Enhancement of Biopolymer Film Properties Using Spermidine, Zinc Oxide, and Graphene Oxide Nanoparticles: A Study of Physical, Thermal, and Mechanical Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Gelatin–Chitosan Film
2.2. Physical and Mechanical Properties of Films
2.2.1. Film Thickness
2.2.2. Mechanical Properties
2.2.3. Water Solubility
2.2.4. Water Vapor Permeability (WVP)
2.2.5. Color and Evaluation of Optical Properties of Films
2.3. Scanning Electron Microscopy (SEM)
2.4. Fourier Transform Infrared Spectroscopy
2.5. X-Ray Diffraction Analysis (XRD)
2.6. Differential Scanning Calorimetry (DSC)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Thickness of Gelatin–Chitosan Films
3.2. Evaluation of Mechanical Properties
3.3. Moisture Content
3.4. Solubility
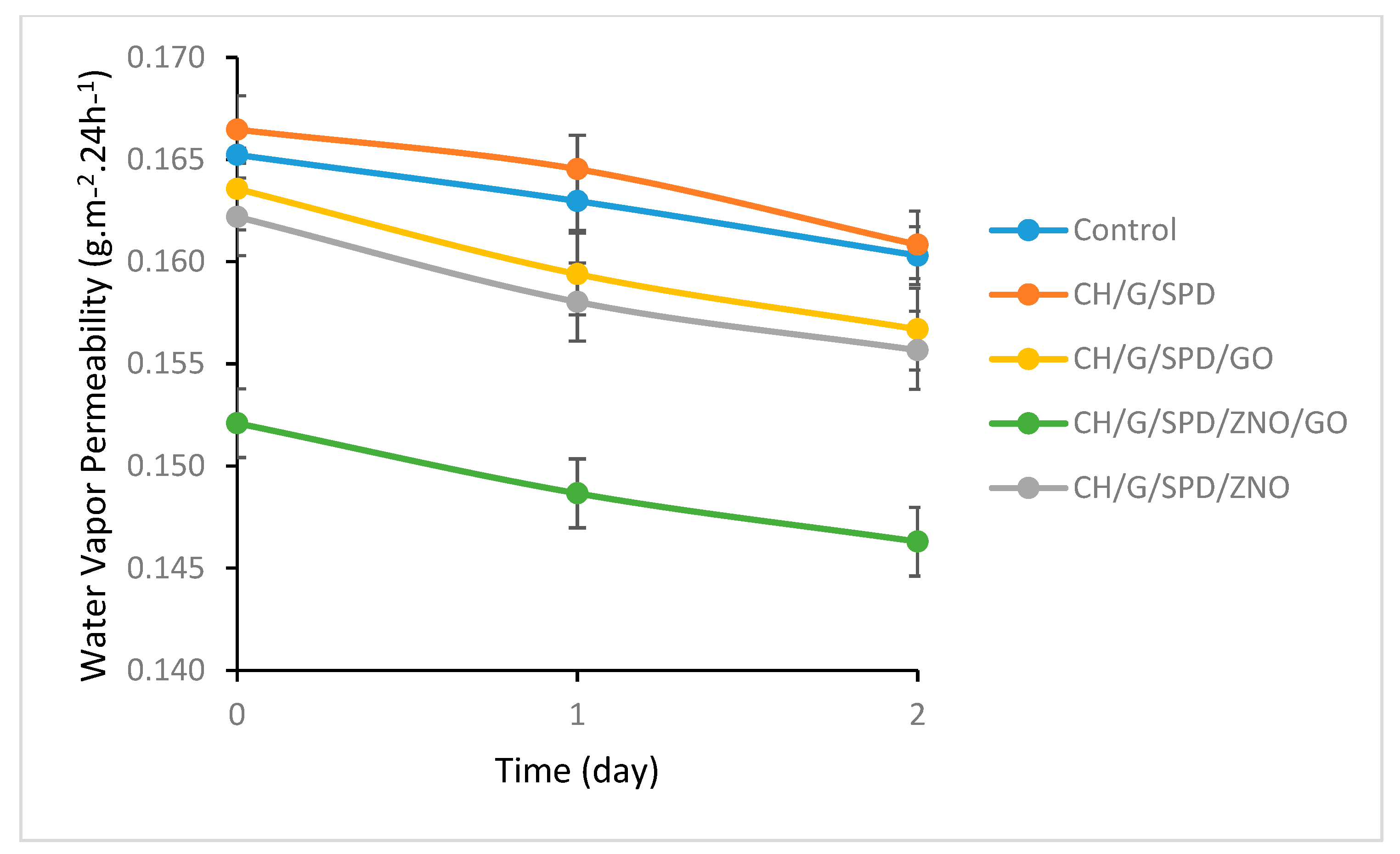
3.5. Water Vapor Permeability (WVP)
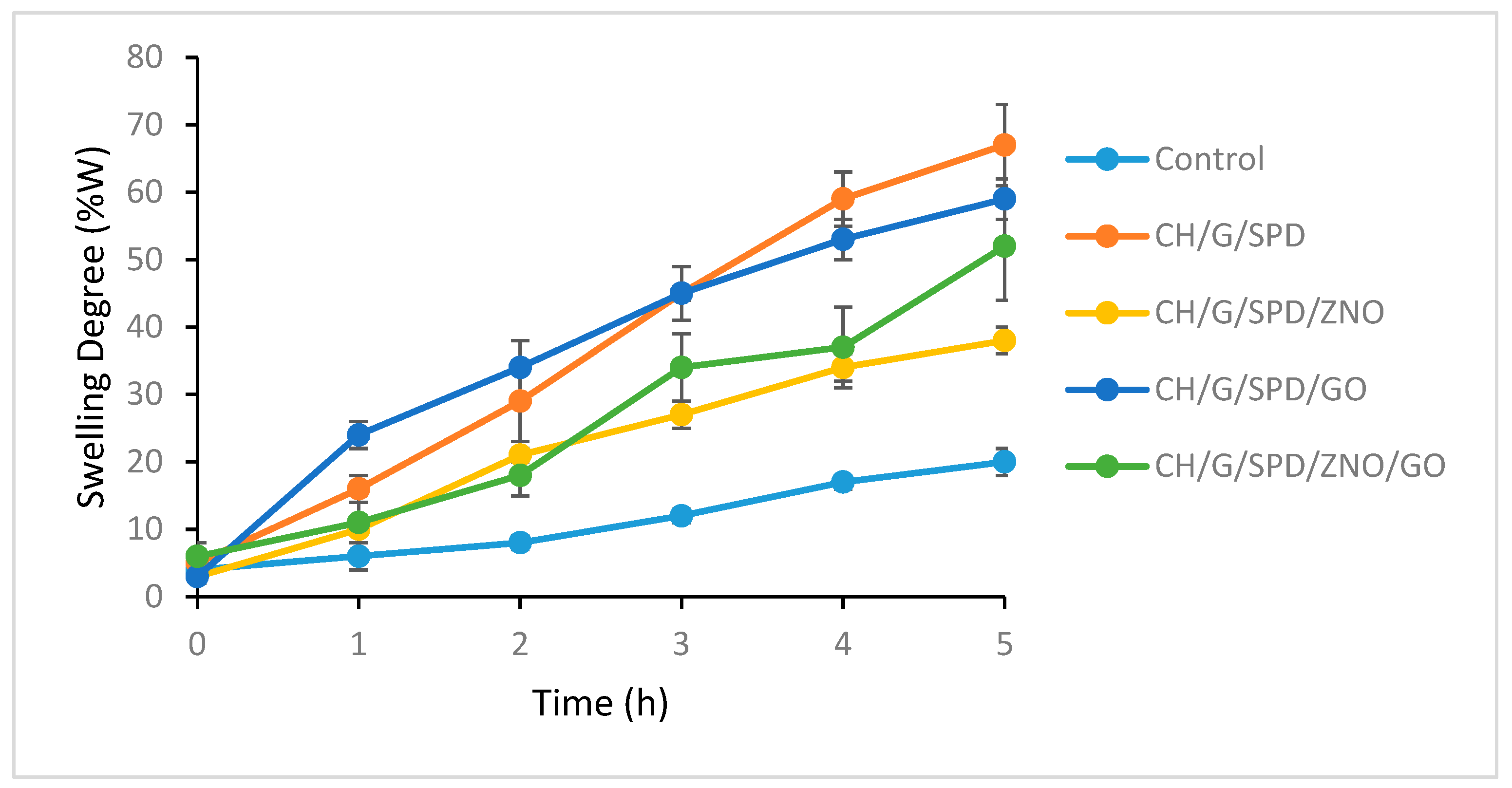
3.6. Swelling Studies
3.7. The Values of L, a*, and b* of the Films
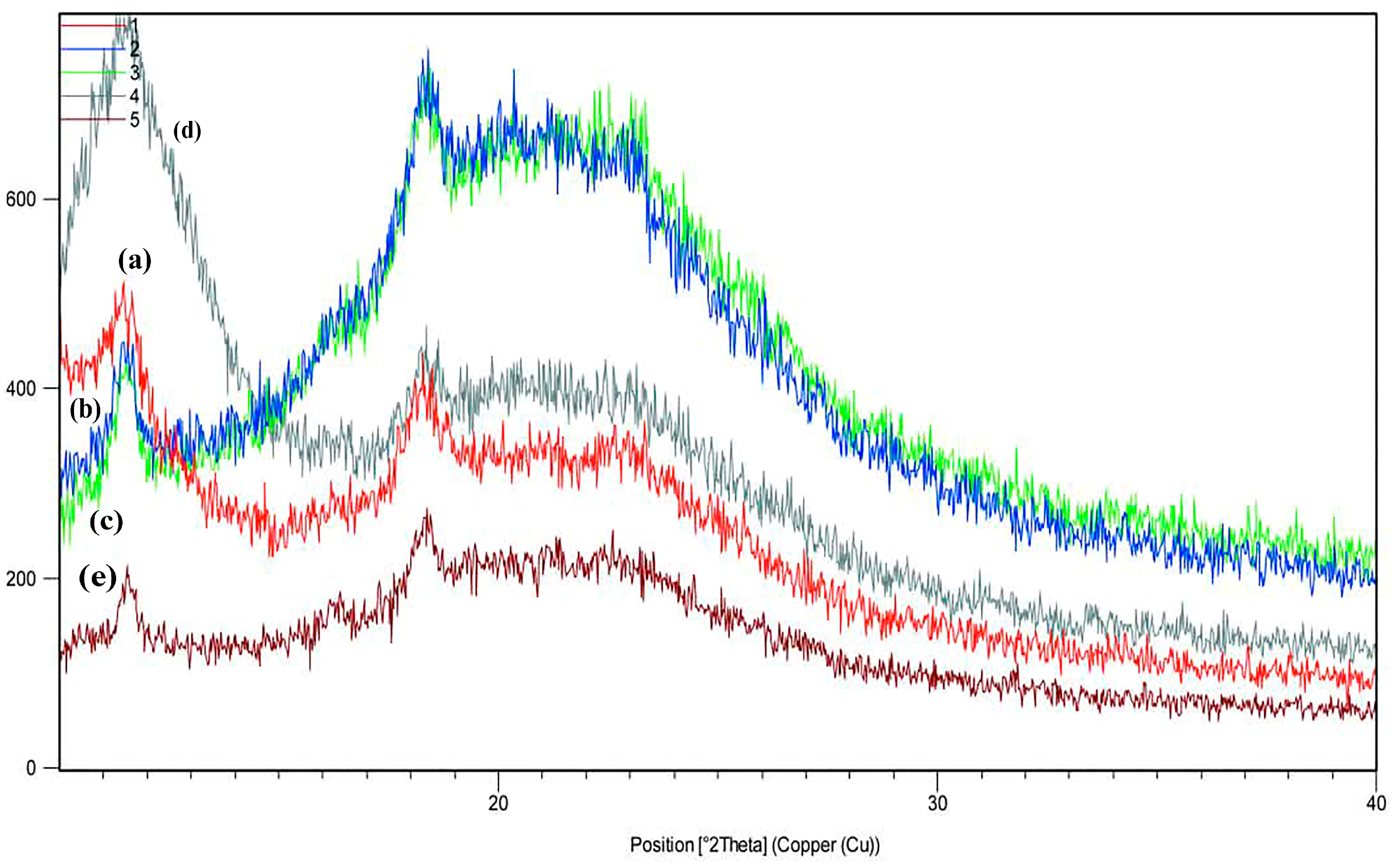
3.8. X-Ray Diffraction (XRD)
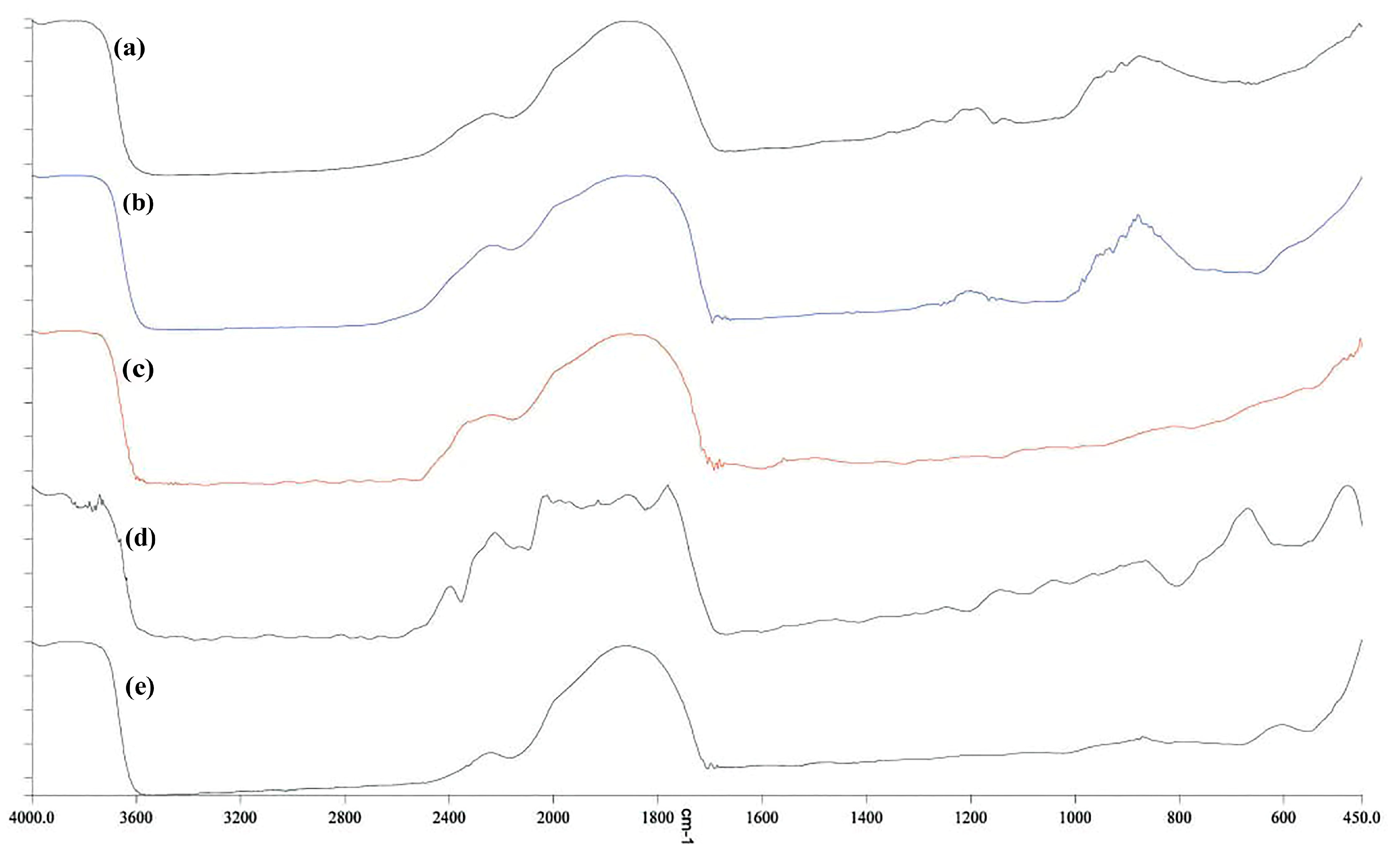
3.9. FTIR Spectroscopy
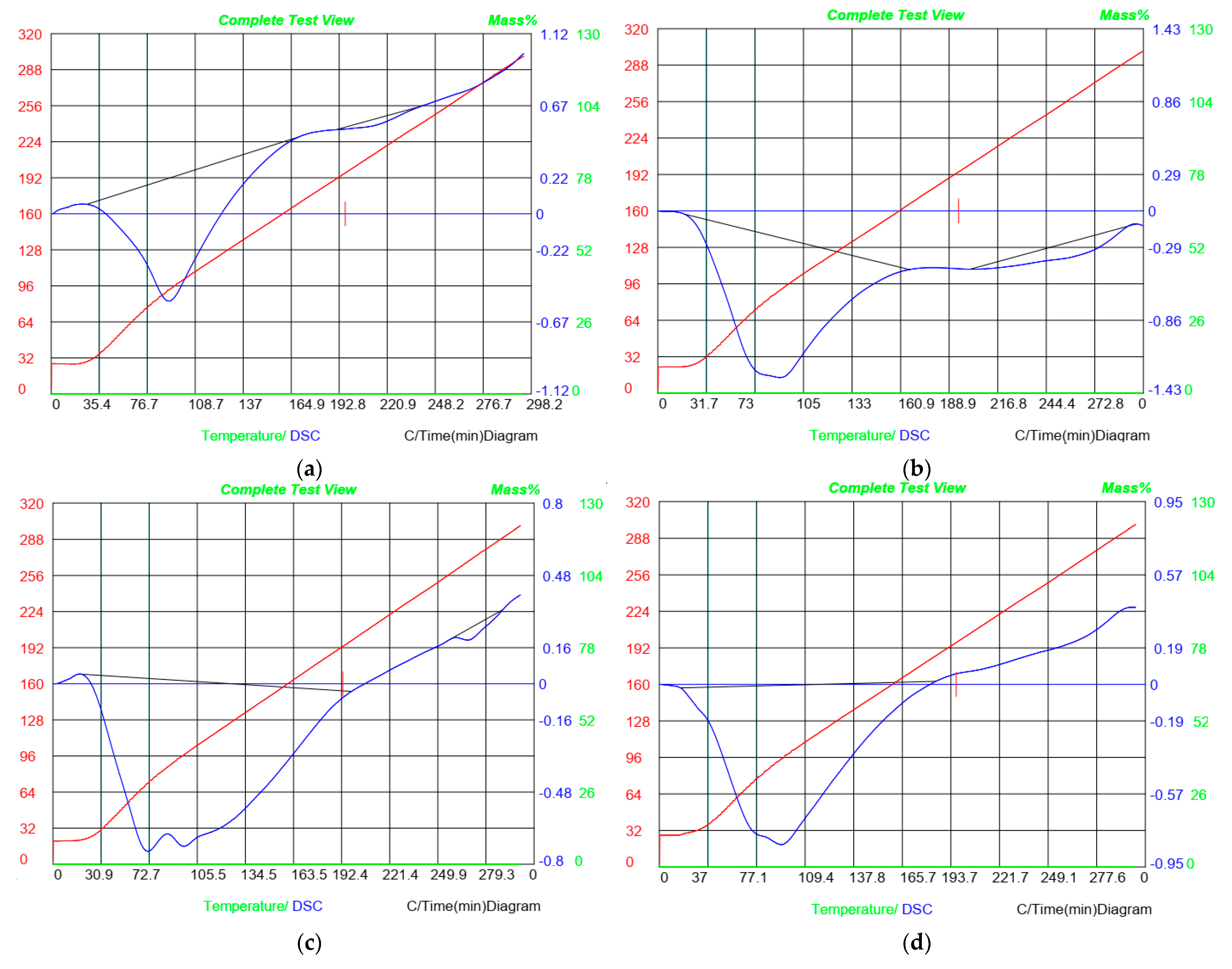
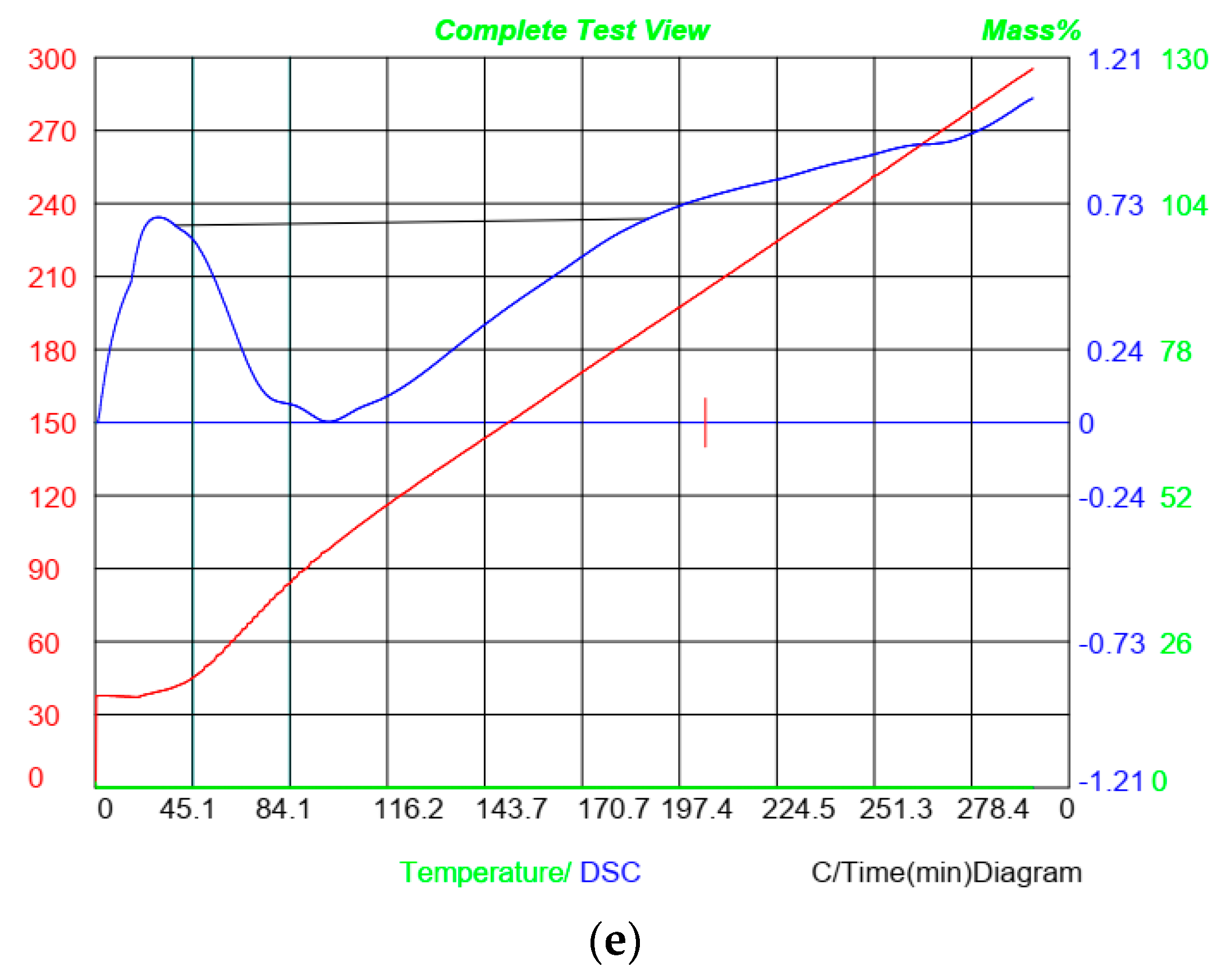
3.10. Differential Scanning Calorimetry (DSC)
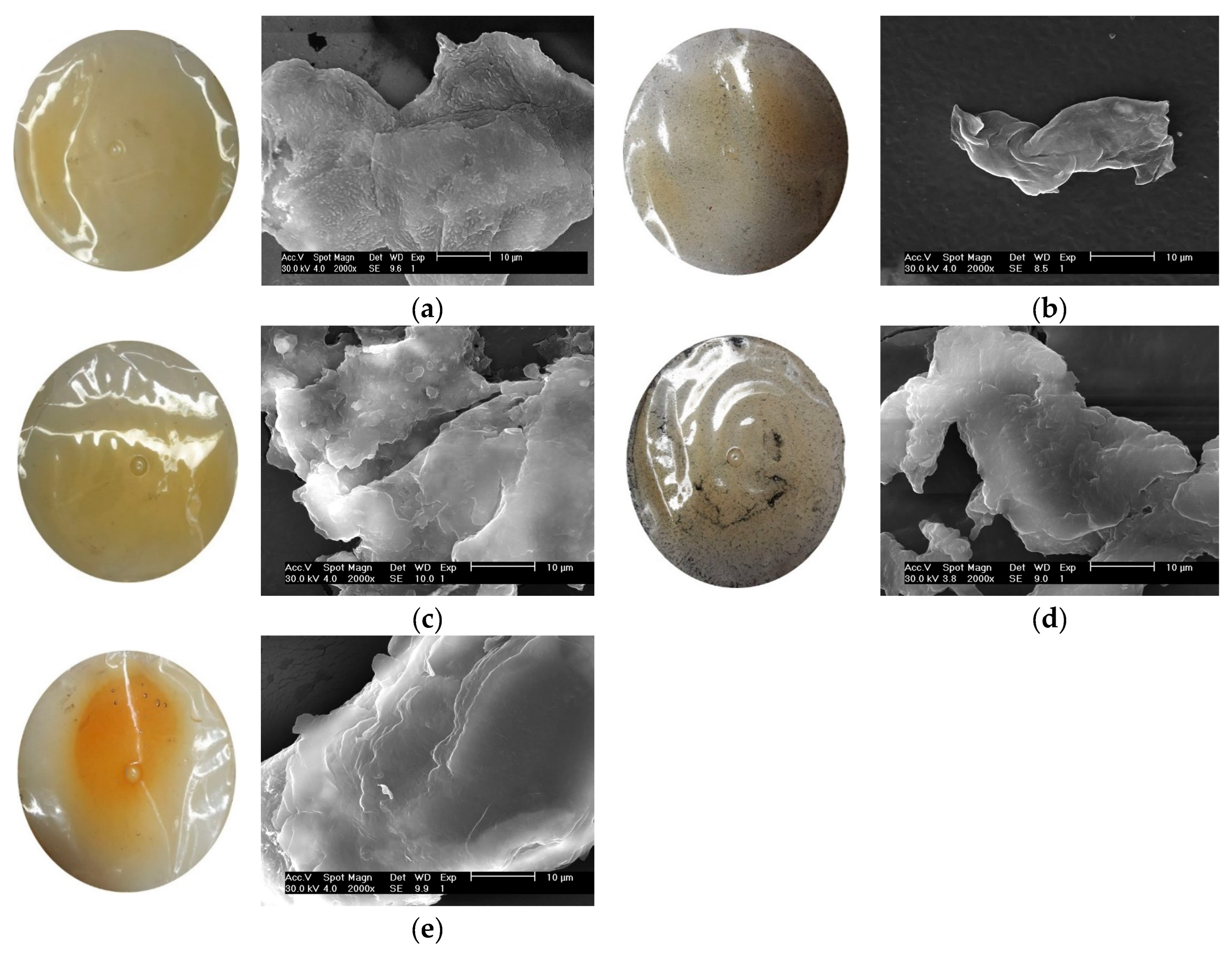
3.11. Surface Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moshayedi, S.H.; Sarpoolaky, H.; Khavandi, A. Fabrication and Characterization of Gelatin/Chitosan/Zinc Oxide Nanocomposite Hydrogels Intended for Biomedical Applications. J. Adv. Mater. Technol. 2022, 11, 57–69. [Google Scholar] [CrossRef]
- Sung, S.; Sin, L.; Tee, T.; Bee, S.; Rahmat, A.; Rahman, W.A.W.A. Antimicrobial agents for food packaging applications. Trends Food Sci. Technol. 2013, 33, 110–123. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y. Combined effects of two kinds of essential oils on physical, mechanical and structural properties of chitosan film. Food Hydrocol. 2014, 36, 287–293. [Google Scholar] [CrossRef]
- Zaferani Tabrizi, M.; Ojagh, S.M.; Alishahi, A.; Kazemi, M. The Effect of Chitosan Type on Biodegradable Films from Gelatin Extracted from Huso Huso Skin Patches and Their Specifications and Characteristics. IFT 2018, 6, 149–159. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; López de Lacey, A.; López-Caballero, M.; Gómez-Guillén, M.; Montero, P. Biodegradable gelatin–chitosan films incorporated with essential oils as antimicrobial agents for fish preservation. Food Microbiol. 2010, 27, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, R.; Pan, Y.; Du, M.; Zhao, Y.; Liu, H. Gelatin/Chitosan Films Incorporated with Curcumin Based on Photodynamic Inactivation Technology for Antibacterial Food Packaging. Polymers 2022, 14, 1600. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Mehrotra, G.K.; Dutta, P.K. Chitosan based ZnO nanoparticles loaded gallic-acid films for active food packaging. Food Chem. 2021, 334, 127605. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Petrisor, G.; Oprea, O.-C.; Trușcǎ, R.-D.; Ficai, A.; Andronescu, E.; Hudita, A.; Holban, A.M. Antimicrobial Hydroxyethyl-Cellulose-Based Composite Films with Zinc Oxide and Mesoporous Silica Loaded with Cinnamon Essential Oil. Pharmaceutics 2024, 16, 1225. [Google Scholar] [CrossRef]
- Hamdan, N.; Khodir, W.K.W.A.; Hamid, S.A.; Nasir, M.H.M.; Hamzah, A.S.; Cruz-Maya, I.; Guarino, V. PCL/Gelatin/Graphene Oxide Electrospun Nanofibers: Effect of Surface Functionalization on In Vitro and Antibacterial Response. Nanomaterials 2023, 13, 488. [Google Scholar] [CrossRef]
- Mekonnen, T.; Mussone, P.; Khalil, H.; Bressler, D. Progress in bio-based plastics and plasticizing modifications. J. Mater. Chem. 2013, 11, 13379–13398. [Google Scholar] [CrossRef]
- Esposito, M.; Di Pierro, P.; Regalado-Gonzales, C.; Mariniello, L.; Giosafatto, C.V.L.; Porta, R. Polyamines as new cationic plasticizers for pectin-based edible films. Carbohydr. Polym. 2016, 153, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Porta, R.; Di Pierro, P.; Roviello, V.; Sabbah, M. Tuning the functional properties of bitter vetch (Vicia ervilia) protein films grafted with spermidine. Int. J. Mol. Sci. 2017, 18, 2658–2669. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, M.; Di Pierro, P.; Giosafatto, C.V.L.; Esposito, M.; Mariniello, L.; Regalado-Gonzales, C.; Porta, R. Plasticizing Effects of Polyamines in Protein-Based Films. Int. J. Mol. Sci. 2017, 18, 1026. [Google Scholar] [CrossRef]
- Sabbah, M.; Di Pierroa, P.; Cammarotac, M.; Dell’Olmoa, E.; Arcielloa, A.; Porta, R. Development and properties of new chitosan-based films plasticized with spermidine and/or glycerol. Food Hydrocol. 2020, 87, 245–252. [Google Scholar] [CrossRef]
- Nafchi, A.M.; Moradpour, M.; Saeidi, M.; Alias, A.K. Effects of nanorod-rich ZnO on rheological, sorption isotherm, and physicochemical properties of bovine gelatin films. LWT-Food Sci. Technol. 2014, 58, 142–149. [Google Scholar] [CrossRef]
- Pouralkhas, M.; Kordjazi, M.; Ojagh, S.M.; Asadi Farsani, O. Physicochemical and functional characterization of gelatin edible film incorporated with fucoidan isolated from Sargassum tenerrimum. Int. J. Food Sci. Nutr. 2023, 11, 4124–4135. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.N.; Zhou, F.Z.; Yang, T.; Yin, S.W.; Tang, C.H.; Yang, X.Q. Fabrication and characterization of pickering high internal phase emulsions (HIPEs) stabilized by chitosan-caseinophosphopeptides nanocomplexes as oral delivery vehicles. Food Hydrocoll. 2019, 93, 34–45. [Google Scholar] [CrossRef]
- Roshandel-hesari, N.; Mokabr, M.; Taleghani, A.; Akbari, R. Investigation of physicochemical properties, antimicrobial and antioxidant activity of edible films based on chitosan/casein containing Origanum vulgare L essential oil and its effect on quality maintenance of cherry tomato. Food Chem. 2022, 396, 133650. [Google Scholar] [CrossRef] [PubMed]
- Poverenov, E.; Rutenberg, R.; Danino, S.H.; Horev, B.; Rodov, V. Gelatin-Chitosan Composite Films and Edible Coatings to Enhance the Quality of Food Products: Layer-by-Layer vs. Blended Formulations. Food Bioprocess Technol. 2014, 7, 3319–3327. [Google Scholar] [CrossRef]
- Lanier, T.C.; Hart, K.; Martin, R.E. A Manual of Standard Methods for Measuring Specifying the Properties of Surimi; National Fisheries Institute: Reston, VR, USA, 1991. [Google Scholar]
- Bhowmik, S.; Agyei, D.; Ali, A. Enhancement of mechanical, barrier, and functional properties of chitosan film reinforced with glycerol, COS, and gallic acid for active food packaging. Sustain. Mater. Technol. 2024, 41, e01092. [Google Scholar] [CrossRef]
- Hoque, M.S.; Benjakul, S.; Prodpran, T. Effects of partial hydrolysis and plasticizer content on the properties of film from cuttlefish (Sepia pharaonis) skin gelatin. Food Hydrocoll. 2011, 25, 82–90. [Google Scholar] [CrossRef]
- Jiang, P.; He, K.; Chen, M.; Yang, Y.; Tang, T.; Tian, Y. Development of multifunctional chitosan packaging film by plasticizing novel essential oil-based hydrophobic deep eutectic solvent: Structure, properties, and application. Carbohydr. Polym. 2025, 347, 122701. [Google Scholar] [CrossRef]
- Xu, M.; Yu, H.; Chen, X.; Yuan, G. Physico-chemical, biological properties of chitosan/gelatin-based films with Finger Millet bran extract. J. Food Meas. Charact. 2022, 16, 2939–2947. [Google Scholar] [CrossRef]
- Kim, I.Y.; Seo, S.J.; Moon, H.S.; Yoo, M.K.; Park, I.Y.; Kim, B.C. Bacterial cellulose-chitosan composite hydrogel beads for enzyme immobilization. Biotechnol. Bioproc. 2017, 22, 89–94. [Google Scholar] [CrossRef]
- Rahman, P.M.; Mujeeb, V.M.A.; Muraleedharan, K. Chitosan/nano ZnO composite films: Enhanced mechanical, antimicrobial and dielectric properties. Arab. J. Chem. 2023, 11, 120–127. [Google Scholar] [CrossRef]
- Indumathi, M.P.; Sarojini, K.S.; Rajarajeswari, G.R. Antimicrobial and biodegradable chitosan/cellulose acetate phthalate/ZnO nano composite films with optimal oxygen permeability and hydrophobicity. Int. J. Biol. Macromol. 2023, 132, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.M.; El-Sayed, S.M.; El-Sayed, H.S.; Salama, H.H.; Dufresne, A. Enhancement of Egyptian Soft White Cheese Shelf Life Using a Novel Chitosan/carboxymethyl Cellulose/zinc Oxide Bio nanocomposite Film. Carbohydr. Polym. 2016, 151, 9–19. [Google Scholar] [CrossRef]
- Hasheminya, M.; Dehghannya, J.; Ehsani, A. Development of basil seed mucilage (a heteropolysaccharide)—Polyvinyl alcohol biopolymers incorporating zinc oxide nanoparticles. Int. J. Biol. Macromol. 2023, 253, 127342. [Google Scholar] [CrossRef]
- Momtaz, M.; Momtaz, E.; Mehrgardi, M.; Momtaz, F.; Narimani, T.; Poursina, F. Preparation and characterization of gelatin/chitosan nanocomposite reinforced by NiO nanoparticles as an active food packaging. Sci. Rep. 2024, 14, 519. [Google Scholar] [CrossRef] [PubMed]
- Devaraju, A.; Sivasamy, P.; Babu Loganathan, G. Mechanical properties of polymer composites with ZnO nano-particle. Mater. Proc. 2020, 22, 531–534. [Google Scholar] [CrossRef]
- Fadaei Heydari, S.H.; Shahgholi, M.; Karimipour, A.; Salehi, M.; Galehdari, S.A. The effects of graphene oxide nanoparticles on the mechanical and thermal properties of polyurethane/polycaprolactone nanocomposites; a molecular dynamics approach. Results Eng. 2024, 24, 102933. [Google Scholar] [CrossRef]
- Kumar, S.; Koh, J. Physiochemical and optical properties of chitosanbased graphene oxide bionanocomposite. Int. J. Biol. Macromol. 2014, 70, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Liew, W.; Muhamad, I.; Wei Chew, J.; Juhanni, K.; Karim, A. Synergistic effect of graphene oxide/zinc oxide nanocomposites on polylactic acid-based active packaging film: Properties, release kinetics and antimicrobial efficiency. Int. J. Biol. Macromol. 2023, 253, 127288. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Q.; Zhang, C.; Li, P. Characterisation and cooperative antimicrobial properties of chitosan/nano-ZnO composite nanofibrous membranes. Food Chem. 2013, 132, 419–427. [Google Scholar] [CrossRef]
- Kumar, M.; Pon Selvan, C.; Santhanam, K.; Kadirvel, A.; Chandraprabu, V.; SampathKumar, L. Effect of Nanomaterials on Tribological and Mechanical Properties of Polymer Nanocomposite Materials. Adv. Mater. Sci. Eng. 2022, 5, 1–16. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Lei, F.; Hussain, Z.; Huang, D.D.; Zhu, S.H. Enhancement of storability and antioxidant systems of sweet cherry fruit by nitric oxide-releasing chitosan nanoparticles (GSNO-CS NPs). Food Chem. 2019, 285, 10–21. [Google Scholar] [CrossRef]
- Li, F.; Long, L.; Weng, Y. A Review on the Contemporary Development of Composite Materials Comprising Graphene/Graphene Derivatives. Adv. Mater. Sci. Eng. 2020, 60, 586–634. [Google Scholar] [CrossRef]
- Moezzi, A.; McDonagh, A.; Cortie, M. Zinc oxide particles: Synthesis, properties and applications. Chem. Eng. J. 2012, 185–186, 1–22. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Ghavi, F.F. Preparation and functional properties of fish gelatin-chitosan blend edible films. Food Chem. 2013, 136, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Nowak, N.; Tkaczewska, J.; Grzebieniarz, W.; Juszczak, L.; Mazur, T.; Szuwarzyński, M.; Guzik, P.; Jamróz, E. Active and Intelligent Four-Layer Films Based on Chitosan, Gelatin, Furcellaran and Active Ingredients—Preparation, Characterisation and Application on Salmon. Food Bioprocess Technol. 2024, 17, 1862–1875. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Oprea, O.; Ficai, A.; Trusca, R.D.; Andronescu, E.; Holban, A.M. Biodegradable alginate films with zno nanoparticles and citronella essential oil—A novel antimicrobial structure. Pharmaceutics 2021, 13, 1020. [Google Scholar] [CrossRef] [PubMed]
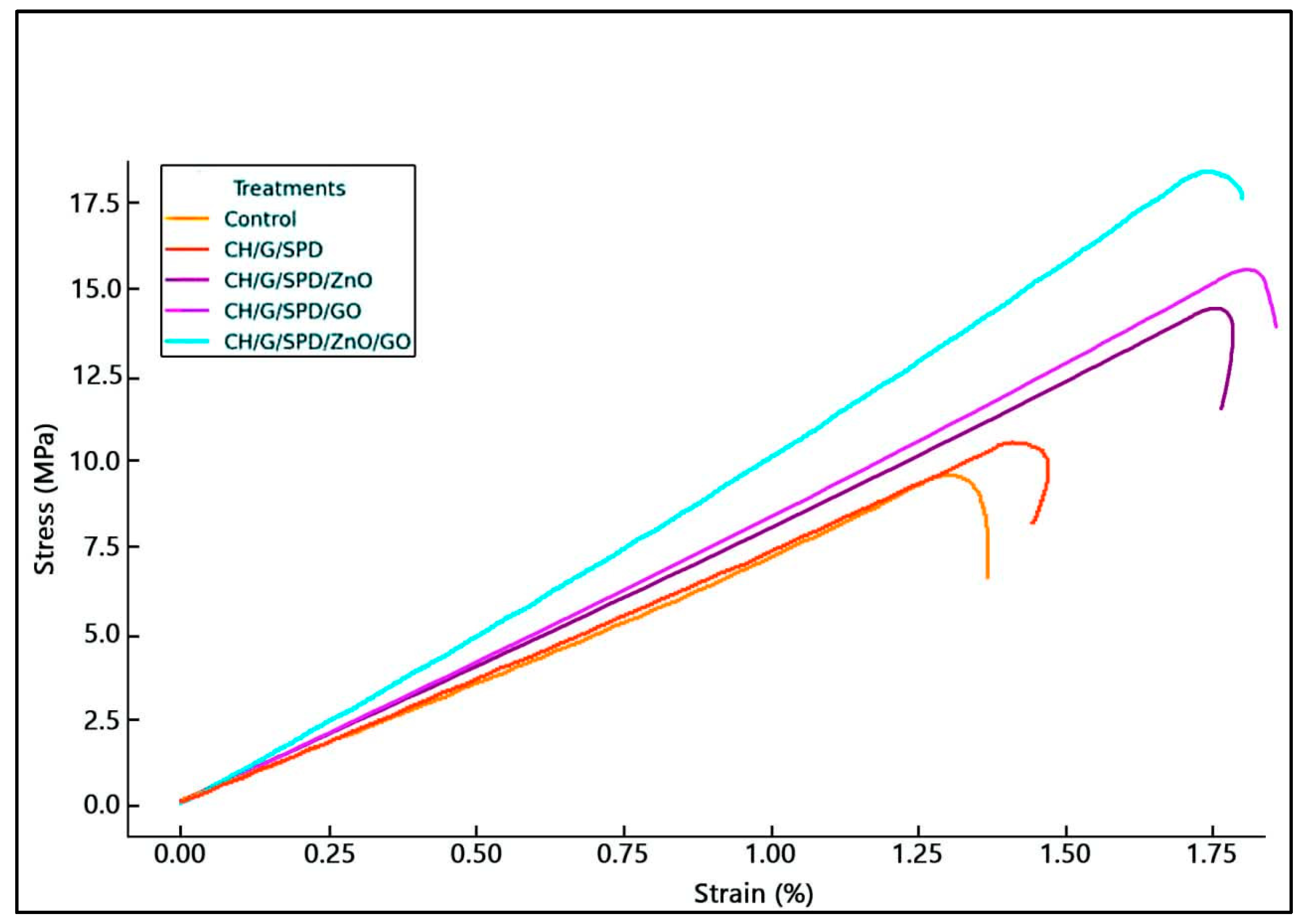
| Thickness (mm) | Tensile Strength (TS) (MPa) | Elongation (%) | Young’s Modulus (Ym) (MPa) | |
|---|---|---|---|---|
| Control | 0.140 ± 0.002 c | 9.203 ± 0.257 d | 124.123 ± 2.888 c | 22.927 ± 0.522 c |
| CH/G/SPD | 0.144 ± 0.004 c | 9.327 ± 0.370 d | 125.467 ± 2.163 c | 24.250 ± 0.171 c |
| CH/G/SPD/ZnO | 0.155 ± 0.003 b | 14.073 ± 0.172 c | 170.010 ± 1.650 b | 52.743 ± 0.444 b |
| CH/G/SPD/GO | 0.154 ± 0.003 b | 15.143 ± 0.262 b | 174.987 ± 1.840 a | 53.877 ± 0.762 b |
| CH/G/SPD/ZnO/GO | 0.171 ± 0.003 a | 17.787 ± 0.391 a | 167.420 ± 1.906 b | 66.617 ± 2.205 a |
| Control | CH/G/SPD | CH/G/SPD/ZnO | CH/G/SPD/GO | CH/G/SPD/ZnO/GO | |
|---|---|---|---|---|---|
| Moisture | 10.54 ± 1.50 a | 6.15 ± 0.10 b | 2.60 ± 0.71 c | 2.80 ± 1.29 c | 3.16 ± 0.60 c |
| Solubility | 35.21 ± 0.10 b | 65.63 ± 0.05 a | 32.12 ± 0.04 bc | 32.26 ± 0.10 bc | 21.7 ± 0.06 c |
| Control | CH/G/SPD | CH/G/SPD/ZnO | CH/G/SPD/GO | CH/G/SPD/ZnO/GO | |
|---|---|---|---|---|---|
| Light | 0.08 ± 0.01 b | 0.07 ± 0.01 b | 0.03 ± 0.00 c | 0.21 ± 0.00 a | 0.08 ± 0.01 b |
| L | 85.47 ± 2.75 a | 86.17 ± 0.46 a | 73.57 ± 4.01 b | 72.67 ± 4.35 b | 83.53 ± 3.76 a |
| a* | 3.67 ± 1.63 a | 3.37 ± 0.46 a | 4.70 ± 0.00 a | 4.97 ± 0.92 a | 4.93 ± 2.51 a |
| b* | 14.10 ± 3.90 ab | 12.80 ± 1.18 ab | 9.40 ± 0.00 b | 9.93 ± 0.92 b | 17.23 ± 4.88 a |
| ΔE | 86.83 ± 2.00 a | 87.17 ± 0.40 a | 74.33 ± 3.95 b | 73.53 ± 4.22 b | 85.60 ± 2.46 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vafaei, E.; Hasani, M.; Salehi, N.; Sabbagh, F.; Hasani, S. Enhancement of Biopolymer Film Properties Using Spermidine, Zinc Oxide, and Graphene Oxide Nanoparticles: A Study of Physical, Thermal, and Mechanical Characteristics. Materials 2025, 18, 225. https://doi.org/10.3390/ma18020225
Vafaei E, Hasani M, Salehi N, Sabbagh F, Hasani S. Enhancement of Biopolymer Film Properties Using Spermidine, Zinc Oxide, and Graphene Oxide Nanoparticles: A Study of Physical, Thermal, and Mechanical Characteristics. Materials. 2025; 18(2):225. https://doi.org/10.3390/ma18020225
Chicago/Turabian StyleVafaei, Esmaeil, Maryam Hasani, Nasrin Salehi, Farzaneh Sabbagh, and Shirin Hasani. 2025. "Enhancement of Biopolymer Film Properties Using Spermidine, Zinc Oxide, and Graphene Oxide Nanoparticles: A Study of Physical, Thermal, and Mechanical Characteristics" Materials 18, no. 2: 225. https://doi.org/10.3390/ma18020225
APA StyleVafaei, E., Hasani, M., Salehi, N., Sabbagh, F., & Hasani, S. (2025). Enhancement of Biopolymer Film Properties Using Spermidine, Zinc Oxide, and Graphene Oxide Nanoparticles: A Study of Physical, Thermal, and Mechanical Characteristics. Materials, 18(2), 225. https://doi.org/10.3390/ma18020225







