Development and Application Prospects of Biomass-Based Organic Binders for Pellets Compared with Bentonite
Abstract
1. Introduction
2. The Binding Mechanism of Biomass-Based Binder in Pellets and Its Application Potential
2.1. The Adhesion Between the Binder and the Surface of Iron Ore
2.2. The Cohesion of the Binder
2.3. The Application Potential of Biomass-Based Binder
3. Application Characteristics of Some Composite Binders as Pellet Additives
3.1. Lignosulfonate
3.1.1. Sources and Structure of Lignosulfonate
3.1.2. Interaction Between Lignosulfonate and Minerals
3.1.3. Process Characteristics and Application Effect of Lignosulfonate
3.2. Carboxymethyl Cellulose (CMC)
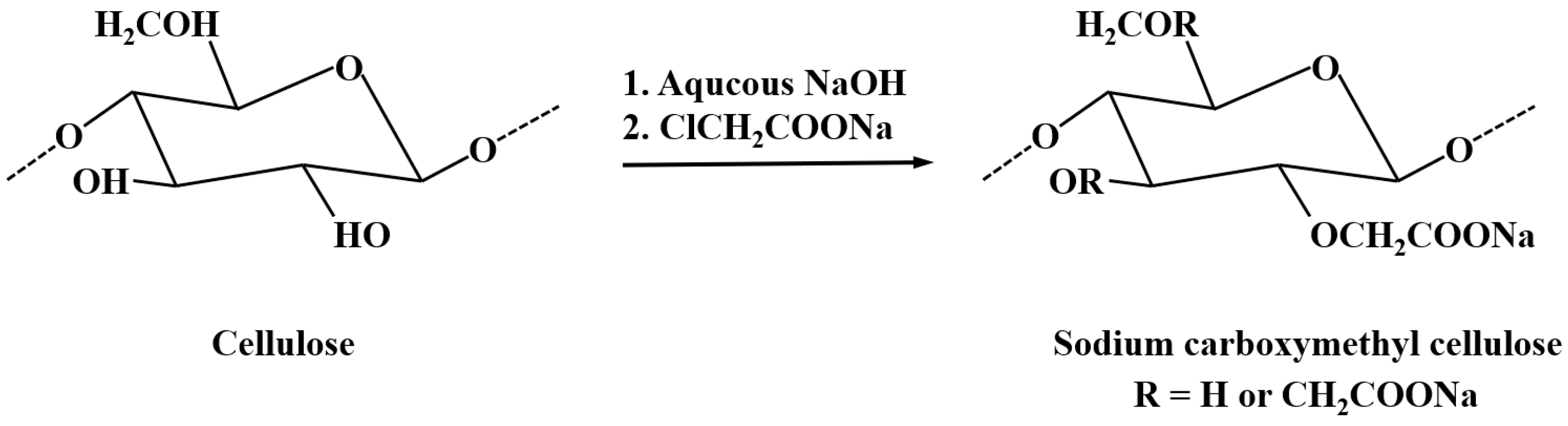
3.2.1. Sources and Structure of CMC
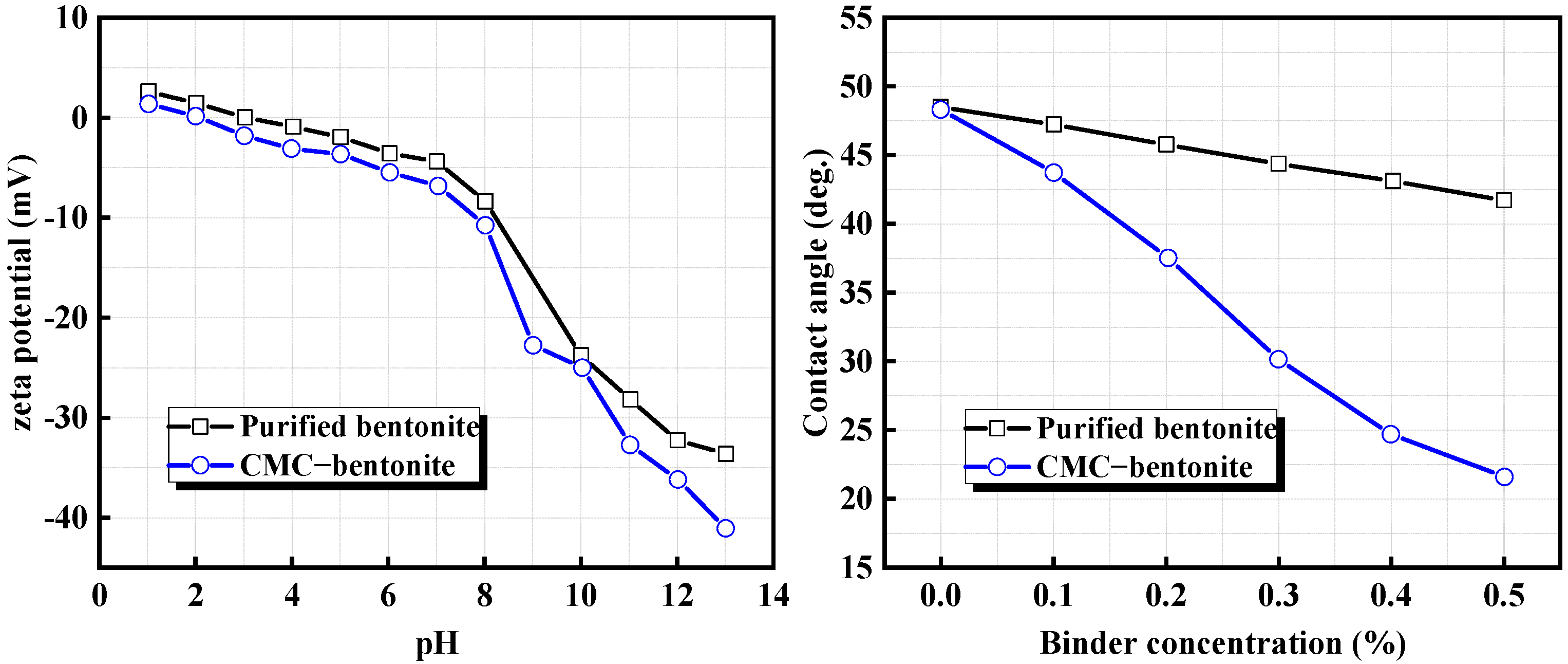
3.2.2. Interaction Between CMC and Minerals
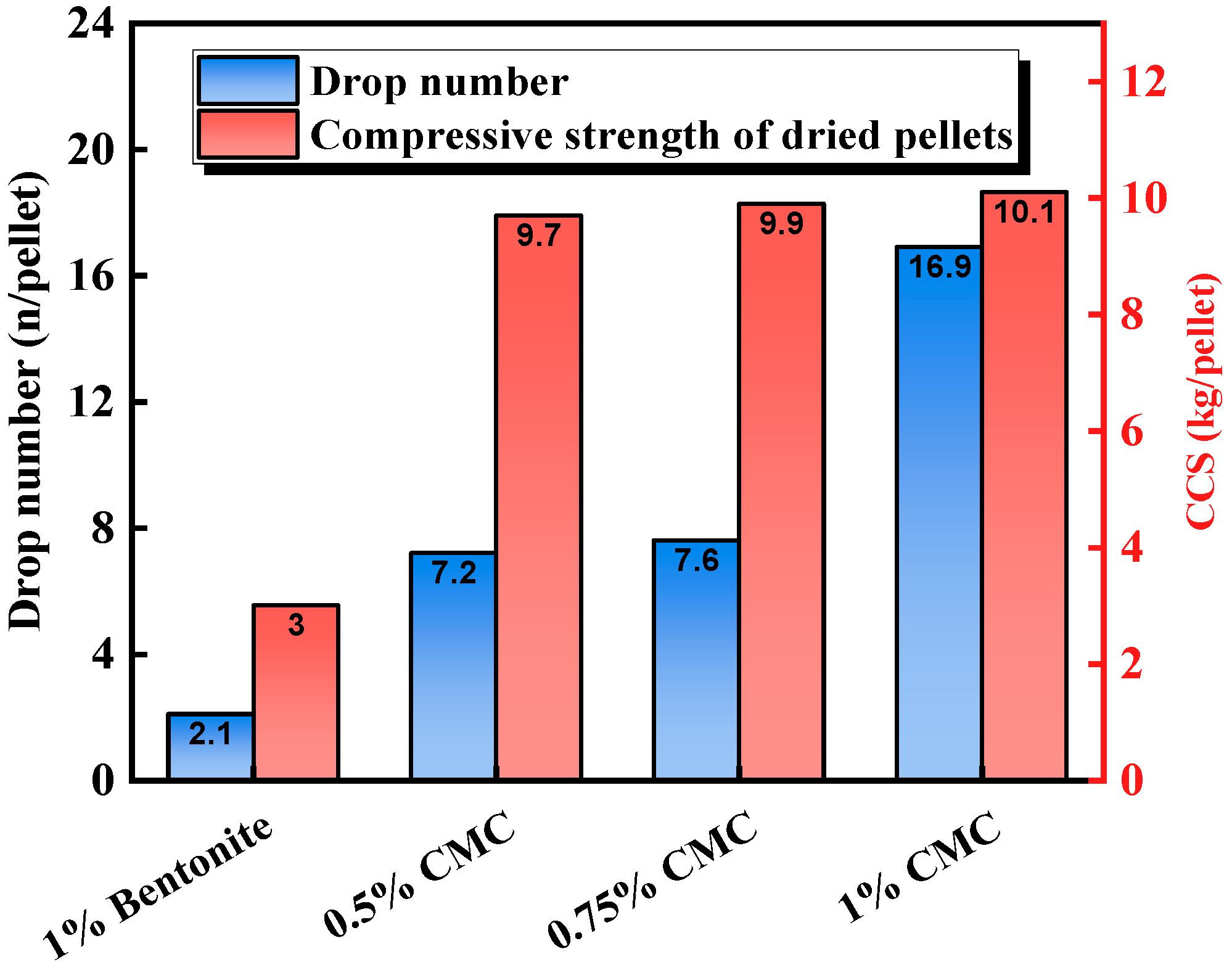
3.2.3. Process Characteristics and Application Effect of CMC
3.3. Carboxymethyl Starch
3.3.1. Sources and Structure of Carboxymethyl Starch
3.3.2. Interaction Between CMS and Minerals
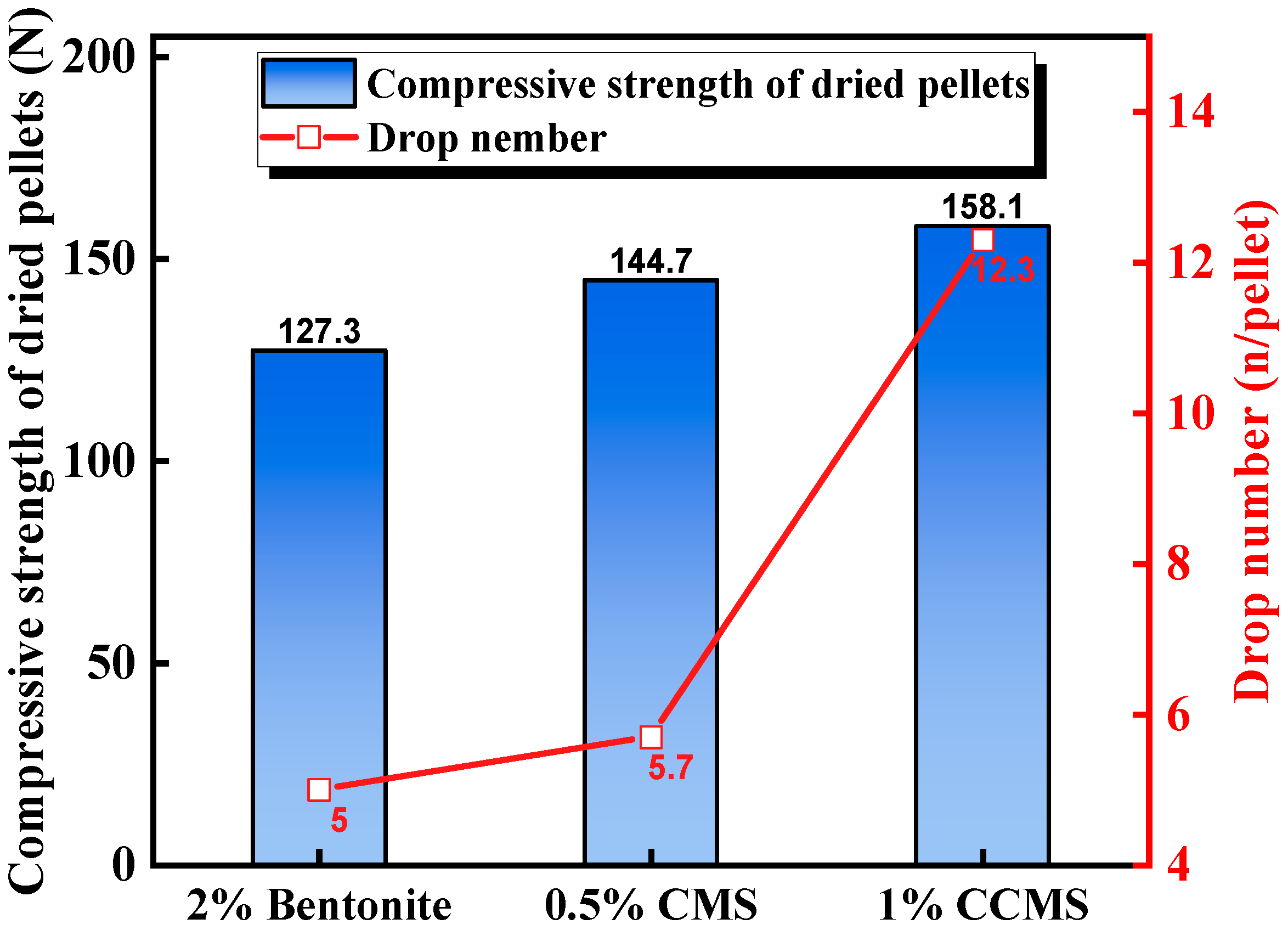
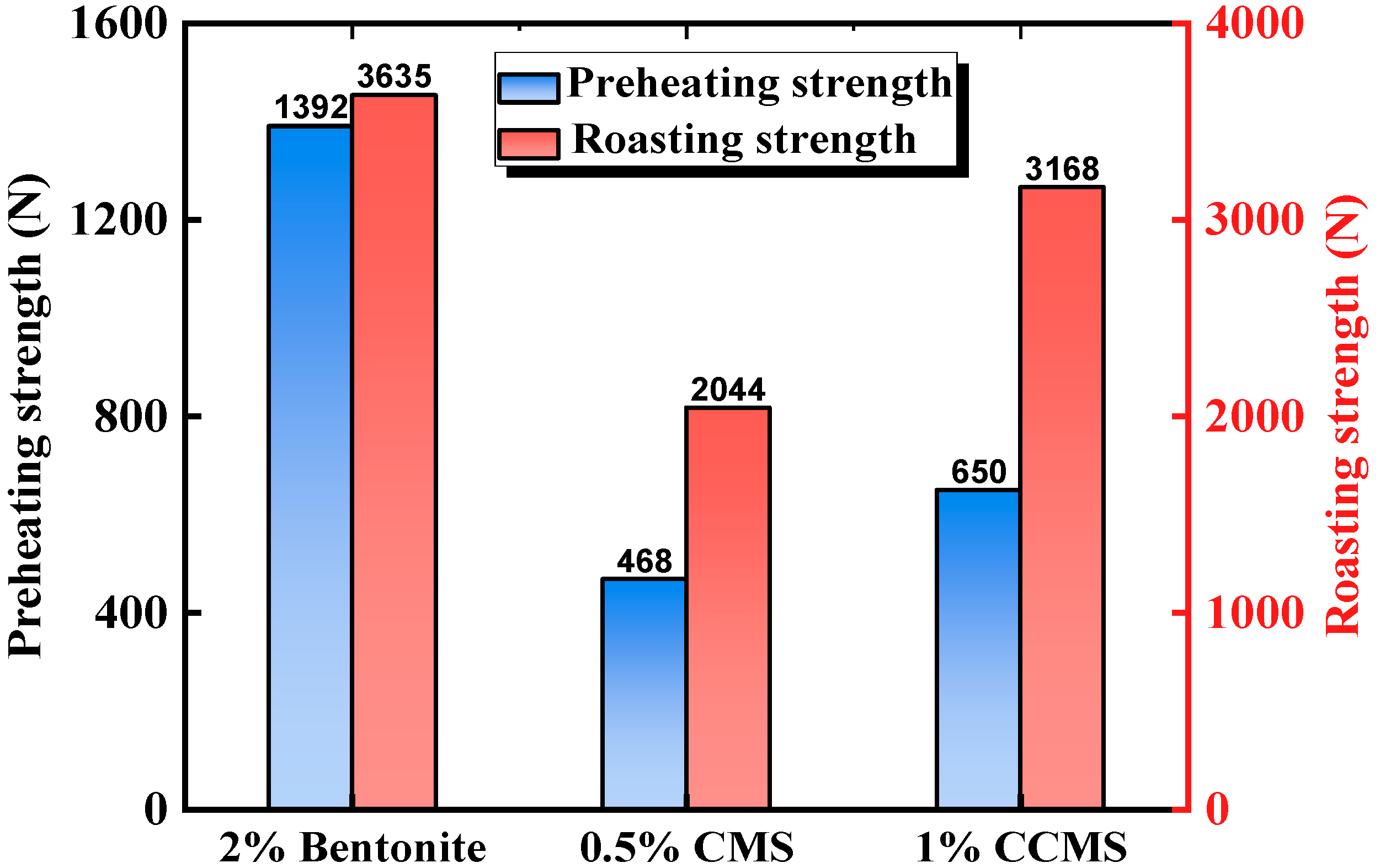
3.3.3. Process Characteristics and Application Effect of CMS
3.4. Discussion
4. Conclusions
- Na-LS relies on the chemical adhesion of sulfonic acid groups but requires the addition of low-iron oxides such as LD sludge/copper slag to compensate for high-temperature strength loss, which can increase the iron grade of pellets by 1–2% and reduce the RSI by more than 30%.
- CMC achieves a strong cohesive force through a high degree of substitution (DS > 0.8) and high polymerization degree, improving green pellet performance. However, nano-CaCO3 needs to be added to form a molten phase that fills pores, addressing the issue of insufficient sintering strength.
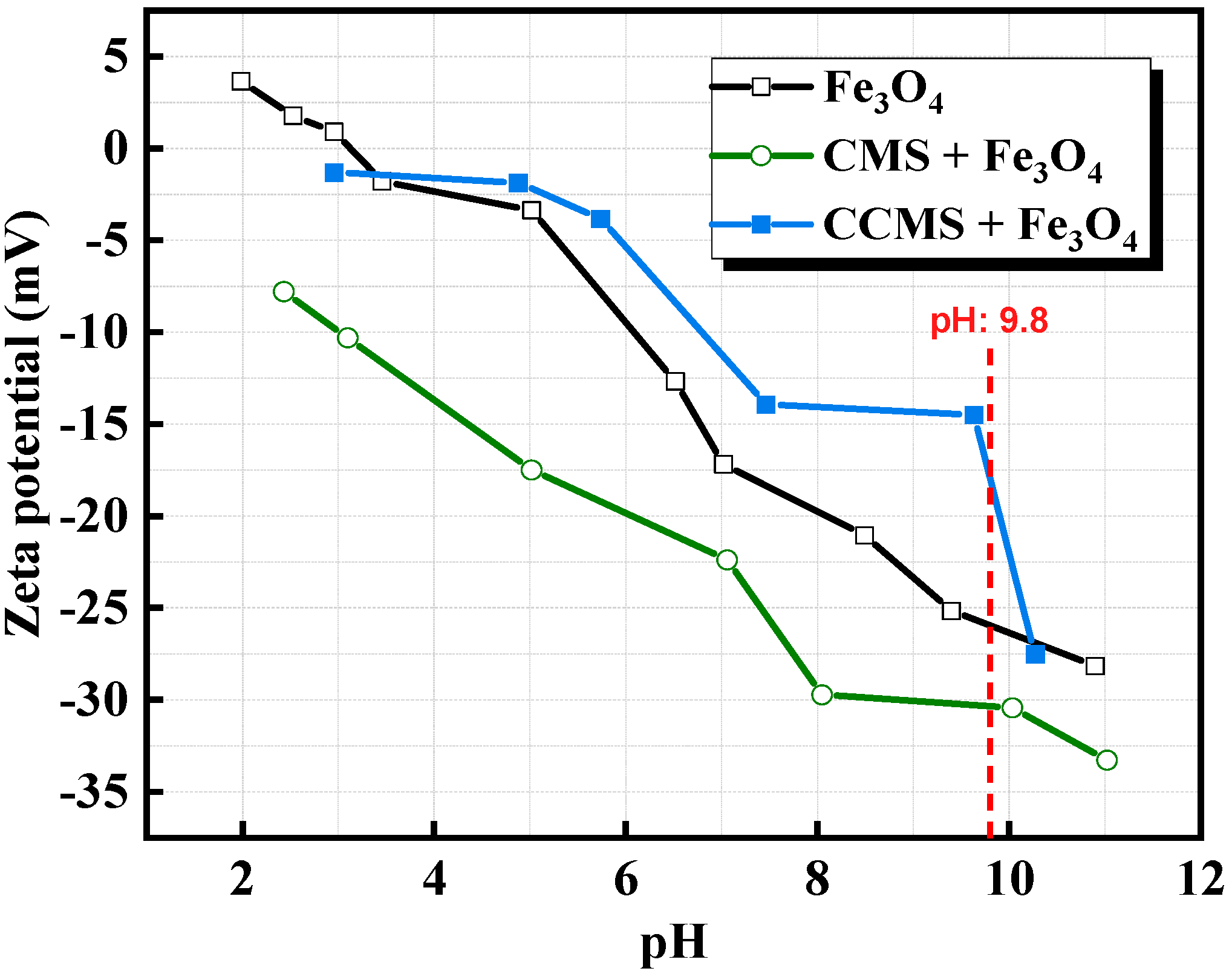
- CMS forms a continuous thick film with iron ore through the action of polar groups. The charge compensation effect of nano-CaCO3 (isoelectric point pH = 9.8) overcomes electrostatic repulsion, increasing the preheating strength of pellet ore by 40%. Both preheating strength and roasting strength meet industrial standards, with the iron grade improved by 1.04%.
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, L.M. Study on the Liquid Phase Formation and Crystallization Mechanism of Medium-High Silica Fluxed Pellets; University of Science and Technology: Beijing, China, 2025. [Google Scholar]
- Zhou, X.L.; Zhen, Z.K.; Wan, J.Y.; Chen, T.J.; Luo, Y.H.; Wang, Z.C. Study on the consolidation mechanism of organic binders high-grade pellets. Sinter. Pelletizing 2025, 50, 70–77. [Google Scholar]
- Xu, C.Y. Research on Reduction Behavior of Fluxed Pellets Under Hydrogen-Rich Conditions in Blast Furnace; University of Science and Technology: Beijing, China, 2022. [Google Scholar]
- Wang, X.D.; Jin, Y.L. Strategy analysis and testing study of high ratio of pellet utilized in blast furnace. Iron Steel 2021, 56, 7–16. [Google Scholar]
- Eisele, T.C.; Kawatra, S.K. A review of binders in iron ore pelletization. Miner. Process. Extr. Metall. Rev. 2003, 24, 1–90. [Google Scholar] [CrossRef]
- Cassola, M.S.; Chaves, A.P. Effect of the addition of organic binders on the behavior of iron ore pellets. KONA Powder Part. J. 1998, 16, 136–142. [Google Scholar] [CrossRef]
- Halt, J.A.; Kawatra, S.K. Review of organic binders for iron ore concentrate agglomeration. Min. Metall. Explor. 2014, 31, 73–94. [Google Scholar] [CrossRef]
- Sivrikaya, O.; Arol, A.I. Use of boron compounds as binders in iron ore pelletization. Open Miner. Process. J. 2010, 3, 25–35. [Google Scholar] [CrossRef]
- Kawatra, S.K.; Claremboux, V. Iron ore pelletization: Part II. Inorg. binders. Miner. Process. Extr. Metall. Rev. 2022, 43, 813–832. [Google Scholar] [CrossRef]
- Hayati-Ashtiani, M.; Jazayeri, S.H.; Ghannadi, M.; Nozad, A. Experimental characterizations and swelling studies of natural and activated bentonites with their commercial applications. J. Chem. Eng. Jpn. 2011, 44, 67–77. [Google Scholar] [CrossRef]
- Devasahayam, S. A novel iron ore pelletization for increased strength under ambient conditions. Sustain. Mater. Technol. 2018, 17, e00069. [Google Scholar] [CrossRef]
- Zhao, H.X.; Zhou, F.S.; Zhao, H.Y.; Ma, C.F.; Zhou, Y. A review on the effect of the mechanism of organic polymers on pellet properties for iron ore beneficiation. Polymers 2022, 14, 4874. [Google Scholar] [CrossRef]
- Zhao, H.X.; Zhou, F.S.; Ma, C.F.; Wei, Z.J.; Long, W.J. Bonding mechanism and process characteristics of special polymers applied in pelletizing binders. Coatings 2022, 12, 1618. [Google Scholar] [CrossRef]
- Mendoza, S.; Yin, B.H.; Zhang, A.; Bumby, C.W. Pelletization and sintering of New Zealand titanomagnetite ironsand. Adv. Powder Technol. 2022, 33, 103837. [Google Scholar] [CrossRef]
- Claremboux, V.; Kawatra, S.K. Iron ore pelletization: Part III. organic binders. Miner. Process. Extr. Metall. Rev. 2023, 44, 138–154. [Google Scholar] [CrossRef]
- Dronnet, V.M.; Renard, C.; Axelos, M.A.V.; Thibault, J.-F. Binding of divalent metal cations by sugar-beet pulp. Carbohydr. Polym. 1997, 34, 73–82. [Google Scholar] [CrossRef]
- Tuisov, A.G.; Kychkin, A.; Kychkin, A.K.; Anan’eva, E.S. Reinforced Epoxy Binder Modified with Borpolymer. Polymers 2023, 15, 2632. [Google Scholar] [CrossRef]
- Halt, J.A.; Kawatra, S.K. Does Zeta Potential Iron Ore Conc. Affect Strength Dustiness Unfired Fired Pellets? Miner. Process. Extr. Metall. Rev. 2017, 38, 132–141. [Google Scholar] [CrossRef]
- De Moraes, S.L.; Lima, J.R.B.; Neto, J.B.F. Effect of colloidal agents in iron ore pelletizing. Miner. Process. Extr. Metall. Rev. 2018, 39, 414–419. [Google Scholar] [CrossRef]
- Lyons, R.G.; Kundrat, D.M.; Myers, J.C.; Wise, D. Evaluation of taconite pellets made with an organic binder. Ironmak. Proc. 1986, 45, 31–36. [Google Scholar]
- De Moraes, S.L.; Lima, J.R.B.; Neto, J.B.F.; Fredericci, C.; Saccoccio, E.M. Binding mechanism in green iron ore pellets with an organic binder. Miner. Process. Extr. Metall. Rev. 2020, 41, 247–254. [Google Scholar] [CrossRef]
- Han, G.; Zhang, Y.; Huang, Y.; Sun, Z.; Li, G. Effects of Binders on Oxidized Pellets Preparation from Vanadium/Titanium-Bearing Magnetite. In 2nd International Symposium on High-Temperature Metallurgical Processing; The Minerals, Metals and Materials Society: Pittsburgh, PA, USA, 2011; pp. 279–287. [Google Scholar]
- Zhang, S.H.; Shao, J.N.; Lan, C.C.; Bi, Z.X.; Lv, Q. Application status and prospect of biomass energy in ironmaking process. Iron Steel 2022, 57, 13–22. [Google Scholar]
- Fu, P.; Xu, G.P.; Li, X.H.; Zhu, T.; Zhang, Z. Analysis of the Development Status and Trends of China’s Biomass Power Industry and Carbon Emission Reduction Potential. Ind. Saf. Environ. Prot. 2021, 47, 48–52. [Google Scholar]
- Garcia, R.; Gil, M.V.; Rubiera, F.; Pevida, C. Pelletization of wood and alternative residual biomass blends for producing industrial quality pellets Science. Fuel 2019, 251, 739–753. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink-habil, H.P.; Bohn, A. Cellulose, chemistry and application. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Zhao, Z.; Hao, S.; Hao, P.; Song, Y.H.; Manivannan, A.; Wu, N.; Liu, H. Lignosulphonate-cellulose derived porous activated carbon for supercapacitor electrode. J. Mater. Chem. A 2015, 3, 15049–15056. [Google Scholar] [CrossRef]
- Watcharakitti, J.; Win, E.E.; Nimnuan, J.; Smith, S.M. Modified starch-based adhesives: A review. Polymers 2022, 14, 2023. [Google Scholar] [CrossRef]
- Qiu, G.Z.; Jiang, T.J.; Li, H.X.; Wang, D.Z. Functions and molecular structure of organic binders for iron ore pelletization. Colloids Surf. A Physicochem. Eng. Asp. 2003, 224, 11–22. [Google Scholar] [CrossRef]
- Li, H.X.; Jang, T.; Qiu, G.Z.; Wang, D.Z. Molecular Structure Mould and Selecting Criterion of Organic Binder for Iron Ore Pellet; Central South University of Technology (Department of Mineral Engineering): Changsha, China, 2000; pp. 17–20. [Google Scholar]
- Han, G.H.; Huang, Y.F.; Li, G.H.; Zhang, Y.B.; Zhou, Y.L.; Jiang, T. Optimizing the mass ratio of two organic active fractions in modified humic acid (MHA) binders for iron ore pelletizing. ISIJ Int. 2012, 52, 378–384. [Google Scholar] [CrossRef]
- Zhu, D.; Pan, J.; Lu, L.; Holmes, R.J. Iron Ore: Mineralogy, Processing and Environmental Sustainability; Woodhead Publishing: Cambridge, UK, 2015; pp. 435–473. [Google Scholar]
- Qiu, G.Z.; Jiang, T.; Fan, X.H.; Zhu, D.Q.; Huang, Z.C. Effects of binders on balling behaviors of iron ore concentrates. Scand. J. Metall. 2004, 33, 39–46. [Google Scholar] [CrossRef]
- Vermeer, A.W.P.; Van Riemsdijk, W.H.; Koopal, L.K. Adsorption of humic acid to mineral particles. 1. Specific and electrostatic interactions. Langmuir 1998, 14, 2810–2819. [Google Scholar] [CrossRef]
- Gu, B.H.; Schmitt, J.; Chen, Z.H.; Liang, L.Y.; McCarthy, J.F. Adsorption and desorption of natural organic matter on iron oxide: Mechanisms and models. Environ. Sci. Technol. 1994, 28, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Li, P.; Zhou, Y.L.; Han, G.H.; Li, G.H.; Xu, B.; Jiang, T. Adsorption of lignite humic acid onto magnetite particle surface. J. Cent. South Univ. 2012, 19, 1967–1972. [Google Scholar] [CrossRef]
- Casey, L. Organic Binders for Iron Ore Pelletization. Master’s Thesis, Kemian Tekniikan Korkeakoulu, Espoo, Finland, 2016. [Google Scholar]
- Forsmo, S. Influence of Green Pellet Properties on Pelletizing of Magnetite Iron Ore. Ph.D. Thesis, Luleå Tekniska Universitet, Luleå, Sweden, 2007. [Google Scholar]
- Pal, J.; Ammasi, A.; Bandyopadhyay, B.S.; Dwarapudi, S.; Paul, I. An innovative approach to replace bentonite in hematite ore pelletizing with organic binder. Miner. Process. Extr. Metall. Rev. 2024, 45, 225–237. [Google Scholar] [CrossRef]
- Fang, H.Y.; Gao, L.; Zhou, X.L.; Yan, H.L.; Wang, Y.P.; Ji, H.H. Enhanced compressive strength of preheated limonite pellets with biomass-derived binders. Adv. Powder Technol. 2023, 34, 104154. [Google Scholar] [CrossRef]
- Tafadzwa, N.; Mavengere, S.; Bright, S.; Mapamba, L. Investigating the effectiveness of organic binders as an alternative to bentonite in the pelletization of low-grade iron ore. Physicochem. Probl. Miner. Process. 2023, 59, 176094. [Google Scholar]
- Ammasi, A.; Pal, J. Replacement of bentonite in hematite ore pelletisation using a combination of sodium lignosulphonate and copper smelting slag. Ironmak. Steelmak. 2016, 43, 203–213. [Google Scholar] [CrossRef]
- Zhou, J.A.; Wang, J.; Wang, B.; Ding, B.; Dang, Y.C.; Li, Y.J. The bonding mechanism and effects of sodium lignosulfonate (SL) in iron ore pelletization. Metall. Res. Technol. 2022, 119, 303. [Google Scholar] [CrossRef]
- Parathodiel, H.; Mousa, E.; Ahmed, H.; Elsadek, M.; Forsberg, K.; Andersson, C. Developing iron ore pellets using novel binders for H2-based direct reduction. Sustainability 2023, 15, 11415. [Google Scholar] [CrossRef]
- Sivrikaya, O.; Arol, A.I. Use of organic binders and borates in pelletizing of iron oxides. In Proceedings of the 4th International Boron Symposium, Eskişehir, Turkey, 15–17 October 2009; pp. 251–256. [Google Scholar]
- Li, C.X.; Bai, Y.; Ren, R.C.; Liu, G.Q.; Zhao, J.Y. Study of the mechanism for improving green pellet performance with compound binders. Physicochem. Probl. Miner. Process. 2019, 55, 153–162. [Google Scholar]
- Lu, J.W.; Zhao, X.; Yuan, Z.T.; Gao, P.C.; Li, L.X. Characterization of the Bonding Effect of Nano-CaCO3 Modified CMC on Magnetite Concentrate Pellets. J. Miner. Met. Mater. Soc. 2019, 71, 2549–2555. [Google Scholar] [CrossRef]
- Lu, S.; Yuan, Z.; Zhang, C. Binding mechanisms of polysaccharides adsorbing onto magnetite concentrate surface. Powder Technol. 2018, 340, 17–25. [Google Scholar] [CrossRef]
- Yuan, Z.T.; Lu, S.S.; Liu, J.T.; Gao, P.C.; Lu, J.W.; Li, L.X. Effects of nano-CaCO3 on the adsorption of carboxymethyl starch onto magnetite concentrate in pelletizing. Powder Technol. 2017, 312, 38–47. [Google Scholar] [CrossRef]
- Lu, S.S.; Yuan, Z.T.; Liu, J.T.; Lu, J.W.; Li, L.X.; Hao, H.Q. Binding effects and mechanisms of the carboxymethyl starch modified with nano-CaCO3 in magnetite concentrate pellets. Powder Technol. 2016, 301, 1183–1192. [Google Scholar] [CrossRef]
- Meister, J.J. Modification of lignin. J. Macromol. Sci. 2002, 42, 235–289. [Google Scholar] [CrossRef]
- Gonçalves, S.; Ferra, J.; Paiva, N.; Martins, J.; Carvalho, L.H.; Magalhães, F.D. Lignosulphonates as an alternative to non-renewable binders in wood-based materials. Polymers 2021, 13, 4196. [Google Scholar] [CrossRef] [PubMed]
- Aro, T.; Fatehi, P. Production and application of lignosulfonates and sulfonated lignin. ChemSusChem 2017, 10, 1861–1877. [Google Scholar] [CrossRef]
- Sixta, H. Handbook of Pulp, 2 Volume Set; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Suhr, M.; Klein, G.; Kourti, I.; Gonzalo, M.R.; Santonja, G.G.; Roudier, S.; Sancho, L.D. Best available techniques (BAT) reference document for the production of pulp, paper and board. Eur. Comm. 2015, 906, 370629. [Google Scholar]
- Northey, R.A. The use of lignosulfonates as water reducing agents in the manufacture of gypsum wallboard. In Chemical Modification, Properties, and Usage of Lignin; Springer: New York, NY, USA, 2002; pp. 139–150. [Google Scholar]
- Stern, T.; Schwarzbauer, P. Wood-based lignosulfonate versus synthetic polycarboxylate in concrete admixture systems: The perspective of a traditional pulping by-product competing with an oil-based substitute in a business-to-business market in central Europe. For. Prod. J. 2008, 58, 81–86. [Google Scholar]
- Myrvold, B.O. A new model for the structure of lignosulphonates: Part 1. Behaviour in dilute solutions. Ind. Crops Prod. 2008, 27, 214–219. [Google Scholar] [CrossRef]
- Salazar Valencia, P.J.; Bolívar Marinez, L.E.; Pérez Merchancano, S.T. Molecular modeling of ammonium, calcium, sulfur, and sodium lignosulphonates in acid and basic aqueous environments. Braz. J. Phys. 2015, 45, 567–574. [Google Scholar] [CrossRef]
- Kun, D.; Pukánszky, B. Polymer/lignin blends: Interactions, properties, applications. Eur. Polym. J. 2017, 93, 618–641. [Google Scholar] [CrossRef]
- Fiorani, G.; Crestini, C.; Selva, M.; Perosa, A. Advancements and complexities in the conversion of lignocellulose into chemicals and materials. Front. Chem. 2020, 8, 797. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; Tang, J.; Zhou, Y.; Lin, Q.; Xiao, R.; Zhang, M. Characterization of goethite-fulvic acid composites and their impact on the immobility of Pb/Cd in soil. Chemosphere 2019, 222, 556–563. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, M.J.; Zhu, D.M.; Lou, H.M.; Pang, Y.X.; Qiu, K.X.; Huang, J.H.; Qiu, X.Q. A simple and rapid method to determine sulfonation degree of lignosulfonates. BioEnergy Res. 2019, 12, 260–266. [Google Scholar] [CrossRef]
- Duval, A.; Molina-Boisseau, S.; Chirat, C. Fractionation of lignosulfonates: Comparison of ultrafiltration and ethanol solubility to obtain a set of fractions with distinct properties. Holzforschung 2015, 69, 127–134. [Google Scholar] [CrossRef]
- Lou, H.M.; Lai, H.R.; Wang, M.X.; Pang, Y.X.; Yang, D.J.; Qiu, X.Q.; Wang, B.; Zhang, H.B. Preparation of lignin-based superplasticizer by graft sulfonation and investigation of the dispersive performance and mechanism in a cementitious system. Ind. Eng. Chem. Res. 2013, 52, 16101–16109. [Google Scholar] [CrossRef]
- Fredheim, G.E.; Braaten, S.M.; Christensen, B.E. Molecular weight determination of lignosulfonates by size-exclusion chromatography and multi-angle laser light scattering. J. Chromatogr. A 2002, 942, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, R.F.; Neal, J.A.; McCarthy, J.L. Some properties of paucidisperse gymnosperm lignin sulfonates of different molecular weights. J. Wood Chem. Technol. 1992, 12, 447–469. [Google Scholar] [CrossRef]
- Fan, J.J.; Qiu, G.Z.; Jiang, T.; Guo, Y.F.; Hao, H.Z.; Yang, Y.B. Mechanism of high pressure roll grinding on compression strength of oxidized hematite pellets. J. Cent. South Univ. 2012, 19, 2611–2619. [Google Scholar] [CrossRef]
- Kukrety, A.; Singh, R.K.; Singh, P.; Ray, S.S. Comprehension on the synthesis of carboxymethylcellulose (CMC) utilizing various cellulose rich waste biomass resources. Waste Biomass Valorization 2018, 9, 1587–1595. [Google Scholar] [CrossRef]
- Slotte, S. Production Process of Carboxymethyl Cellulose. Bachelor’s Thesis, University of Toronto, Toronto, ON, Canada, 2021. [Google Scholar]
- Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.S.; Shiddiky, M.J.A.; Ahmed, M.B. Recent developments of carboxymethyl cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef] [PubMed]
- Aggrey, W.N.; Asiedu, N.Y.; Tackie-Otoo, B.N.; Adjei, S.; Mensah-Bonsu, E. Performance of carboxymethyl cellulose produced from cocoa pod husk as fluid loss control agent at high temperatures and variable (low and high) differential pressure conditions-Part 1. J. Pet. Sci. Technol. 2019, 9, 22–38. [Google Scholar]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Kopania, E.; Wietecha, J.; Ciechańska, D. Studies on isolation of cellulose fibres from waste plant biomass. Fibres Text. East. Eur. 2012, 96, 167–172. [Google Scholar]
- Michelin, M.; Gomes, D.G.; Romaní, A.; Polizeli, M.D.L.T.; Teixeira, J.A. Nanocellulose production: Exploring the enzymatic route and residues of pulp and paper industry. Molecules 2020, 25, 3411. [Google Scholar] [CrossRef]
- Abdel Ghaffar, A.M.; El-Arnaouty, M.B.; Abdel Baky, A.A.; Shama, S.A. Radiation-induced grafting of acrylamide and methacrylic acid individually onto carboxymethyl cellulose for removal of hazardous water pollutants. Des. Monomers Polym. 2016, 19, 706–718. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Kamel, S.; Salama, A.; Sarhan, H.A. Carboxymethyl cellulose acetate butyrate: A review of the preparations, properties, and applications. J. Drug Deliv. 2014, 2014, 575969. [Google Scholar] [CrossRef]
- Kunjalukkal Padmanabhan, S.; Lamanna, L.; Friuli, M.; Sannino, A.; Demitri, C.; Licciulli, A. Carboxymethylcellulose-based hydrogel obtained from bacterial cellulose. Molecules 2023, 28, 829. [Google Scholar] [CrossRef] [PubMed]
- Yaradoddi, J.S.; Banapurmath, N.R.; Ganachari, S.V.; Soudagar, M.E.M.; Mubarak, N.M.; Hallad, S.; Hugar, S.; Fayaz, H. Biodegradable carboxymethyl cellulose based material for sustainable packaging application. Sci. Rep. 2020, 10, 21960. [Google Scholar] [CrossRef]
- Hashem, A.; Farag, S.; Badawy, S.M. Carboxymethyl cellulose: Past innovations, present applications, and future horizons. Results Chem. 2025, 17, 102534. [Google Scholar] [CrossRef]
- Pinto, E.; Aggrey, W.N.; Boakye, P.; Amenuvor, G.; Sokama-Neuyam, Y.A.; Fokuo, M.K.; Karimaie, H.; Sarkodie, K.; Adenutsi, C.D.; Erzuah, S.; et al. Cellulose processing from biomass and its derivatization into carboxymethylcellulose: A review. Sci. Afr. 2022, 15, e01078. [Google Scholar] [CrossRef]
- Hoogendam, C.W.; De Keizer, A.; Cohen Stuart, M.A.; Bijsterbosch, B.H.; Batelaan, J.G.; Van der Horst, P.M. Adsorption mechanisms of carboxymethyl cellulose on mineral surfaces. Langmuir 1998, 14, 3825–3839. [Google Scholar] [CrossRef]
- Saha, B.; Patra, A.S.; Mukherjee, A.K.; Paul, I. Interaction and thermal stability of carboxymethyl cellulose on α-Fe2O3 (001) surface: ReaxFF molecular dynamics simulations study. J. Mol. Graph. Model. 2021, 102, 107787. [Google Scholar] [CrossRef] [PubMed]
- Zhivkov, A.M. Electric properties of carboxymethyl cellulose. In Cellulose-Fundamental Aspects; IntechOpen: London, UK, 2013; pp. 1–31. [Google Scholar]
- Jalil, R.; Sarif, M.; Elham, P.; Hashim, S. Synthesis of carboxymethyl cellulose (CMC) from delignified Dyera Costulata. Malays. J. Chem. Eng. Technol. 2022, 5, 172–179. [Google Scholar] [CrossRef]
- Casaburi, A.; Rojo, Ú.M.; Cerrutti, P.; Vázquez, A.; Foresti, M.L. Carboxymethyl cellulose with tailored degree of substitution obtained from bacterial cellulose. Food Hydrocoll. 2018, 75, 147–156. [Google Scholar] [CrossRef]
- Yang, G.; Fan, X.; Chen, X.; Yuan, L.S.; Huang, X.X.; Li, X. Interaction mechanism between carboxymethyl cellulose and iron ore concentrates in iron ore agglomeration. J. Cent. South Univ. 2015, 22, 1241–1246. [Google Scholar] [CrossRef]
- Li, D.G. Properties of sodium carboxymethyl cellulose and its application in papermaking industry. Heilongjiang Pap. Mak. 2008, 36, 50–52. [Google Scholar]
- Fan, X.H.; Yang, G.M.; Chen, X.L.; He, X.N.; Huang, X.X.; Gao, L. Effect of carboxymethyl cellulose on the drying dynamics and thermal cracking performance of iron ore green pellets. Powder Technol. 2014, 267, 11–17. [Google Scholar] [CrossRef]
- De Britto, D.; Assis, O.B.G. Thermal degradation of carboxymethylcellulose in different salty forms. Thermochim. Acta 2009, 494, 115–122. [Google Scholar] [CrossRef]
- Srivastava, U.; Kawatra, S.K.; Eisele, T.C. Study of organic and inorganic binders on strength of iron oxide pellets. Metall. Mater. Trans. B 2013, 44, 1000–1009. [Google Scholar] [CrossRef]
- Dwarapudi, S.; Ghosh, T.K.; Shankar, A.; Tathavadkar, V.; Bhattacharjee, D.; Venugopal, R. Effect of pellet basicity and MgO content on the quality and microstructure of hematite pellets. Int. J. Miner. Process. 2011, 99, 43–53. [Google Scholar] [CrossRef]
- Copeland, L.; Blazek, J.; Salman, H.; Tang, M.C. Form and functionality of starch. Food Hydrocoll. 2009, 23, 1527–1534. [Google Scholar] [CrossRef]
- Bertoft, E. Understanding starch structure: Recent progress. Agronomy 2017, 7, 56. [Google Scholar] [CrossRef]
- Seidel, C.; Kulicke, W.H.; Heß, C.; Hartmann, B.; Lechner, M.D.; Lazik, W. Synthesis and characterization of cross-linked carboxymethyl potato starch ether gels. Starch-Stärke 2004, 56, 157–166. [Google Scholar] [CrossRef]
- Spychaj, T.; Wilpiszewska, K.; Zdanowicz, M. Medium and high substituted carboxymethyl starch: Synthesis, characterization and application. Starch-Stärke 2013, 65, 22–33. [Google Scholar] [CrossRef]
- Heinze, T.; Pfeiffer, K.; Liebert, T.; Heinze, U. Effective approaches for estimating the functionalization pattern of carboxymethyl starch of different origin. Starch-Stärke 1999, 51, 11–16. [Google Scholar] [CrossRef]
- Stojanović, Ž.; Jeremić, K.; Jovanović, S. Synthesis of carboxymethyl starch. Starch-Stärke 2000, 52, 413–419. [Google Scholar] [CrossRef]
- Thomson, R.A.M. Chemistry and Technology of Water-Soluble Polymers; Springer: New York, NY, USA, 1983. [Google Scholar]
- Sidar, A.; Albuquerque, E.D.; Voshol, G.P.; Ram, A.F.; Vijgenboom, E.; Punt, P.J. Carbohydrate binding modules: Diversity of domain architecture in amylases and cellulases from filamentous microorganisms. Front. Bioeng. Biotechnol. 2020, 8, 871. [Google Scholar] [CrossRef]
- Lemieux, M.; Gosselin, P.; Mateescu, M.A. Influence of drying procedure and of low degree of substitution on the structural and drug release properties of carboxymethyl starch. AAPS PharmSciTech 2010, 11, 775–785. [Google Scholar] [CrossRef]
- Li, H.X.; Wang, D.Z.; Hu, Y.H.; Qiu, G.Z.; Jiang, T. The mechanism of improving pellet strength by carboxyl methlatedamylum. Cent. South Univ. Technol. (Dep. Miner. Eng.) 2001, 32, 351–354. [Google Scholar]







| Binders | Additives | Specific Performance | Ref. |
|---|---|---|---|
| Ca-lignosulphonate (Ca-LS) | LD sludge | Pellets containing 0.4% Ca-LS and 5% LD sludge were comparable with bentonite-added pellets. | Pal et al. [39] |
| Na-lignosulphonate (Na-LS) | / | The compressive strength of the preheated pellets reached 342.55 N/P at 1100 °C. | Fang et al. [40] |
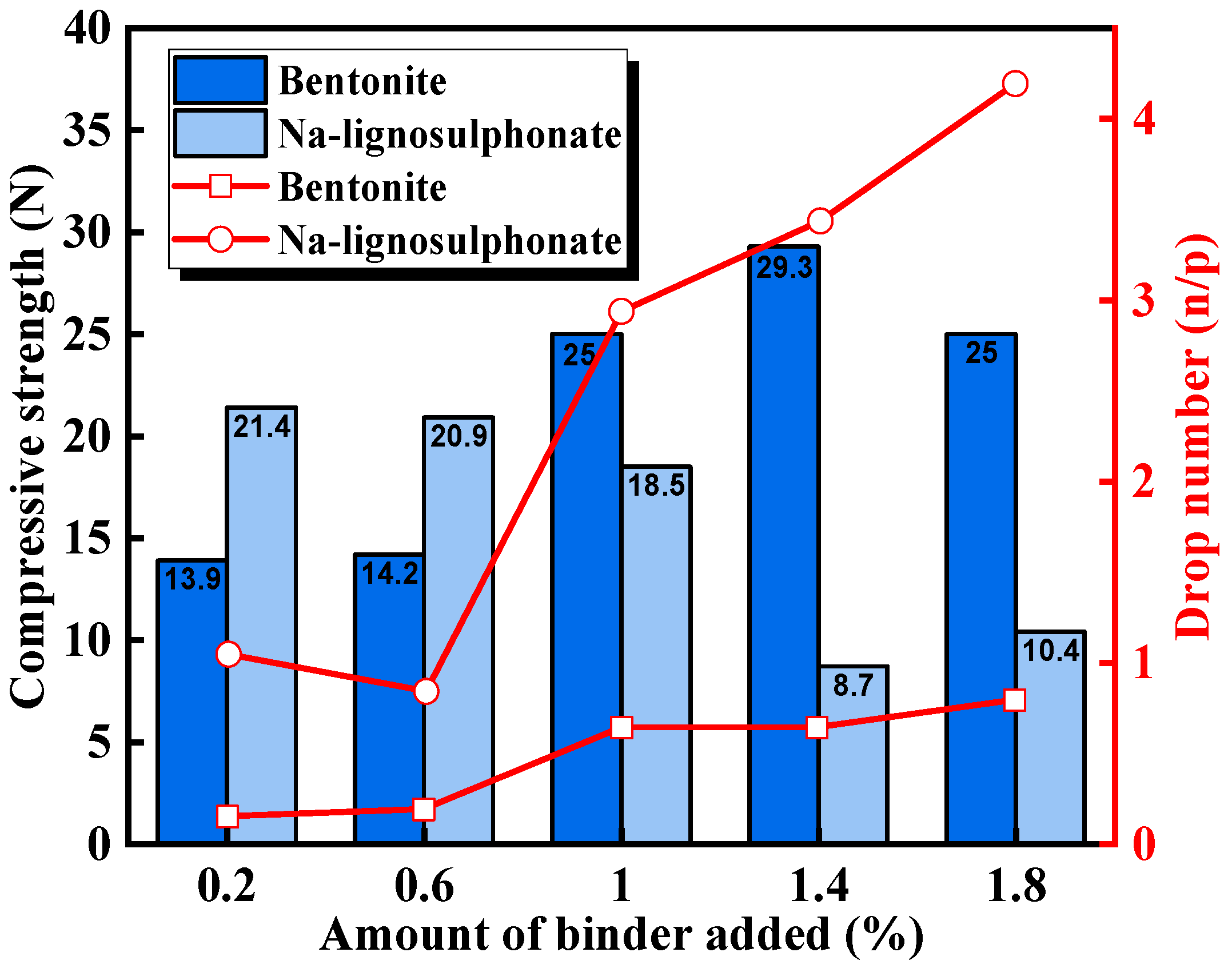
| Na-lignosulphonate (Na-LS) | / | The drop number, compressive strength, and porosity were better than those of the bentonite-added pellets. | Tafadzwa et al. [41] |
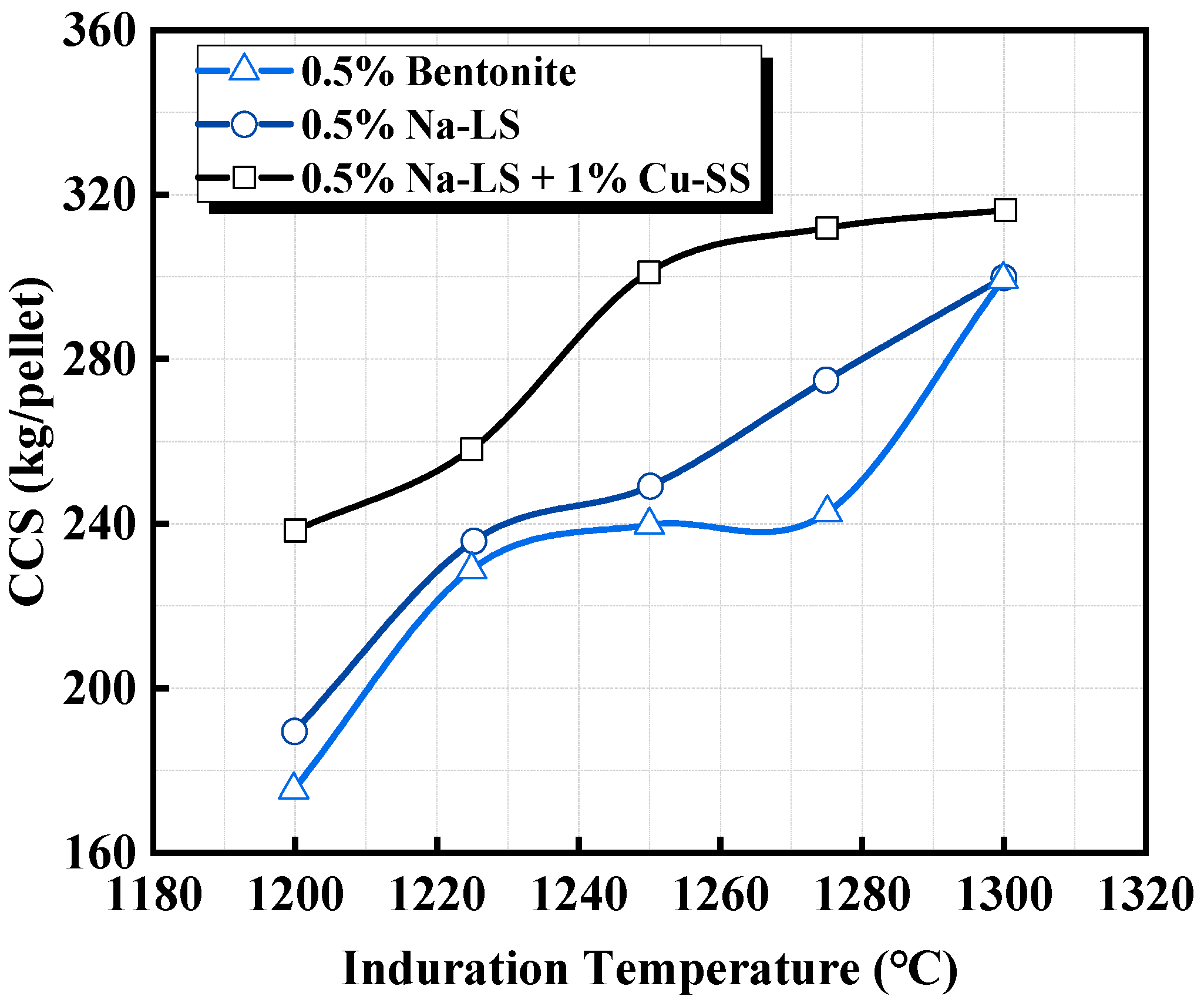
| Na-lignosulphonate (Na-LS) | Copper slag, Limestone | Using only 1.0% copper slag and 0.5% Na-LS, good CCS (300 kg/pellet) at 1250 °C, the highest RI (82.5%), low RDI (18%), and low swelling index (11%) were achieved. | Ammasi et al. [42] |
| Na-lignosulphonate (Na-LS) | CaCO3 | The CaCO3 promoted the binding ability of LS and had good compressive strength after preheating and induration. | Zhou et al. [43] |
| Carboxymethyl cellulose (CMC) | / | Green pellets with only CMC added exhibited excellent performance. | Parathodiel et al. [44] |
| Carboxymethyl cellulose (CMC) | Calcined colemanite | Compared with traditional bentonite production particles, its performance was superior. | Sivrikaya et al. [45] |
| Carboxymethyl cellulose (CMC) | Bentonite | CMC increased the hydrophilicity of bentonite and significantly improved green pellet performance. | Li et al. [46] |
| Carboxymethyl cellulose (CMC) | Nano-CaCO3 | The compressive strength of both preheated pellets and sintered pellets exceeded industrial standards. | Lu et al. [47] |
| Carboxymethyl starch (CMS) | / | Drop strength and compression strengths of dry pellets exceeded industry standards. | Lu et al. [48] |
| Carboxymethyl starch (CMS) | Nano-CaCO3 | CMS and nano-CaCO3 had high binding capacity and a higher adsorption rate. | Yuan et al. [49] |
| Carboxymethyl starch (CMS) | Nano-CaCO3 | CCMS (nano-CaCO3 and CMS) pellets had better preheating and sintering strength, and the total iron content increased by 1.04%. | Lu et al. [50] |
| Temperature (°C) | 100 | 200 | 250 | 300 | 350 | 400 | 500 | 700 |
|---|---|---|---|---|---|---|---|---|
| Compressive strength of bentonite (kg/pellet) | 7.19 | 7.70 | 7.98 | 7.88 | 6.36 | 7.31 | 8.00 | 9.28 |
| Compressive strength of 0.2% Ca-LS (kg/pellet) | 5.91 | 6.90 | 6.91 | 6.48 | 5.47 | 6.61 | 7.99 | 8.31 |
| Compressive strength of 0.2% Ca-LS+3% LDS (kg/pellet) | 5.85 | 6.08 | 6.21 | 5.20 | 4.42 | 4.66 | 7.18 | 7.78 |
| Sample | Fetotal | RI, % | RDI, % | Swelling Index, % | Ref. |
|---|---|---|---|---|---|
| Bentonite | 60.93 | 65.6 | 3.4 | 7.4 | Pal et al. [39] |
| 0.3% Ca-LS + 5% LDS | 62.53 | 64.5 | 1.5 | 6.8 | |
| 0.4% Ca-LS + 5% LDS | 63.13 | 66.1 | 1.8 | 5.8 | |
| Bentonite | 65.22 | 77.06 | 18.5 | 9.09 | Ammasi et al. [42] |
| 0.5% Na-LS | \ | 75.93 | 27.17 | 13.09 | |
| 0.5% Na-LS + 1% Cu-SS | 65.7 | 82.9 | 17.97 | 10.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, W.; Peng, Z.; Wang, J.; Xue, Q.; Zuo, H. Development and Application Prospects of Biomass-Based Organic Binders for Pellets Compared with Bentonite. Materials 2025, 18, 4553. https://doi.org/10.3390/ma18194553
Liu Y, Liu W, Peng Z, Wang J, Xue Q, Zuo H. Development and Application Prospects of Biomass-Based Organic Binders for Pellets Compared with Bentonite. Materials. 2025; 18(19):4553. https://doi.org/10.3390/ma18194553
Chicago/Turabian StyleLiu, Yu, Wenguo Liu, Zile Peng, Jingsong Wang, Qingguo Xue, and Haibin Zuo. 2025. "Development and Application Prospects of Biomass-Based Organic Binders for Pellets Compared with Bentonite" Materials 18, no. 19: 4553. https://doi.org/10.3390/ma18194553
APA StyleLiu, Y., Liu, W., Peng, Z., Wang, J., Xue, Q., & Zuo, H. (2025). Development and Application Prospects of Biomass-Based Organic Binders for Pellets Compared with Bentonite. Materials, 18(19), 4553. https://doi.org/10.3390/ma18194553






