Behavior of YSZ (High Y2O3 Content) Layer on Inconel to Electro-Chemical Corrosion
Abstract
:1. Introduction
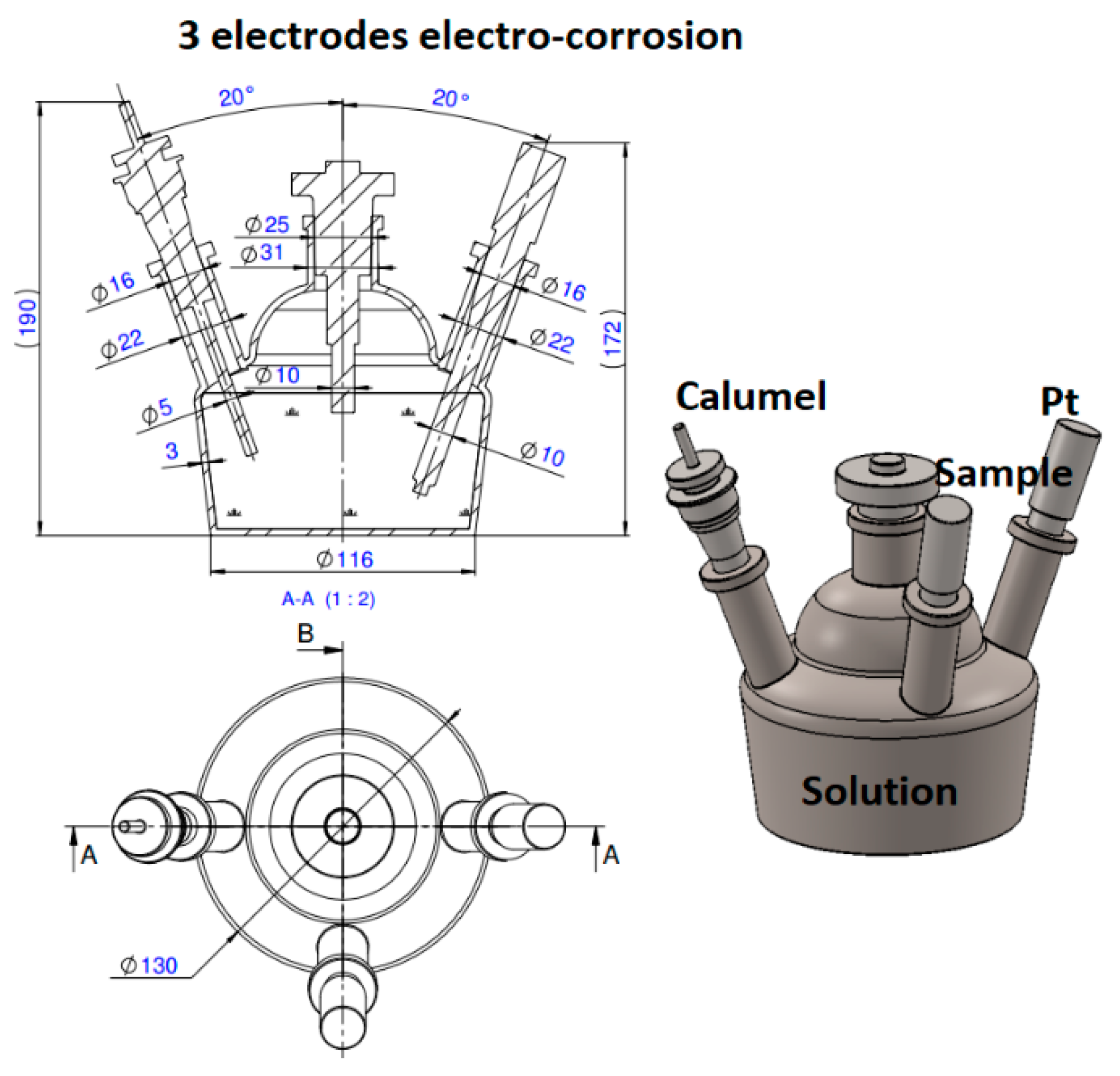
2. Materials and Methods
- -
- Linear anodic polarization for Tafel method: potential range (−400)–(+400) mV for the open-circuit potential, potential scanning rate: dE/dt = 0.1 mV/s;
- -
- cyclic polarization: potential range (−200)–(+500) mV, potential scanning rate = 10 mV/s;
- -
3. Results and Discussion
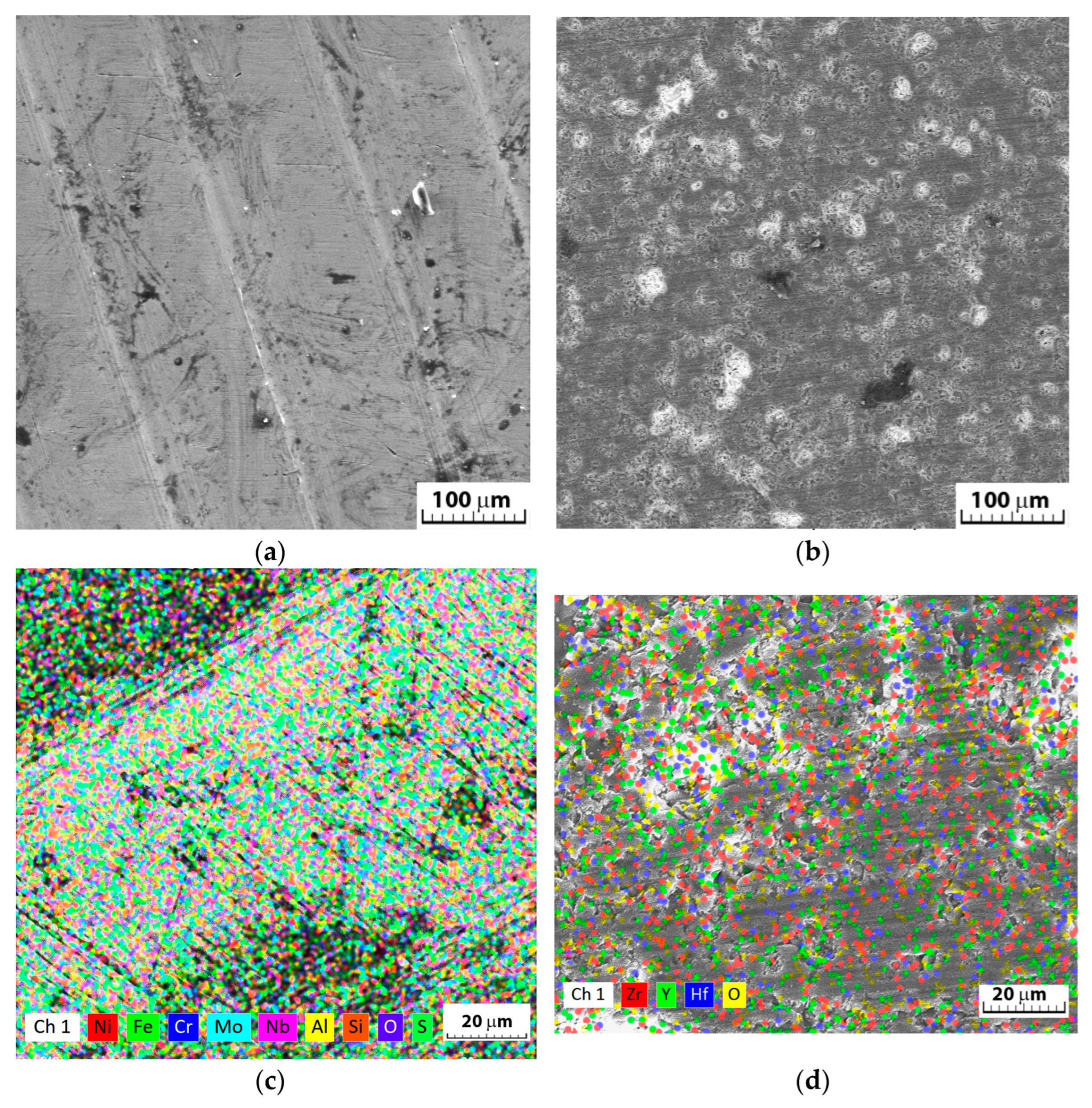
3.1. Structural and Chemical Analysis of YSZ Coatings
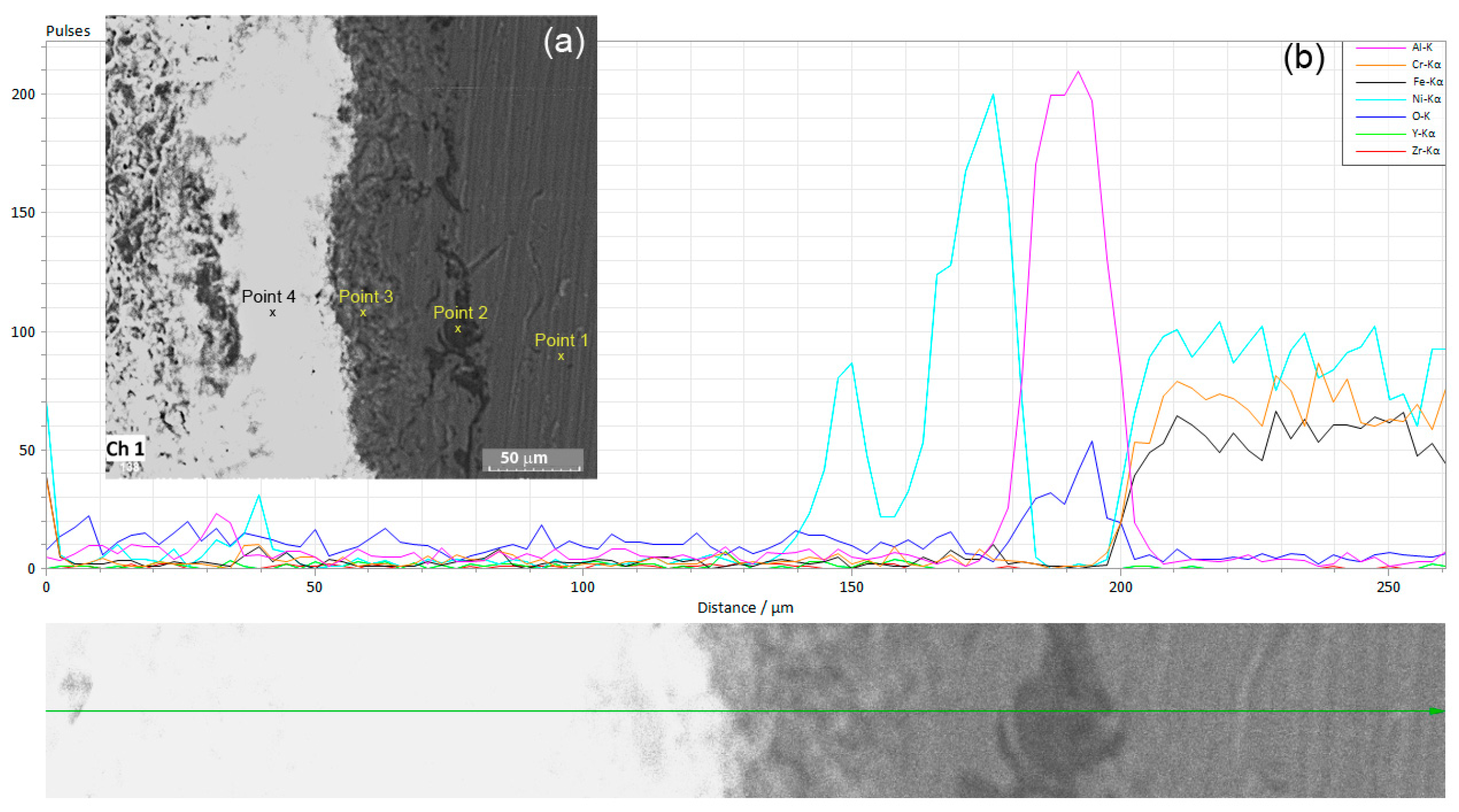
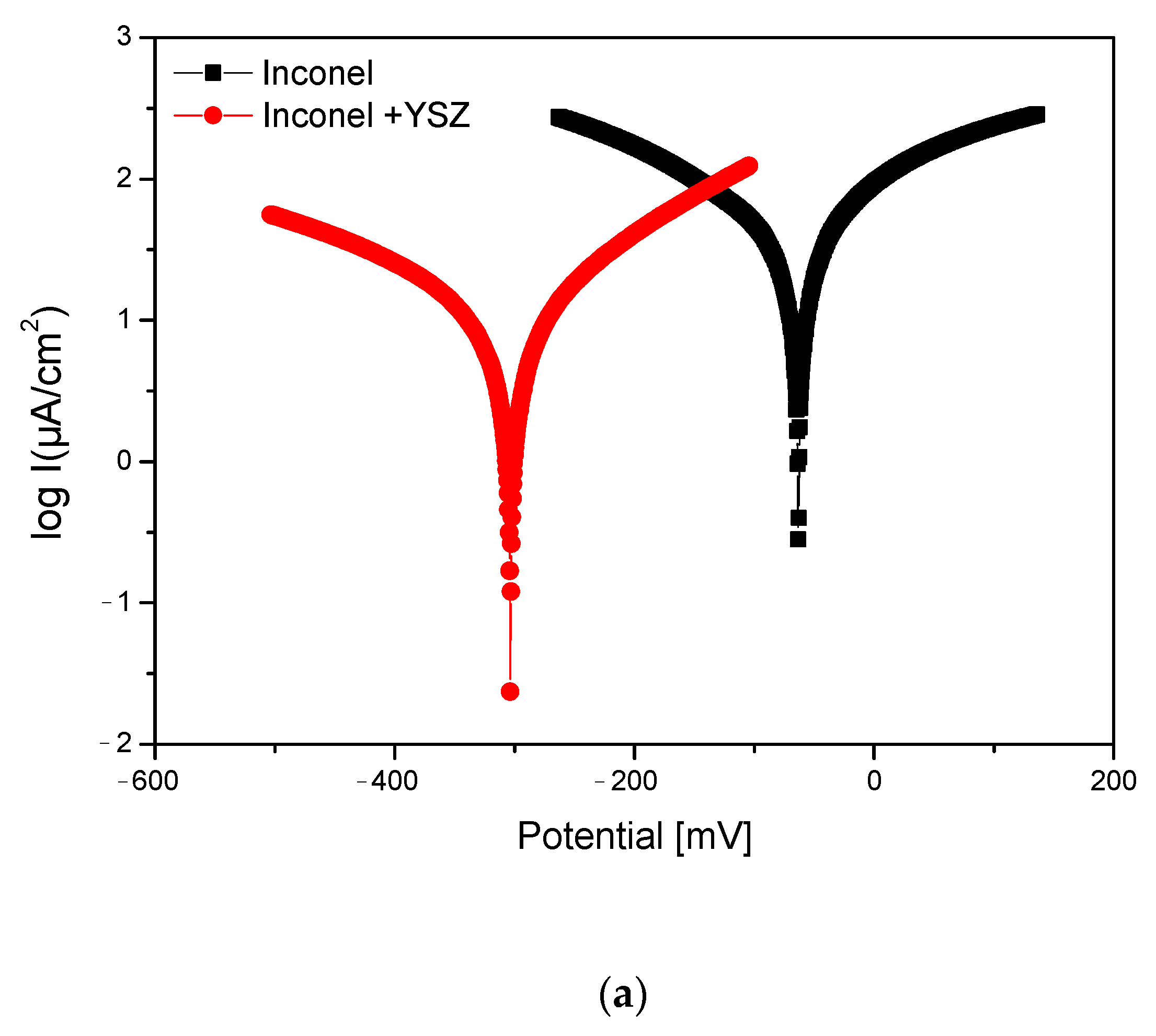
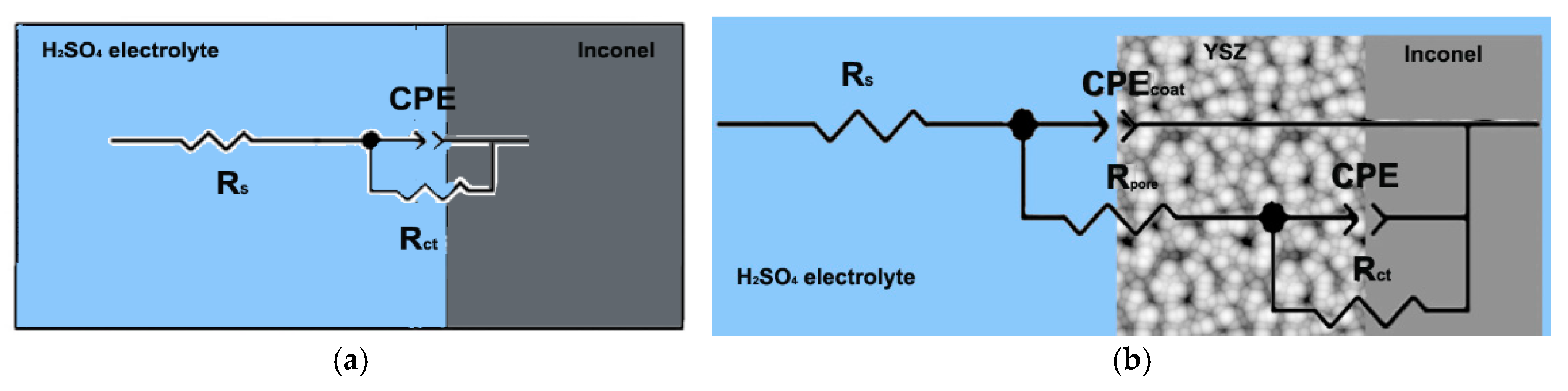
3.2. Linear and Cyclic Potentiometry and Electro-Impedance Spectroscopy (EIS)
4. Conclusions
- -
- New ceramic coatings of stabilized zirconia with 38 wt% Y2O3 powders were obtained by atmospheric plasma spraying on Inconel substrate (the ceramic coating showed good coverage of the surface substrate, using a NiAl-based bonding layer).
- -
- The coated Inconel showed a lower corrosion potential in an acidic solution compared to the substrate sample (the ICORR value was 6.8 times lower for the coated sample). The corrosion resistance of the substrate was improved by the protective layer formed by the bonding material (deposited from Metco 410 powders) and the ceramics (grown from Metco 207 powders). As the ceramic coating was chemically inert, the role of this layer was to reduce the contact of the electrolyte solution with the anchoring layer (Al2O3 30(Ni 20Al)), and the protection was achieved by the oxide layer at the interface between this anchoring layer and the ceramic layer.
- -
- After the electrochemical corrosion tests, no cracks, pits, or compounds were observed on the coated sample, and the differences in the corrosion micro-morphologies of the surfaces (Inconel and Inconel + YSZ) were mostly caused by the galvanic coupling between the γ-matrix and the different secondary phases of the substrate.
- -
- Based on the working environment (electrolyte solution), different bonding layers (chemical composition and thickness) can be proposed to improve the resistance of the ceramic coating.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, K.; Huang, W.; Deng, P.; Zhong, R.; Tan, Z.; Hu, Y.; Li, J.; Mao, W. Mechanical properties, thermal shock resistance and stress evolution of plasma-sprayed 56 wt% Y2O3-stabilized ZrO2 thick thermal barrier coatings. Surf. Coat. Technol. 2024, 494, 131352. [Google Scholar] [CrossRef]
- Widyastuti; Zulfa, L.L.; Rizaldi, W.A.; Azhar, J.; Safrida, N.; Pratama, A.D.; Wahyuono, R.A.; Sulistijono; Fajarina, R.; Hakim, A.N. A study on thermal behaviour of thermal barrier coating: Investigation of particle size, YSZ/polysilazane, time and temperature curing effect. RSC Adv. 2024, 14, 24687. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.-Y.; Meng, G.H.; Chen, L.; Li, G.R.; Liu, M.J.; Zhang, W.X.; Zhao, L.N.; Zhang, Q.; Zhang, X.D.; Wan, C.L.; et al. Progress in ceramic materials and structure design toward advanced thermal barrier coatings. J. Adv. Ceram. 2022, 11, 985–1068. [Google Scholar] [CrossRef]
- Zhou, Q.; Wen, J.; Li, Y.; Han, J.; Zhou, B.; Guo, H.; Li, J.; He, J. Molten salt corrosion behavior of laser remelted PS-PVD YSZ thermal barrier coatings. J. Mater. Res. Technol. 2024, 30, 2666–2679. [Google Scholar] [CrossRef]
- Xiang, Y.; Yan, K.; Yu, H.; Guo, Y.; Ying, Y.; Li, Z.; Sun, J.; Fang, C. Comparative investigation on the hot corrosion failure of YSZ and GdYb-YSZ double-ceramic-layer thermal barrier coatings under Na2SO4+V2O5 molten salts. Ceram. Int. 2023, 49, 18678–18688. [Google Scholar] [CrossRef]
- Abedi, H.R.; Salehi, M.; Shafyei, A. Microstructural, mechanical and thermal shock properties of triple-layer TBCs with different thicknesses of bond coat and ceramic top coat deposited onto polyimide matrix composite. Ceram. Int. 2018, 44, 6212–6222. [Google Scholar] [CrossRef]
- Lee, J.-K.; Kim, H.G. YSZ atmospheric plasma coating method for improved high temperature corrosion and wear resistance. J. Mech. Sci. Technol. 2020, 34, 3629–3633. [Google Scholar] [CrossRef]
- Tao, S.; Yang, J.; Shao, F.; Zhao, H.; Zhong, Z.; Zhuang, Y.; Sheng, J.; Ni, J.; Li, Q.; Tao, S. Atmospheric plasma sprayed thick thermal barrier coatings: Microstructure, thermal shock behaviors and failure mechanism. Eng. Fail. Anal. 2022, 131, 105819. [Google Scholar] [CrossRef]
- Predescu, C.; Pantilimon, C.; Sohaciu, M.; Matei, E.; Savastru, D.; Berbecaru, A.; Predescu, A.; Anton, M.G.; Coman, G. Investigation of the corrosion cracks in a C4 heavy transport pipeline by microscopy, fluorescence and diffraction techniques. J. Optoelectron. Adv. Mater. 2016, 8, 873–877. [Google Scholar]
- Istrate, B.; Mareci, D.; Munteanu, C.; Stanciu, S.; Luca, D.; Crimu, C.I.; Kamel, E. In vitro electrochemical properties of biodegradable ZrO2 -CaO coated MgCa alloy using atmospheric plasma spraying. J. Optoelectron. Adv. Mater. 2015, 17, 1186–1192. [Google Scholar]
- Istrate, B.; Mareci, D.; Munteanu, C.; Stanciu, S.; Crimu, C.I.; Trinca, L.C.; Kamel, E. In vitro electrochemical properties of biodegradable YSZ-coated MgCa alloy. Environ. Eng. Manag. J. 2016, 15, 955–963. [Google Scholar]
- Pavithran, N.R.; Harichandran, R.; Kumar, D.D. Effect of Yttria-stabilized zirconia coating on the corrosion and thermal behaviour of additive manufactured Inconel 718 alloy. J. Alloys Compd. 2023, 968, 171877. [Google Scholar] [CrossRef]
- Alaneme, K.K.; Olubambi, P.A. Corrosion and wear behaviour of rice husk ash—Alumina reinforced Al–Mg–Si alloy matrix hybrid composites. J. Mater. Res. Technol. 2013, 2, 188–194. [Google Scholar] [CrossRef]
- Shu, C.; Wang, J.; Lu, X.; Chen, T.; Xu, M.; Du, G.; Rao, W.; Luo, Z.; Duan, Z.; Xie, Y.; et al. Investigation of corrosion resistance of YSZ coating with sacrificial aluminum oxide protective layer against CMAS and composite corrosives. J. Eur. Ceram. Soc. 2024, 44, 2537–2549. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, W.; Dou, M.; Han, J.; Wu, D.; Zou, Y. Highly-durable plasma-sprayed Al2O3-YSZ/YSZ double ceramic layer TBCs against CMAS corrosion. J. Eur. Ceram. Soc. 2024, 44, 7328–7338. [Google Scholar] [CrossRef]
- Afrasiabi, A.; Saremi, M.; Kobayashi, A. A comparative study on hot corrosion resistance of three types of thermal barrier coatings: YSZ, YSZ +Al2O3 and YSZ/Al2O3. Mater. Sci. Eng. A 2008, 478, 264–269. [Google Scholar] [CrossRef]
- Amaya, C.; Aperador, W.; Caicedo, J.C.; Espinoza-Beltrán, F.J.; Muñoz-Saldaña, J.; Zambrano, G.; Prieto, P. Corrosion study of Alumina/Yttria-Stabilized Zirconia (Al2O3/YSZ) nanostructured Thermal Barrier Coatings (TBC) exposed to high temperature treatment. Corros. Sci. 2009, 51, 2994–2999. [Google Scholar] [CrossRef]
- Hernández-Acosta, M.A.; Martines-Arano, H.; Soto-Ruvalcaba, L.; Martínez-González, C.L.; Martínez-Gutiérrez, H.; Torres-Torres, C. Fractional thermal transport and twisted light induced by an optical two-wave mixing in single-wall carbon nanotubes. Int. J. Therm. Sci. 2020, 147, 106136. [Google Scholar] [CrossRef]
- Barbero, G.; Evangelista, L.R.; Zola, R.S.; Lenzi, E.K.; Scarfone, A.M. A Brief Review of Fractional Calculus as a Tool for Applications in Physics: Adsorption Phenomena and Electrical Impedance in Complex Fluids. Fractal Fract. 2024, 8, 369. [Google Scholar] [CrossRef]
- Liu, L.; Maeda, K.; Onda, T.; Chen, Z.C. Effect of YSZ with different Y2O3 contents on toughening behavior of Al2O3/Ba-β-Al2O3/ZrO2 composites. Ceram. Int. 2019, 45, 18037–18043. [Google Scholar] [CrossRef]
- Mauer, G.; Vaßen, R.; Stöver, D. Atmospheric plasma spraying of yttria-stabilized zirconia coatings with specific porosity. Surf. Coat. Technol. 2009, 204, 172–179. [Google Scholar] [CrossRef]
- Dejang, N.; Limpichaipanit, A.; Watcharapasorn, A.; Wirojanupatump, S.; Niranatlumpong, P.; Jiansirisomboon, S. Fabrication and Properties of Plasma-Sprayed Al2O3/ZrO2 Composite Coatings. J. Therm. Spray Technol. 2011, 20, 1259–1268. [Google Scholar] [CrossRef]
- Chen, D.; Jordan, E.; Gell, M. Microstructure of Suspension Plasma Spray and Air Plasma Spray Al2O3-ZrO2 Composite Coatings. J. Therm. Spray Technol. 2009, 18, 421–426. [Google Scholar] [CrossRef]
- Owoseni, T.; Bai, M.; Hussain, T.; Faisal, N.H.; Lee, T.L.; Kelleher, L. Neutron diffraction residual stress measurements in suspension HVOF sprayed Al2O3 and YSZ coatings. In Proceedings of the Conference: International Thermal Spray Conference, Orlando, FL, USA, 7–10 May 2018; pp. 490–495. [Google Scholar]
- Available online: https://powersteel.eu/material/inconel-718-2-4668-uns-n07718-alloy-718-sb-637-bohler/ (accessed on 1 May 2022).
- Luțcanu, M.; Coteață, M.; Bernevig, M.A.; Nechifor, C.D.; Cazacu, M.M.; Paraschiv, P.; Istrate, B.; Bădărău, G.; Cimpoeșu, N. Obtaining and analyze of Al2O3-ZrO2 ceramic layers on metallic substrate. Arch. Metall. Mater. 2022, 67, 479–485. [Google Scholar]
- Cotrut, C.M.; Vladescu, A.; Dinu, M.; Vranceanu, D.M. Influence of deposition temperature on the properties of hydroxyapatite obtained by electrochemical assisted deposition. Ceram. Int. 2018, 44, 669–677. [Google Scholar] [CrossRef]
- Moga, S.; Malinovschi, V.; Marin, A.; Coaca, E.; Negrea, D.; Craciun, V.; Lungu, M. Mechanical and corrosion-resistant coatings prepared on AZ63 Mg alloy by plasma electrolytic oxidation. Surf. Coat. Technol. 2023, 462, 129464. [Google Scholar] [CrossRef]
- Yang, J.; Yang, H.; Yu, H.; Wang, Z.; Zeng, X. Corrosion behavior of additive manufactured Ti-6Al-4V alloy in NaCl solution. Metall. Mater. Trans. A 2017, 48, 3583–3593. [Google Scholar] [CrossRef]
- Lutcanu, M.; Cimpoesu, R.; Abrudeanu, M.; Munteanu, C.; Moga, S.G.; Coteata, M.; Zegan, G.; Benchea, M.; Cimpoesu, N.; Murariu, A.M. Mechanical Properties and Thermal Shock Behavior of Al2O3-YSZ Ceramic Layers Obtained by Atmospheric Plasma Spraying. Crystals 2023, 13, 614. [Google Scholar] [CrossRef]
- Zamani, P.; Valefi, Z.; Jafarzadeh, K. Comprehensive study on corrosion protection properties of Al2O3, Cr2O3 and Al2O3–Cr2O3 ceramic coatings deposited by plasma spraying on carbon steel. Ceram. Int. 2022, 48, 1574–1588. [Google Scholar] [CrossRef]
- Dwi Desiati, R.; Sugiarti, E.; Hermantos, B.; Timuda, G.E.; Izzuddin, H.; Salsabilla, F.A.; Anawati, A. Corrosion behavior of YSZ and YSZ/NiCo coatings on Inconel 625 exposed alkali chlorides. J. Met. Mater. Miner. 2024, 34, 1879. [Google Scholar] [CrossRef]
- Sun, K.; Zhong, W.; Qiu, S.; Cai, W.; Xie, X.; Wang, H.; Zhang, S.; Li, W. Study on the Microstructure, Corrosion Resistance and Dielectric Properties of Atmospheric Plasma-Sprayed Y2O3 Ceramic Coatings. Coatings 2024, 14, 377. [Google Scholar] [CrossRef]
- Cimpoeșu, R.; Luțcanu, M.; Cazac, A.M.; Adomniței, I.; Bejinariu, C.; Andrușcă, L.; Prelipceanu, M.; Cioca, L.I.; Chicet, D.L.; Radu, A.M.; et al. Electrochemical Corrosion Resistance of Al2O3–YSZ Coatings on Steel Substrates. Appl. Sci. 2024, 14, 10877. [Google Scholar] [CrossRef]
- Amin, M.A.; El-Bagoury, N.; Saracoglu, M.; Ramadan, M. Electrochemical and Corrosion Behavior of cast Re-containing Inconel 718 Alloys in Sulphuric Acid Solutions and the Effect of Cl−. Int. J. Electrochem. Sci. 2014, 9, 5352–5374. [Google Scholar] [CrossRef]
- Bacaita, E.S.; Bejinariu, C.; Zoltan, B.; Peptu, C.; Andrei, G.; Popa, M.; Magop, D.; Agop, M. Nonlinearities in Drug Release Process from Polymeric Microparticles: Long-Time-Scale Behaviour. J. Appl. Math. 2012, 2012, 653720. [Google Scholar] [CrossRef]
- Buzea, C.G.; Bejinariu, C.; Boris, C.; Vizureanu, P.; Agop, M. Motion of Free Particles in Fractal Space-time, International Journal of Nonlinear Sciences and Numerical Simulation. Int. J. Nonlinear Sci. Numer. Simul. 2009, 10, 1399–1414. [Google Scholar] [CrossRef]
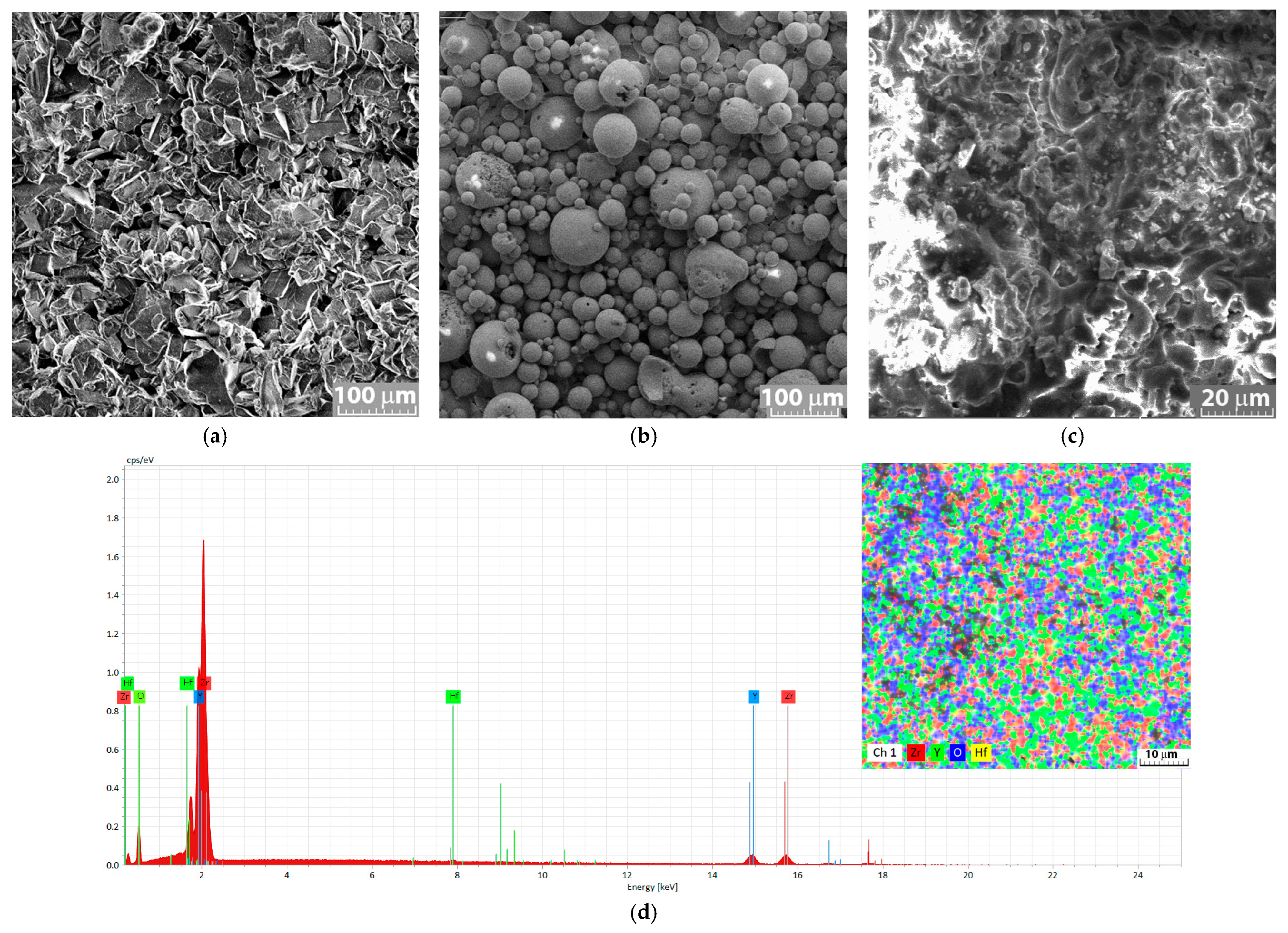



| Chem. Elem. | Zr % | Y % | O % | Hf % | Ni % | Fe % | Cr % | Al % | Nb % | Other % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt | at | wt | at | wt | at | wt | at | wt | at | wt | at | wt | at | wt | at | wt | at | ||
| Coating Surface | 44.9 | 20.7 | 29.2 | 13.8 | 25 | 65.4 | 0.9 | 0.2 | - | - | - | - | - | - | - | - | - | - | - |
| Coating surface without bonding layer | 43.1 | 19 | 29 | 13.1 | 26.9 | 67.7 | 0.9 | 0.2 | - | - | - | - | - | - | - | - | - | - | - |
| Point 1 (substrate) | - | - | - | - | - | - | - | - | 53.7 | 52.4 | 19.3 | 19.8 | 17.5 | 19.2 | 0.8 | 1.6 | 7.5 | 4.6 | S῀1 |
| Point 2 (bonding) | - | - | - | - | 43.7 | 57 | - | - | 1.2 | 0.4 | - | - | - | - | 55 | 42.5 | - | - | - |
| Point 3 (bonding) | 16 | 6.9 | 10.1 | 4.5 | 21.7 | 53.2 | - | - | 51.6 | 34.5 | - | - | - | - | 0.6 | 0.9 | - | - | - |
| Point 4 (coating) | 48.8 | 24.4 | 29.1 | 14.9 | 21.2 | 60.4 | 0.9 | 0.2 | - | - | - | - | - | - | - | - | - | - | - |
| EDS error % | 1.7 | 1.2 | 5 | 0.2 | 1.5 | 0.6 | 0.5 | 1.5 | 0.2 | ||||||||||
| Material | Corrosion Process Parameters | |||||
|---|---|---|---|---|---|---|
| E (I = 0) (mV) | icorr µA/cm | Rp (ohm.cm2) | vcorr (mm/year) | βc (mV/dec) | βa (mV/dec) | |
| Inconel | −63.1 | 100.10 | 685.93 | 1.16 | −452 | 435 |
| Inconel + YSZ | −303.4 mV | 14.61 | 3230 | 0.17 | 339 | 214 |
| Rs Ohm.cm2 | CPE | Rct Ohm.cm2 | CPE | Rpore Ohm.cm2 | |||
|---|---|---|---|---|---|---|---|
| Q Ssn/cm2 | n | Q Ssn/cm2 | n | ||||
| Inconel | 68 | 3.89 × 10−5 | 0.78 | 4001 | - | - | - |
| Inconel + YSZ | 334 | 6.049 × 10−5 | 0.5 | 1007 | 24.64 × 10−5 | 0.7 | 1142 |
| Elements/ Samples | Ni% | Fe% | Cr% | Nb% | Zr% | Y% | O% | Hf% | Others | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt | at | wt | at | wt | at | wt | at | wt | at | wt | at | wt | at | wt | at | wt% | |
| Initial Inconel | 53.1 | 52.9 | 19.7 | 20.6 | 19.2 | 21.6 | 3.9 | 2.4 | - | - | - | - | - | - | - | - | Mo: 4.2 |
| Inconel after corrosion | 49.2 | 35.5 | 18.2 | 13 | 17.4 | 14 | 4.1 | 1.9 | - | - | - | - | 1 | 1.3 | - | - | Al: 0.5, S: 0.2 |
| Inconel + YSZ coating | - | - | - | - | - | - | - | - | 46.4 | 22.4 | 29.9 | 14.8 | 22.8 | 62.6 | 0.9 | 0.2 | - |
| EDS error % | 1.26 | 0.5 | 0.5 | 0.4 | 2.35 | 1.6 | 0.5–4 | 0.2 | Mo: 0.01, Al: 0.07 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adomniței, I.; Cimpoeșu, R.; Chicet, D.L.; Coteață, M.; Lupu, F.C.; Bejinariu, C.; Andrușcă, L.; Paraschiv, P.; Axinte, M.; Bădărău, G.; et al. Behavior of YSZ (High Y2O3 Content) Layer on Inconel to Electro-Chemical Corrosion. Materials 2025, 18, 400. https://doi.org/10.3390/ma18020400
Adomniței I, Cimpoeșu R, Chicet DL, Coteață M, Lupu FC, Bejinariu C, Andrușcă L, Paraschiv P, Axinte M, Bădărău G, et al. Behavior of YSZ (High Y2O3 Content) Layer on Inconel to Electro-Chemical Corrosion. Materials. 2025; 18(2):400. https://doi.org/10.3390/ma18020400
Chicago/Turabian StyleAdomniței, Ionut, Ramona Cimpoeșu, Daniela Lucia Chicet, Margareta Coteață, Fabian Cezar Lupu, Costică Bejinariu, Liviu Andrușcă, Petronela Paraschiv, Mihai Axinte, Gheorghe Bădărău, and et al. 2025. "Behavior of YSZ (High Y2O3 Content) Layer on Inconel to Electro-Chemical Corrosion" Materials 18, no. 2: 400. https://doi.org/10.3390/ma18020400
APA StyleAdomniței, I., Cimpoeșu, R., Chicet, D. L., Coteață, M., Lupu, F. C., Bejinariu, C., Andrușcă, L., Paraschiv, P., Axinte, M., Bădărău, G., & Cimpoeșu, N. (2025). Behavior of YSZ (High Y2O3 Content) Layer on Inconel to Electro-Chemical Corrosion. Materials, 18(2), 400. https://doi.org/10.3390/ma18020400












