Unveiling the Influence of Hot Carriers on Photovoltage Formation in Perovskite Solar Cells
Abstract
:1. Introduction
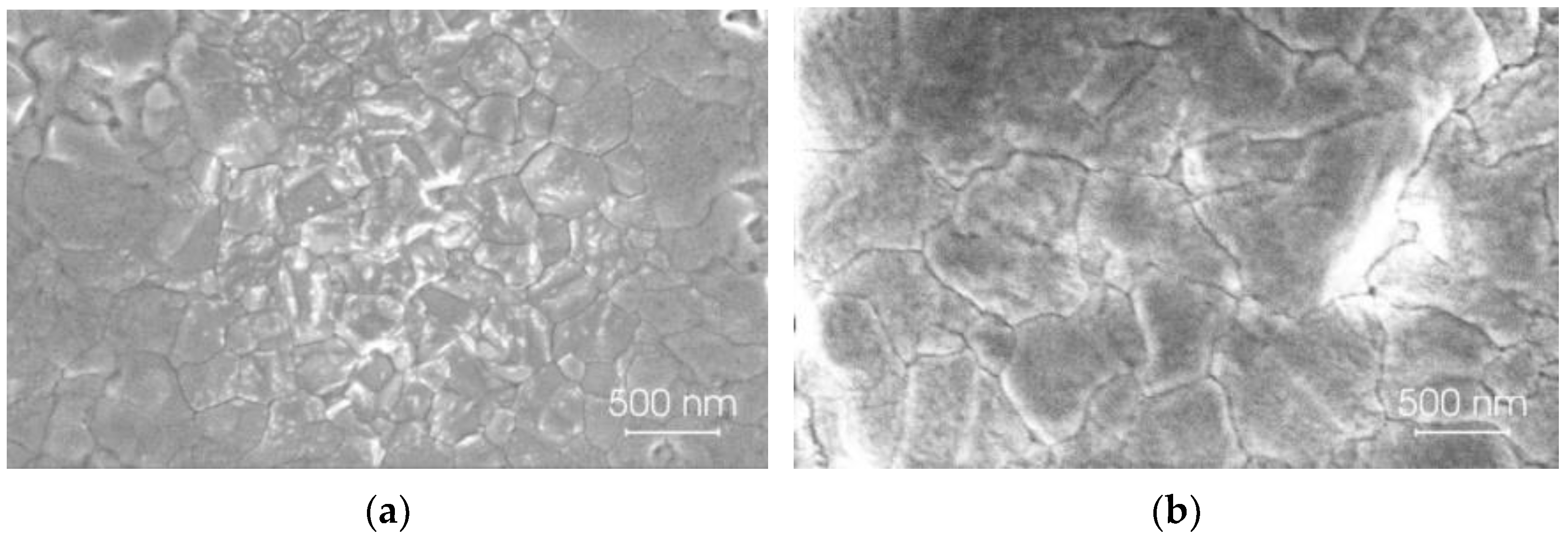
2. Fabrication and Characterization of Perovskite Films
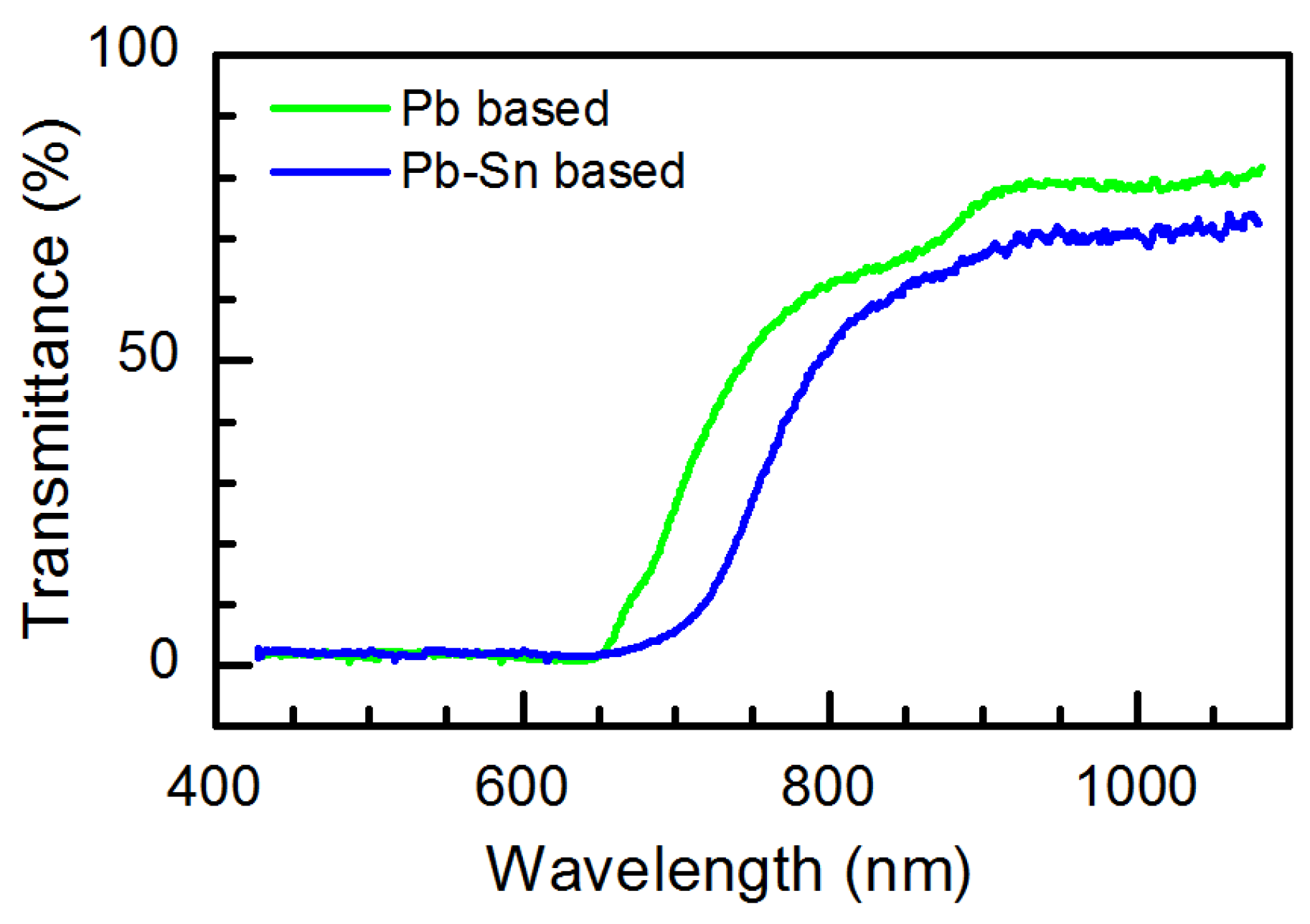
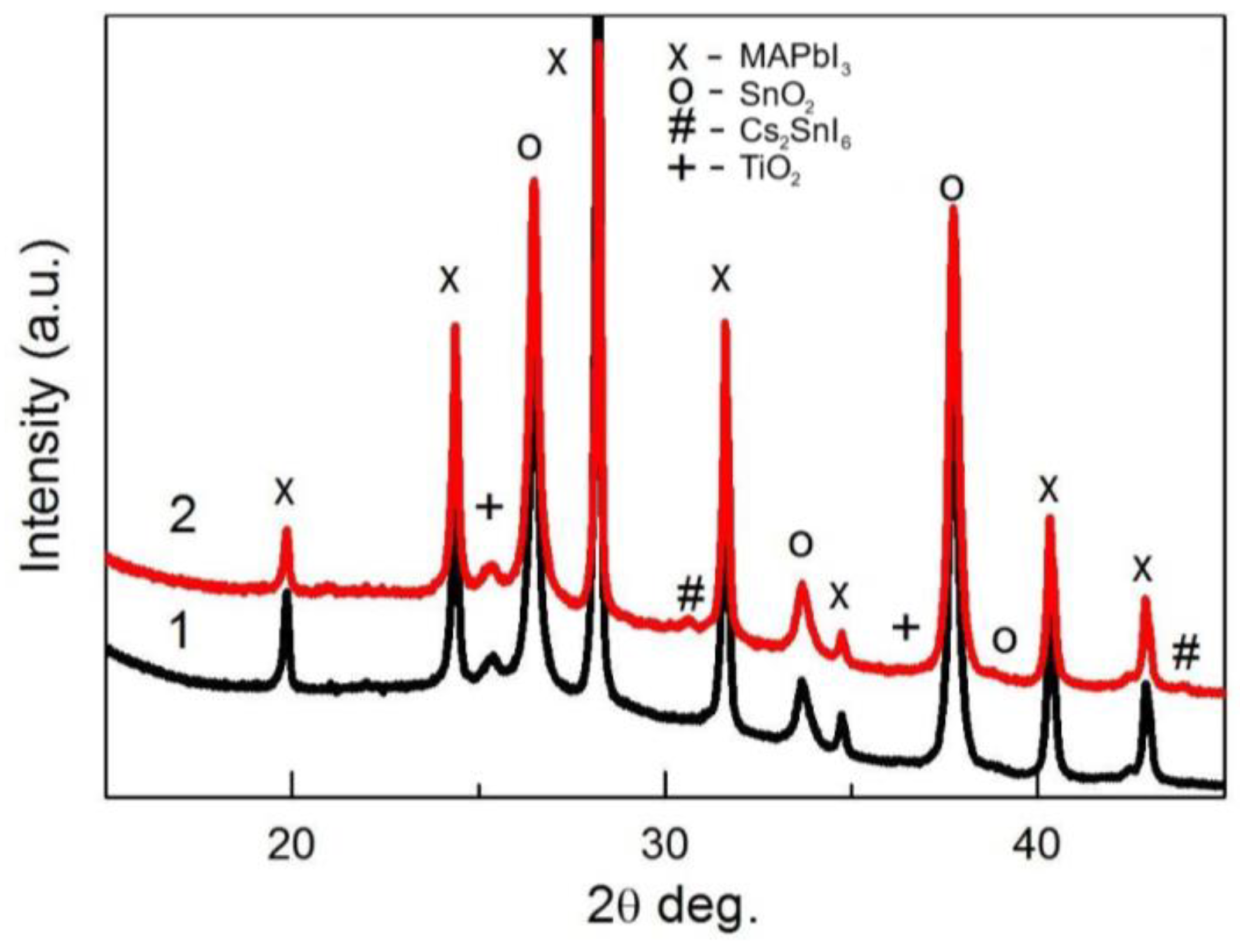
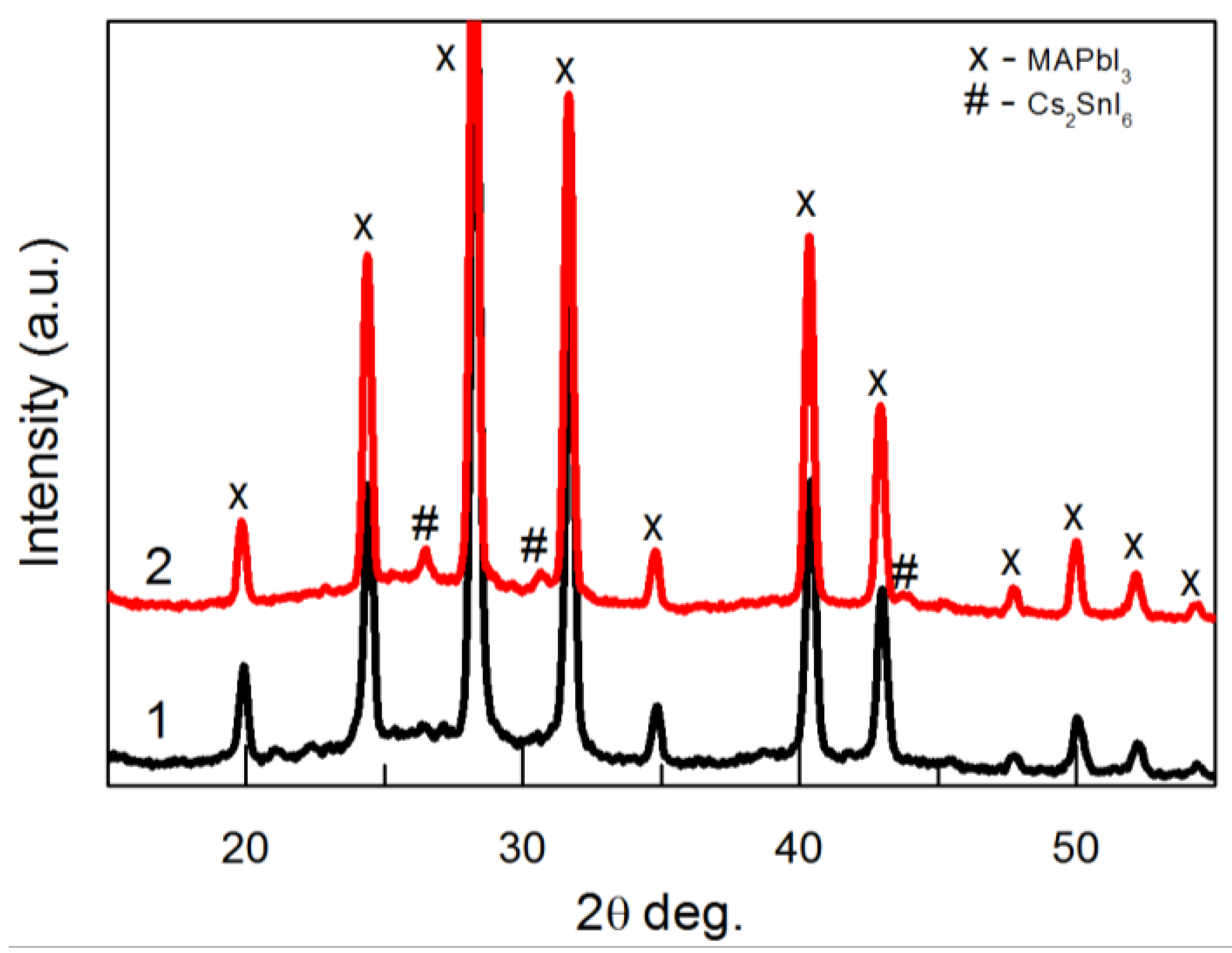
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mozaffari, N.; Walter, D.; White, T.P.; Bui, A.D.; Tabi, G.D.; Weber, K.; Catchpole, K.R. Unraveling the role of energy band alignment and mobile ions on interfacial recombination in perovskite solar cells. Sol. RRL 2022, 6, 2101087. [Google Scholar] [CrossRef]
- Yolthida, K.; Long, D.X.; Jang, Y.H.; Hong, J. Enhancing photovoltaic performance through optimized antisolvent strategy for low-bandgap Pb−Sn perovskites. ACS Appl. Energy Mater. 2023, 6, 12285–12295. [Google Scholar] [CrossRef]
- Kim, M.; Jeong, J.; Lu, H.; Lee, T.K.; Eickemeyer, F.T.; Liu, Y.; Choi, I.W.; Choi, S.J.; Jo, Y.; Kim, H.B.; et al. Conformal quantum dot-SnO2 layers as electron transporters for efficient perovskite solar cells. Science 2022, 375, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhu, K. Advances in mixed tin-lead narrow-band perovskites for single-junction and all-perovskite tandem solar cells. Adv. Mater. 2024, 36, 2314241. [Google Scholar] [CrossRef]
- Quan, L.N.; Rand, B.P.; Friend, R.H.; Mhaisalkar, S.G.; Lee, T.W.; Sargent, E.H. Perovskites for next-generation optical sources. Chem. Rev. 2019, 119, 7444–7478. [Google Scholar] [CrossRef] [PubMed]
- Kothandaraman, R.K.; Jiang, Y.; Feurer, T.; Tiwari, A.N.; Fu, F. Near-infrared-transparent perovskite solar cells and peroskite-based tandem photovoltaics. Small Methods 2020, 4, 2000395. [Google Scholar] [CrossRef]
- Wehrenfennig, C.; Eperon, G.E.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. High carrier mobilities and lifetimes in organolead trihalide perovskites. Adv. Mater. 2014, 26, 1584–11589. [Google Scholar] [CrossRef]
- Karakus, M.; Jensen, S.A.; D’Angelo, F.; Turchinovich, D.; Bonn, M.; Cánovas, E. Phonon-electron scattering limits free charge mobility in methylammonium lead iodide perovskites. J. Phys. Chem. Lett. 2015, 6, 4991–4996. [Google Scholar] [CrossRef]
- Song, W.; Chen, J.; Li, Z.; Fang, X. Self-Powered MXene/GaN van der Waals Heterojunction Ultraviolet Photodiodes with Superhigh Efficiency and Stable Current Outputs. Adv Mater. 2021, 33, e2101059. [Google Scholar] [CrossRef] [PubMed]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an Organometal trihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Electron-hole diffusion lengths > 175 µm in solution-grown CH3NH3PbI3 single crystals. Sci. Express 2015, 347, 967–969. [Google Scholar] [CrossRef]
- Staub, F.; Hempel, H.; Hebig, J.C.; Mock, J.; Paetzold, U.W.; Rau, U.; Unold, T.; Kirchartz, T. Beyond bulk lifetimes: Insights into lead halide perovskite films from time-resolved photoluminescence. Phys. Rev. Appl. 2016, 6, 044017. [Google Scholar] [CrossRef]
- Solanki, A.; Yadav, P.; Turren-Cruz, S.H.; Lim, S.S.; Saliba, M.; Sum, T.C. Cation influence on carrier dynamics in perovskite solar cells. Nano Energy 2019, 58, 604–611. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Hong, E.; Yan, T.; Fang, X. Effective Dual Cation Release in Quasi-2D Perovskites for Ultrafast UV Light-Powered Imaging. Adv. Mater. 2024, 2412014. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.; Li, C.; Xie, L.; Yuan, Y.; Wang, S.; Cao, Z.; Ding, L.; Hao, F. Hot-Casting Large-Grain Perovskite Film for Efficient Solar Cells: Film Formation and Device Performance. Nanomicro. Lett. 2020, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- Moghadamzadeh, S.; Hossain, I.M.; Duong, T.; Gharibzadeh, S.; Abzieher, T.; Pham, H.; Hu, H.; Fassl, P.; Lemmer, U.; Nejand, B.A.; et al. Triple-cation low-bandgap perovskite thin-films for high-efficiency four-terminal all-perovskite tandem solar cells. J. Mater. Chem. A 2020, 8, 24608–24619. [Google Scholar] [CrossRef]
- Ankireddy, K.; Ghahremani, A.H.; Martin, B.; Gupta, G.; Druffel, T. Rapid thermal annealing of CH3NH3PbI3 perovskite thin films by intense pulsed light with aid of diiodomethane additive. J. Mater. Chem. A 2018, 6, 9378–9383. [Google Scholar] [CrossRef]
- Long, J.; Sheng, W.; Dai, R.; Huang, Z.; Yang, J.; Zhang, J.; Li, X.; Tan, L.; Chen, Y. Understanding the mechanism between antisolvent dripping and additive doping strategies on the passivation effects in perovskite solar cells. ACS Appl. Mater. Interfaces 2020, 12, 56151–56160. [Google Scholar] [CrossRef] [PubMed]
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Gratzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Q.; Shi, Y.-L.; Li, M.; Zhang, L.; Wang, Z.-K.; Liao, L.-S. Vacuum-evaporated all-inorganic cesium leads bromine perovskites for high-performance light-emitting diodes. J. Mater. Chem. C 2017, 5, 8144–8149. [Google Scholar] [CrossRef]
- Yuan, S.; Qiu, Z.; Gao, C.; Zhang, H.; Jiang, Y.; Li, C.; Yu, J.; Cao, B. High-quality perovskite films grown with a fast solvent- assisted molecule inserting strategy for highly efficient and stable solar cells. ACS Appl. Mater. Interfaces 2016, 8, 22238–22245. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. Two-step sequential deposition of organometal halide perovskite for photovoltaic application. Adv. Funct. Mater. 2017, 27, 1605654. [Google Scholar] [CrossRef]
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.P.; et al. Efficient perovskite solar cells via improved carrier management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Shockley, W.; Queisser, H. Detailed Balance Limit of Efficiency of p–n Junction Solar Cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
- Luo, D.; Su, R.; Zhang, W.; Gong, Q.; Zhu, R. Minimizing non-radiative recombination losses in perovskite solar cells. Nat. Rev. Mater. 2020, 5, 44–60. [Google Scholar] [CrossRef]
- Du, Y.; Wu, J.; Zhang, X.; Zhu, Q.; Zhang, M.; Liu, X.; Zou, Y.; Wang, S.; Sun, W. Surface passivation using pyridinium iodide for highly efficient planar perovskite solar cells. J. Energy Chem. 2021, 52, 84–91. [Google Scholar] [CrossRef]
- Wetzelaer, G.J.H.; Scheepers, M.; Sempere, A.M.; Momblona, C.; Avila, J.; Bolink, H.J. Trap-assisted non-radiative recombination in organic-inorganic perovskite solar cells. Adv. Mater. 2015, 27, 1837–1841. [Google Scholar] [CrossRef] [PubMed]
- Aydin, E.; Bastiani, M.D.; Wolf, S.D. Defect and contact passivation for perovskite solar cells. Adv. Mater. 2019, 31, 1900428. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Wang, Y.; Yang, X.; Tang, Q. Alkyl-chain-regulated charge transfer in fluorescent inorganic CsPbBr3 perovskite solar cells. Angew. Chem. Int. 2020, 59, 4391–4395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ji, X.; Yao, H.; Fan, Q.; Yu, B.; Li, J. Review on efficiency improvement effort of perovskite solar cell. Sol. Energy 2022, 233, 421–434. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Liu, J.; Qi, J.; He, Y.; Wang, Y.; Hu, W.; Cheng, Y.; Chen, K.; Hu, Y.; et al. Reducing optical reflection loss for perovskite solar cell via printable mesoporous SiO2 antireflection coatings. Adv. Funct. Mater. 2022, 32, 2203872. [Google Scholar] [CrossRef]
- Kumar, B.K.; Gupta, N.D. Nanostructured anti-reflection coating for absorption enhancement in perovskite silicon tandem solar cells. Opt. Mater Express. 2024, 14, 139–154. [Google Scholar] [CrossRef]
- Shaker, L.M.; Al-Amiery, A.A.; Hanoon, M.M.; Al-Azzawi, W.K.; Kadhum, A.A. Examining the influence of thermal effects on solar cells: A comprehensive review. Sustain. Energy Res. 2024, 11, 1–30. [Google Scholar] [CrossRef]
- Ašmontas, S.; Gradauskas, J.; Sužiedėlis, A.; Šilėnas, A.; Širmulis, E.; Švedas, V.; Vaičikauskas, V.; Žalys, O. Hot carrier impact on photovoltage formation in solar cells. Appl. Phys. Lett. 2018, 113, 071103–071106. [Google Scholar] [CrossRef]
- Ašmontas, S.; Gradauskas, J.; Sužiedėlis, A.; Šilėnas, A.; Širmulis, E.; Vaičikauskas, V.; Vaičiūnas, V.; Žalys, O.; Fedorenko, L.; Bulat, L. Photovoltage formation across GaAs p-n junction under illumination of intense laser radiation. Opt. Quant. Electron. 2016, 48, 448. [Google Scholar] [CrossRef]
- Ašmontas, S.; Olekas, A. Thermoelectromotive force and diffusion of hot electrons in metal-semiconductor Schottky barrier. Phys. Tech. Semicond. 1980, 14, 2196. [Google Scholar]
- Ašmontas, S. Electrogradient Phenomena in Semiconductors; Mokslas: Vilnius, Lithuania, 1984; p. 192. [Google Scholar]
- Fu, J.; Li, M.; Han, G.; Wu, B.; Huan, C.H.A.; Leek, M.; Sum, T.C. Hot carrier cooling mechanisms in halide perovskites. Nat. Commun. 2017, 8, 1300. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fu, J.; Xu, Q.; Sum, T.C. Slow hot-carrier cooling in halide perovskites: Prospects for hot-carrier solar cells. Adv. Mater. 2019, 31, 1802486. [Google Scholar] [CrossRef] [PubMed]
- Kahman, S.; Loi, M.A. Hot carrier solar cells and the potential of perovskites for breaking the Shockley-Queisser limit. J. Mater. Chem. C 2019, 7, 2471–2486. [Google Scholar] [CrossRef]
- Sekiguchi, F.; Hirori, H.; Yumoto, G.; Shimazaki, A.; Nakamura, T.; Wakamiya, A.; Kanemitsu, Y. Enhancing the hot-phonon bottleneck effects in a metal halide perovskite by terahertz phonon excitation. Phys. Rev. Lett. 2021, 126, 077401. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.T.; Nozil, A.J. Efficiency of hot-carrier solar energy converters. J. Appl. Phys. 1982, 53, 3813–3818. [Google Scholar] [CrossRef]
- Neges, M.; Schwarzburg, K.; Willig, F. Monte Carlo simulation of charge loss and collection of hot charge carriers, first step towards a more realistic hot-carrier solar energy converter. Sol. Energy Mater. Sol. Cells 2006, 90, 2107–2128. [Google Scholar] [CrossRef]
- Conibeer, G.J.; Jiang, C.W.; Köning, D.; Shrestha, S.; Walsh, T.; Green, M.A. Slowing of carrier cooling in hot carrier solar cells. Thin Solid Film. 2008, 516, 6948–6953. [Google Scholar] [CrossRef]
- Conibeer, G.J.; Ekins-Daukes, N.; Guillemoles, J.F.; Köning, D.; Cho, E.C.; Jiang, C.W.; Shrestha, S.; Green, M. Progress of hot carrier cells. Sol. Energy Mater. Sol. Cells 2009, 93, 713–719. [Google Scholar] [CrossRef]
- Kempa, K.; Naughton, M.J.; Ren, Z.F.; Herczynski, A.; Kirpatrick, T.; Rybczynski, J.; Gao, Y. Hot electron effect in nanoscopically thin photovoltaic junctions. Appl. Phys. Lett. 2009, 95, 233121. [Google Scholar] [CrossRef]
- Le Bris, A.; Guillemoles, J.F. Hot carrier solar cells: Achievable efficiency accounting for heat losses in the absorber and through contacts. Appl. Phys. Lett. 2010, 97, 113506. [Google Scholar] [CrossRef]
- Takeda, Y.; Motohiro, T. Highly efficient solar cells using hot carriers generated by two-step excitation. Sol. Energy Mater. Sol. Cells 2011, 95, 2638–2644. [Google Scholar] [CrossRef]
- Kirk, A.P.; Fischetti, M.V. Fundumental limitations of hot-carrier solar cells. Phys. Rev. 2012, 86, 165206. [Google Scholar] [CrossRef]
- Dimmock, A.R.; Day, S.; Kauer, M.; Smith, K.; Heffernan, J. Demonstration of a hot-carrier photovoltaic cell. Prog. Photovol. Res. Appl. 2014, 22, 151–160. [Google Scholar] [CrossRef]
- Yao, Y.; Köning, D. Comparison of bulk material candidates for hot carrier absorber. Sol. Energy Mater. Sol. Cells 2015, 140, 422–427. [Google Scholar] [CrossRef]
- Ferry, D.K.; Goodnick, S.M.; Whiteside, V.R.; Sellers, I.R. Challenges, myths, and opportunities in hot carrier solar cells. J. Appl. Phys. 2020, 128, 220903. [Google Scholar] [CrossRef]
- Sogabe, T.; Shiba, K.; Sakamoto, K. Hydrodinamic and energy transport model-based hot-carrier effect in GaAs pin solar cell. Electron. Mater. 2022, 3, 185–200. [Google Scholar] [CrossRef]
- Sharma, A.S.; Hanif, M.; Bremner, S.P.; Nielsem, M.P. Heat flow through nonideal contacts in hot-carrier solar cells. Phys. Rev. Appl. 2023, 20, 034001. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.Y.; Domaski, K.; Correa-Baena, J.P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef]
- Singh, T.; Miyasaka, T. Stabilizing the efficiency beyond 20% with a mixed cation perovskite solar cell fabricated in ambient air under controlled humidity. Adv. Energy Mater. 2018, 8, 1700677. [Google Scholar] [CrossRef]
- Domanski, K.; Alharbi, E.A.; Hagfeldt, A.; Grätzel, M.; Tress, W. Systematic investigation of the impact of operation conditions on the degradation behavior of perovskite solar cells. Nat. Energy. 2018, 3, 6167. [Google Scholar] [CrossRef]
- Ašmontas, S.; Čerškus, A.; Gradauskas, J.; Grigucevičienė, A.; Leinartas, K.; Lučun, A.; Petrauskas, K.; Selskis, A.; Sužiedelis, A.; Širmulis, E.; et al. Cesium-containing triple cation perovskite solar cells. Coatings 2021, 11, 279. [Google Scholar] [CrossRef]
- Ašmontas, S.; Čerškus, A.; Gradauskas, J.; Grigucevičienė, A.; Juškėnas, R.; Leinartas, K.; Lučun, A.; Petrauskas, K.; Selskis, A.; Staišiūnas, L.; et al. Photoelectric properties of planar and mesoporous structured perovskite solar cells. Materials 2022, 15, 4300. [Google Scholar] [CrossRef]
- Wang, J.; Zou, X.; Zhu, J.; Cheng, J.; Chen, D.; Bai, X.; Yao, Y.; Chang, C.; Yu, X.; Liu, B.; et al. Effect of optimization of TiO2 electron transport layer on performance of perovskite solar cells with rough FTO substrates. Materials 2020, 13, 2272. [Google Scholar] [CrossRef]
- Sun, X.; Xu, J.; Xiao, L.; Chen, J.; Zhang, B.; Yao, J.; Dai, S. Influence of the porosity of the TiO2 film on the performance of the perovskite solar cells. Int. J. Photoenergy 2017, 2017, 4935265. [Google Scholar] [CrossRef]
- Jiang, T.; Chen, Z.; Chen, X.; Liu, T.; Chen, X.; Sha, W.E.I.; Zhu, H.; Yang, Y.M. Realizing high efficiency over 20% of low-bandgap Pb-Sn-alloyed perovskite solar cells by in situ reduction of Sn4+. Sol. RRL 2020, 4, 1900467. [Google Scholar] [CrossRef]
- Savill, K.J.; Ulatowski, A.M.; Herz, L.M. Optoelectronic properties of tin-lead halid perovskites. ACS Energy Lett. 2021, 6, 2413–2426. [Google Scholar] [CrossRef] [PubMed]
- Ruf, F.; Aygüler, M.F.; Giesbrecht, N.; Rendenbach, B.; Magin, A.; Docampo, P.; Kalt, H.; Hetterich, M. Temperature-dependent studies of exciton binding energy and phase-transition suppression in (Cs,FA,MA)Pb(I,Br)3 perovskites. APL Mater. 2019, 7, 031113. [Google Scholar] [CrossRef]
- Poli, I.; Ambrosio, F.; Treglia, A.; Berger, F.J.; Prato, M.; Albaqami, M.D.; De Angelis, F.; Petrozza, A. Photoluminescence intensity enhancement in tin halide perovskites. Adv. Sci. 2022, 9, e2202795. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhao, R.; Sun, F.; Zhang, P.; Zhang, R.; Chen, Z.; Li, S. Wavelength-Tuneable Near-Infrared luminescence in mixed Tin–Lead halide perovskites. Front. Chem. 2022, 10, 887983. [Google Scholar] [CrossRef] [PubMed]
- Berberan-Santos, M.N. A luminescence decay function encompassing the stretched exponential and the compressed hyperbola. Chem. Phys. Lett. 2008, 460, 146–150. [Google Scholar] [CrossRef]
- Hydayat, R.; Nurunnizar, A.A.; Fariz, A.; Herman; Rosa, E.S.; Shobin; Oizumi, T.; Fujii, A.; Ozaki, M. Revealing the charge carrier kinetics in perovskite solar cells affected by mesoscopic structures and defect states from simple transient photovoltage measurements. Sci. Rep. 2020, 10, 19197. [Google Scholar] [CrossRef]
- Ščajev, P.; Qin, C.; Aleksiejūnas, R.; Baronas, P.; Miasojedovas, S.; Fujihara, T.; Matsushima, T.; Adachi, C.; Juršėnas, S. Diffusion enhancement in highly excited MAPbI3 perovskite layers with additives. J. Phys. Chem. Lett. 2018, 6, 3167–3172. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ji, L.; Liu, R.; Zhang, C.; Mak, C.H.; Zou, X.; Shen, H.H.; Leu, S.Y.; Hsu, H.Y. A review on morphology engineering for highly efficient and stable hybrid perovskite solar cells. J. Mater. Chem. A 2018, 6, 12842–12875. [Google Scholar] [CrossRef]
- Vollbrecht, J.; Brus, V.V. On the recombination order of surface recombination under open circuit condition. Org. Electr. 2020, 86, 105905. [Google Scholar] [CrossRef]
- Jošt, M.; Ajdič, Ž.; Topič, M. Performance of Triple-Cation Perovskite Solar Cells under Different Indoor Operating Conditions. ACS Appl. Mater. Interfaces 2024, 16, 62195–62202. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, R.K.; Jung, J.; Yang, S.W.; Yun, J.; Yun, Y.; Vidyasagar, D.; Choi, W.C.; Lee, C.L.; Noh, J.H.; Kim, D.H.; et al. High-throughput compositional mapping of triple-cation tin–lead perovskites for high-efficiency solar cells. InfoMat 2023, 5, e12393. [Google Scholar] [CrossRef]
- Lee, H.; Kang, S., Lee; Zu, K.; Kim, D.H. Progress and outlook of Sn–Pb mixed perovskite solar cells. Nano Converg. 2023, 10, 27. [Google Scholar] [CrossRef] [PubMed]


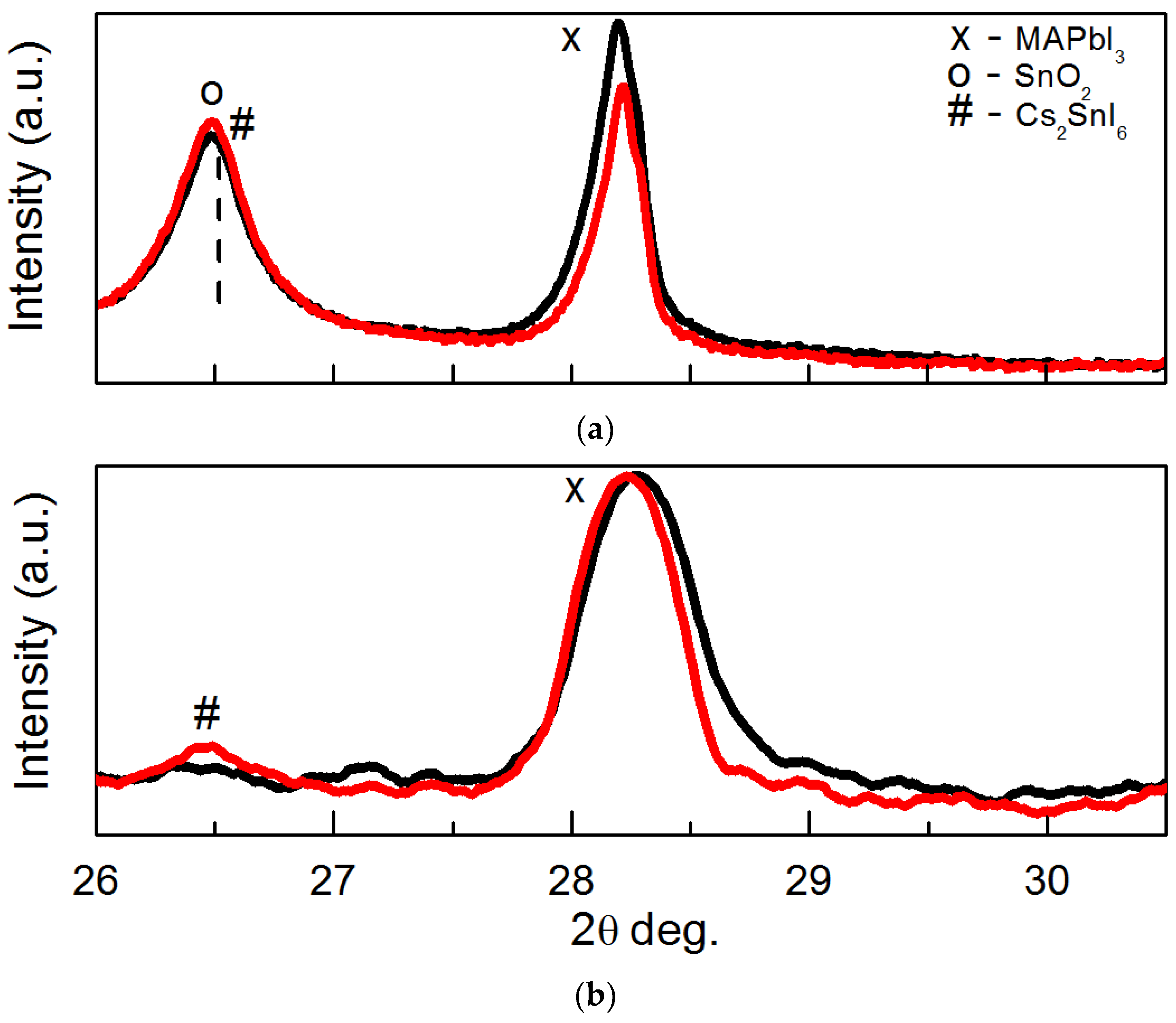
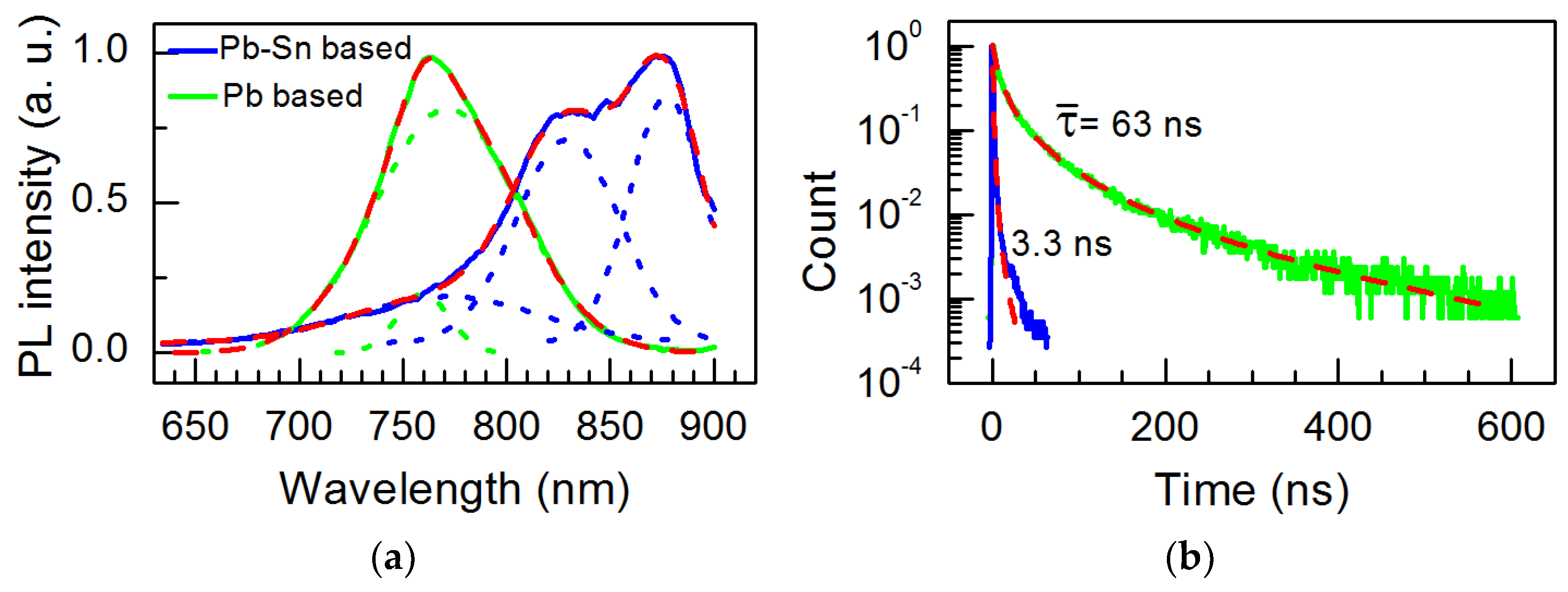

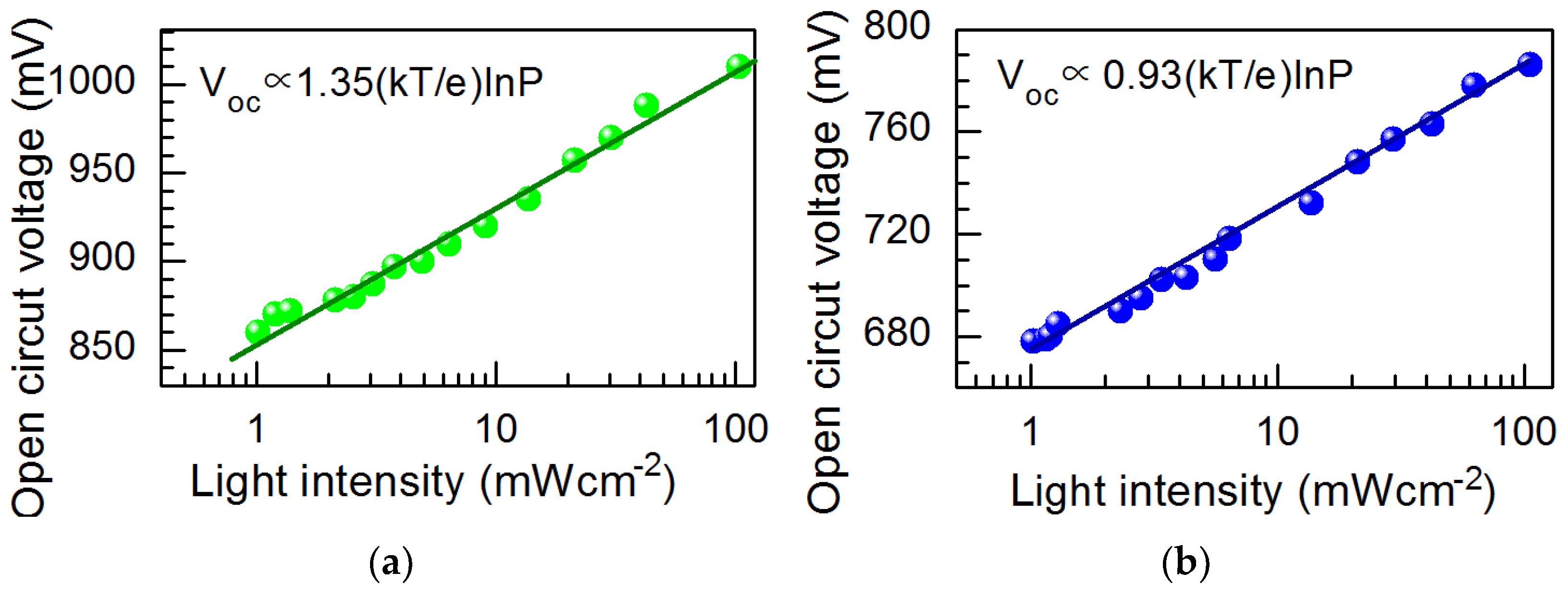
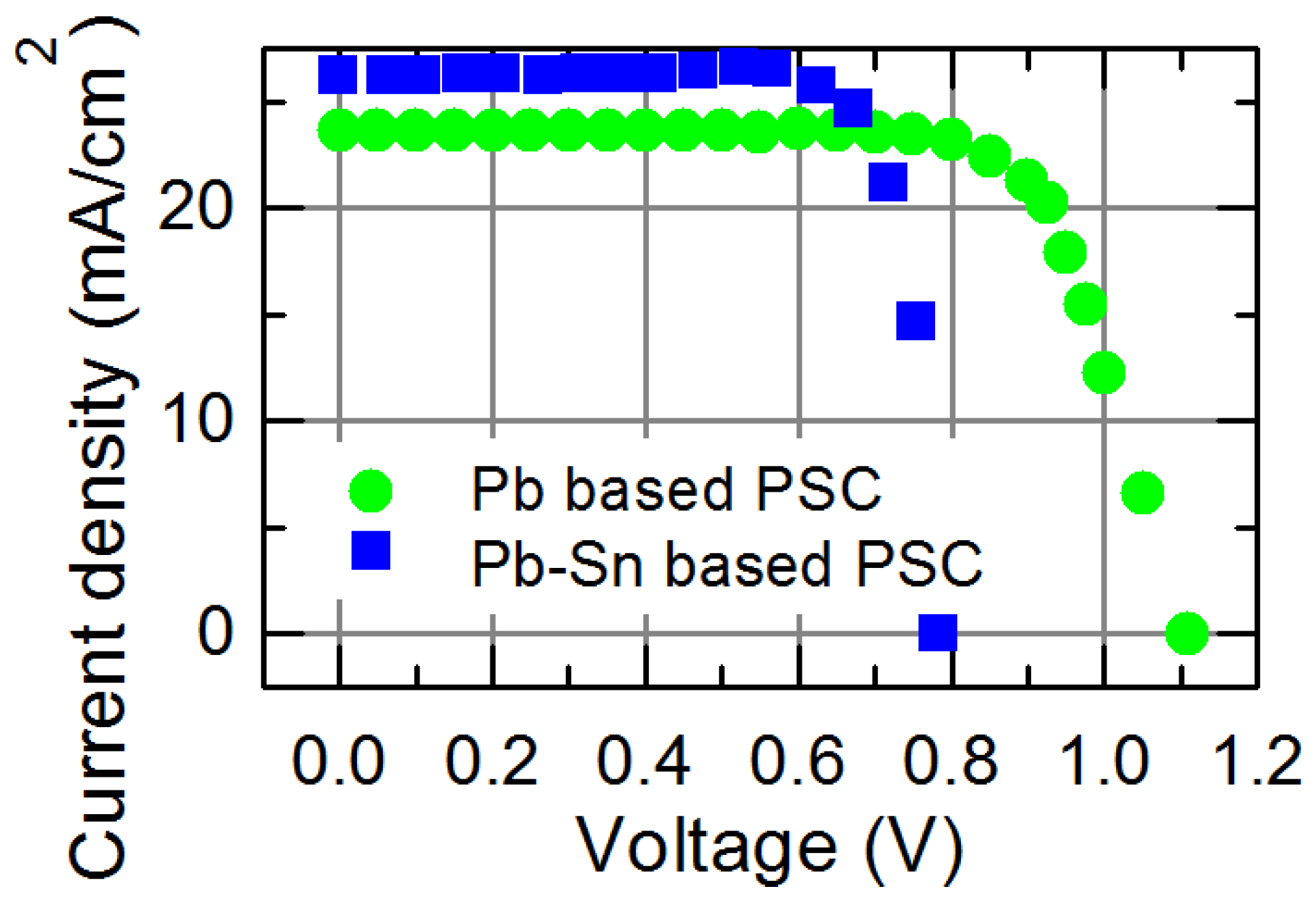
| Perovskite Solar Cell | Voc, V | Jsc, mA·cm−2 | FF, % | PCE, % |
|---|---|---|---|---|
| Pb based | 1.11 | 23.6 | 73 | 19.1 |
| Pb-Sn based | 0.785 | 26.2 | 70 | 14.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mujahid, M.; Čerškus, A.; Gradauskas, J.; Grigucevičienė, A.; Giraitis, R.; Leinartas, K.; Lučun, A.; Petrauskas, K.; Selskis, A.; Sužiedėlis, A.; et al. Unveiling the Influence of Hot Carriers on Photovoltage Formation in Perovskite Solar Cells. Materials 2025, 18, 85. https://doi.org/10.3390/ma18010085
Mujahid M, Čerškus A, Gradauskas J, Grigucevičienė A, Giraitis R, Leinartas K, Lučun A, Petrauskas K, Selskis A, Sužiedėlis A, et al. Unveiling the Influence of Hot Carriers on Photovoltage Formation in Perovskite Solar Cells. Materials. 2025; 18(1):85. https://doi.org/10.3390/ma18010085
Chicago/Turabian StyleMujahid, Muhammad, Aurimas Čerškus, Jonas Gradauskas, Asta Grigucevičienė, Raimondas Giraitis, Konstantinas Leinartas, Andžej Lučun, Kazimieras Petrauskas, Algirdas Selskis, Algirdas Sužiedėlis, and et al. 2025. "Unveiling the Influence of Hot Carriers on Photovoltage Formation in Perovskite Solar Cells" Materials 18, no. 1: 85. https://doi.org/10.3390/ma18010085
APA StyleMujahid, M., Čerškus, A., Gradauskas, J., Grigucevičienė, A., Giraitis, R., Leinartas, K., Lučun, A., Petrauskas, K., Selskis, A., Sužiedėlis, A., Šilėnas, A., Širmulis, E., & Ašmontas, S. (2025). Unveiling the Influence of Hot Carriers on Photovoltage Formation in Perovskite Solar Cells. Materials, 18(1), 85. https://doi.org/10.3390/ma18010085








