Fluorescent Neutron Track Detectors for Boron-10 Microdistribution Measurement in BNCT: A Feasibility Study
Abstract
:1. Introduction
2. Materials and Methods
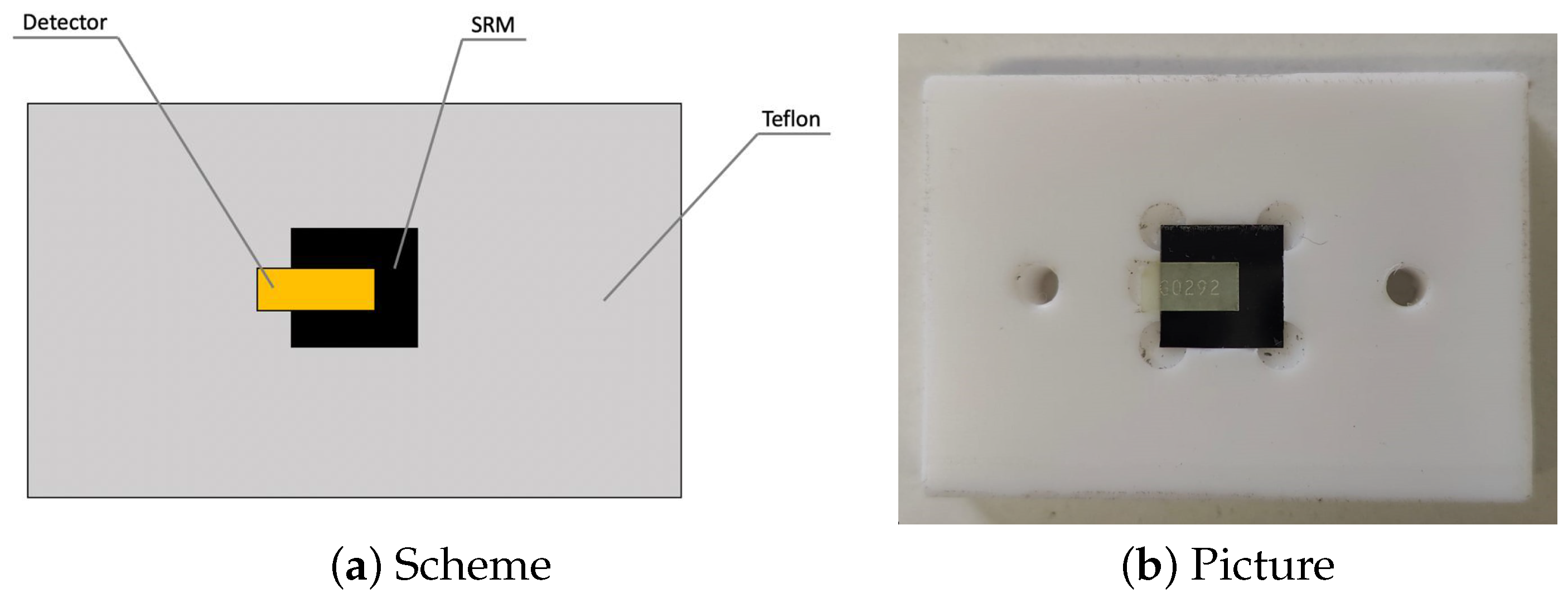
2.1. The Fluorescent Neutron Track Detector
2.2. Readout System
2.3. Data Processing
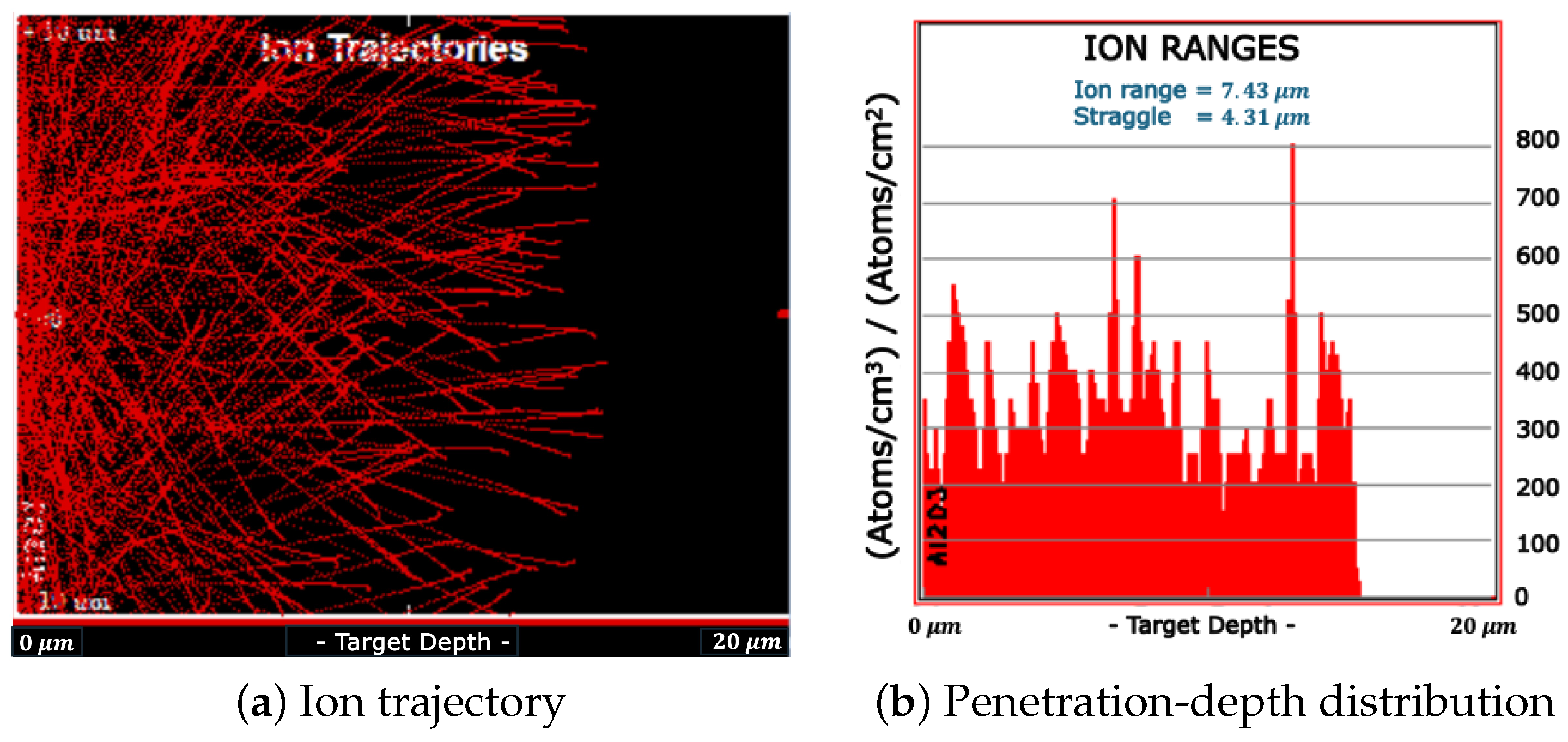
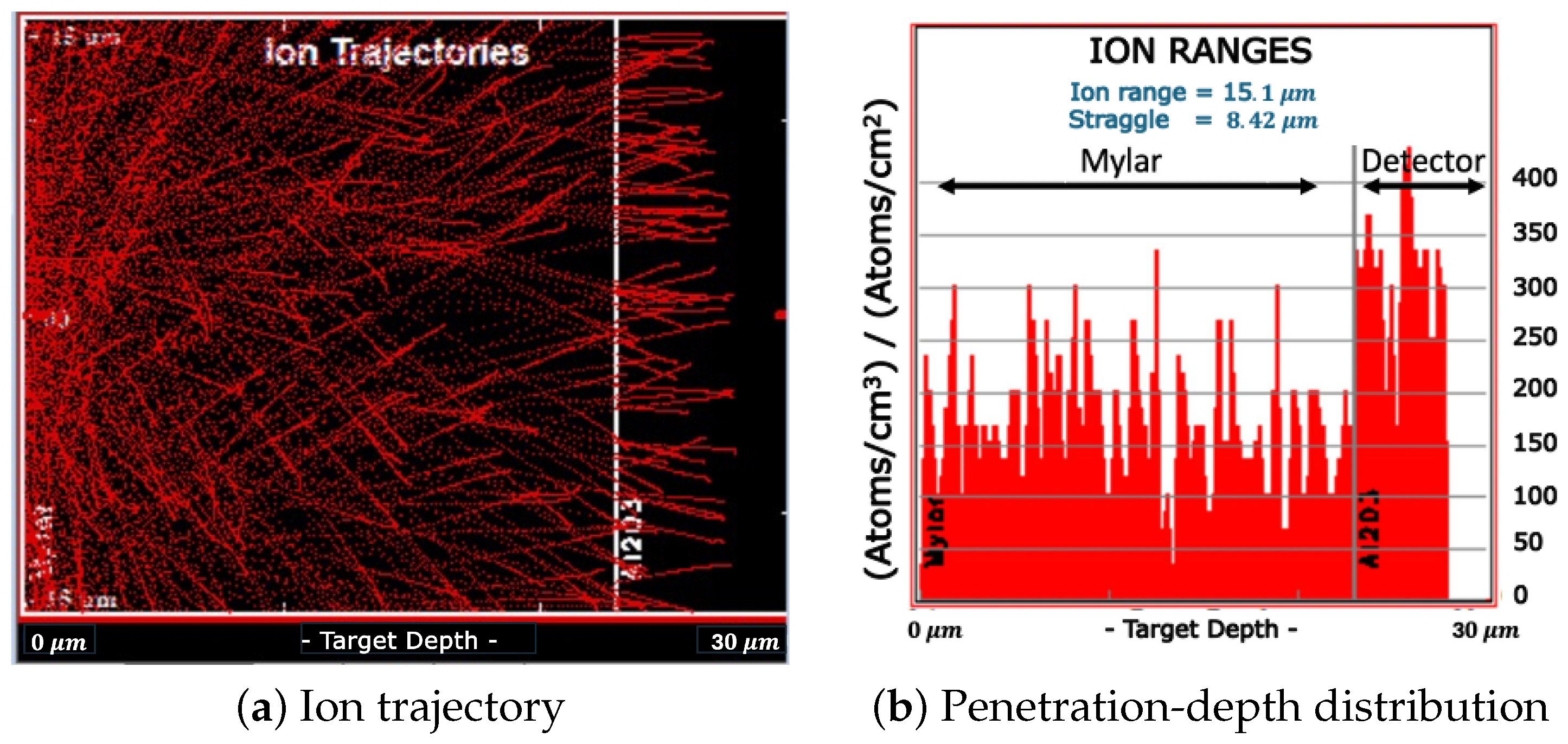
2.4. The Experiments
3. Results
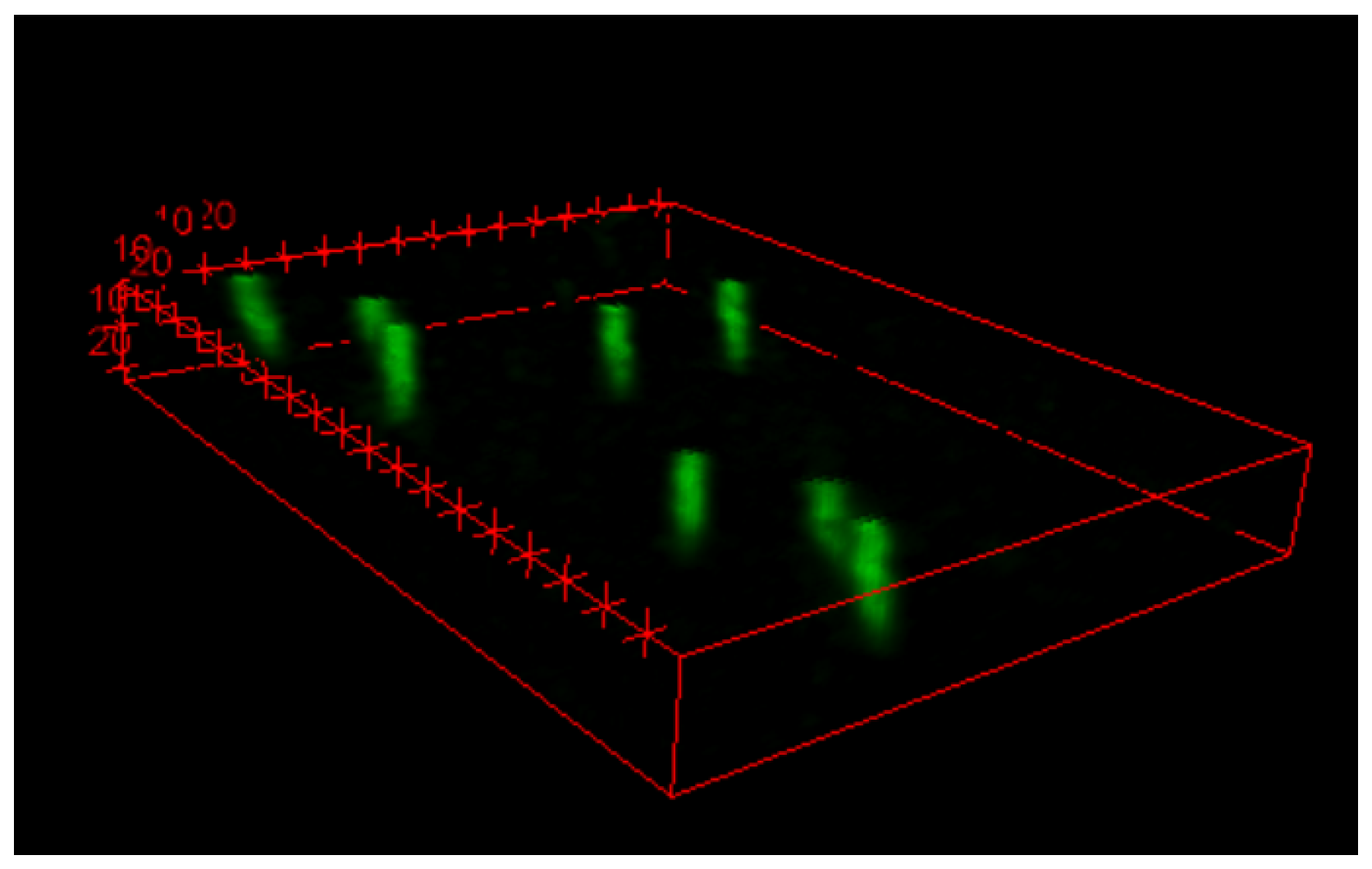
3.1. Preliminary Tests with Am-241
3.2. Measurement of a Reference Boron Neutron-Capture Therapy Radiation Field
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Atomic Energy Agency. Advances in Boron Neutron Capture Therapy; IAEA: Vienna, Austria, 2023. [Google Scholar]
- Xu, H.; Liu, J.; Li, R.; Lin, J.; Gui, L.; Wang, Y.; Jin, Z.; Xia, W.; Liu, Y.; Cheng, S.; et al. Novel promising boron agents for boron neutron capture therapy: Current status and outlook on the future. Coord. Chem. Rev. 2024, 511, 215795. [Google Scholar] [CrossRef]
- Wittig, A.; Michel, J.; Moss, R.L.; Stecher-Rasmussen, F.; Arlinghaus, H.F.; Bendel, P.; Mauri, P.L.; Altieri, S.; Hilger, R.; Salvadori, P.A.; et al. Boron analysis and boron imaging in biological materials for Boron Neutron Capture Therapy (BNCT). Crit. Rev. Oncol./Hematol. 2008, 68, 66–90. [Google Scholar] [CrossRef]
- Bennett, B.D.; Zha, X.; Gay, I.; Morrison, G.H. Intracellular boron localization and uptake in cell cultures using imaging secondary ion mass spectrometry (ion microscopy) for neutron capture therapy for cancer. Biol. Cell 1992, 74, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.; Chandra, S.; Barth, R.F.; Yang, W.; Joel, D.D.; Coderre, J.A. Quantitative imaging and microlocalization of boron-10 in brain tumors and infiltrating tumor cells by SIMS ion microscopy: Relevance to neutron capture therapy. Cancer Res. 2001, 61, 8179–8187. [Google Scholar]
- Fartmann, M.; Kriegeskotte, C.; Dambach, S.; Wittig, A.; Sauerwein, W.; Arlinghaus, H. Quantitative imaging of atomic and molecular species in cancer cell cultures with TOF-SIMS and Laser-SNMS. Appl. Surf. Sci. 2004, 231–232, 428–431. [Google Scholar] [CrossRef]
- Chandra, S.; Smith, D.R.; Morrison, G.H. Peer Reviewed: A Subcellular Imaging by Dynamic SIMS Ion Microscopy. Anal. Chem. 2000, 72, 104A–114A. [Google Scholar] [CrossRef]
- Chandra, S. SIMS ion microscopy as a novel, practical tool for subcellular chemical imaging in cancer research. Appl. Surf. Sci. 2003, 203–204, 679–683. [Google Scholar] [CrossRef]
- Chandra, S.; Ahmad, T.; Barth, R.F.; Kabalka, G.W. Quantitative evaluation of boron neutron capture therapy (BNCT) drugs for boron delivery and retention at subcellular-scale resolution in human glioblastoma cells with imaging secondary ion mass spectrometry (SIMS). J. Microsc. 2014, 254, 146–156. [Google Scholar] [CrossRef]
- Hoppe, P.; Cohen, S.; Meibom, A. N ano SIMS: Technical Aspects and Applications in Cosmochemistry and Biological Geochemistry. Geostand. Geoanalytical Res. 2013, 37, 111–154. [Google Scholar] [CrossRef]
- Aldossari, S.; McMahon, G.; Lockyer, N.P.; Moore, K.L. Microdistribution and quantification of the boron neutron capture therapy drug BPA in primary cell cultures of human glioblastoma tumour by NanoSIMS. Analyst 2019, 144, 6214–6224. [Google Scholar] [CrossRef]
- Edwards, L.C. Autoradiography by neutron activation: The cellular distribution of boron-10 in the transplanted mouse brain tumour. Int. J. Appl. Radiat. Isot. 1956, 1, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Amano, K.; Kitamura, K.; Tateishi, J.; Hatanaka, H. Boron distribution analysis by alpha-autoradiography. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1986, 27, 677–684. [Google Scholar]
- Solares, G.R.; Zamenhof, R.G. A novel approach to the microdosimetry of neutron capture therapy. Part I. High-resolution quantitative autoradiography applied to microdosimetry in neutron capture therapy. Radiat. Res. 1995, 144, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Sakurai, Y.; Suzuki, M.; Masunaga, S.I.; Takamiya, K.; Maruhashi, A.; Ono, K. Development of a simple and rapid method of precisely identifying the position of 10B atoms in tissue: An improvement in standard alpha autoradiography. J. Radiat. Res. 2014, 55, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Fairchild, R.G.; Tonna, E.A.; Seibold, C.T.; Straub, R.F. Neutron autoradiographic determination of boron-10 concentration and distribution in mammalian tissue. Radiat. Res. 1968, 36, 87–97. [Google Scholar] [CrossRef]
- Fairchild, R.G.; Gabel, D.; Laster, B.H.; Greenberg, D.; Kiszenick, W.; Micca, P.L. Microanalytical techniques for boron analysis using the 10B(n,α)7Li reaction. Med. Phys. 1986, 13, 50–56. [Google Scholar] [CrossRef]
- Gabel, D.; Holstein, H.; Larsson, B.; Gille, L.; Ericson, G.; Sacker, D.; Som, P.; Fairchild, R.G. Quantitative neutron capture radiography for studying the biodistribution of tumor-seeking boron-containing compounds. Cancer Res. 1987, 47, 5451–5454. [Google Scholar] [PubMed]
- Alfassi, Z.B.; Probst, T.U. On the calibration curve for determination of boron in tissue by quantitative neutron capture radiography. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 1999, 428, 502–507. [Google Scholar] [CrossRef]
- Pugliesi, R.; Pereira, M.A.S. Study of the neutron radiography characteristics for the solid state nuclear track detector Makrofol-DE. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2002, 484, 613–618. [Google Scholar] [CrossRef]
- Roveda, L.; Prati, U.; Bakeine, J.; Trotta, F.; Marotta, P.; Valsecchi, P.; Zonta, A.; Nano, R.; Facoetti, A.; Chiari, P.; et al. How to Study Boron Biodistribution in Liver Metastases from Colorectal Cancer. J. Chemother. 2004, 16, 15–18. [Google Scholar] [CrossRef]
- Portu, A.; Carpano, M.; Dagrosa, A.; Cabrini, R.; Martin, G.S. Qualitative autoradiography with polycarbonate foils enables histological and track analyses on the same section. Biotech. Histochem. 2013, 88, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Postuma, I.; Sommi, P.; Vitali, A.; Shu, D.; di Martino, G.; Cansolino, L.; Ferrari, C.; Ricci, V.; Magni, C.; Protti, N.; et al. Colocalization of tracks from boron neutron capture reactions and images of isolated cells. Appl. Radiat. Isot. 2021, 167, 109353. [Google Scholar] [CrossRef]
- Wu, Y.; Shu, D.; Geng, C.; Postuma, I.; Tang, X.; Liu, Y.H. Optimization of subcellular boron distribution measurement using UV-C imprint and neutron autoradiography in boron neutron capture therapy. Radiat. Meas. 2025, 181, 107351. [Google Scholar] [CrossRef]
- Isaacson, M.; Johnson, D. The microanalysis of light elements using transmitted energy loss electrons. Ultramicroscopy 1975, 1, 33–52. [Google Scholar] [CrossRef]
- Zhu, Y.; Egerton, R.; Malac, M. Concentration limits for the measurement of boron by electron energy-loss spectroscopy and electron-spectroscopic imaging. Ultramicroscopy 2001, 87, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.; Sauerwein, W.; Wittig, A.; Balossier, G.; Zierold, K. Subcellular localization of boron in cultured melanoma cells by electron energy-loss spectroscopy of freeze-dried cryosections. J. Microsc. 2003, 210, 25–34. [Google Scholar] [CrossRef]
- Michel, J.; Balossier, G.; Wittig, A.; Sauerwein, W.; Zierold, K. EELS Spectrum-Imaging for Boron Detection in Biological Cryofixed Tissues. Instrum. Sci. Technol. 2005, 33, 631–644. [Google Scholar] [CrossRef]
- Leapman, R.; Kocsis, E.; Zhang, G.; Talbot, T.; Laquerriere, P. Three-dimensional distributions of elements in biological samples by energy-filtered electron tomography. Ultramicroscopy 2004, 100, 115–125. [Google Scholar] [CrossRef]
- Arlinghaus, H.F.; Spaar, M.T.; Switzer, R.C.; Kabalka, G.W. Imaging of Boron in Tissue at the Cellular Level for Boron Neutron Capture Therapy. Anal. Chem. 1997, 69, 3169–3176. [Google Scholar] [CrossRef]
- Arlinghaus, H.; Kriegeskotte, C.; Fartmann, M.; Wittig, A.; Sauerwein, W.; Lipinsky, D. Mass spectrometric characterization of elements and molecules in cell cultures and tissues. Appl. Surf. Sci. 2006, 252, 6941–6948. [Google Scholar] [CrossRef]
- Motto-Ros, V.; Sancey, L.; Ma, Q.L.; Lux, F.; Bai, X.S.; Wang, X.C.; Yu, J.; Panczer, G.; Tillement, O. Mapping of native inorganic elements and injected nanoparticles in a biological organ with laser-induced plasma. Appl. Phys. Lett. 2012, 101, 223702. [Google Scholar] [CrossRef]
- Sancey, L.; Motto-Ros, V.; Kotb, S.; Wang, X.; Lux, F.; Panczer, G.; Yu, J.; Tillement, O. Laser-induced breakdown spectroscopy: A new approach for nanoparticle’s mapping and quantification in organ tissue. J. Vis. Exp. JoVE 2014, e51353. [Google Scholar] [CrossRef]
- Busser, B.; Moncayo, S.; Coll, J.L.; Sancey, L.; Motto-Ros, V. Elemental imaging using laser-induced breakdown spectroscopy: A new and promising approach for biological and medical applications. Coord. Chem. Rev. 2018, 358, 70–79. [Google Scholar] [CrossRef]
- Leprince, M.; Sancey, L.; Coll, J.L.; Motto-Ros, V.; Busser, B. L’imagerie élémentaire par spectroscopie LIBS. Méd./Sci. 2019, 35, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Liu, H.; Ozeki, Y.; Sato, S.; Hayashi, T.; Nakamura, H. Imaging of cellular uptake of boron cluster compound by stimulated Raman scattering microscopy. Appl. Phys. Express 2019, 12, 112004. [Google Scholar] [CrossRef]
- Zuo, C.S.; Prasad, P.V.; Busse, P.; Tang, L.; Zamenhof, R.G. Proton nuclear magnetic resonance measurement of p-boronophenylalanine (BPA): A therapeutic agent for boron neutron capture therapy. Med. Phys. 1999, 26, 1230–1236. [Google Scholar] [CrossRef]
- Bendel, P.; Margalit, R.; Salomon, Y. Optimized 1 H MRS and MRSI methods for the in vivo detection of boronophenylalanine. Magn. Reson. Med. 2005, 53, 1166–1171. [Google Scholar] [CrossRef]
- Timonen, M.; Kankaanranta, L.; Lundbom, N.; Collan, J.; Kangasmäki, A.; Kortesniemi, M.; Häkkinen, A.M.; Lönngren, A.; Karjalainen, S.; Rasilainen, M.; et al. 1H MRS studies in the Finnish boron neutron capture therapy project: Detection of 10B-carrier, l-p-boronophenylalanine-fructose. Eur. J. Radiol. 2005, 56, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Bendel, P.; Koudinova, N.; Salomon, Y. In vivo imaging of the neutron capture therapy agent BSH in mice using 10B MRI. Magn. Reson. Med. 2001, 46, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Kunz, U.; Lehmann, H.; Gabel, D. Determination of the Subcellular Distribution of Mercaptoundecahydro-closo-dodecaborate (BSH) in Human Glioblastoma Multiforme by Electron Microscopy. J. Neuro-Oncol. 2002, 57, 97–104. [Google Scholar] [CrossRef]
- Akselrod, M.; Kouwenberg, J. Fluorescent nuclear track detectors—Review of past, present and future of the technology. Radiat. Meas. 2018, 117, 35–51. [Google Scholar] [CrossRef]
- Schlegel, J.; Liew, H.; Rein, K.; Dzyubachyk, O.; Debus, J.; Abdollahi, A.; Niklas, M. Biosensor Cell-Fit-HD4D for correlation of single-cell fate and microscale energy deposition in complex ion beams. STAR Protoc. 2022, 3, 101798. [Google Scholar] [CrossRef]
- Niklas, M.; Greilich, S.; Melzig, C.; Akselrod, M.S.; Debus, J.; Jäkel, O.; Abdollahi, A. Engineering cell-fluorescent ion track hybrid detectors. Radiat. Oncol. 2013, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, J.F.; Ziegler, M.D.; Biersack, J.P. SRIM—The stopping and range of ions in matter (2010). Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2010, 268, 1818–1823. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- SRM-2137; Boron Implant in Silicon Standard for Calibration of Concentration in a Depth Profile. National Institute of Standards & Technology: Gaithersburg, MD, USA, 2010. Available online: https://tsapps.nist.gov/srmext/certificates/archives/2137.pdf (accessed on 15 January 2025).
- Kouwenberg, J.J.M.; Wolterbeek, H.T.; Denkova, A.G.; Bos, A.J.J. Fluorescent nuclear track detectors for alpha radiation microdosimetry. Radiat. Oncol. 2018, 13, 107. [Google Scholar] [CrossRef]
- Niklas, M.; Henrich, M.; Jäkel, O.; Engelhardt, J.; Abdollahi, A.; Greilich, S. STED microscopy visualizes energy deposition of single ions in a solid-state detector beyond diffraction limit. Phys. Med. Biol. 2017, 62, 180–190. [Google Scholar] [CrossRef] [PubMed]





| Technique | Qualitative Spatial Resolution | |
|---|---|---|
| FNTD | Subcellular | |
| EELS | Subcellular | tens of |
| SNMS | Subcellular | hundreds of |
| SIMS | Subcellular | a few |
| Nano-SIMS | Subcellular | hundreds of |
| Autoradiography | Subcellular | |
| LIBS | Cellular/Subcellular | 10 |
| MRI | Organs | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galuzzi, L.; Parisi, G.; Pascali, V.; Niklas, M.; Bortot, D.; Protti, N.; Altieri, S. Fluorescent Neutron Track Detectors for Boron-10 Microdistribution Measurement in BNCT: A Feasibility Study. Materials 2025, 18, 621. https://doi.org/10.3390/ma18030621
Galuzzi L, Parisi G, Pascali V, Niklas M, Bortot D, Protti N, Altieri S. Fluorescent Neutron Track Detectors for Boron-10 Microdistribution Measurement in BNCT: A Feasibility Study. Materials. 2025; 18(3):621. https://doi.org/10.3390/ma18030621
Chicago/Turabian StyleGaluzzi, Laura, Gabriele Parisi, Valeria Pascali, Martin Niklas, Davide Bortot, Nicoletta Protti, and Saverio Altieri. 2025. "Fluorescent Neutron Track Detectors for Boron-10 Microdistribution Measurement in BNCT: A Feasibility Study" Materials 18, no. 3: 621. https://doi.org/10.3390/ma18030621
APA StyleGaluzzi, L., Parisi, G., Pascali, V., Niklas, M., Bortot, D., Protti, N., & Altieri, S. (2025). Fluorescent Neutron Track Detectors for Boron-10 Microdistribution Measurement in BNCT: A Feasibility Study. Materials, 18(3), 621. https://doi.org/10.3390/ma18030621







