Abstract
Zircaloy-4 is extensively used in nuclear reactors as fuel element cladding and core structural material. However, the safety concerns post-Fukushima underscore the need for further enhancing its high-temperature and high-pressure water-side corrosion resistance. Therefore, this study aimed to investigate the effects of high-current pulsed electron beam (HCPEB) irradiation on the microstructures and corrosion resistance of Zircaloy-4, with the goal of improving its performance in nuclear applications. Results showed that after irradiation, the cross-section of the sample could be divided into three distinct layers: the outermost melted layer (approximately 4.80 μm), the intermediate heat-affected zone, and the bottom normal matrix. Large numbers of twin martensites were induced within the melted layer, which became finer with increasing irradiation times. Additionally, plenty of ultrafine/nanoscale grains were observed on the surface of the sample pulsed 25 times. Zr(Fe, Cr)2 second-phase particles (SPPs) were dissolved throughout the modified layer and Fe and Cr elements were uniformly distributed under the action of HCPEB. As a result, the corrosion resistance of the sample pulsed 25 times was significantly improved compared to the initial one. Research results confirmed that HCPEB irradiation is an effective method in improving the service life of Zircaloy-4 under extreme environmental conditions.
1. Introduction
With the increasing scarcity of fossil fuels, nuclear energy that has the advantages of high efficiency, low carbon, and cleanliness has attracted widespread attention and become one of the most promising energy sources [1,2]. However, due to certain risks when using nuclear energy, nuclear safety has become an important issue around the globe [3]. Zircaloy-4, a zirconium-based alloy, possesses a unique combination of properties, such as a low neutron absorption cross-section, good high-temperature strength, and excellent high-temperature and high-pressure water-side corrosion resistance, making it ideal for use in nuclear reactors, particularly as cladding material in fuel assemblies. Its critical role in ensuring the performance, safety, and longevity of nuclear reactors cannot be overstated [4,5]. While the Fukushima nuclear accident was caused, at least in part, by the reacted between zirconium alloy and high-temperature steam, which released a large amount of hydrogen gas that triggered a hydrogen explosion, leading to a series of serious consequences. Therefore, it is important to explore effective ways to improve the corrosion resistance of Zircaloy-4 against high-temperature and high-pressure water-side corrosion to further promote the safety of nuclear reactors.
At present, researchers mainly improve the corrosion resistance of zirconium alloys through the following approaches: developing new types of zirconium alloys [6,7], adjusting thermal processing techniques [8,9], and surface modification of existing zirconium alloys [10,11,12,13,14,15,16]. Compared to the other two ways, surface modification methods have garnered the most discussion and research among scholars as they do not affect the comprehensive performance of the alloy matrix and have various methods. Among the surface modification methods for zirconium alloys, the most common is surface coating technology [10,11], followed by laser surface treatment [12], ion implantation [13,14,15], ion irradiation [16], etc. However, each method inevitably has certain issues. For instance, surface coatings will increase the material’s thickness and reduce the thermal conductivity of zirconium alloys, and there are concerns about the adhesion between the coating and the matrix; laser treatment technology has issues such as a small beam spot, low effective energy utilization, and susceptibility to oxidation of the modified surface; and ion beams have limitations such as limited penetration depth and the high cost of equipment.
In recent years, a high-current pulsed electron beam (HCPEB) surface modification technology with the advantages of high energy utilization, large depth of penetration, low cost, high efficiency, simplicity, and reliability has been gradually developed and has attracted more and more scholars’ attention. Its main characteristic is that under vacuum conditions, the accelerating voltage accelerates the electrons emitted by the electron gun and converges them into a high-energy electron beam (with an energy density up to 108–109 W/cm2). Within a few microseconds or even nanoseconds, this beam penetrates the material surface, causing rapid heating (at a rate up to 109 K/s), melting, or even vaporization. Subsequently, it relies on the heat conduction of the substrate itself to cool down (at a rate up to 107 K/s) and solidify (at speeds of 2–10 m/s). At the same time, the extremely high temperature gradient on the material surface (up to 108 K/m) also induces high-magnitude thermal stresses (up to hundreds of MPa-GPa). Under the combined action of the non-equilibrium temperature field and the dynamic thermal stress field, the material surface can achieve special modification effects that are difficult to achieve with conventional surface modification methods, such as purification and homogenization of the sample surface, formation of a large number of fine crystals, supersaturated solid solutions, crystal defects, deformed structures, etc. [17,18,19,20,21,22]. A large number of previous studies have shown that HCPEB technology is an effective method to improve the surface corrosion resistance of various materials [23,24,25,26,27,28,29,30,31,32,33,34].
In view of this, the authors also attempted to use HCPEB to modify the surface of Zircaloy-4 in a previous work [35]. However, due to the limitation of the test conditions at that time, only the electrochemical workstation was used to test the corrosion resistance of the samples before and after modification at room temperature. Considering the actual service conditions of Zircaloy-4, in the present work, the corrosion resistance of the sample after HCPEB modification was investigated under 500 °C/10.3 MPa superheated steam, and the microstructures and corrosion behavior after irradiation will be discussed in detail by means of more observation and analysis with a transmission electron microscope (TEM). The research results will provide the necessary theoretical and experimental data for the study of improving the service life of Zircaloy-4 under extreme environmental conditions through HCPEB surface modification.
2. Materials and Methods
An annealed Zircaloy-4 plate (Sn 1.38, Fe 0.22, Cr 0.12, Zr balance, wt%) was used in this study. Initially, the plate was cut into numerous 10 mm × 10 mm × 6 mm samples. Then, one of the 10 × 10 mm surfaces of each sample was sanded and polished for use. Subsequently, the irradiation treatment was carried out on the polished surfaces using the “HOPE-I”-type HCPEB equipment. More details about the principles of the HCPEB equipment are in references [20,22]. The irradiation parameters used are shown in Table 1. A corrosion test of samples before and after surface modification was examined by using a high-temperature and high-pressure static reactor in the corrosion environment of 500 °C/10.3 MPa superheated steam, and the corrosion time was 24 h. After surface modification and corrosion test, X-ray diffractometry (XRD, Rigaku D/max-2500/pc, Tokyo, Japan, CuKα, scan speed: 4°/min) was employed to examine the surface phase structures of the samples, a scanning electron microscope (SEM, JEOL, JSM-7800 F, Tokyo, Japan) was utilized to analyze the surface and cross-section morphologies of the samples, a transmission electron microscope (TEM, JEOL, JEM-2100 F, Tokyo, Japan, equipped with a scanning TEM (STEM) attachment and an energy dispersive spectrometer (EDS)) was adopted to study the internal structures within the modified layers and the oxide films. The TEM samples of the oxide films were prepared by focused ion beam (FIB).

Table 1.
HCPEB irradiation parameters.
3. Results
3.1. XRD Analysis
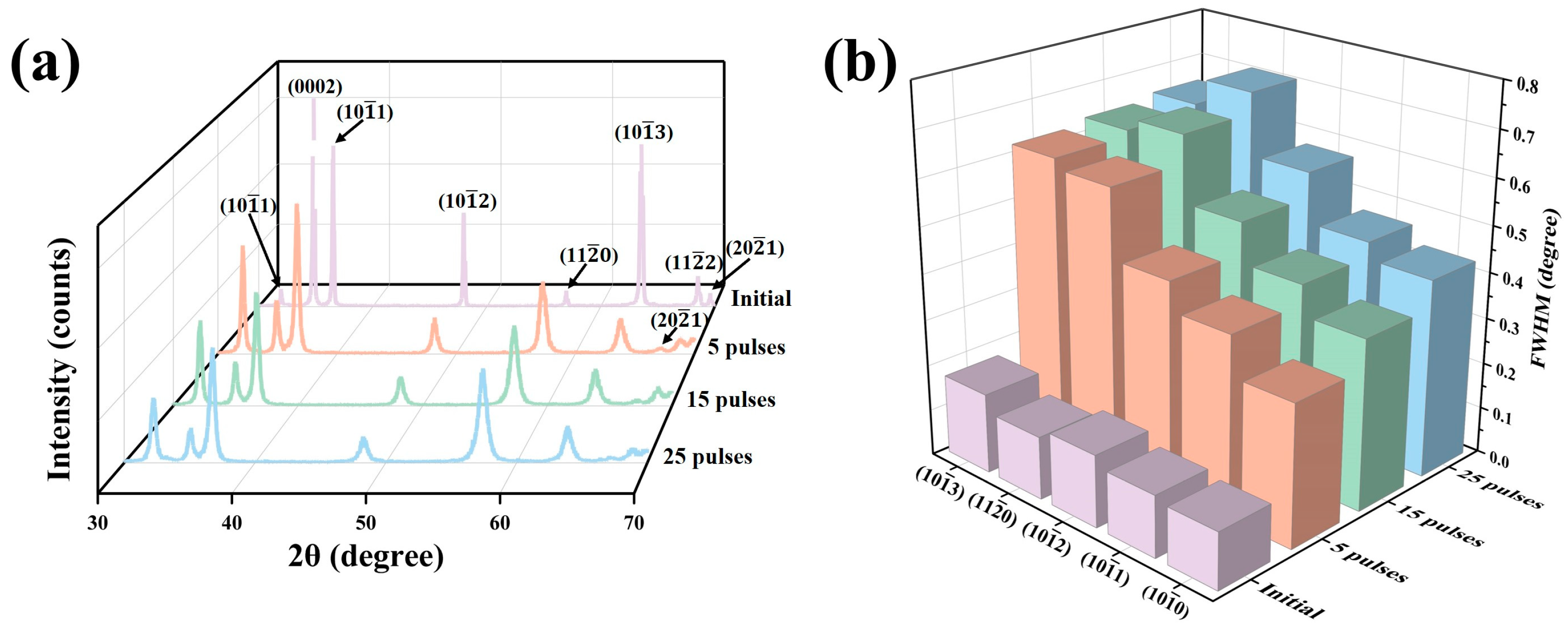
Figure 1a shows the XRD patterns of the sample surfaces before and after HCPEB irradiation. During the process of HCPEB irradiation, the instantaneous high temperature melted the surface layer. However, due to the extremely short melting and cooling time, the rapid cooling process induced a martensitic transformation, forming a large number of α′ martensites [36]. Because the α′-Zr phase has the same crystal structure as the α-Zr phase, it could not be accurately identified here and will be further explored in the following text [37,38,39]. Figure 1b shows the change regularities of the full width at half maximum (FWHM) for partial crystal planes before and after HCPEB irradiation. It can be clearly seen that compared with the initial sample, the XRD diffraction peaks of the irradiated samples were significantly broadened, and the FWHM increased gradually with the increase in irradiation times, indicating that the substructures within the surface layer were refined continuously under the action of the HCPEB irradiation [40,41,42].
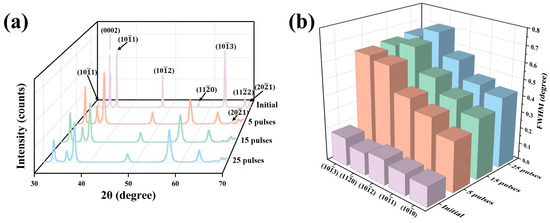
Figure 1.
(a) XRD patterns of the sample surfaces before and after HCPEB irradiation, (b) change regularities of the FWHM for partial crystal planes before and after HCPEB irradiation.
3.2. Microstructural Analysis
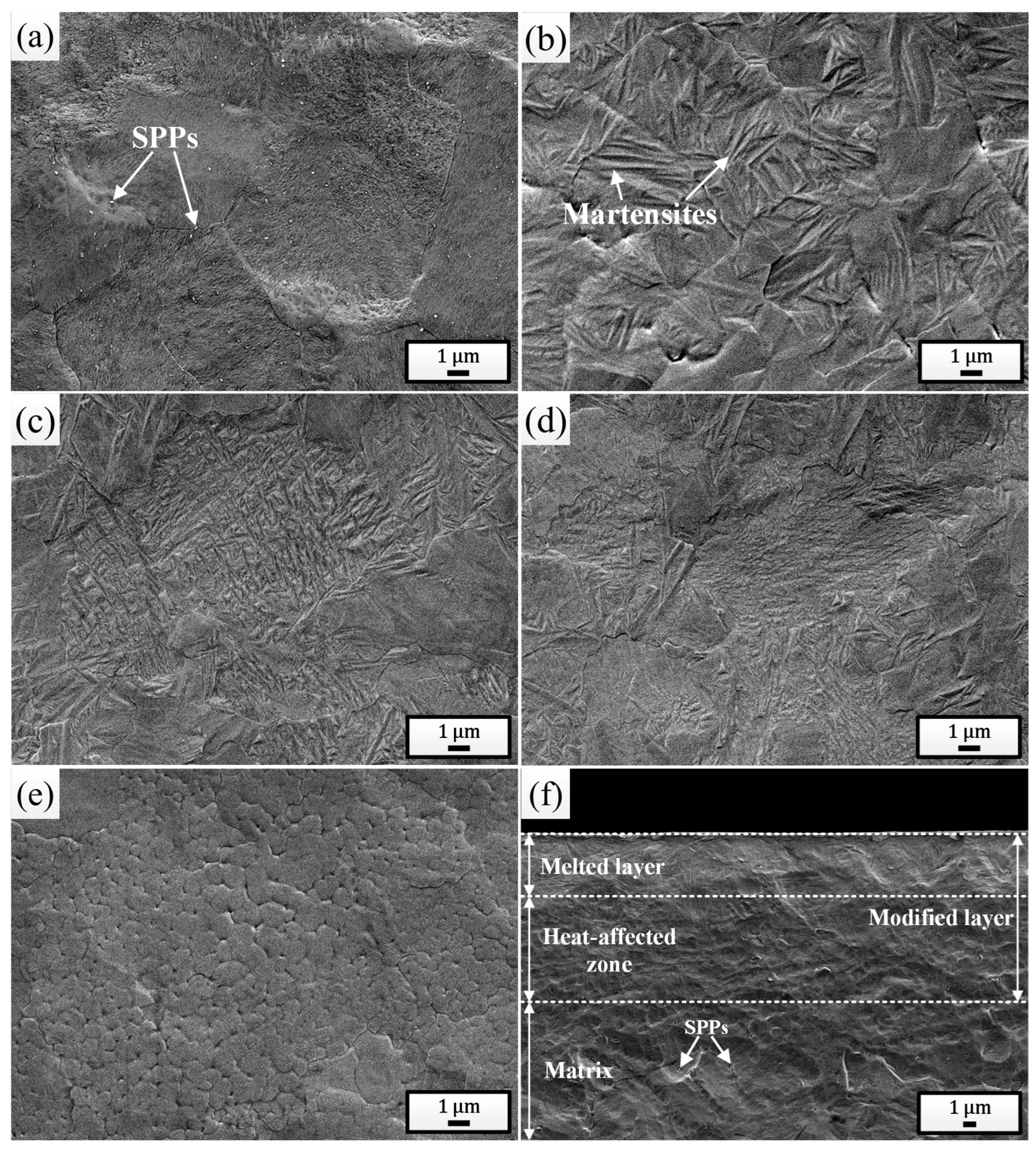
Figure 2a shows the surface SEM morphology of the initial sample. Prior to observation, the polished surface was etched using a metallographic corrosion solution consisting of 10% HF + 45% HNO3 + 45% H2O (volume fraction). It can be clearly seen that the sample surface was composed of α-Zr grains with a large number of Zr(Fe, Cr)2 secondary phase particles (SPPs) distributed within them and along the grain boundaries, and other microstructures were not found. Figure 2b shows the surface SEM morphology of the sample pulsed 5 times. It is evident that a significant amount of martensites were formed on the irradiated surface with an average width of 0.11 μm, confirming the occurrence of martensitic phase transformation. With increase in irradiation times (Figure 2c–d), the martensites became finer and finer [43,44]. After irradiation with 25 pulses, the average width of martensites was as low as 0.02 μm. Additionally, large numbers of ultrafine grains were also observed on the surface of the sample pulsed 25 times, with an average size of 0.49 μm, as shown in Figure 2e. This is due to the increased heat accumulation in the surface layer, which extended the melting time to some extent, promoting the nucleation of local primary grains, whereas limited cooling time prevented these grains from growing larger, ultimately returning to room temperature [45]. It can also be observed that martensites were rarely found within these ultrafine grains, as the martensitic transformation would be inhibited when the grain size was sufficiently small [46]. Figure 2f provides the cross-sectional SEM morphology of the sample pulsed 25 times. It can be seen clearly that the cross-section was composed of a distinct three-layer structure: the outermost white, bright melted layer (approximately 4.80 μm), the intermediate heat-affected zone, and the bottom normal matrix. It is also noticeable that the SPPs were almost absent in both the melted layer and the heat-affected zone, indicating that under the action of HCPEB, the Zr(Fe, Cr)2 SPPs were significantly dissolved. Especially for the heat-affected zone, although the heat conducted to this layer was not sufficient to melt the matrix, it was enough to cause the dissolution of the SPPs.
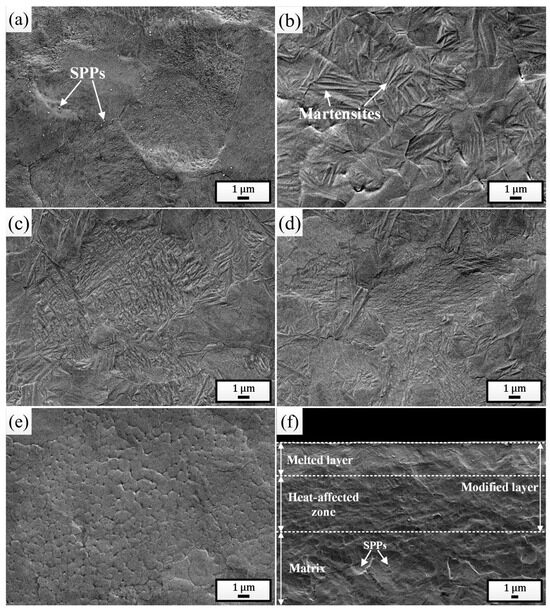
Figure 2.
Surface SEM morphologies of the initial sample (a) and the samples pulsed 5 times (b), 15 times (c), and 25 times (d,e). (f) Cross-sectional SEM morphology of the sample pulsed 25 times.
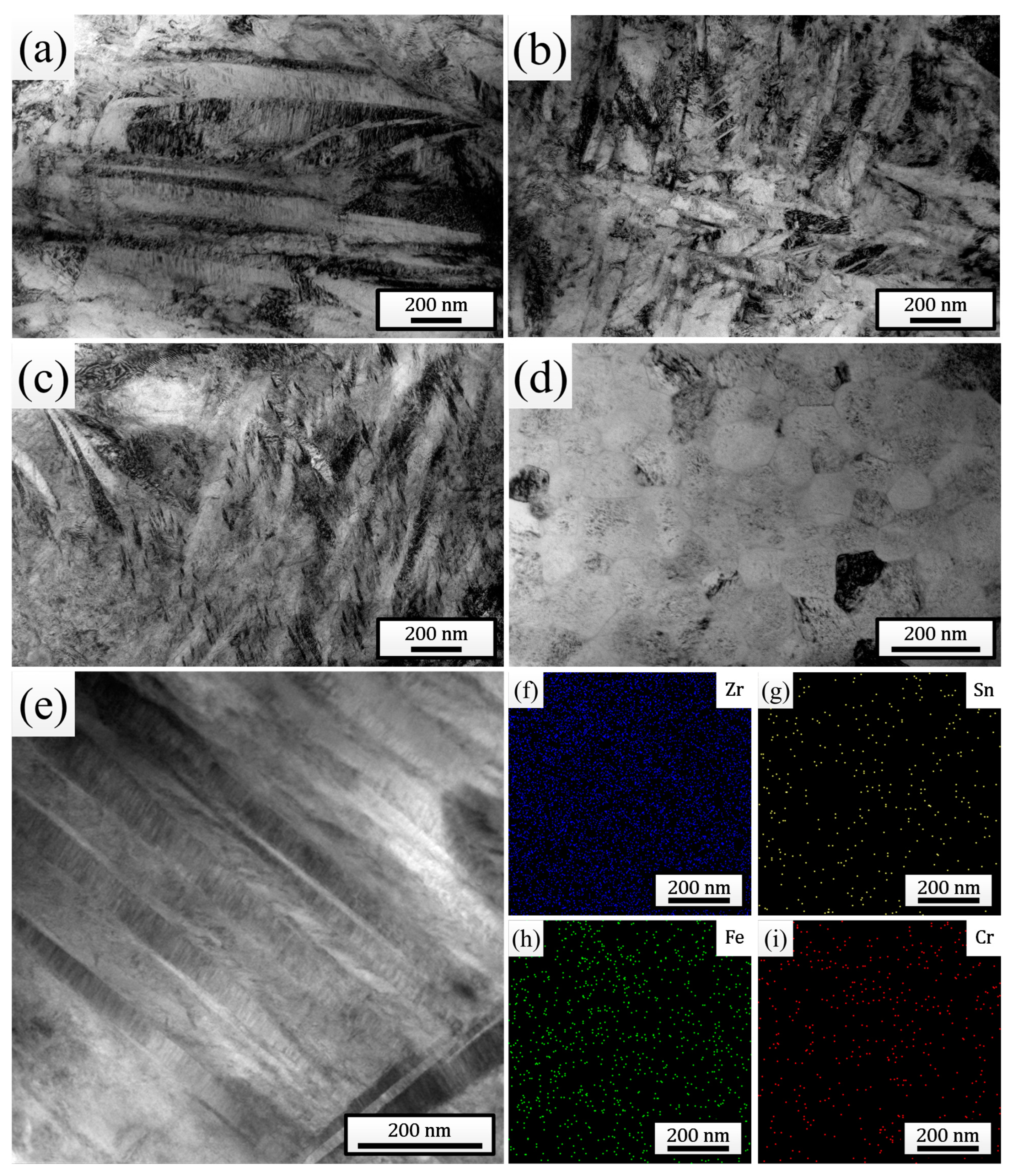
Figure 3a–d show the TEM bright-field morphologies of the melted layers after different times of HCPEB irradiation. The observation results were completely consistent with the XRD and SEM analysis results, both clearly indicating the dissolution of the SPPs and the continuous refinement of the martensites after irradiation. For the sample pulsed 25 times, a large number of nanoscale martensites with widths even lower than 10 nm were distinctly observed (Figure 3c). In addition, plenty of twin substructures were found within the martensitic plates, indicating that the martensitic type within the melted layer was mainly twin martensite. Additionally, for the sample pulsed 25 times, plenty of nanoscale grains with sizes less than 100 nm (further refined compared to that observed in Figure 2e, with an average size of 70.50 nm) were also observed (Figure 3d), within which martensites indeed could not be found, confirming the point in the previous paragraph. To study the distribution of elements inside the melted layer, a local area of the sample pulsed 25 times was selected for EDS mapping analysis (Figure 3e), and the results are shown in Figure 3f–i. It can be clearly seen that all the elements dissolved in the matrix were distributed uniformly, and the phenomenon of local evident aggregation did not occur, indicating that HCPEB irradiation promoted the uniform distribution of elements within the sample surface.
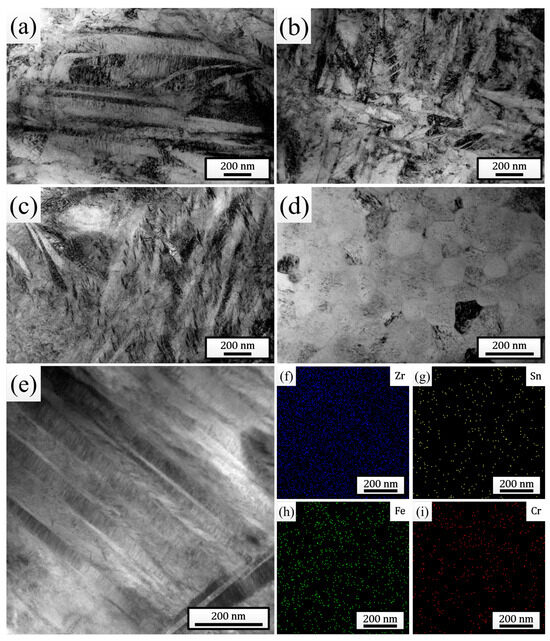
Figure 3.
TEM bright-field morphologies of the melted layers after irradiation with 5 pulses (a), 15 pulses (b), and 25 pulses (c,d). (e) STEM bright-field morphology of the melted layer after 25 pulses of HCPEB irradiation. (f–i) EDS mapping results of elements in (e).
3.3. Corrosion Behavior
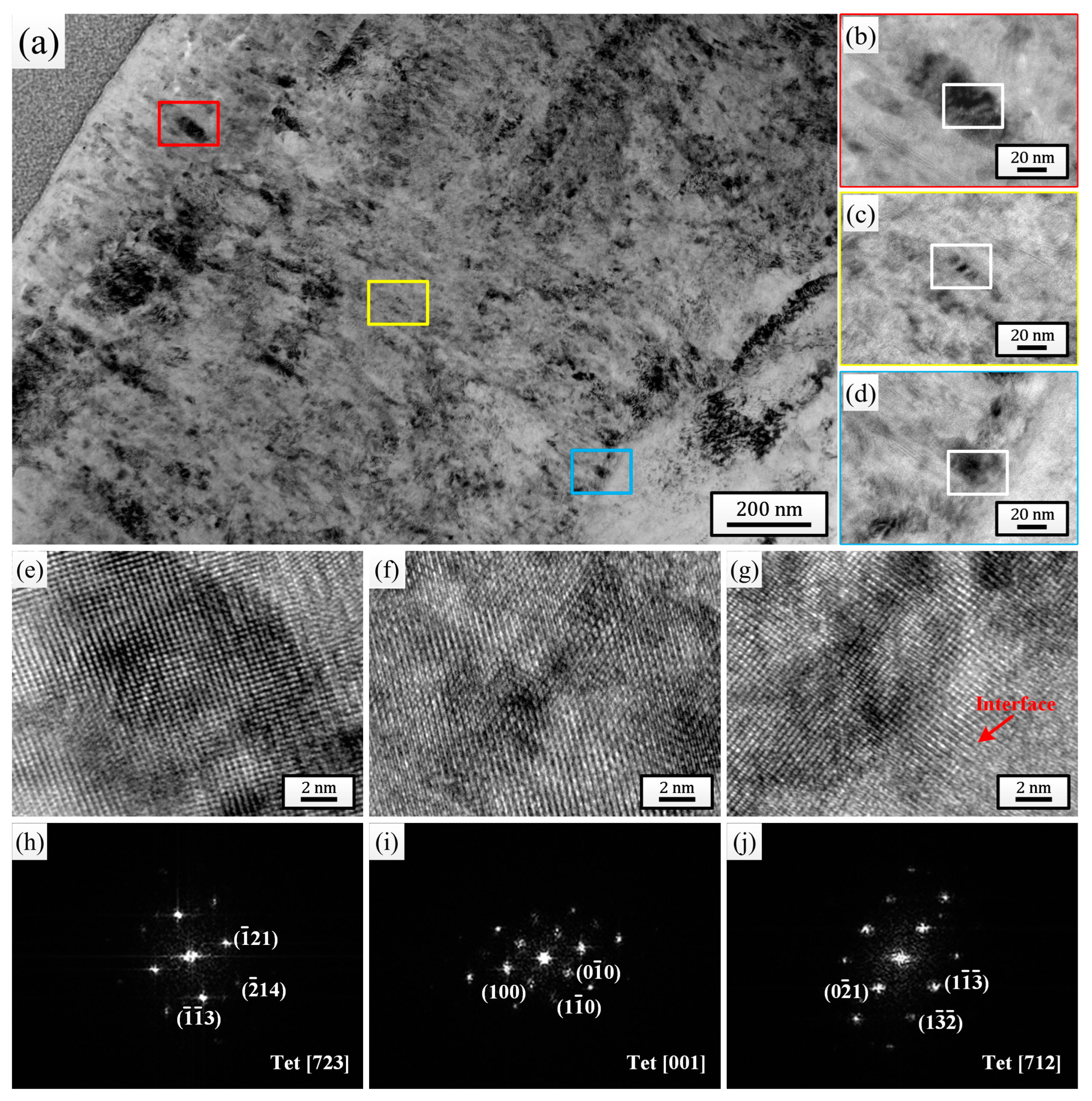
For the comprehensive microstructure analysis results, the sample pulsed 25 times was chosen to investigate the effect of HCPEB irradiation on the corrosion behavior of Zircaloy-4. After 24 h exposure to 500 °C/10.3 MPa superheated steam, the cross-sectional TEM bright-field morphologies of the oxide films grown on the initial sample (Figure 4) and the sample pulsed 25 times (Figure 5) were observed and analyzed. By comparing Figure 4a and Figure 5a, it can be observed that the thickness of the oxide film grown on the sample pulsed 25 times (approximately 1.57 μm) was evidently lower than that grown on the initial one (approximately 1.88 μm). And after only 24 h of corrosion, the outermost layers of both oxide films exhibited significant loose areas, characterized by the presence of many small-sized micro-pores and horizontal micro-cracks. But, it is evident that the thickness of the loose area for the sample pulsed 25 times was significantly lower than that of the initial one, and the size and quantity of micro-pores and micro-cracks within this area were also much lower. Of course, apart from the outermost layer, a small number of micro-pores and micro-cracks could also be observed in other areas of the oxide films for both samples, but without a doubt, the oxide film of the sample pulsed 25 times displayed a better compactness in every area. Figure 4b–d and Figure 5b–d are high-magnification images of the regions marked with red (outer layer), yellow (middle layer), and blue (bottom layer) squares corresponding to each figure, respectively. It is well known that the ZrO2 growing on the Zr matrix can mainly be divided into two types: m-ZrO2 and t-ZrO2. The t-ZrO2 phase possesses good chemical stability and low lattice mismatch, which can enhance the adhesion and crack resistance of the oxide film. This contributes to the formation of a uniform and compact oxide film, thereby enhancing the corrosion resistance of the zirconium alloys. In contrast, m-ZrO2 may lead to the embrittlement of the oxide film and the formation of cracks, resulting in a less compact oxide film and reducing the corrosion resistance of the zirconium alloys [47]. To test the phase composition of the ZrO2 grains at different oxide film depths for the two samples, high-resolution observation was performed on the regions marked with white squares in Figure 4b–d and Figure 5b–d, and the high-resolution TEM (HRTEM) images and their corresponding fast Fourier transform (FFT) images are shown in Figure 4e–j and Figure 5e–j, respectively. It can be seen that for the initial sample, the grains selected at the outer layer, middle layer, and oxide/matrix (O/M) interface were all m-ZrO2 phase, while for the sample pulsed 25 times, the grains selected were all the t-ZrO2 phase. This result further confirmed that the sample pulsed 25 times had better high-temperature and high-pressure corrosion resistance. Moreover, the dispersively distributed white, bright lines between the diffraction spots in Figure 4h–j could intuitively indicate that there existed many stacking faults in the selected grains, while almost no bright lines are observed between the diffraction spots in Figure 5h–j, indicating that there existed fewer defects inside the grains.

Figure 4.
Cross-sectional TEM bright field morphology of the oxide film grown on the initial sample after 24 h exposure to 500 °C/10.3 MPa superheated steam. (b–d) High-magnification images of the regions marked with red, yellow and blue squares in (a), respectively. (e–g) HRTEM images of the regions marked with white squares in (b–d), respectively. (h–j) Corresponding FFT patterns of (e–g), respectively.
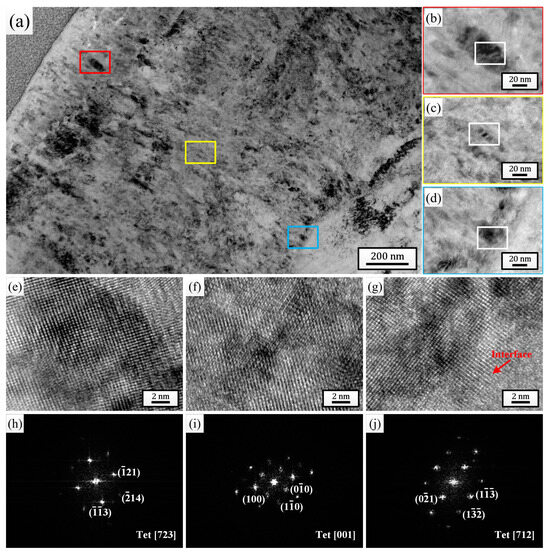
Figure 5.
Cross-sectional TEM bright field morphology of the oxide film grown on sample pulsed 25 times after 24 h exposure to 500 °C/10.3 MPa superheated steam. (b–d) High-magnification images of the regions marked with red, yellow and blue squares in (a), respectively. (e–g) HRTEM images of the regions marked with white squares in (b–d), respectively. (h–j) Corresponding FFT patterns of (e–g), respectively.
4. Discussion
The Pilling–Bedworth ratio (P.B. ratio, the ratio of the volume of the metal oxide to the volume of the metal consumed in forming that oxide) of ZrO2/Zr is 1.56, which means that the volume of the oxide film will expand during the oxidation process. Due to the constraint of the Zr matrix, significant compressive stress will be generated within the oxide film [48]. Consequently, the formation of ZrO2 is accompanied by the generation of defects such as vacancies, dislocations, interstitial atoms, etc. Under the combined effects of high temperature, compressive stress, and time, these defects will migrate and coalesce, promoting the formation of micro-pores and micro-cracks. At the same time, this process will also accelerate the transformation of the t-ZrO2 phase to the m-ZrO2 phase [47,49]. In other words, the protective characteristics of the oxide film will gradually weaken.
Research indicated that the presence of higher concentrations of alloying elements Fe and Cr in the oxide film could inhibit the formation of micro-pores and micro-cracks in ZrO2 grains by reducing the stress concentration in the oxide film, stabilizing the t-ZrO2 phase, enhancing the adhesion between the oxide film and the matrix, hindering the grain boundary migration, altering the growth dynamics of the oxide film, etc. [9,50]. Obviously, during the corrosion process, alloying elements dissolved in the Zr matrix were more likely to dissolve into the oxide film than those in the SPPs. That is to say, due to the substantial dissolution of SPPs in the Zr matrix, the concentration of alloying elements in the oxide film grown on the HCPEB irradiated sample was significantly higher than that grown on the initial sample. At the same time, the distribution of alloying elements in the matrix after repeated remelting was more uniform, which undoubtedly played a better role in enhancing the corrosion resistance of the alloy. In contrast, the oxidation of SPPs in the matrix would generate local additional stresses in the oxide film, accelerating the process of corrosion [6].
Additionally, after multiple irradiations, a high-volume fraction of boundaries was provided by large numbers of ultrafine/nanoscale grains, martensites, and twins. These boundaries collectively served as rapid diffusion pathways for Fe and Cr atoms to the sample surface, playing an extremely important role in the formation of a dense protective oxide film.
Research results provide a new method for the surface modification of Zircaloy-4 to improve its service life in nuclear reactors and also provide a new idea for the modification research of other zirconium alloys and even other alloys in industrial applications. Of course, the applicability and effectiveness of HCPEB technology may vary when dealing with different materials, which requires the adjustment and optimization of process parameters according to the characteristics of different materials. At the same time, the long-term stability and reliability of HCPEB technology in industrial applications also need to be ensured through testing and verification in actual production.
5. Conclusions
HCPEB surface treatment was applied to Zircaloy-4 with varying times of irradiation. During irradiation, the sample surface was melted, within which martensitic phase transformation occurred, resulting in the formation of large numbers of twin martensites. With the increasing of irradiation times, these martensites became finer and finer. After 25 pulses of irradiation, the average width of the martensites was as low as 0.02 μm, and plenty of ultrafine/nanoscale grains were also observed. Zr(Fe, Cr)2 SPPs were dissolved both in the melted layer and the heat-affected zone, and the alloying elements Fe and Cr were uniformly distributed under the action of HCPEB irradiation. After 24 h corrosion in 500 °C/10.3 MPa superheated steam, the thickness of the oxide film grown on the sample pulsed 25 times was reduced by 19.75% compared to that grown on the initial one, and the oxide film was significantly improved in uniformity and compactness.
The research results provide necessary theoretical and experimental data for the study of improving the service life of Zircaloy-4 under extreme environmental conditions through HCPEB surface modification. In future work, more characterization methods will be employed for a detailed analysis of the modified surface, and corrosion tests will be conducted over extended exposure times to provide further data support for this research.
Author Contributions
Conceptualization, S.Y. and Z.H.; methodology, H.Y.; validation, T.C.; investigation, H.Y.; data curation, S.Y. and H.Y.; writing—original draft preparation, S.Y.; writing—review and editing, S.Y., H.Y., Z.H. and T.C.; supervision, T.C.; funding acquisition, S.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Jilin Province Scientific and Technological Development Program, grant number YDZJ202201ZYTS382.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All research data supporting this publication are directly available within this publication.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Al Mubarak, F.; Rezaee, R.; Wood, D.A. Economic, societal, and environmental impacts of available energy sources: A review. Eng 2024, 5, 1232–1265. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. A review on clean energy solutions for better sustainability. Int. J. Energy Res. 2015, 39, 585–606. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Z.; Wang, Z.; Chen, S.; Ge, D.; Chen, C.; Jia, J.; Li, Y.; Jin, M.; Zhou, T. Nuclear safety in the unexpected second nuclear era. Proc. Natl. Acad. Sci. USA 2019, 116, 17673–17682. [Google Scholar] [CrossRef]
- Diniasi, D.; Golgovici, F.; Marin, A.H.; Negrea, A.D.; Fulger, M.; Demetrescu, I. Long-term corrosion testing of Zy-4 in a LiOH solution under high pressure and temperature conditions. Materials 2021, 14, 4586. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Jian, W.; Ge, X.; Wang, H.; Zhang, D.; Chen, X.; Zhang, Q.; Suo, X. Fouling behavior on the zircaloy-4 alloy cladding tube: An experimental and simulation study. Appl. Surf. Sci. 2024, 670, 160724. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, B.K.; Yoo, S.J.; Jeong, Y.H. Corrosion behavior and oxide properties of Zr–1.1 wt% Nb–0.05 wt% Cu alloy. J. Nucl. Mater. 2006, 359, 59–68. [Google Scholar] [CrossRef]
- Park, J.Y.; Yoo, S.J.; Choi, B.K.; Jeong, Y.H. Oxide microstructures of advanced Zr alloys corroded in 360 C water loop. J. Alloys Compd. 2007, 437, 274–279. [Google Scholar] [CrossRef]
- Yao, M.; Zhou, B.; Li, Q.; Liu, W.; Geng, X.; Lu, Y. A superior corrosion behavior of Zircaloy-4 in lithiated water at 360 °C/18.6 MPa by β-quenching. J. Nucl. Mater. 2008, 374, 197–203. [Google Scholar] [CrossRef]
- Yao, M.; Shen, Y.; Li, Q.; Peng, J.; Zhou, B.; Zhang, J. The effect of final annealing after β-quenching on the corrosion resistance of Zircaloy-4 in lithiated water with 0.04 M LiOH. J. Nucl. Mater. 2013, 435, 63–70. [Google Scholar] [CrossRef]
- Fazi, A.; Stiller, K.; Andrén, H.O.; Thuvander, M. Cold sprayed Cr-coating on Optimized ZIRLO™ claddings: The Cr/Zr interface and its microstructural and chemical evolution after autoclave corrosion testing. J. Nucl. Mater. 2022, 560, 153505. [Google Scholar] [CrossRef]
- Alat, E.; Motta, A.T.; Comstock, R.J.; Partezana, J.M.; Wolfe, D.E. Multilayer (TiN, TiAlN) ceramic coatings for nuclear fuel cladding. J. Nucl. Mater. 2016, 478, 236–244. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J. The effect of laser surface treatment on the nodular corrosion of Zircaloy-4. Rare. Metal. Mat. Eng 1996, 25, 44–46. [Google Scholar]
- Peng, D.; Bai, X.; Pan, F.; Sun, H.; Chen, B. Influence of aluminum ions implanted on oxidation behavior of ZIRLO alloy at 500 C. Vacuum 2006, 80, 530–536. [Google Scholar] [CrossRef]
- Liu, Y.; Zu, X.; Qiu, S.; Li, C.; Ma, W.; Huang, X. Surface characteristics and oxidation behavior of nitrogen ion-implanted Zr–Sn–Nb alloy. Surf. Coat. Technol. 2006, 200, 5631–5635. [Google Scholar] [CrossRef]
- Ryabchikov, A.; Kashkarov, E.; Shevelev, A.; Syrtanov, M. High-intensity chromium ion implantation into Zr-1Nb alloy. Surf. Coat. Technol. 2020, 383, 125272. [Google Scholar] [CrossRef]
- Zhang, Y.; Weber, W.J. Ion irradiation and modification: The role of coupled electronic and nuclear energy dissipation and subsequent nonequilibrium processes in materials. Appl. Phys. Rev. 2020, 7, 041307. [Google Scholar] [CrossRef]
- Grosdidier, T.; Zou, J.; Bolle, B.; Hao, S.; Dong, C. Grain refinement, hardening and metastable phase formation by high current pulsed electron beam (HCPEB) treatment under heating and melting modes. J. Alloys Compd. 2010, 504, S508–S511. [Google Scholar] [CrossRef]
- Meisner, S.; Vlasov, I.; Yakovlev, E.; Panin, S.; Meisner, L.; D’yachenko, F. Impact of electron beam surface modification on deformation behavior and fracture properties of TiNi shape memory alloy. Mater. Sci. Eng. A 2019, 740, 381–389. [Google Scholar] [CrossRef]
- Ozur, G.; Proskurovsky, D.; Karlik, K. A wide-aperture, low-energy, and high-current electron beam source with a plasma anode based on a reflective discharge. Instrum. Exp. Tech. 2005, 48, 753–760. [Google Scholar] [CrossRef]
- Dong, C.; Wu, A.M.; Hao, S.Z.; Zou, J.; Liu, Z.; Zhong, P.; Zhang, A.; Xu, T.; Chen, J.; Xu, J. Surface treatment by high current pulsed electron beam. Surf. Coat. Technol. 2003, 163, 620–624. [Google Scholar] [CrossRef]
- Proskurovsky, D.; Rotshtein, V.; Ozur, G.; Ivanov, Y.F.; Markov, A. Physical foundations for surface treatment of materials with low energy, high current electron beams. Surf. Coat. Technol. 2000, 125, 49–56. [Google Scholar] [CrossRef]
- Zou, J.; Grosdidier, T.; Zhang, K.; Dong, C. Mechanisms of nanostructure and metastable phase formations in the surface melted layers of a HCPEB-treated D2 steel. Acta Mater. 2006, 54, 5409–5419. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, K.; Zou, J.; Liu, D.; Zhang, T. Effect of the high current pulsed electron beam treatment on the surface microstructure and corrosion resistance of a Mg-4Sm alloy. J. Alloys Compd. 2018, 741, 65–75. [Google Scholar] [CrossRef]
- Song, L.; Zhang, K.; Zou, J.; Yan, P. Surface modifications of a hyperperitectic Zn-10 wt% Cu alloy by pulsed electron beam treatment. Surf. Coat. Technol. 2020, 388, 125530. [Google Scholar] [CrossRef]
- Wu, P.P.; Deng, K.K.; Nie, K.B.; Zhang, Z.Z. Corrosion resistance of AZ91 Mg alloy modified by high-current pulsed electron beam. Acta Metall. Sin. 2019, 32, 218–226. [Google Scholar] [CrossRef]
- Cai, J.; Li, X.; Lyu, P.; Guan, Q.; Lu, J.; Xue, W. Hot corrosion behavior of NiCoCrAlYSi laser cladding coating modified using high-current pulsed electron beam in different corrosive salt environments. Mater. Charact. 2024, 208, 113565. [Google Scholar] [CrossRef]
- Wang, M.; Du, L.; Xu, Y.; Zhang, X.; Qi, P.; Xu, P.; Peng, W. Surface microstructure evolution mechanism of WC-Co hard alloy treated by high current pulsed electron beam. Vacuum 2022, 202, 111139. [Google Scholar] [CrossRef]
- Lyu, P.; Peng, T.; Miao, Y.; Liu, Z.; Gao, Q.; Zhang, C.; Jin, Y.; Guan, Q.; Cai, J. Microstructure and properties of CoCrFeNiMo0. 2 high-entropy alloy enhanced by high-current pulsed electron beam. Surf. Coat. Technol. 2021, 410, 126911. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, C.-T.; Shi, J.; Jin, Y.; Lu, R. Influence of high current pulsed electron beam on microstructure and properties of Ni–W alloy coatings. J. Alloys Compd. 2020, 828, 154460. [Google Scholar] [CrossRef]
- Duan, R.; Tian, M.; Li, Y.; Bai, P.; Zhao, Z.; Wang, J.; Wei, S. Hot corrosion behavior of NiCoCrAlY laser cladding coating on 17-4PH stainless steel before and after high-current pulsed electron beam irradiation. J. Vac. Sci. Technol. A 2023, 41, 053103. [Google Scholar] [CrossRef]
- Lv, P.; Sun, X.; Cai, J.; Zhang, C.; Liu, X.; Guan, Q. Microstructure and high temperature oxidation resistance of nickel based alloy GH4169 irradiated by high current pulsed electron beam. Surf. Coat. Technol. 2017, 309, 401–409. [Google Scholar] [CrossRef]
- Samih, Y.; Marcos, G.; Stein, N.; Allain, N.; Fleury, E.; Dong, C.; Grosdidier, T. Microstructure modifications and associated hardness and corrosion improvements in the AISI 420 martensitic stainless steel treated by high current pulsed electron beam (HCPEB). Surf. Coat. Technol. 2014, 259, 737–745. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, S.; Lv, P.; Li, Y.; Wang, X.; Hou, X.; Guan, Q. The microstructures and corrosion properties of polycrystalline copper induced by high-current pulsed electron beam. Appl. Surf. Sci. 2014, 294, 9–14. [Google Scholar] [CrossRef]
- Grosdidier, T.; Zou, J.; Stein, N.; Boulanger, C.; Hao, S.; Dong, C. Texture modification, grain refinement and improved hardness/corrosion balance of a FeAl alloy by pulsed electron beam surface treatment in the “heating mode”. Scr. Mater. 2008, 58, 1058–1061. [Google Scholar] [CrossRef]
- Yang, S.; Guo, Z.; Zhang, M.; Guan, Q.; Jin, Y.; Liu, Y. Effect of high current pulsed electron beam surface irradiation on the microstructure and electrochemical behavior of zircaloy-4. Nucl. Instrum. Methods Phys. Res. Sect. B 2018, 434, 81–87. [Google Scholar] [CrossRef]
- Chai, L.; Chen, B.; Wang, S.; Zhang, Z.; Murty, K.L. Microstructural, textural and hardness evolution of commercially pure Zr surface-treated by high current pulsed electron beam. Appl. Surf. Sci. 2016, 390, 430–434. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, S.; Li, X.; Dong, C.; Grosdidier, T. Surface modification of pure titanium by pulsed electron beam. Appl. Surf. Sci. 2011, 257, 5899–5902. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Y.; Jiang, H.; Peng, Q. Effect of heat treatment and Nb and H contents on the phase transformation of N18 and N36 zirconium alloys. J. Alloys Compd. 2008, 462, 103–108. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, J.; Weber, S.; Hao, S.; Dong, C.; Grosdidier, T. Microstructure and property modifications in a near α Ti alloy induced by pulsed electron beam surface treatment. Surf. Coat. Technol. 2011, 206, 295–304. [Google Scholar] [CrossRef]
- Cai, J.; Lv, P.; Guan, Q.; Xu, X.; Lu, J.; Wang, Z.; Han, Z. Thermal cycling behavior of thermal barrier coatings with MCrAlY bond coat irradiated by high-current pulsed electron beam. ACS Appl. Mater. Interfaces 2016, 8, 32541–32556. [Google Scholar] [CrossRef]
- Hao, S.; Gao, B.; Wu, A.; Zou, J.; Qin, Y.; Dong, C.; An, J.; Guan, Q. Surface modification of steels and magnesium alloy by high current pulsed electron beam. Nucl. Instrum. Methods Phys. Res. Sect. B 2005, 240, 646–652. [Google Scholar] [CrossRef]
- Xu, F.; Tang, G.; Guo, G.; Ma, X.; Ozur, G. Influence of irradiation number of high current pulsed electron beam on the structure and properties of M50 steel. Nucl. Instrum. Methods Phys. Res. Sect. B 2010, 268, 2395–2399. [Google Scholar] [CrossRef]
- Kajiwara, S.; Ohno, S.; Honma, K. Martensitic transformations in ultra-fine particles of metals and alloys. Philos. Mag. A 1991, 63, 625–644. [Google Scholar] [CrossRef]
- Grosdidier, T.; Combres, Y.; Gautier, E.; Philippe, M.-J. Effect of microstructure variations on the formation of deformation-induced martensite and associated tensile properties in a β metastable Ti alloy. Metall. Mater. Trans. A 2000, 31, 1095–1106. [Google Scholar] [CrossRef]
- Meng, Q.; Rong, Y.; Hsu, T. Nucleation barrier for phase transformations in nanosized crystals. Phys. Rev. B 2002, 65, 174118. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, D.; Zou, J.; Grosdidier, T.; Dong, C. Improved in vitro corrosion resistance of a NiTi alloy by high current pulsed electron beam treatment. Surf. Coat. Technol. 2006, 201, 3096–3102. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, J.; Yao, M.; Zhou, B.; Peng, J.; Liang, X. Oxide microstructural evolution of Zr–0.7 Sn–0.35 Nb–0.3 Fe alloys containing Ge corroded in lithiated water. J. Nucl. Mater. 2014, 451, 255–263. [Google Scholar] [CrossRef]
- Evans, H. Stress effects in high temperature oxidation of metals. Int. Mater. Rev. 1995, 40, 1–40. [Google Scholar] [CrossRef]
- Xie, S.; Zhou, B.; Liang, X.; Liu, W.; Li, H.; Li, Q.; Yao, M.; Zhang, J. A novel mechanism for nodular corrosion of Zircaloy-4 corroded in 773 K superheated steam. Corros. Sci. 2017, 126, 44–54. [Google Scholar] [CrossRef]
- Pecheur, D.; Lefebvre, F.; Motta, A.T.; Lemaignan, C.; Wadier, J. Precipitate evolution in the Zircaloy-4 oxide layer. J. Nucl. Mater. 1992, 189, 318–332. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).