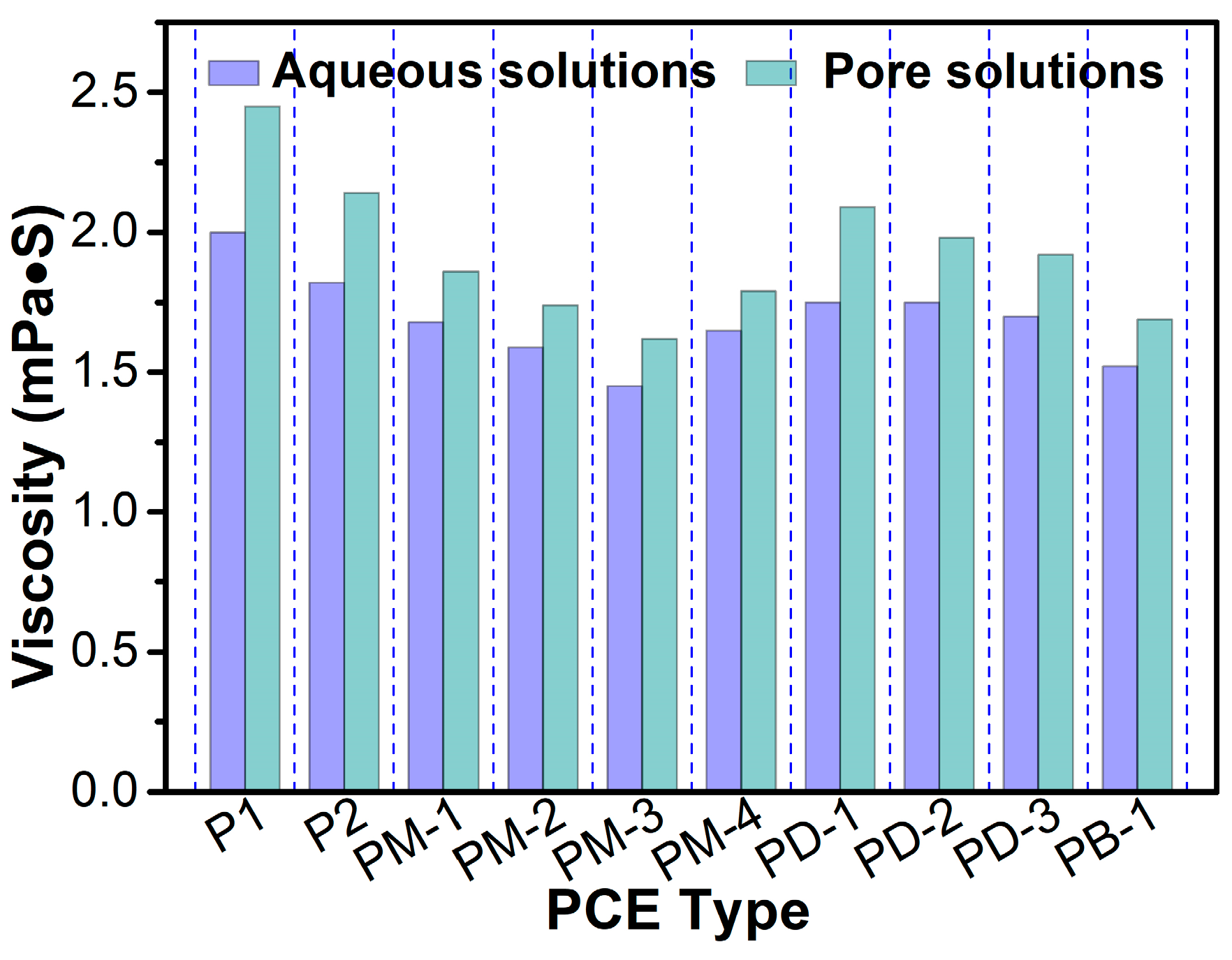
3.5. Viscosity of Aqueous Solution and Simulated Pore Solution
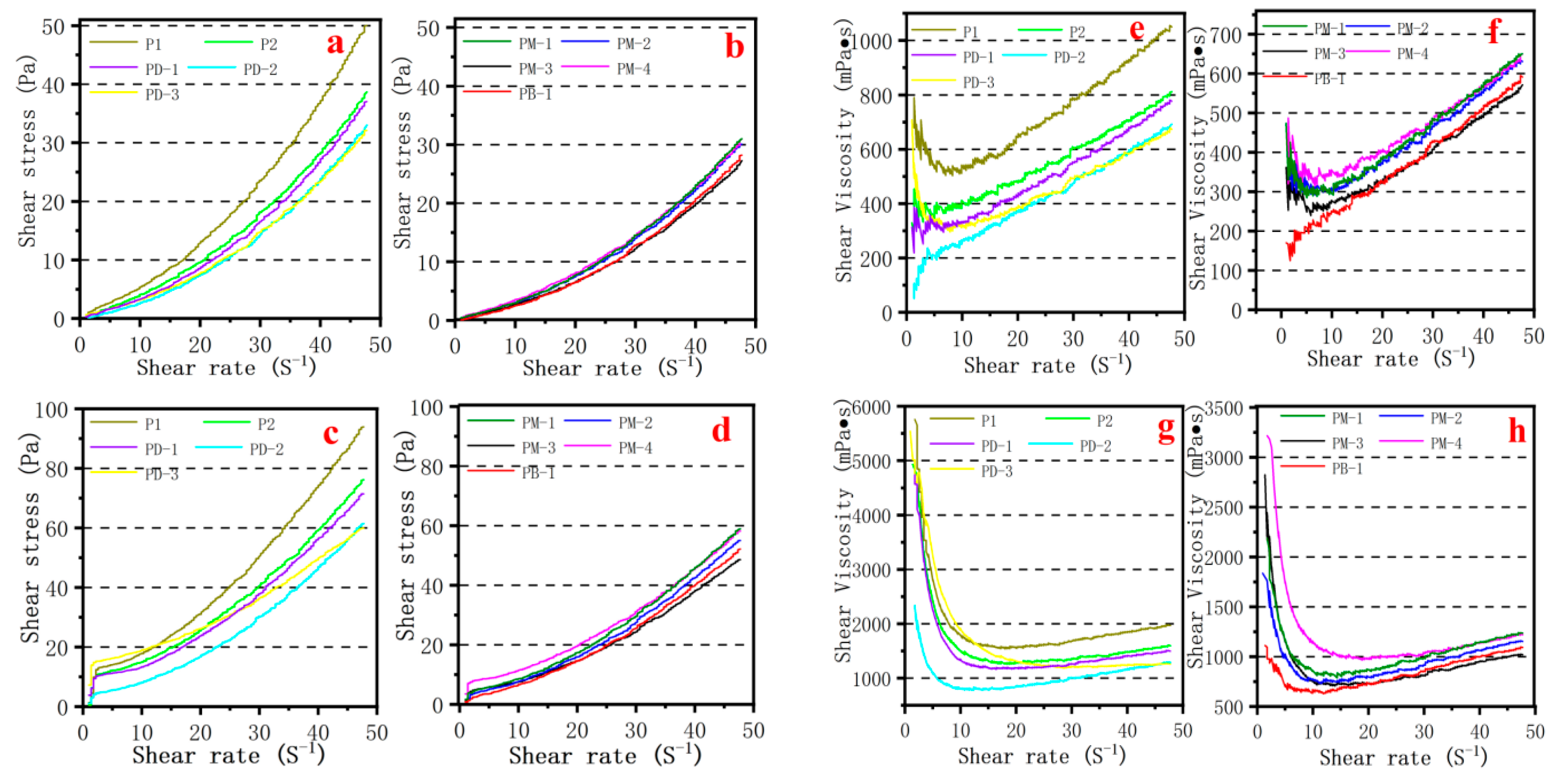
3.6. Rheology of Cement Pastes
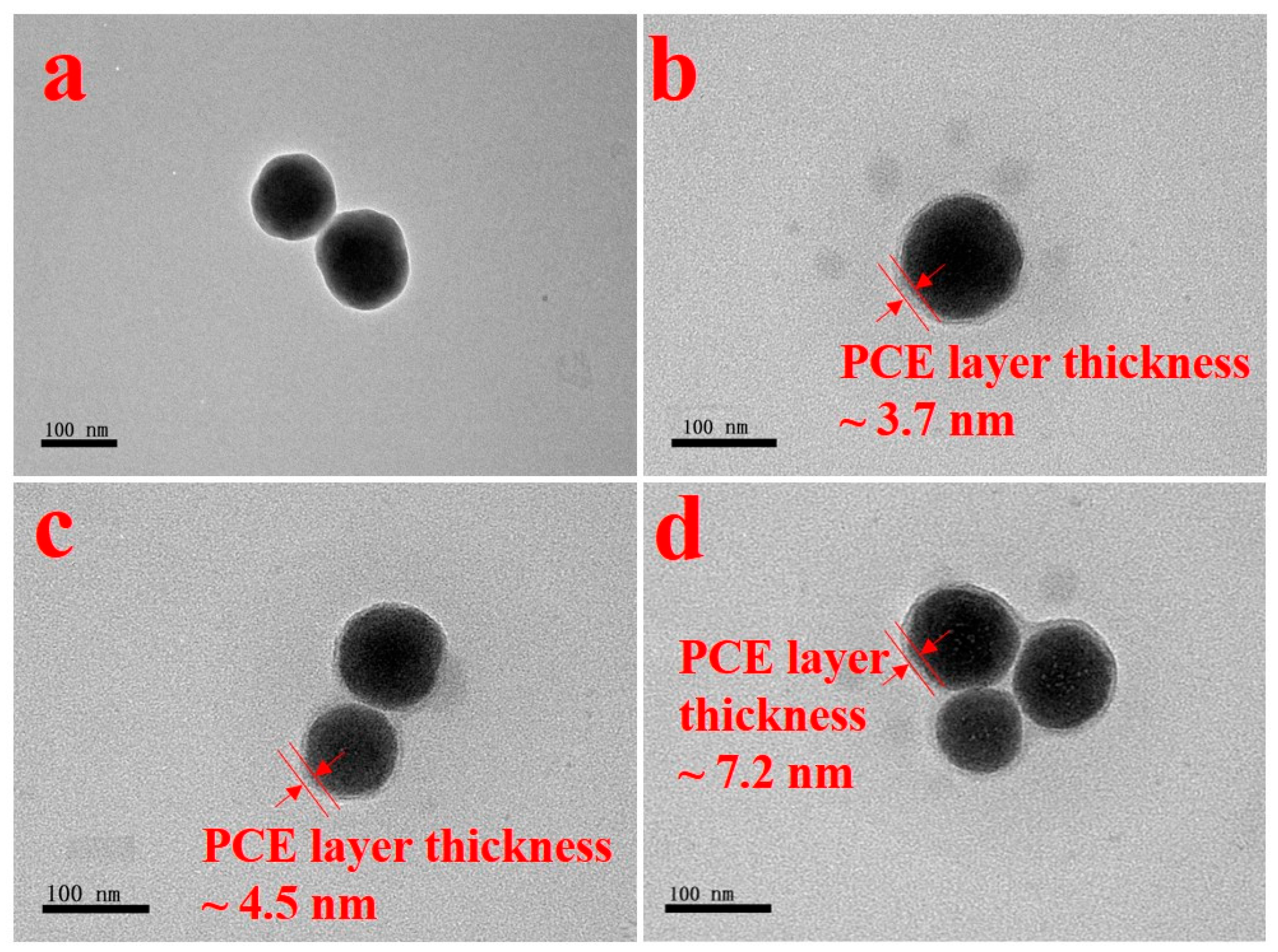
3.7. Water Film Thickness and Adsorption Layer Thickness
3.8. Adsorption Behavior
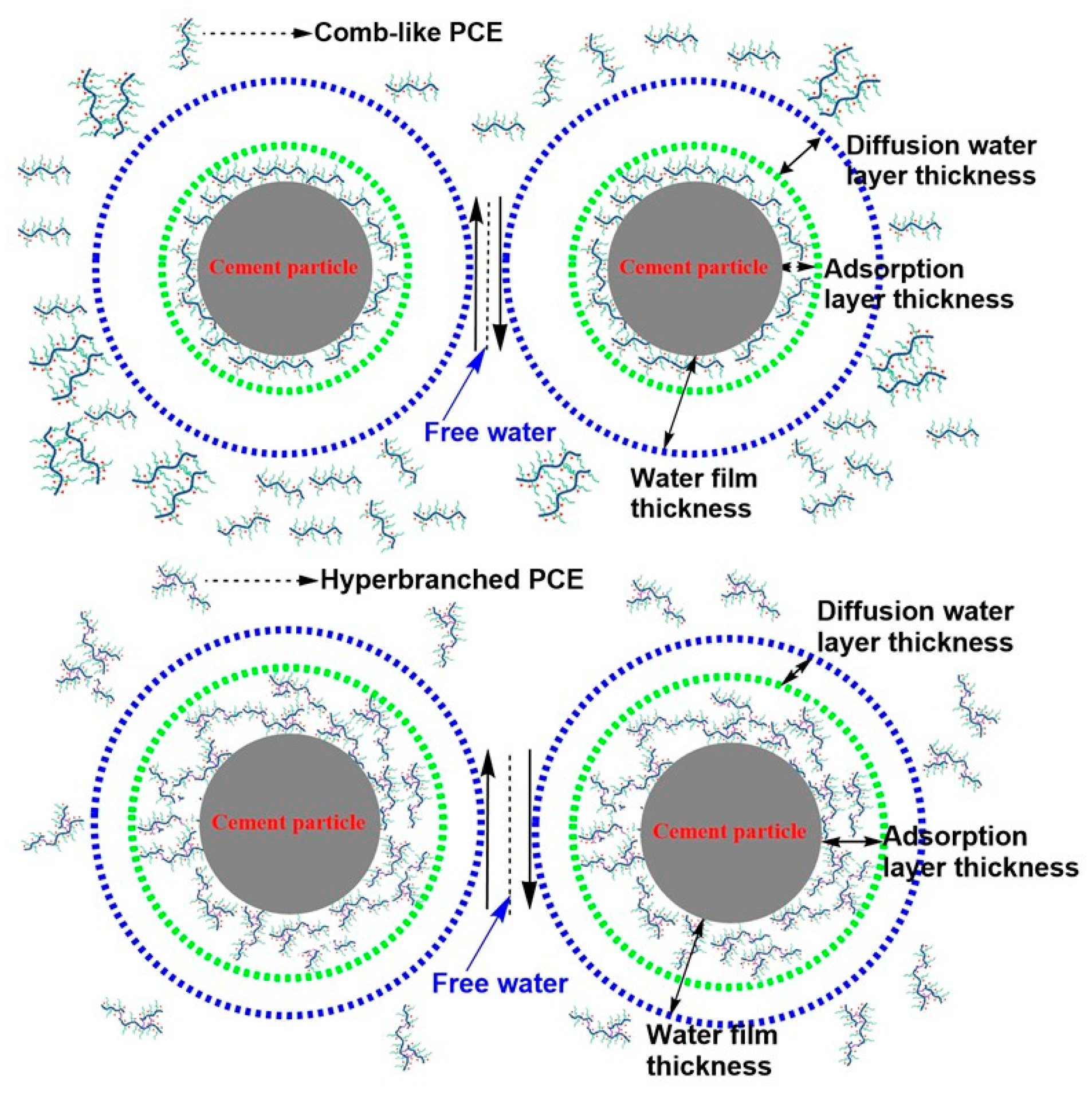
3.9. Viscosity-Reducing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, H.; Plank, J.; Sun, Z. Investigation on the optimal chemical structure of methacrylate ester based polycarboxylate superplasticizers to be used as cement grinding aid under laboratory conditions: Effect of anionicity, side chain length and dosage on grinding efficiency, mortar workability and strength development. Constr. Build. Mater. 2019, 224, 1018–1025. [Google Scholar]
- Ma, B.; Qi, H.; Tan, H.; Su, Y.; Li, X.; Liu, X.; Li, C.; Zhang, T. Effect of aliphatic-based superplasticizer on rheological performance of cement paste plasticized by polycarboxylate superplasticizer. Constr. Build. Mater. 2020, 233, 117181. [Google Scholar] [CrossRef]
- Makul, N. Modern sustainable cement and concrete composites: Review of current status, challenges and guidelines. Sustain. Mater. Technol. 2020, 25, e155. [Google Scholar] [CrossRef]
- Revilla-Cuesta, V.; Skaf, M.; Faleschini, F.; Manso, J.M.; Ortega-López, V. Self-compacting concrete manufactured with recycled concrete aggregate: An overview. J. Clean. Prod. 2020, 262, 121362. [Google Scholar] [CrossRef]
- Yaphary, Y.L.; Lam, R.H.; Lau, D. Chemical technologies for modern concrete production. Procedia Eng. 2017, 172, 1270–1277. [Google Scholar] [CrossRef]
- Banfill, P.F.G. Additivity effects in the rheology of fresh concrete containing water-reducing admixtures. Constr. Build. Mater. 2011, 25, 2955–2960. [Google Scholar] [CrossRef]
- Haach, V.G.; Vasconcelos, G.; Lourenço, P.B. Influence of aggregates grading and water/cement ratio in workability and hardened properties of mortars. Constr. Build. Mater. 2011, 25, 2980–2987. [Google Scholar] [CrossRef]
- Živica, V. Effects of the very low water/cement ratio. Constr. Build. Mater. 2009, 23, 3579–3582. [Google Scholar] [CrossRef]
- Houst, Y.F.; Bowen, P.; Perche, F.; Kauppi, A.; Borget, P.; Galmiche, L.; Le Meins, J.-F.; Lafuma, F.; Flatt, R.J.; Schober, I.; et al. Design and function of novel superplasticizers for more durable high performance concrete (superplast project). Cem. Concr. Res. 2008, 38, 1197–1209. [Google Scholar] [CrossRef]
- Erdoǧdu, Ş. Effect of retempering with superplasticizer admixtures on slump loss and compressive strength of concrete subjected to prolonged mixing. Cem. Concr. Res. 2005, 35, 907–912. [Google Scholar] [CrossRef]
- Huang, Z.; Yang, Y.; Ran, Q.; Liu, J. Preparing hyperbranched polycarboxylate superplasticizers possessing excellent viscosity-reducing performance through in situ redox initialized polymerization method. Cem. Concr. Compos. 2018, 93, 323–330. [Google Scholar] [CrossRef]
- Lombois-Burger, H.; Colombet, P.; Halary, J.L.; Van Damme, H. On the frictional contribution to the viscosity of cement and silica pastes in the presence of adsorbing and non adsorbing polymers. Cem. Concr. Res. 2008, 38, 1306–1314. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Liu, J.; Han, F.; Lin, W. Effect of Superplasticizers on Apparent Viscosity of Cement-Based Material with a Low Water—Binder Ratio. J. Mater. Civil. Eng. 2016, 28, 4016085. [Google Scholar] [CrossRef]
- He, Y.; Zhang, G.; He, S.; Liu, S.; Jiang, M. Effect of C-S-H-PCE and TEA on performances of lithium slag-cement binder. J. Build. 2023, 78, 107659. [Google Scholar] [CrossRef]
- Ran, Q.; Ma, J.; Wang, T.; Zhao, H.; Song, F.; Fan, S.; Yang, Y.; Lyu, Z.; Liu, J. Synthesis, characterization and dispersion properties of a series of bis(phosphonic acid)amino-terminated polymers. Colloid Polym. Sci. 2016, 294, 189–198. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Hooton, R.; Zhang, X.; He, S. Effects of TEA on rheological property and hydration performance of lithium slag-cement composite binder. Constr. Build. Mater. 2022, 318, 125757. [Google Scholar] [CrossRef]
- Hot, J.; Bessaies-Bey, H.; Brumaud, C.; Duc, M.; Castella, C.; Roussel, N. Adsorbing polymers and viscosity of cement pastes. Cem. Concr. Res. 2014, 63, 12–19. [Google Scholar] [CrossRef]
- He, Y.; Zhang, X.; Hooton, R.D. Effects of organosilane-modified polycarboxylate superplasticizer on the fluidity and hydration properties of cement paste. Constr. Build. Mater. 2017, 132, 112–123. [Google Scholar] [CrossRef]
- Kwan, A.K.H.; Li, L.G. Combined effects of water film, paste film and mortar film thicknesses on fresh properties of concrete. Constr. Build. Mater. 2014, 50, 598–608. [Google Scholar] [CrossRef]
- Liu, X.; Guan, J.; Lai, G.; Wang, Z.; Zhu, J.; Cui, S.; Lan, M.; Li, H. Performances and working mechanism of a novel polycarboxylate superplasticizer synthesized through changing molecular topological structure. J. Colloid Interf. Sci. 2017, 54, 12–24. [Google Scholar] [CrossRef]
- He, Y.; Ding, H.; He, S.; Jiang, M.; Hooton, R. Influence of β-cyclodextrin modified PCE on fluidity and hydration behavior of cement paste. Colloid Surface A. 2024, 688, 133512. [Google Scholar] [CrossRef]
- Amin, A.; Darweesh, H.H.M.; Ramadan, A.M.; Morsi, S.M.M.; Ayoub, M.M.H. Employing of some hyperbranched polyesteramides as new polymeric admixtures for cement. J. Appl. Polym. Sci. 2011, 121, 309–320. [Google Scholar] [CrossRef]
- Liu, X.; Guan, J.; Lai, G.; Zheng, Y.; Wang, Z.; Cui, S.; Lan, M.; Li, H. Novel designs of polycarboxylate superplasticizers for improving resistance in clay-contaminated concrete. J. Ind. Eng. Chem. 2017, 55, 80–90. [Google Scholar] [CrossRef]
- Navarro-Blasco, I.; Pérez-Nicolás, M.; Fernández, J.M.; Duran, A.; Sirera, R.; Alvarez, J.I. Assessment of the interaction of polycarboxylate superplasticizers in hydrated lime pastes modified with nanosilica or metakaolin as pozzolanic reactives. Constr. Build. Mater. 2014, 73, 1–12. [Google Scholar] [CrossRef]
- Fréchet, J.M.J.; Hawker, C.J. Hyperbranched polyphenylene and hyperbranched polyesters: New soluble, three-dimensional, reactive polymers. React. Funct. Polym. 1995, 26, 127–136. [Google Scholar] [CrossRef]
- Grinshpun, V.; Rudin, A. Measurement of Mark-Houwink constants by size exclusion chromatography with a low angle laser light scattering detector. Die Makromol. Chem. Rapid Commun. 1985, 6, 219–223. [Google Scholar] [CrossRef]
- Stecher, J.; Plank, J. Novel concrete superplasticizers based on phosphate esters. Cem. Concr. Res. 2019, 119, 36–43. [Google Scholar] [CrossRef]
- Tramaux, A.; Azéma, N.; El Bitouri, Y.; David, G.; Negrell, C.; Poulesquen, A.; Haas, J.; Remond, S. Diphosphonated comb-like copolymers synthesis as suspensions dispersants and their resistance to ionic competition phenomenon. Powder Technol. 2018, 335, 334–343. [Google Scholar] [CrossRef]
- Plank, J.; Sakai, E.; Miao, C.W.; Yu, C.; Hong, J.X. Chemical admixtures—Chemistry, applications and their impact on concrete microstructure and durability. Cem. Concr. Res. 2015, 78, 81–99. [Google Scholar] [CrossRef]
- He, Y.; Zhang, X.; Hooton, R.; Wang, Y.; Kong, Y.; Wang, X.; Wang, X. Influence of PCE on rheological and hydration performances of cement paste. J. Mater. Civil Eng. 2020, 32, 04020002. [Google Scholar] [CrossRef]
- Ilg, M.; Plank, J. Effect of non-ionic auxiliary dispersants on the rheological properties of mortars and concretes of low water-to-cement ratio. Constr. Build. Mater. 2020, 259, 119780. [Google Scholar] [CrossRef]
- Altun, M.G.; Özen, S.; Mardani-Aghabaglou, A. Effect of side chain length change of polycarboxylate-ether based high range water reducing admixture on properties of self-compacting concrete. Constr. Build. Mater. 2020, 246, 118427. [Google Scholar] [CrossRef]
- Ran, Q.; Somasundaran, P.; Miao, C.; Liu, J.; Wu, S.; Shen, J. Effect of the length of the side chains of comb-like copolymer dispersants on dispersion and rheological properties of concentrated cement suspensions. J. Colloid Interf. Sci. 2009, 336, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, X.; Zhang, Y. Effect of free water on the flowability of cement paste with chemical or mineral admixtures. Constr. Build. Mater. 2016, 111, 571–579. [Google Scholar] [CrossRef]
- Ye, H.; Gao, X.; Wang, R.; Wang, H. Relationship among particle characteristic, water film thickness and flowability of fresh paste containing different mineral admixtures. Constr. Build. Mater. 2017, 153, 193–201. [Google Scholar] [CrossRef]
- Krieger, I.M.; Dougherty, T.J. A Mechanism for Non-Newtonian Flow in Suspensions of Rigid Spheres. Trans. Soc. Rheol. 1959, 3, 137–152. [Google Scholar] [CrossRef]
- Hodnea, H.; Saasen, A. The effect of the cement zeta potential and slurry conductivity on the consistency of oilwell cement slurries. Cem. Concr. Res. 2000, 30, 1767–1772. [Google Scholar] [CrossRef]

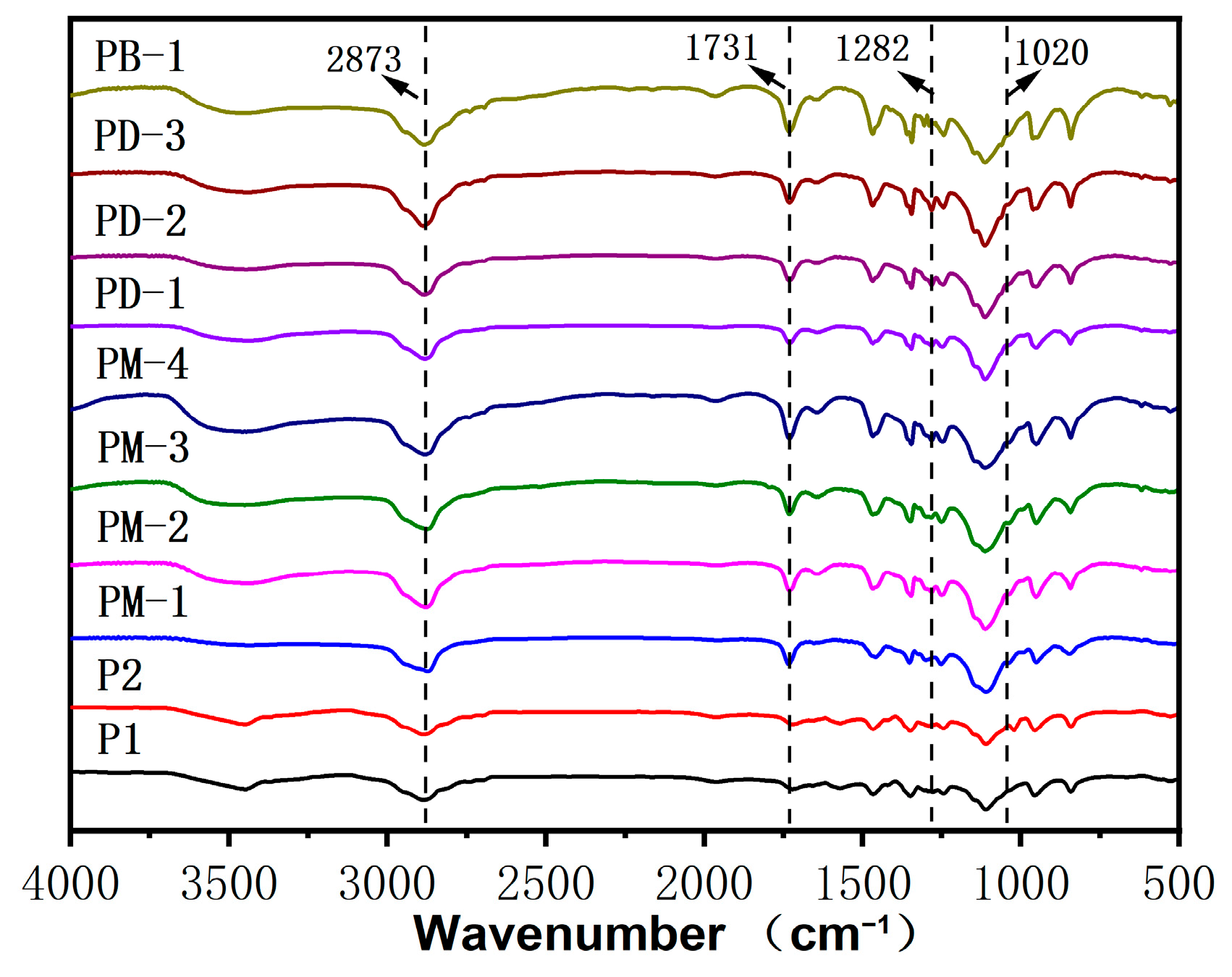
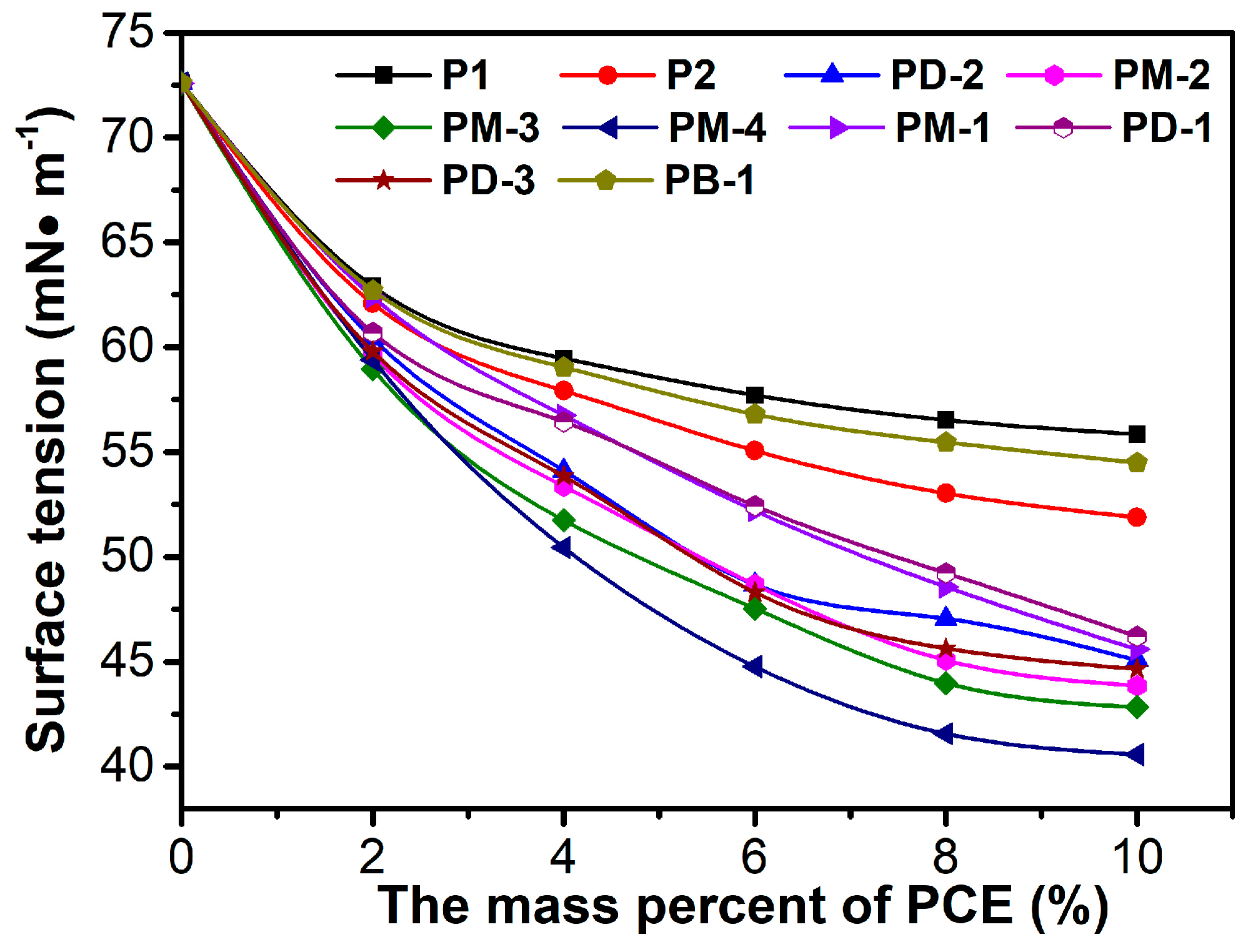
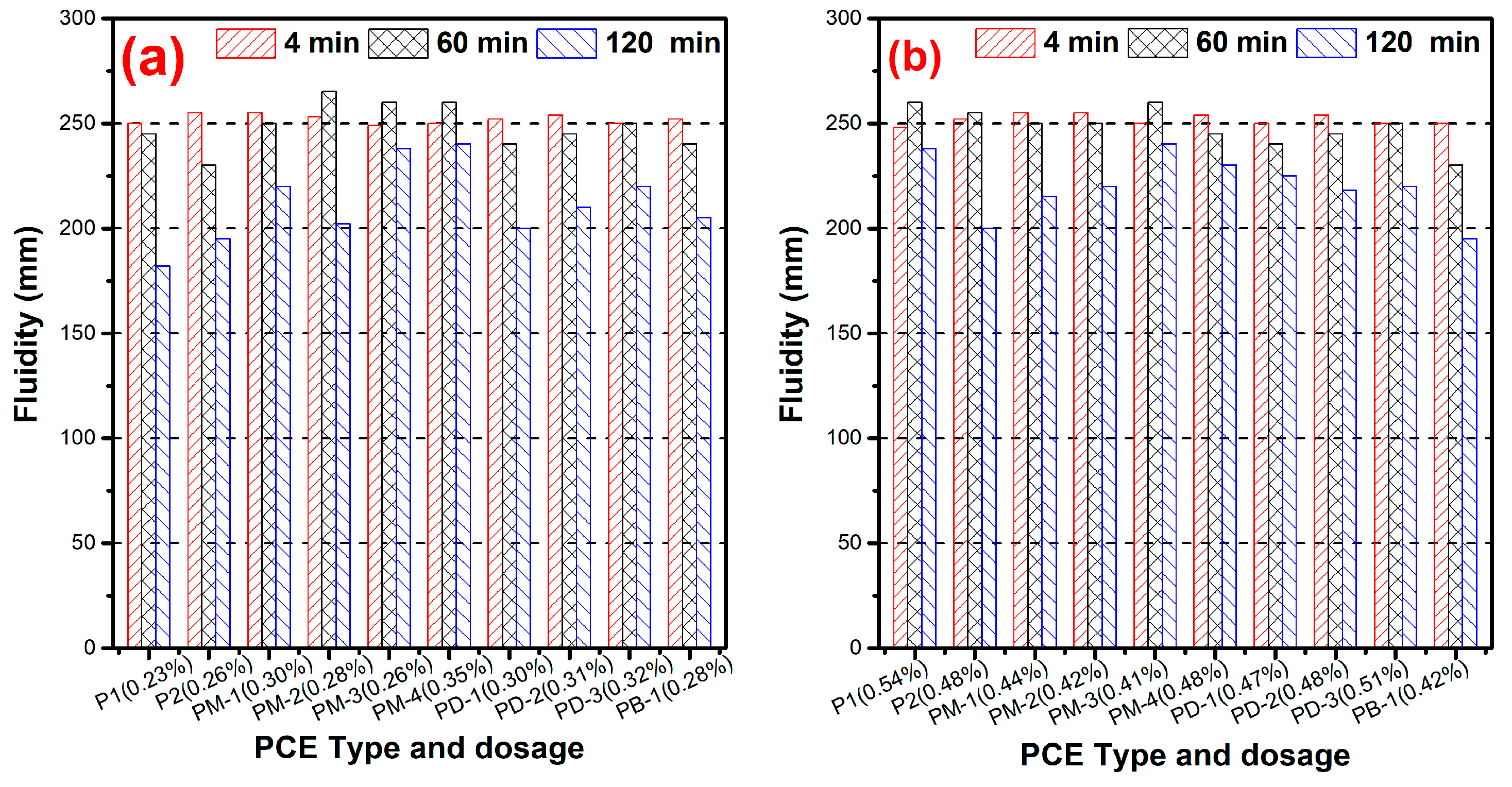
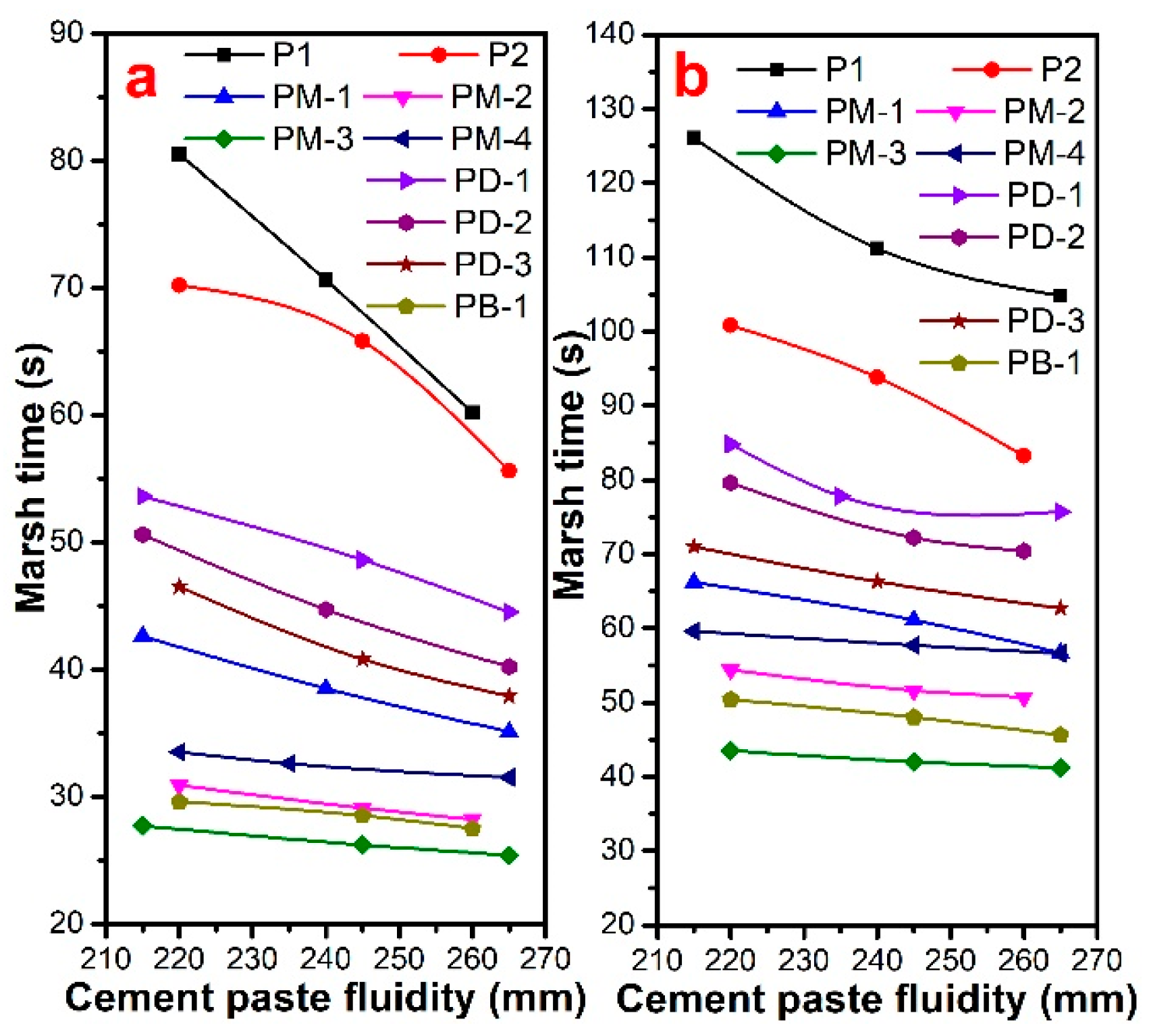


| Samples | Phosphate Monoester/% | Phosphate Diester/% | Free Phosphoric Acid/% | Acid Value 1 | Acid Value 2 |
|---|---|---|---|---|---|
| MOEP-1 | 37.09 | 59.87 | 3.04 | 208.07 | 76.14 |
| MOEP-2 | 46.18 | 49.53 | 4.30 | 181.54 | 91.60 |
| MOEP-3 | 43.88 | 37.92 | 18.19 | 272.43 | 169.12 |
| HPEP | 33.33 | 63.94 | 2.74 | 90.75 | 32.73 |
| Al2O3 (%) | CaO (%) | Fe2O3 (%) | K2O (%) | MgO (%) | Na2O (%) | SO3 (%) | SiO2 (%) | TiO2 (%) |
|---|---|---|---|---|---|---|---|---|
| 4.94 | 63.05 | 2.92 | 0.66 | 1.33 | 0.15 | 3.83 | 19.95 | 0.27 |
| PCE Type | Phosphate Ester (g) | MBA (g) | TGA (g) | Mn (104 g/mol) | Mw (104 g/mol) | PDI | [η] (dL/g) | Mark–Houwink α | Tc (°C) | Tm (°C) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MOEP-1 | MOEP-2 | MOEP-3 | HPEP | ||||||||||
| P1 | - | - | - | - | - | 0.36 | 3.42 | 5.41 | 1.58 | 0.31 | 0.52 | 18.6 | 43.8 |
| P2 | - | - | - | - | - | 0.40 | 3.35 | 5.36 | 1.60 | 0.26 | 0.44 | 22.1 | 45.5 |
| PM-1 | 3.62 | - | - | - | - | 0.48 | 3.38 | 5.58 | 1.65 | 0.25 | 0.40 | 23.2 | 44.8 |
| PM-2 | - | 3.44 | - | - | - | 0.50 | 3.53 | 6.99 | 1.98 | 0.18 | 0.34 | 23.9 | 46.4 |
| PM-3 | - | - | 4.29 | - | - | 0.55 | 3.68 | 7.80 | 2.12 | 0.16 | 0.30 | 26.4 | 46.9 |
| PM-4 | - | - | - | 5.72 | - | 0.46 | 3.46 | 6.37 | 1.84 | 0.19 | 0.33 | 26.4 | 45.7 |
| PD-1 | - | 2.78 | - | - | - | 0.46 | 3.45 | 5.69 | 1.65 | 0.25 | 0.42 | 26.4 | 49.2 |
| PD-2 | - | - | 2.30 | - | - | 0.42 | 3.41 | 6.34 | 1.86 | 0.24 | 0.38 | 25.9 | 50.2 |
| PD-3 | - | - | - | 2.71 | - | 0.42 | 3.65 | 6.02 | 1.65 | 0.22 | 0.36 | 25.8 | 48.2 |
| PB-1 | - | - | - | - | 1.23 | 0.48 | 3.69 | 7.05 | 1.91 | 0.17 | 0.32 | 27.1 | 50.8 |
| PCE Type | Concentration before Adsorption | Concentration after Adsorption | Adsorption Amount (mg/g) | Adsorption Efficiency | ||||
|---|---|---|---|---|---|---|---|---|
| w/c = 0.29 | w/c = 0.20 | w/c = 0.29 | w/c = 0.20 | w/c = 0.29 | w/c = 0.20 | w/c = 0.29 | w/c = 0.20 | |
| P1 | 0.23% | 0.54% | 0.16% | 0.46% | 0.28 | 0.32 | 30.4% | 14.8% |
| P2 | 0.26% | 0.48% | 0.18% | 0.34% | 0.32 | 0.40 | 30.8% | 20.8% |
| PM-1 | 0.30% | 0.44% | 0.20% | 0.33% | 0.40 | 0.44 | 33.3% | 25.0% |
| PM-2 | 0.28% | 0.42% | 0.15% | 0.28% | 0.52 | 0.56 | 46.4% | 33.3% |
| PM-3 | 0.26% | 0.41% | 0.08% | 0.21% | 0.72 | 0.80 | 69.2% | 48.8% |
| PM-4 | 0.35% | 0.48% | 0.17% | 0.29% | 0.72 | 0.76 | 51.4% | 39.6% |
| PD-1 | 0.30% | 0.42% | 0.20% | 0.30% | 0.40 | 0.48 | 33.3% | 28.6% |
| PD-2 | 0.31% | 0.47% | 0.19% | 0.34% | 0.48 | 0.52 | 38.7% | 27.7% |
| PD-3 | 0.32% | 0.48% | 0.17% | 0.32% | 0.60 | 0.64 | 46.9% | 33.3% |
| PB-1 | 0.28% | 0.51% | 0.09% | 0.30% | 0.76 | 0.84 | 67.9% | 41.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Yang, C.; He, Y.; Wang, F.; Zeng, C. Effects and Mechanism of Hyperbranched Phosphate Polycarboxylate Superplasticizers on Reducing Viscosity of Cement Paste. Materials 2024, 17, 1896. https://doi.org/10.3390/ma17081896
Chen J, Yang C, He Y, Wang F, Zeng C. Effects and Mechanism of Hyperbranched Phosphate Polycarboxylate Superplasticizers on Reducing Viscosity of Cement Paste. Materials. 2024; 17(8):1896. https://doi.org/10.3390/ma17081896
Chicago/Turabian StyleChen, Jing, Changhui Yang, Yan He, Futao Wang, and Chao Zeng. 2024. "Effects and Mechanism of Hyperbranched Phosphate Polycarboxylate Superplasticizers on Reducing Viscosity of Cement Paste" Materials 17, no. 8: 1896. https://doi.org/10.3390/ma17081896
APA StyleChen, J., Yang, C., He, Y., Wang, F., & Zeng, C. (2024). Effects and Mechanism of Hyperbranched Phosphate Polycarboxylate Superplasticizers on Reducing Viscosity of Cement Paste. Materials, 17(8), 1896. https://doi.org/10.3390/ma17081896





