CO2-Responsive Worm-like Micelle Based on Double-Tailed Surfactant
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Preparation of Surfactant Solution
2.3. pH Measurement
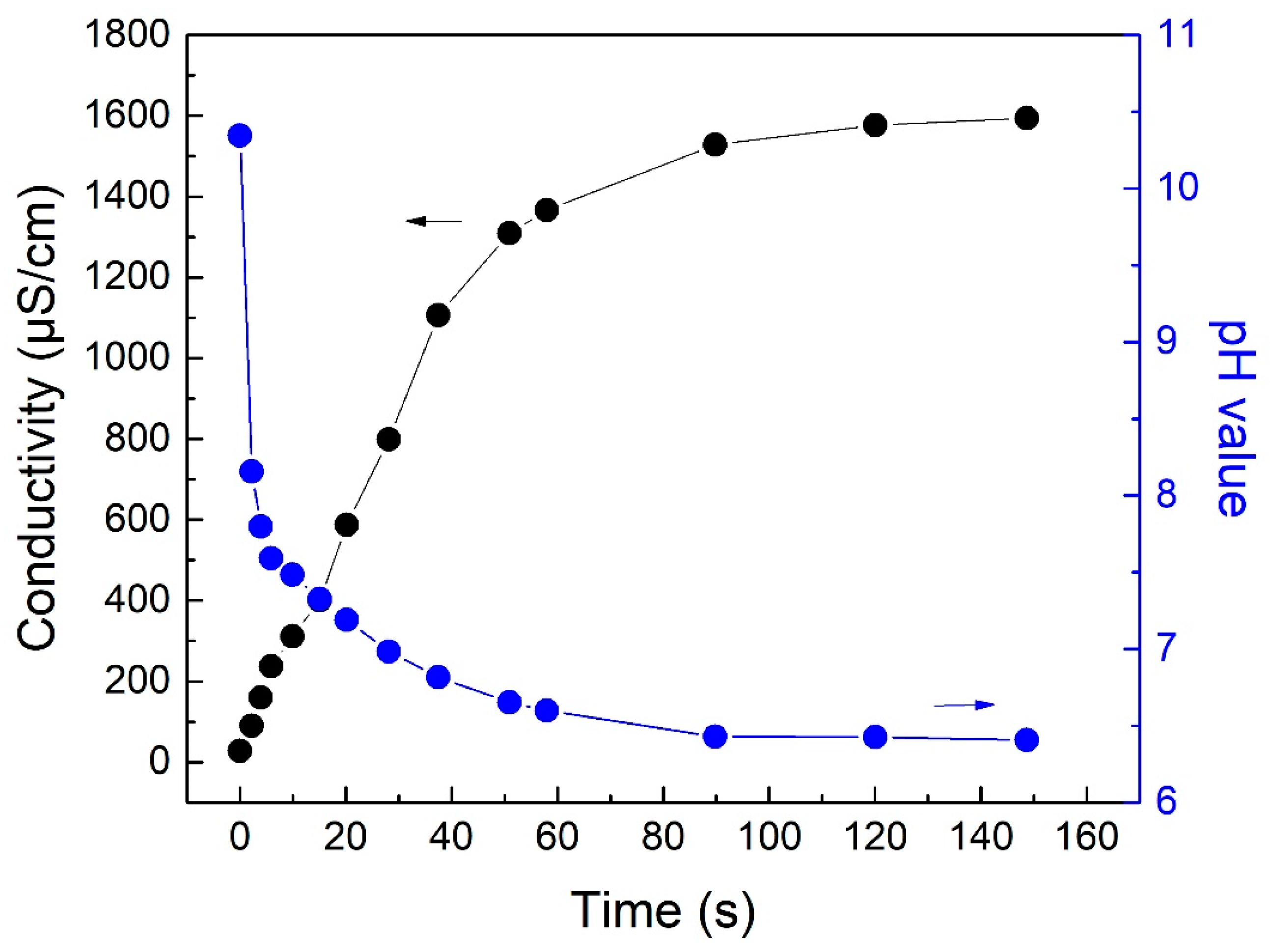
2.4. Conductivity Measurement
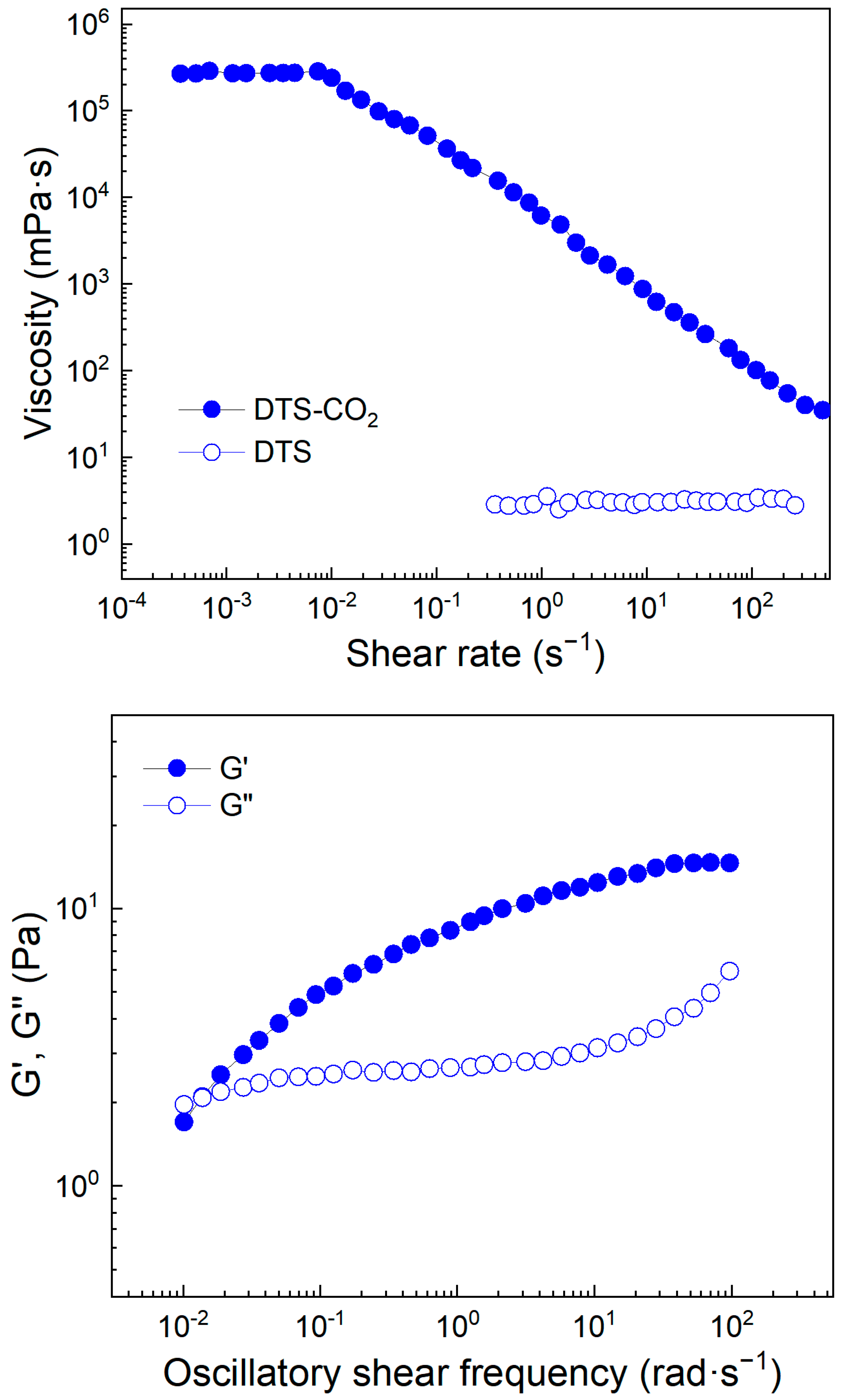
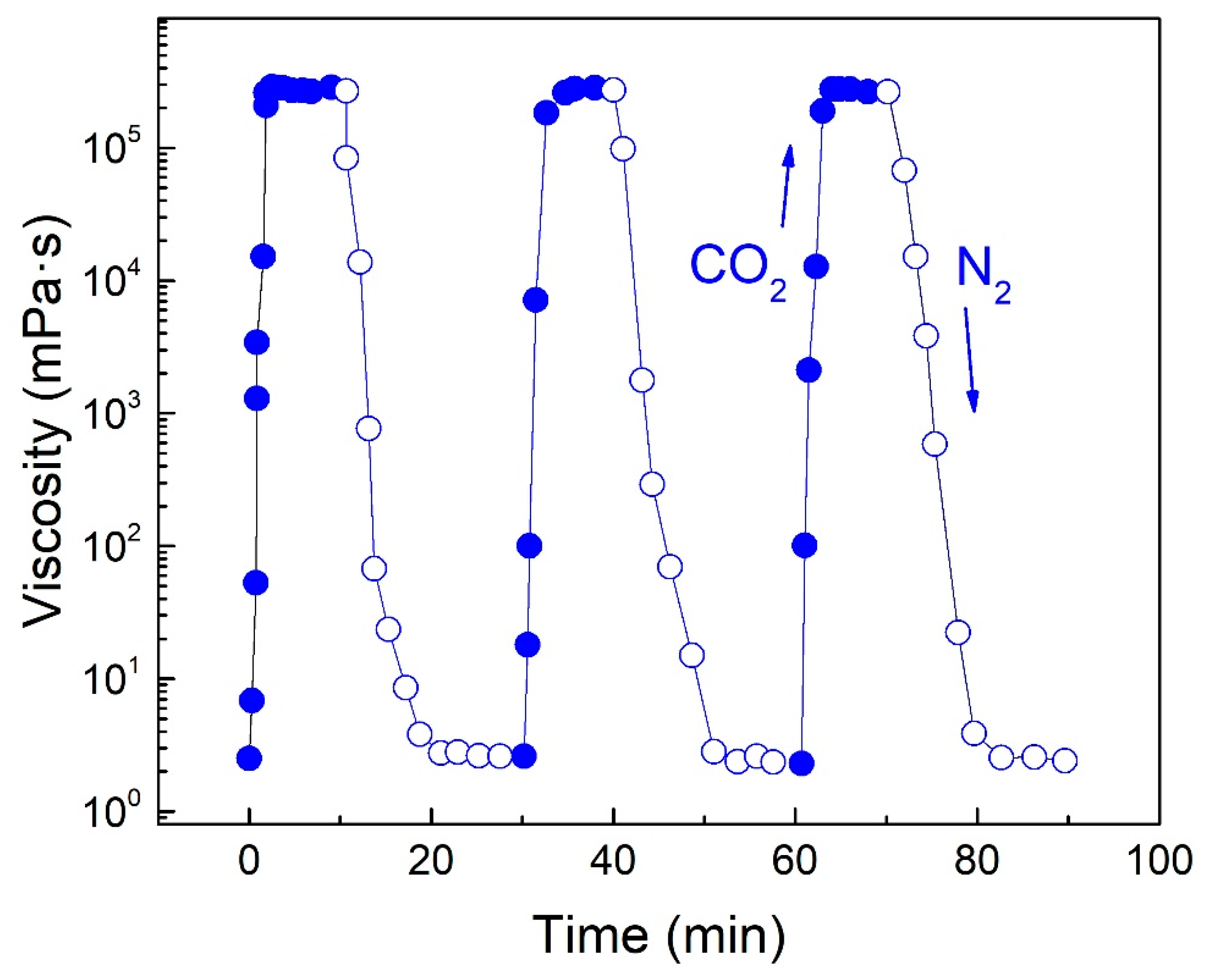
2.5. Rheological Property Testing
2.6. Cryo-TEM Observation
3. Results
3.1. CO2-Responsive Properties of DTS
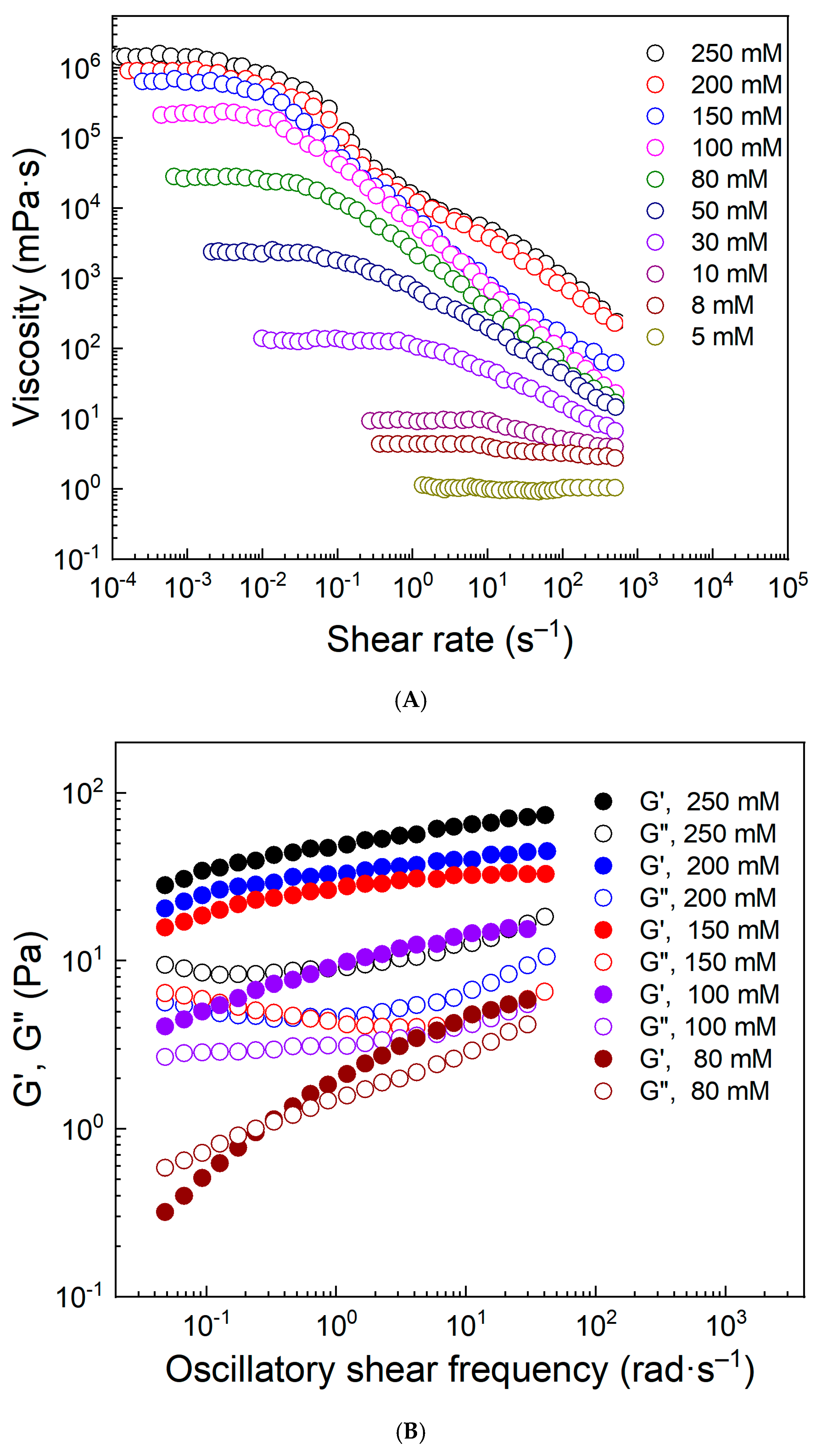
3.2. Factors Affecting the Rheological Properties of Worm-like Micelles
3.2.1. CO2 Sparging Time
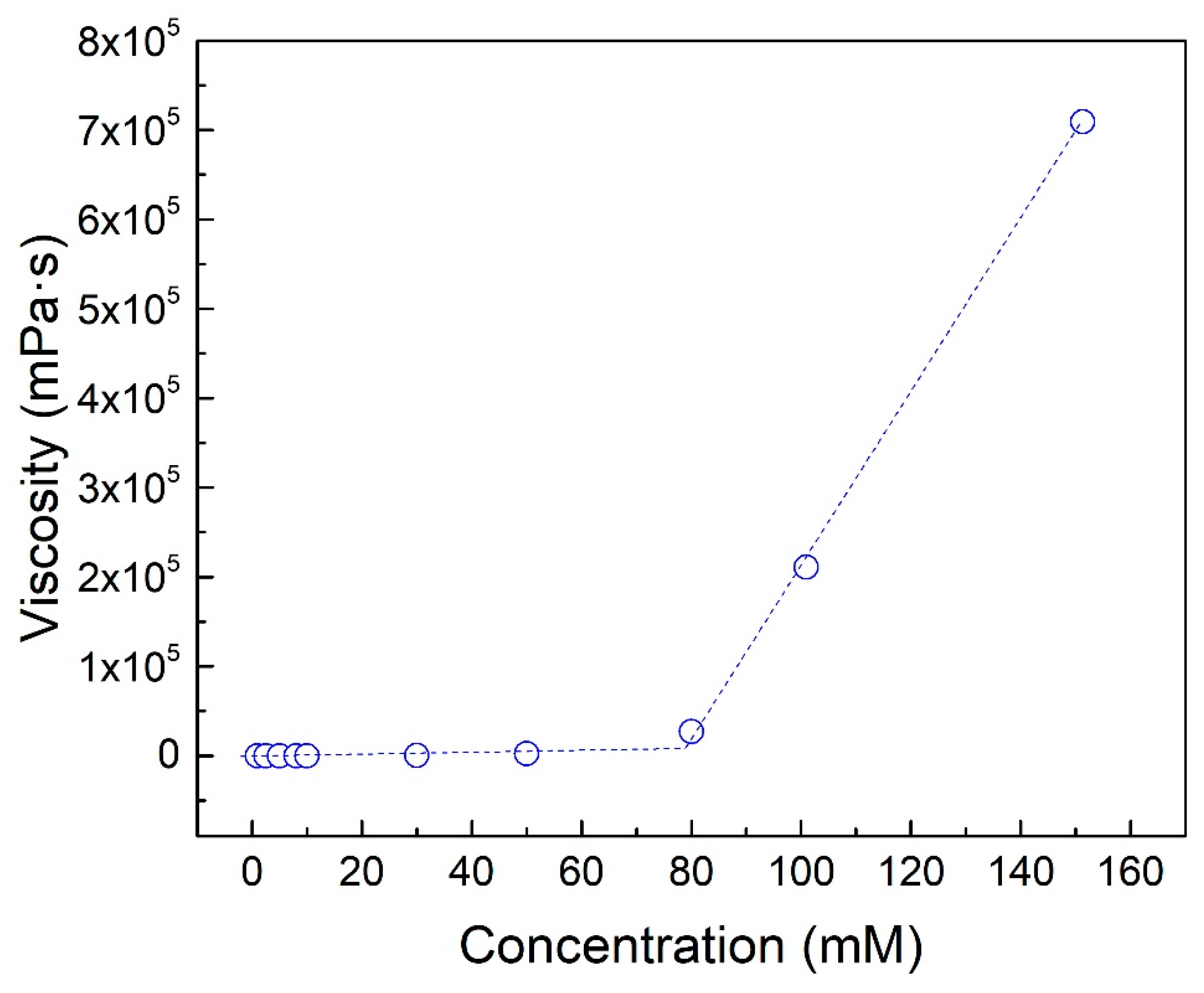
3.2.2. Concentration of DTS
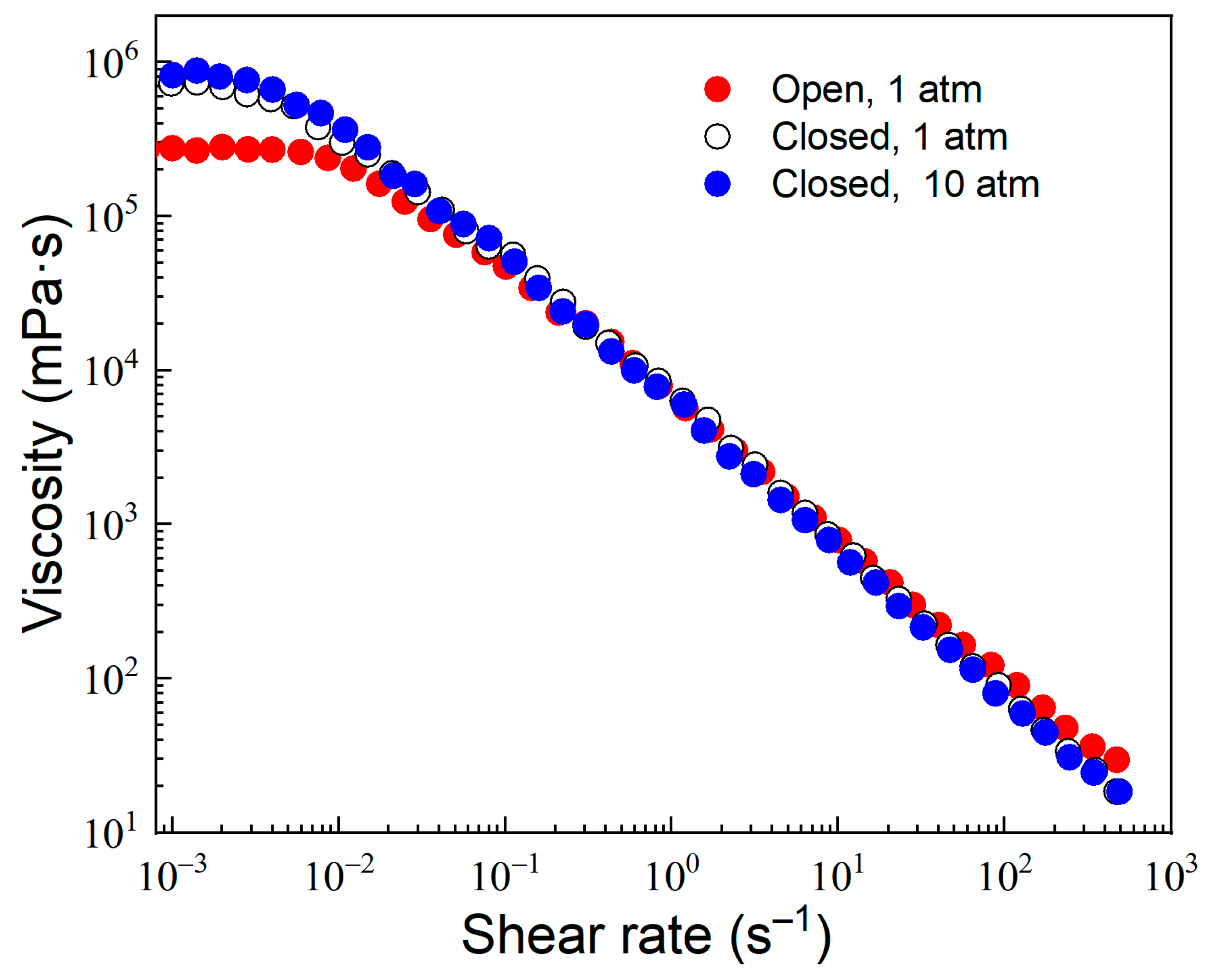
3.2.3. Pressure and Temperature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Lin, Z.; Chen, Z.; Cui, Z.; Song, B. Wormlike micellar solutions formed by an anionic surfactant and a cationic surfactant with two head groups. Soft Matter 2024, 20, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.R.; Daneshi, M.; Frigaard, I.; Elfring, G. Shear layers and plugs in the capillary flow of wormlike micellar gels. Soft Matter 2024, 20, 4715–4733. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xu, Z.; Wang, D.; Chen, X.; Zhang, Z.; Yin, Q.; Li, Y. Intracellular pH-activated PEG-b-PDPA wormlike micelles for hydrophobic drug delivery. Polym. Chem. 2013, 4, 5052–5055. [Google Scholar] [CrossRef]
- Zeng, L.; Zou, L.; Yu, H.; He, X.; Cao, H.; Zhang, Z.; Yin, Q.; Zhang, P.; Gu, W.; Chen, L.; et al. Treatment of malignant brain tumor by tumor-triggered programmed wormlike micelles with precise targeting and deep penetration. Adv. Funct. Mater. 2016, 26, 4201–4212. [Google Scholar] [CrossRef]
- McCoy, T.M.; Valiakhmetova, A.; Pottage, M.J.; Garvey, C.J.; de Campo, L.; Rehm, C.; Kuryashov, D.A.; Tabor, R.F. Structural evolution of wormlike micellar fluids formed by erucyl amidopropyl betaine with oil, salts, and surfactants. Langmuir 2016, 32, 12423–12433. [Google Scholar] [CrossRef]
- Du, D.; Pu, W.; Chen, B.; Varfolomeev, M.A.; Liu, R. Experimental study on EOR potential of water-in-oil emulsion via CO2/N2 triggered wormlike micelle solution. Fuel 2021, 288, 119639. [Google Scholar] [CrossRef]
- Numin, M.S.; Jumbri, K.; Ramli, A.; Borhan, N. Microemulsion rheological analysis of alkaline, surfactant, and polymer in oil-water interface. Processes 2020, 8, 762. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Y.; Fang, B.; Talmon, Y.; Ge, W.; Raghavan, S.R.; Zakin, J.L. Light-Responsive Threadlike Micelles as Drag Reducing Fluids with Enhanced Heat-Transfer Capabilities. Langmuir 2011, 27, 5806–5813. [Google Scholar] [CrossRef]
- Souza, R.N.; Duarte, L.G.T.A.; Jora, M.Z.; Atvars, T.D.Z.; Sabadini, E. Thermal behavior of wormlike micelles under turbulent and quiescent regimes. Colloids Surf. A 2020, 603, 125271. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, F.; Liu, D.; Chen, F.; Wei, J. Energy analysis of a surfactant micelle’s deformation by coarse-grained molecular dynamics simulations. Chem. Eng. Sci. 2019, 202, 138–145. [Google Scholar] [CrossRef]
- Yan, Z.; Dai, C.; Zhao, M.; Zhao, G.; Li, Y.; Wu, X.; Du, M.; Liu, Y. pH-switchable wormlike micelle formation by N-alkyl-N-methylpyrrolidinium bromide-based cationic surfactant. Colloids Surf. A 2015, 482, 283–289. [Google Scholar] [CrossRef]
- Liu, P.; Pei, X.; Li, C.; Li, R.; Chen, Z.; Song, B.; Cui, Z.; Xie, D. pH-switchable wormlike micelles with high viscoelasticity formed by pseudo-oligomeric surfactants. J. Mol. Liq. 2021, 334, 116499. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, Y.; Chu, Z.; He, S.; Zhang, J.; Feng, Y. Thermally induced structural transitions from fluids to hydrogels with pH-switchable anionic wormlike micelles. J. Colloid Interface Sci. 2013, 394, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.; Jain, M.; Kumar, S.; Kasoju, N.; Kumar, S.; Aswal, V.K.; Seoud, O.E.; Malek, N. Light responsive microstructural transitions in photo-responsive wormlike micelle mediated viscoelastic material based on cationic surfactant and photo-responsive organic acids. J. Mol. Liq. 2024, 394, 123798. [Google Scholar] [CrossRef]
- Bi, Y.; Wang, T.; Xiao, J.; Yu, L. Wormlike micelles with photo and pH dual-stimuli-responsive behaviors formed by aqueous mixture of cationic and anionic surfactants. Colloids Surf. A 2023, 668, 131441. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, W.; Wang, C.; An, P.; Fang, Y.; Feng, Y.; Qin, Z.; Liu, X. Switching wormlike micelles of selenium-containing surfactant using redox reaction. Soft Matter 2015, 11, 7469–7473. [Google Scholar] [CrossRef]
- Hu, T.; Zheng, X.; Huang, M.; Zhou, X.; Chen, S.; Zhang, H. Preparation and response behaviour of wormlike micelles in response to CO2 stimulation. Colloids Surf. A 2024, 685, 133072. [Google Scholar] [CrossRef]
- Liu, Y.; Jessop, P.G.; Cunningham, M.; Eckert, C.A.; Liotta, C.L. Switchable surfactants. Science 2006, 313, 958–960. [Google Scholar] [CrossRef]
- Lin, S.; Theato, P. CO2-responsive polymers. Macromol. Rapid Commun. 2013, 34, 1118–1133. [Google Scholar] [CrossRef]
- Jessop, P.G.; Mercer, S.M.; Heldebrant, D.J. CO2-triggered switchable solvents, surfactants, and other materials. Energy Environ. Sci. 2012, 5, 7240–7253. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Wang, J.; He, S.; Guo, Z.; Chu, Z.; Dreiss, C.A. CO2-switchable wormlike micelles. Chem. Commun. 2013, 49, 4902–4904. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Cunningham, M.F.; Jessop, P.G. Switchable viscosity triggered by CO2 using smart worm-like micelles. Chem. Commun. 2013, 49, 2655–2657. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; He, X.; Zhang, D.; Su, X. CO2-responsive wormlike micelles based on pseudo-tetrameric surfactant. Molecules 2022, 27, 7922. [Google Scholar] [CrossRef] [PubMed]
- Greaves, T.L.; Drummond, C.J. Solvent nanostructure, the solvophobic effect and amphiphile self-assembly in ionic liquids. Chem. Soc. Rev. 2013, 42, 1096–1120. [Google Scholar] [CrossRef]
- Yang, H.; Wang, J.; Yang, S.; Zhang, W. Aggregate conformation and rheological properties of didodecyldimethylammonium bromide in aqueous solution. J. Dispers. Sci. Technol. 2010, 31, 650–653. [Google Scholar] [CrossRef]
- Gonçalves, R.A.; Lam, Y.; Lindman, B. Double-chain cationic surfactants: Swelling, structure, phase transitions and additive effects. Molecules 2021, 26, 3946. [Google Scholar] [CrossRef]
- Cui, Z.; Yang, L.; Cui, Y.; Binks, B.P. Effects of surfactant structure on the phase inversion of emulsions stabilized by mixtures of silica nanoparticles and cationic surfactant. Langmuir 2010, 26, 4717–4724. [Google Scholar] [CrossRef]
- Biswal, N.R.; Paria, S. Wetting of PTFE and glass surfaces by aqueous solutions of cationic and anionic double-chain surfactants. Ind. Eng. Chem. Res. 2012, 51, 10172–10178. [Google Scholar] [CrossRef]
- Heinz, H.; Pramanik, C.; Heinz, O.; Ding, Y.; Mishra, R.K.; Marchon, D.; Flatt, R.J.; Estrela-Lopis, I.; Llop, J.; Moya, S.; et al. Nanoparticle decoration with surfactants: Molecular interactions, assembly, and applications. Surf. Sci. Rep. 2017, 72, 1–58. [Google Scholar] [CrossRef]
- Veeralakshmi, S.; Sabapathi, G.; Nehru, S.; Venuvanalingam, P.; Arunachalam, S. Surfactant–cobalt (III) complexes: The impact of hydrophobicity on interaction with HSA and DNA–insights from experimental and theoretical approach. Colloids Surf. B 2017, 153, 85–94. [Google Scholar] [CrossRef]
- Lin, Z.; Bi, Z.; Li, H.; Pei, X.; Chen, Z.; Cui, Z.; Song, B. Wormlike micellar glycerol solutions formed from a double-tailed surfactant with two quaternary ammonium head groups. Langmuir 2024, 40, 19954–19963. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Huang, H.; Zhang, M.; Mu, M.; Chen, R.; Su, X. CO2-Responsive Worm-like Micelle Based on Double-Tailed Surfactant. Materials 2025, 18, 902. https://doi.org/10.3390/ma18040902
Liu F, Huang H, Zhang M, Mu M, Chen R, Su X. CO2-Responsive Worm-like Micelle Based on Double-Tailed Surfactant. Materials. 2025; 18(4):902. https://doi.org/10.3390/ma18040902
Chicago/Turabian StyleLiu, Fanghui, Huiyu Huang, Mingmin Zhang, Meng Mu, Rui Chen, and Xin Su. 2025. "CO2-Responsive Worm-like Micelle Based on Double-Tailed Surfactant" Materials 18, no. 4: 902. https://doi.org/10.3390/ma18040902
APA StyleLiu, F., Huang, H., Zhang, M., Mu, M., Chen, R., & Su, X. (2025). CO2-Responsive Worm-like Micelle Based on Double-Tailed Surfactant. Materials, 18(4), 902. https://doi.org/10.3390/ma18040902




