Mechanisms Underlying Phase Transition and Regulation of Tantalum Powder Properties During Magnesium Thermal Reduction of Ta2O5 in a Molten Salt Medium
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Thermomechanical Analysis
3.2. Evolution of the Physical Phase During Magnesium Reduction
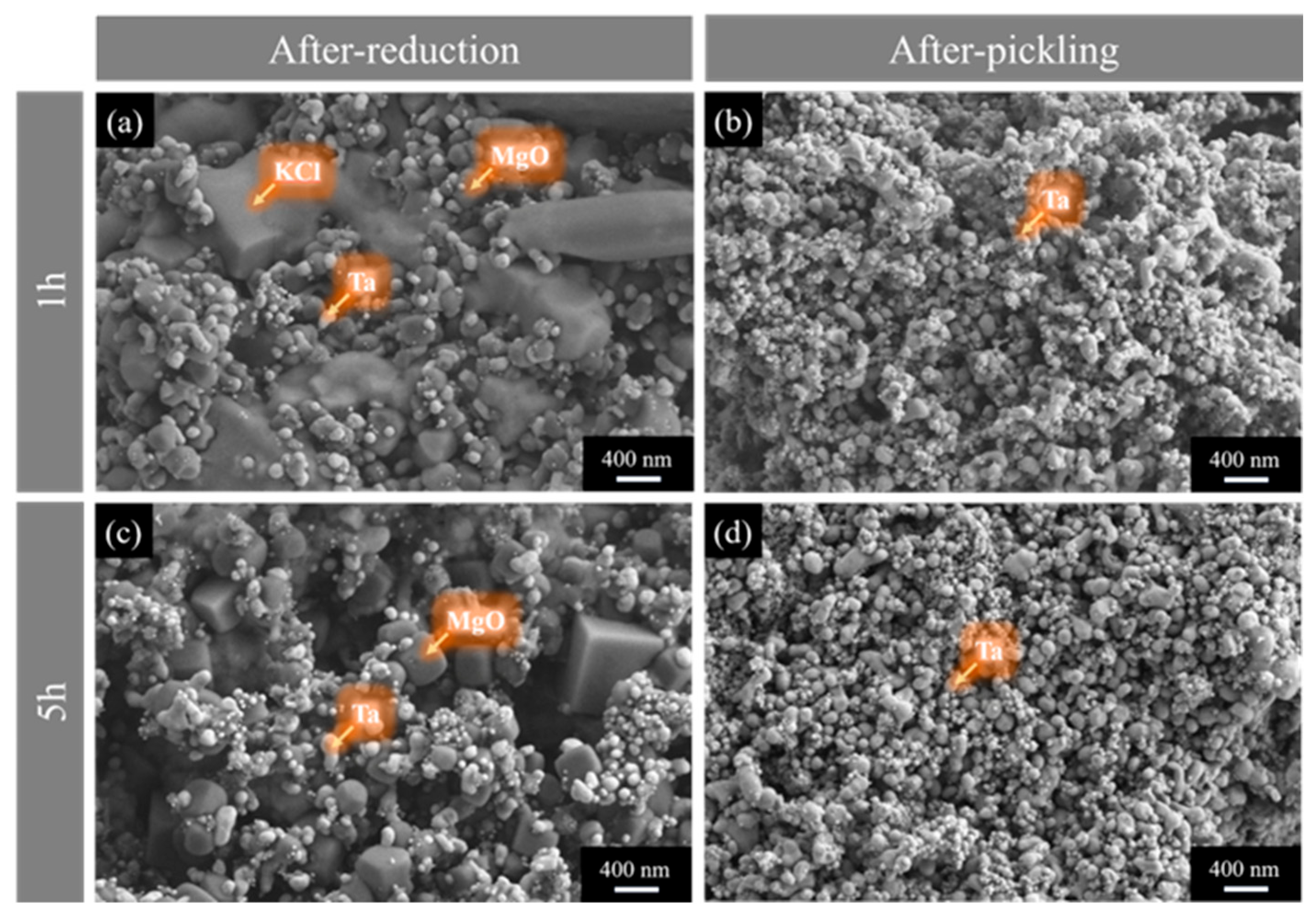
3.3. Effect of Reduction Temperature and Time on the Physicochemical Properties of Tantalum Powder
4. Conclusions
- (1)
- During the magnesium reduction process, no lower-valence tantalum oxides were formed, consistent with thermodynamic calculations. However, in placement methods A and B, regions with insufficient magnesium were present during the reaction, where the by-product magnesium oxide was easily combined with tantalum pentoxide to form magnesium tantalate (MgTa2O6 and Mg4Ta2O9). As the reaction proceeded in magnesium-rich conditions, these magnesium tantalates (MgTa2O6 and Mg4Ta2O9) were further reduced to tantalum, resulting in multiple reaction pathways (Ta2O5 → Ta and Ta2O5 → MgTaxOy → Ta). In contrast, in placement method C, tantalum pentoxide was directly reduced to high-purity metallic tantalum, following a single reaction pathway (Ta2O5 → Ta).
- (2)
- Tantalum powder obtained via the single pathway (placement method C) had a more concentrated particle size distribution, with fewer small particles and better particle uniformity, compared to the powder obtained via multiple pathways (placement method B). To ensure uniformity in tantalum powder particles, maintaining consistency in the reaction pathway during the reduction process is essential.
- (3)
- Under placement method C, the magnesium reduction of Ta2O5 occurred only at temperatures above 600 °C. At lower temperatures, the resulting particles were finer and had higher activity, with increased oxygen content after water washing, resulting in the presence of Ta2O and Ta phases instead of pure Ta. At 900 °C, a pure tantalum phase was obtained. As the reduction temperature increased from 600 °C to 900 °C, the particle size of the tantalum powder gradually increased from 30 nm to 150 nm, but the particle uniformity decreased. However, extending the holding time improved the uniformity of the tantalum powder morphology, and the oxygen content in the tantalum powder decreased to 1.79% after a holding time of 3 h.
- (4)
- The in-depth study of the phase transformation laws during the magnesium reduction of tantalum pentoxide and the influence of process parameters on the properties of tantalum powder will provide an important theoretical basis and technical support for the production and application of tantalum powder. In addition, this research can serve as a reference for studies on other metal reduction processes. Furthermore, by focusing on the magnesium tantalate formed during the reaction, our team aims to further synthesize a single phase of magnesium tantalate by solid-state synthesis, refine its thermodynamic parameters, and reduce it in order to further investigate its effect on the properties of tantalum powder.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buckman, R.W. New applications for tantalum and tantalum alloys. JOM 2000, 52, 40–41. [Google Scholar] [CrossRef]
- Mohsan, A.U.H.; Wei, D. Advancements in Additive Manufacturing of Tantalum via the Laser Powder Bed Fusion (PBF-LB/M): A Comprehensive Review. Materials 2023, 16, 6419. [Google Scholar] [CrossRef] [PubMed]
- Ogi, T.; Horiuchi, H.; Makino, T.; Arif, A.F.; Okuyama, K. Simple, Rapid, and Environmentally Friendly Method for Selectively Recovering Tantalum by Guanidine-Assisted Precipitation. ACS Sustain. Chem. Eng. 2018, 6, 9585–9590. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, X.; Lv, N.; Gao, L.; Sun, C.; Tong, Z.; Li, D. Microstructure Optimization for Design of Porous Tantalum Scaffolds Based on Mechanical Properties and Permeability. Materials 2023, 16, 7568. [Google Scholar] [CrossRef] [PubMed]
- He, J.L.; Zhang, X.Q.; Yang, G.Q.; Zheng, A.G. The Key Technique and R&D of Capacitor Grade Tantalum Powder. Materials China 2014, 33, 545–553. [Google Scholar]
- Niu, B.; Chen, Z.; Xu, Z. Recovery of Valuable Materials from Waste Tantalum Capacitors by Vacuum Pyrolysis Combined with Mechanical-Physical Separation. ACS Sustain. Chem. Eng. 2017, 5, 2639–2647. [Google Scholar] [CrossRef]
- Yang, Q.; Furigay, M.H.; Chaudhuri, S.; Gau, M.R.; Schatz, G.C.; Schelter, E.J. Toward Redox- and Photoredox-Based Niobium/Tantalum Separations. ACS Sustain. Chem. Eng. 2024, 12, 8503–8511. [Google Scholar] [CrossRef]
- Freeman, Y.; Lessner, P. Evolution of Polymer Tantalum Capacitors. Appl. Sci. 2021, 11, 5514. [Google Scholar] [CrossRef]
- Micheau, C.; Arrachart, G.; Turgis, R.; Lejeune, M.; Draye, M.; Michel, S.; Legeai, S.; Pellet-Rostaing, S. Ionic Liquids as Extraction Media in a Two-Step Eco-Friendly Process for Selective Tantalum Recovery. ACS Sustain. Chem. Eng. 2020, 8, 1954–1963. [Google Scholar] [CrossRef]
- Pan, L.T.; Li, B.; Zheng, A.G.; Lu, J.B.; Li, H.J.; Wang, Q.Y. Application of tantalum to lsic. Chin. J. Rare Met. 2003, 27, 28–34. [Google Scholar]
- Niu, B.; Chen, Z.; Xu, Z. Recovery of Tantalum from Waste Tantalum Capacitors by Supercritical Water Treatment. ACS Sustain. Chem. Eng. 2017, 5, 4421–4428. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, H.; Kwon, N.; Sim, J.; Lee, M.; Oh, S.; Seo, S.; Shin, J.; Park, K. Effects of reduction temperature and diluent content on the properties of high-purity tantalum powder prepared using the Hunter process. Int. J. Refract. Met. Hard Mater. 2023, 112, 106128. [Google Scholar] [CrossRef]
- Sim, J.-J.; Choi, S.-H.; Lee, Y.-K.; Heo, S.G.; Kim, T.-S.; Seo, S.-J.; Park, K.-T. Consideration of Diluents Selection and Input Amounts of the Hunter Process for Tantalum Production. Met. Mater. Int. 2021, 27, 1980–1987. [Google Scholar] [CrossRef]
- Yoon, J.S.; Kim, B.I. Characteristics and production of tantalum powders for solid-electrolyte capacitors. J. Power Sources 2007, 164, 959–963. [Google Scholar] [CrossRef]
- Hwang, S.-M.; Park, S.-J.; Wang, J.-P.; Park, Y.-H.; Lee, D.-W. Preparation of tantalum metal powder by magnesium gas reduction of tantalum pentoxide with different initial particle size. Int. J. Refract. Met. Hard Mater. 2021, 100, 105620. [Google Scholar] [CrossRef]
- Ryu, H.S.; Nersisyan, H.; Park, K.T.; Lee, J.H. Porous tantalum network structures exhibiting high electrochemical performance as capacitors. J. Energy Storage 2021, 34, 102222. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Sim, J.-J.; Byeon, J.-S.; Lee, Y.-T.; Cho, Y.-W.; Kim, H.-C.; Heo, S.-G.; Lee, K.-A.; Seo, S.-J.; Park, K.-T. Production of high-purity tantalum metal powder for capacitors using self-propagating high-temperature synthesis. Arch. Metall. Mater. 2021, 66, 935–939. [Google Scholar] [CrossRef]
- Brar, L.K.; Singla, G.; Kaur, N.; Pandey, O.P. Thermal stability and structural properties of Ta nanopowder synthesized via simultaneous reduction of Ta2O5 by hydrogen and carbon. J. Therm. Anal. Calorim. 2015, 119, 175–182. [Google Scholar] [CrossRef]
- Liu, D.; Liu, J.; Ye, P.; Zhang, H.; Zhang, S. Low-Temperature, Efficient Synthesis of Highly Crystalline Urchin-like Tantalum Diboride Nanoflowers. Materials 2022, 15, 2799. [Google Scholar] [CrossRef]
- Hwang, S.-M.; Hong, J.-W.; Park, Y.-H.; Lee, D.-W. Synthesis of Tantalum Carbide Using Purified Hexane by Titanium Powder. Materials 2022, 15, 7510. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.P.; Xiao, Y.; Liang, X.M.; Fan, Y.Y.; Wang, L.Q.; Yang, S.; He, J.L.; Chen, Y.R. Facile synthesis of nanoscale tantalum powder by electro-deoxidation of Ta2O5. Int. J. Refract. Met. Hard Mater. 2023, 114, 106269. [Google Scholar] [CrossRef]
- Wang, Y.T.; Xue, Y.D.; Zhang, C.H. Electrochemical product engineering towards sustainable recovery and manufacturing of critical metals. Green Chem. 2021, 23, 6301–6321. [Google Scholar]
- Park, K.Y.; Kim, H.J.; Suh, Y.J. Preparation of tantalum nanopowders through hydrogen reduction of TaCl5 vapor. Powder Technol. 2007, 172, 144–148. [Google Scholar] [CrossRef]
- Niu, B.; Chen, Z.; Xu, Z. Method for Recycling Tantalum from Waste Tantalum Capacitors by Chloride Metallurgy. ACS Sustain. Chem. Eng. 2017, 5, 1376–1381. [Google Scholar] [CrossRef]
- Zhu, H.M.; Sadoway, D.R. Synthesis of nanoscale particles of Ta and Nb3Al by homogeneous reduction in liquid ammonia. J. Mater. Res. 2001, 16, 2544–2549. [Google Scholar]
- Nersisyan, H.; Ryu, H.S.; Lee, J.H.; Suh, H.; Won, H.I. Tantalum network nanoparticles from a Ta2O5+kMg system by liquid magnesium controlled combustion. Combust. Flame 2020, 219, 136–146. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, S.; Yang, G.; Xie, Q.; Zuo, J.; Luo, W. Microstructure and Electrical Properties of Tantalum Powder with Different Calcining Technology. Chin. J. Rare Met. 2018, 42, 67–74. [Google Scholar]
- Yue, R.; Zhou, L. Effect of Refiner Addition on Particle Size and Electrical Properties of FTB-48 Capacitor Tantalum Powder. Rare Met. Cem. Carbides 2020, 48, 18–21. [Google Scholar]
- Shekhter, L.N.; Tripp, T.B.; Lanin, L.L.; Conlon, A.M. Metalothermic Reduction of Refractory Metal Oxides. U.S. Patent 2004/0163491, 26 August 2004. [Google Scholar]
- Shekhter, L.N.; Tripp, T.B.; Lanin, L.L.; Reichert, K.; Thomas, O.; Vieregge, J. Metal Powders Produced by the Reduction of the Oxides with Gaseous Magnesium. U.S. Patent 6558447, 6 May 2003. [Google Scholar]
- Hagymási, M.; Otterstedt, R.D.; Schnitter, C.; Thomas, O.; Reifenheiser, R. Pushing Tantalum capacitors to the limit: View to 300 V anodisations and beyond. In Proceedings of the Space Passive Component Days (SPCD), Noordwijk, The Netherlands, 9–12 October 2018. [Google Scholar]
- Hagymási, M.; Schnitter, C.; Reifenheiser, R.; Thomas, O. Highest capacitance at higher voltages: Pushing the limits of tantalum high voltage capacitors. In Proceedings of the Space Passive Component Days (SPCD), Noordwijk, The Netherlands, 12–14 October 2016. [Google Scholar]
- Shekhter, L.N.; Simkins, L.F.; Greville, H.P.; Lanin, L. Method of Preparing Primary Refractory Metal. U.S. Patent 7399335, 15 July 2008. [Google Scholar]
- Yan, J.P.; Dou, Z.H.; Zhang, T.A.; Yan, J.S.; Zhou, X.Y. Primary Reduction Mechanism in the Process of Multi-stage Reduction to Prepare Titanium Powder. Rare Met. Mater. Eng. 2022, 51, 1470–1477. [Google Scholar]
- Orlov, V.M.; Kryzhanov, M.V. Magnesiothermic Reduction of Mg4Ta2O9 in the Combustion Regime. Inorg. Mater. 2018, 54, 910–914. [Google Scholar] [CrossRef]
- Kryzhanov, M.V.; Orlov, V.M.; Sukhorukov, V.V. Thermodynamic modeling of magnesiothermic reduction of niobium and tantalum from pentoxides. Russ. J. Appl. Chem. 2010, 83, 379–383. [Google Scholar] [CrossRef]
- Wu, H.T.; Yang, C.H.; Wu, W.B.; Yue, Y.L. Study on synthesis and evolution of nanocrystalline Mg4Ta2O9 by aqueous sol-gel process. Surf. Rev. Lett. 2012, 19, 1250024. [Google Scholar]
- Dang, M.Z.; Lin, H.X.; Yao, X.G.; Ren, H.S.; Xie, T.Y.; Peng, H.Y.; Tan, Z.Y. Effects of B2O3 and MgO on the microwave dielectric properties of MgTa2O6 ceramics. Ceram. Int. 2019, 45, 24244–24247. [Google Scholar]
- Baskin, Y.; Schell, D.C. Phase Studies in the Binary System MgO–Ta2O5. J. Am. Ceram. Soc. 1963, 46, 174–177. [Google Scholar] [CrossRef]
- Orlov, V.M.; Kryzhanov, M.V. Production of tantalum powders by the magnesium reduction of tantalates. Russ. Metall. Met. 2015, 2015, 590–593. [Google Scholar] [CrossRef]
- Orlov, V.M.; Kiselev, E.N. Magnesium Vapor Reduction of Tantalum Oxide Compounds in the Temperature Range 540–680 °C. Inorg. Mater. 2022, 58, 799–805. [Google Scholar]
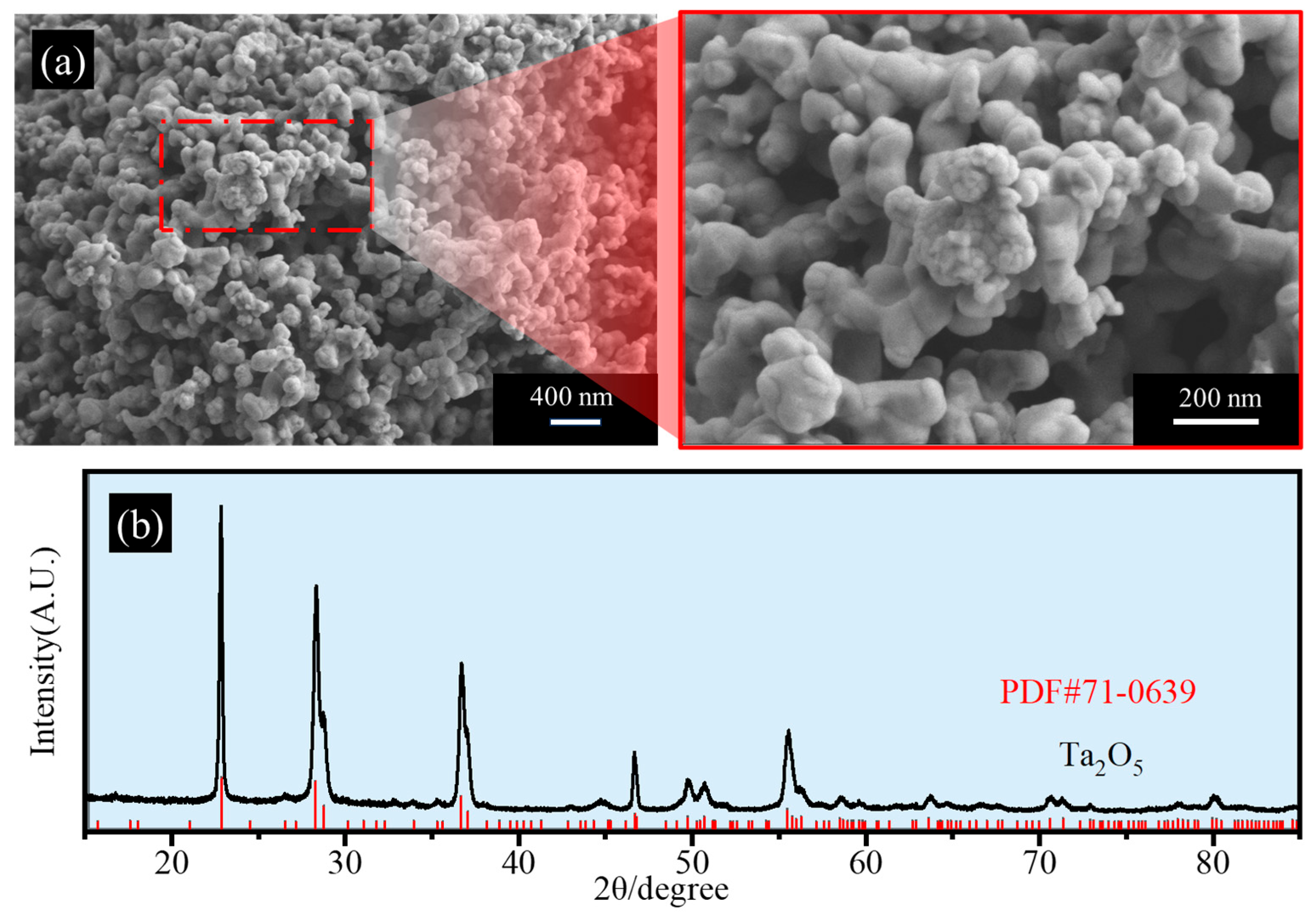

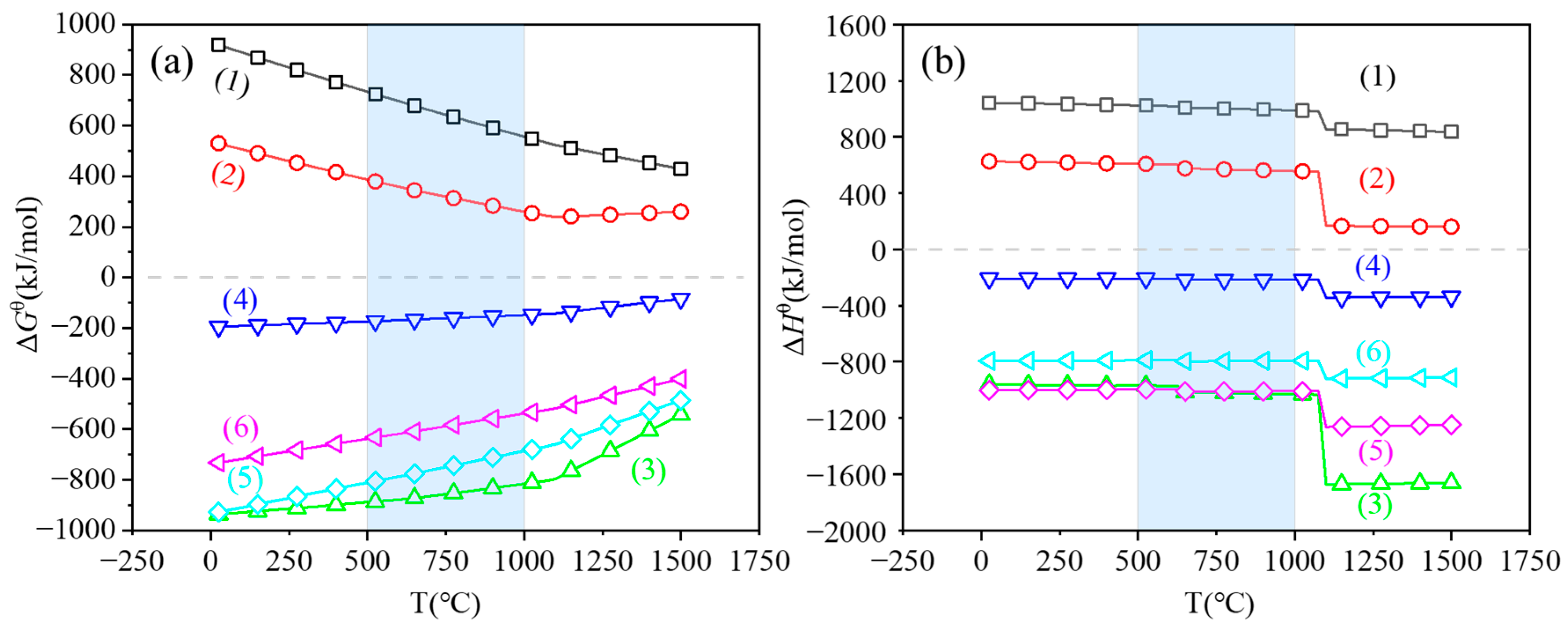
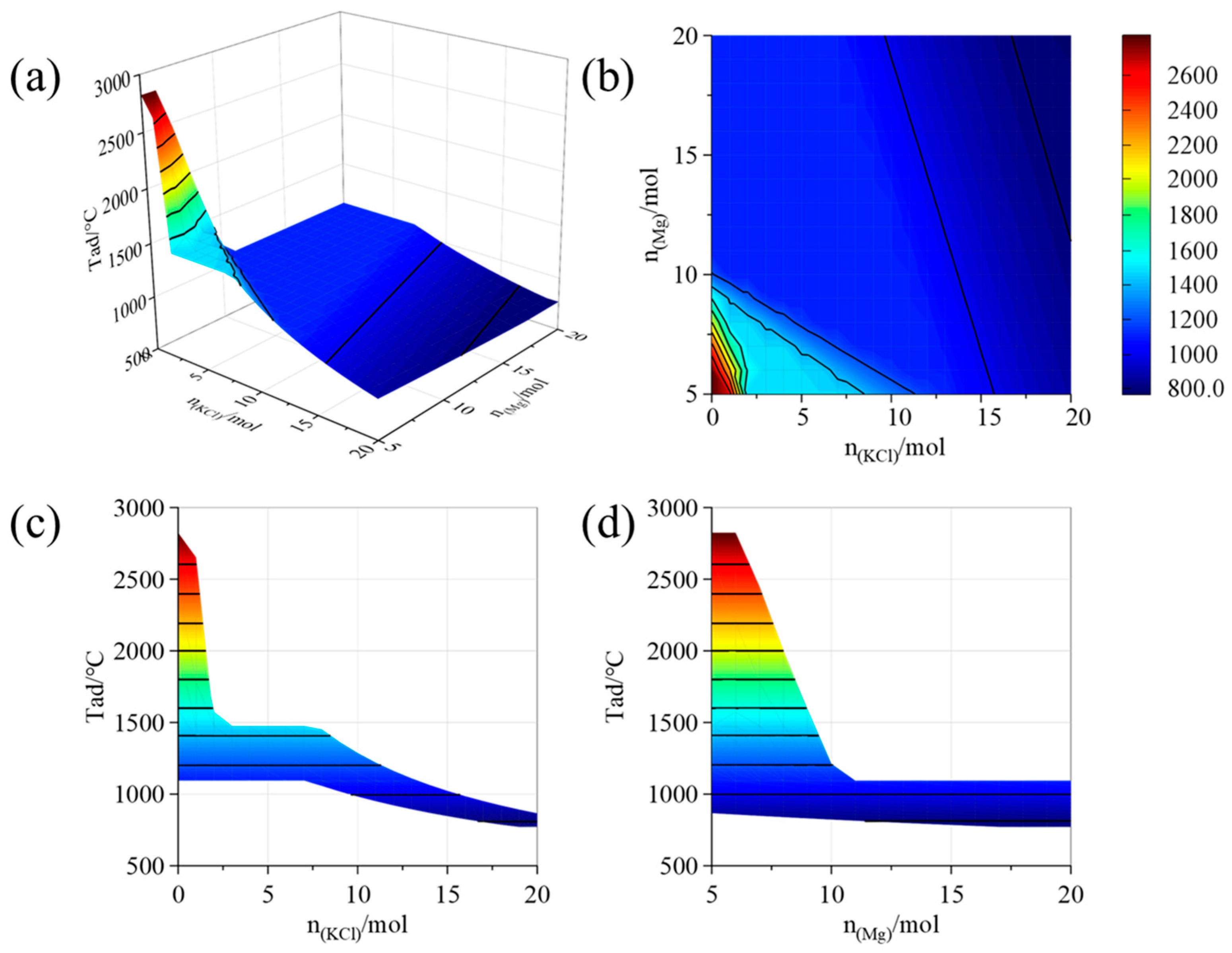
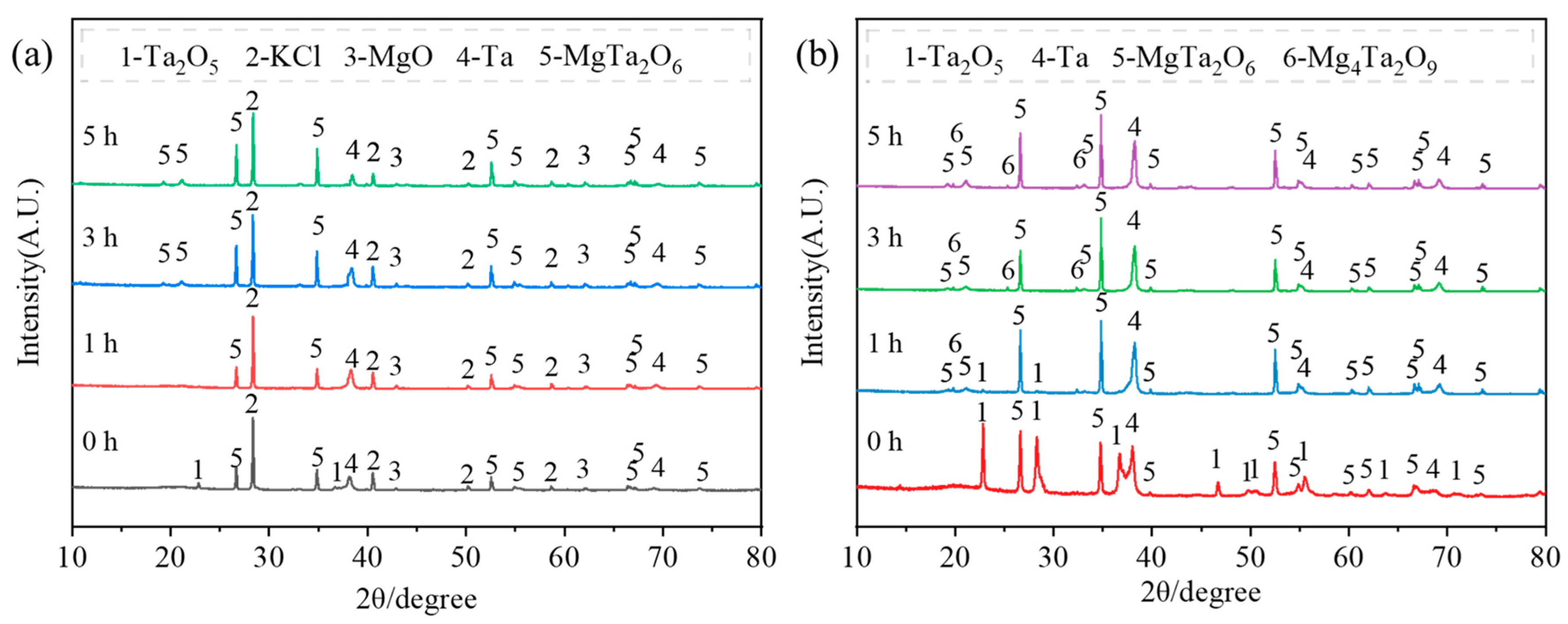



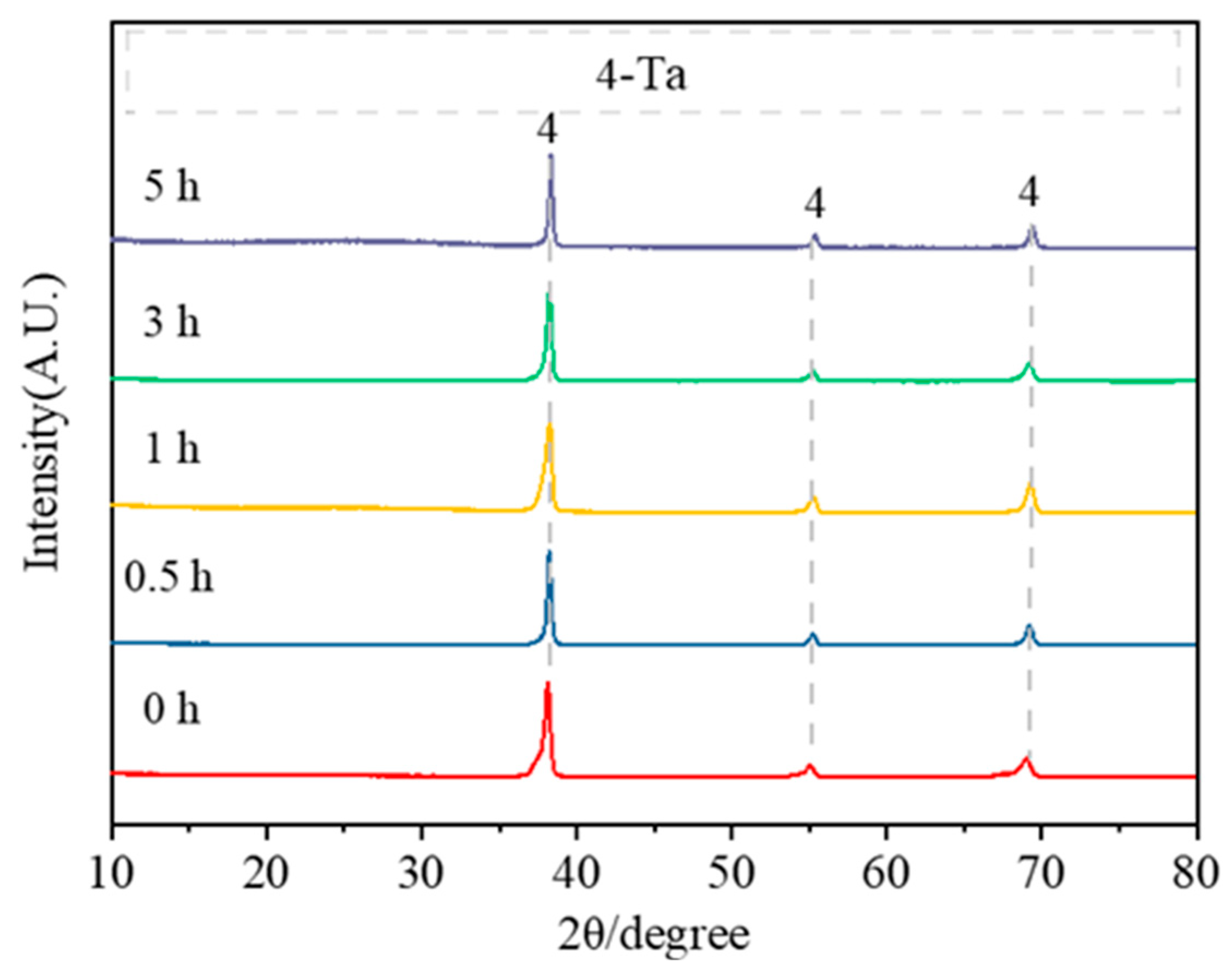
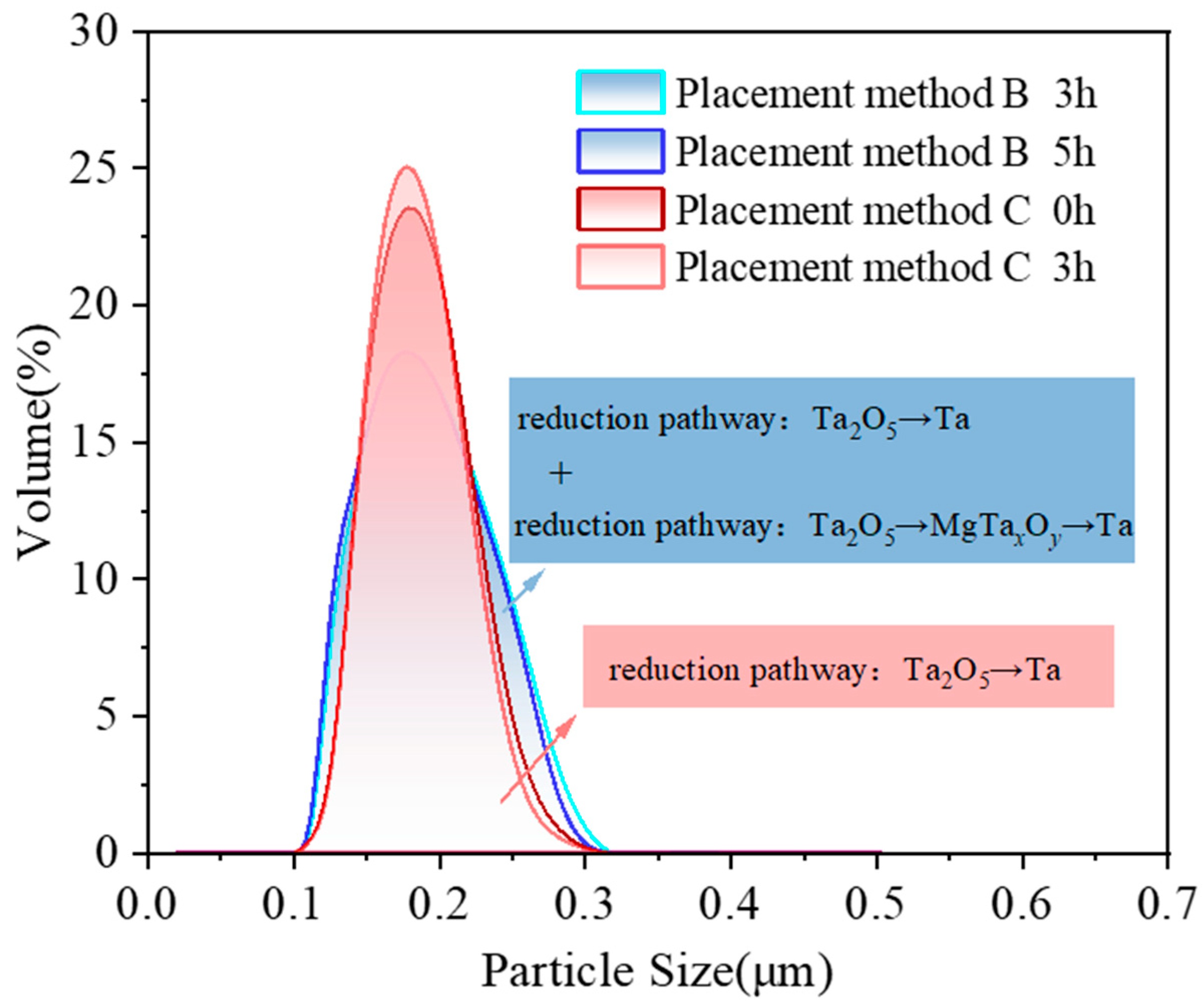

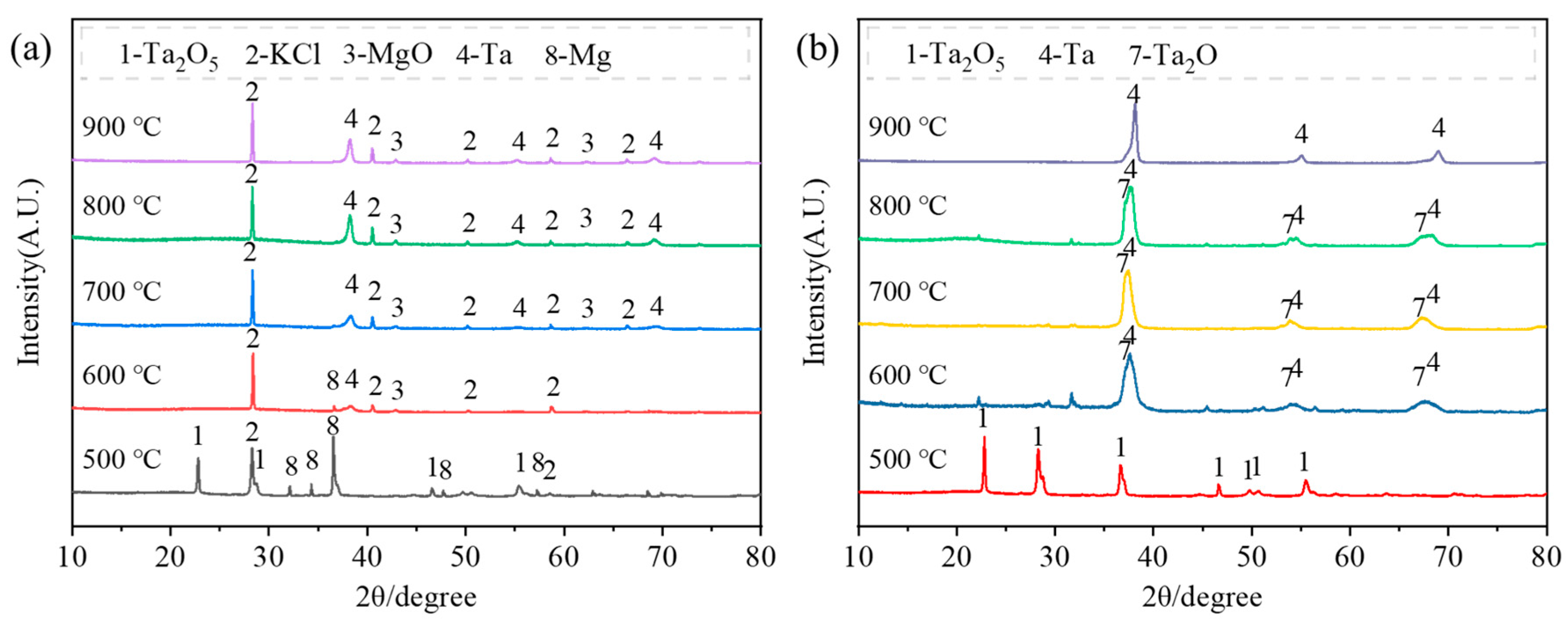
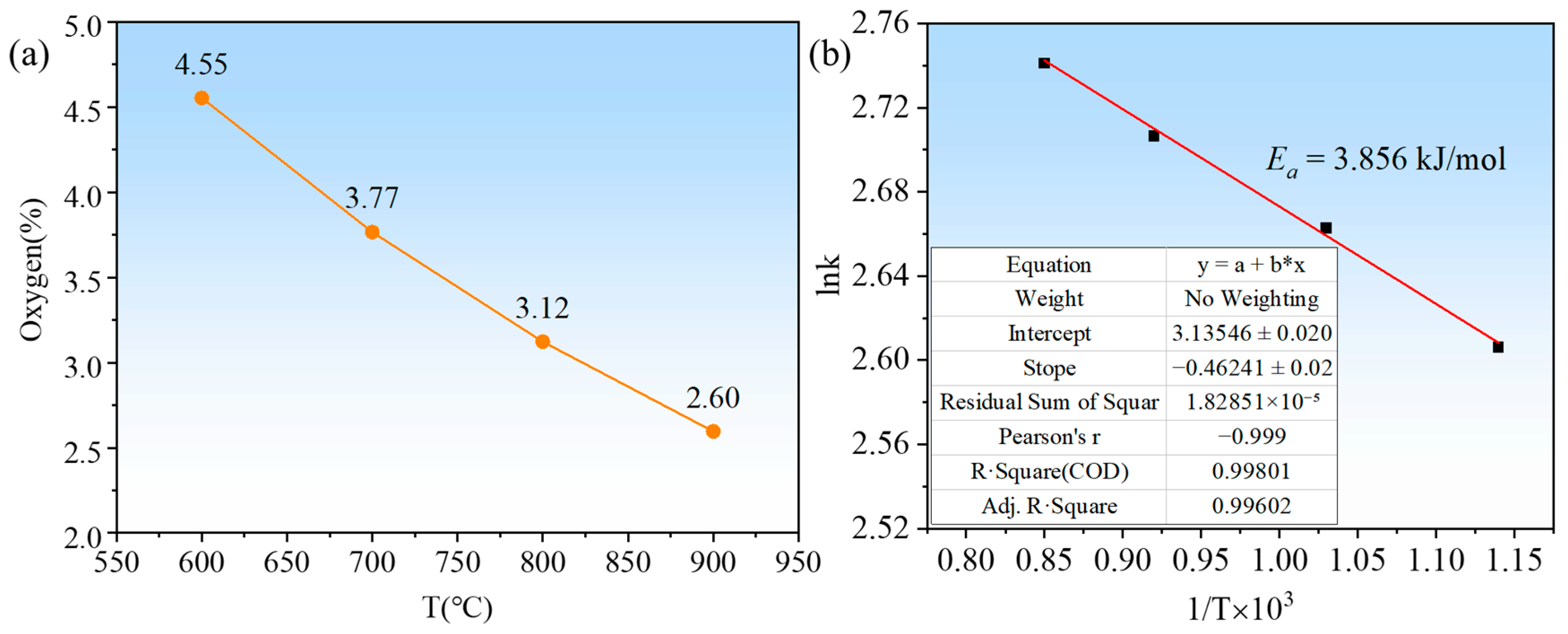

| Placement Methods | Time (h) | D10 (μm) | D50 (μm) | D90 (μm) | SPAN |
|---|---|---|---|---|---|
| B | 3 | 0.127 | 0.170 | 0.229 | 0.60 |
| B | 5 | 0.129 | 0.170 | 0.226 | 0.57 |
| C | 1 | 0.135 | 0.169 | 0.213 | 0.46 |
| C | 3 | 0.136 | 0.169 | 0.209 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Han, Z.; Li, T.; Wang, R.; Zhang, C.; Che, Y.; He, J. Mechanisms Underlying Phase Transition and Regulation of Tantalum Powder Properties During Magnesium Thermal Reduction of Ta2O5 in a Molten Salt Medium. Materials 2025, 18, 1115. https://doi.org/10.3390/ma18051115
Chen Y, Han Z, Li T, Wang R, Zhang C, Che Y, He J. Mechanisms Underlying Phase Transition and Regulation of Tantalum Powder Properties During Magnesium Thermal Reduction of Ta2O5 in a Molten Salt Medium. Materials. 2025; 18(5):1115. https://doi.org/10.3390/ma18051115
Chicago/Turabian StyleChen, Yi, Zhenghao Han, Tianchen Li, Ruifang Wang, Chao Zhang, Yusi Che, and Jilin He. 2025. "Mechanisms Underlying Phase Transition and Regulation of Tantalum Powder Properties During Magnesium Thermal Reduction of Ta2O5 in a Molten Salt Medium" Materials 18, no. 5: 1115. https://doi.org/10.3390/ma18051115
APA StyleChen, Y., Han, Z., Li, T., Wang, R., Zhang, C., Che, Y., & He, J. (2025). Mechanisms Underlying Phase Transition and Regulation of Tantalum Powder Properties During Magnesium Thermal Reduction of Ta2O5 in a Molten Salt Medium. Materials, 18(5), 1115. https://doi.org/10.3390/ma18051115





