Effect of Yb on Microstructure and Mechanical Properties of Al-Cu-Mn Heat-Resistant Aluminum Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results
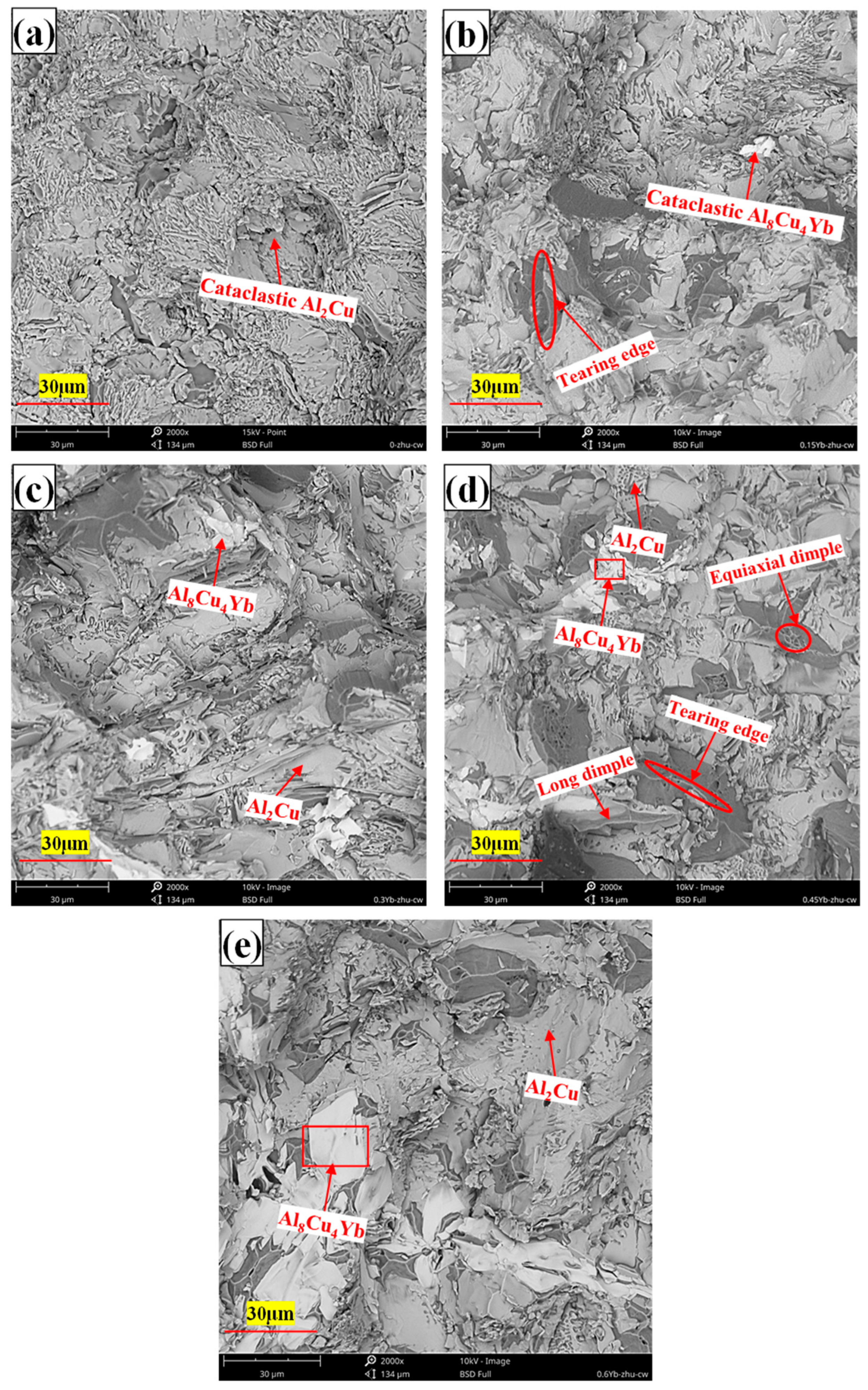
3.1. Microstructure of As-Cast Alloys
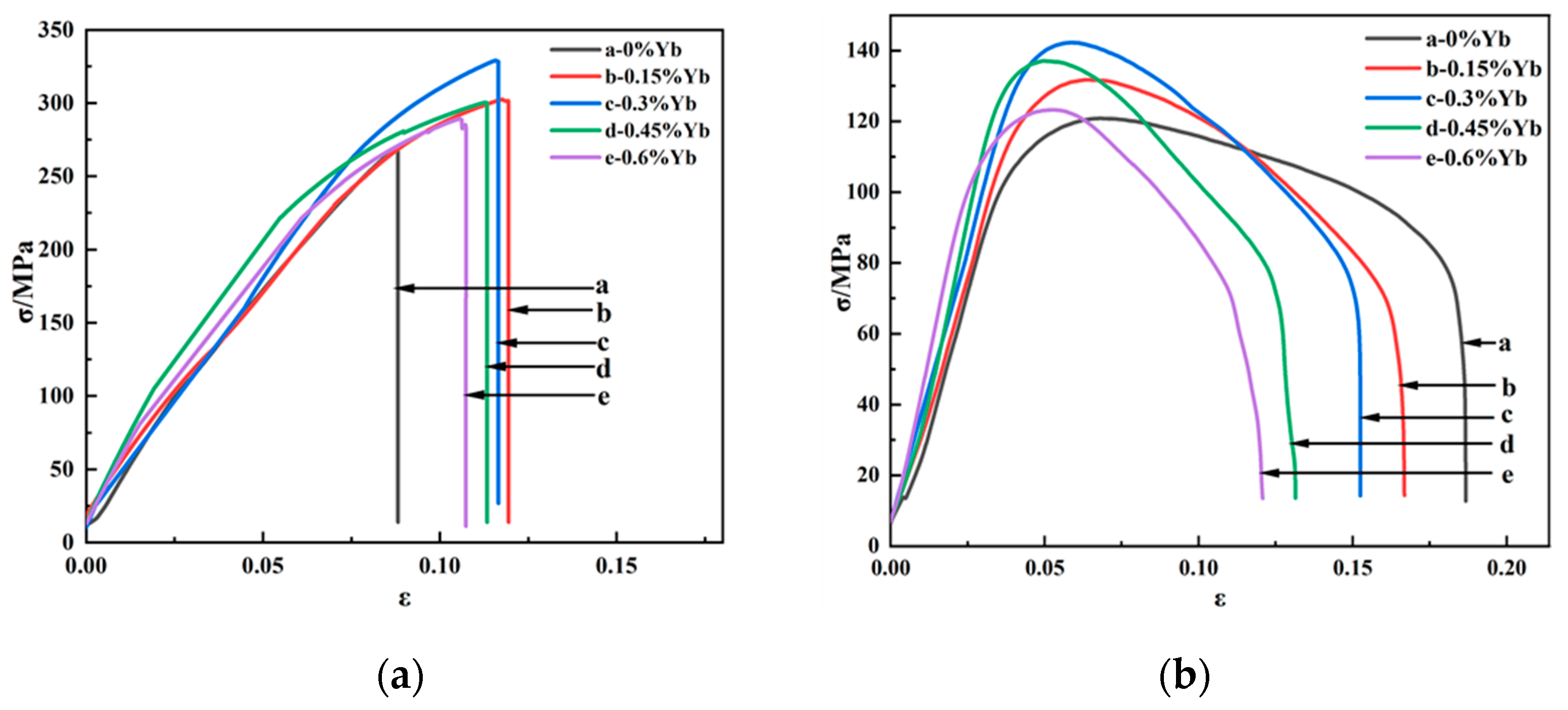
3.2. Mechanical Properties of the As-Cast Alloys
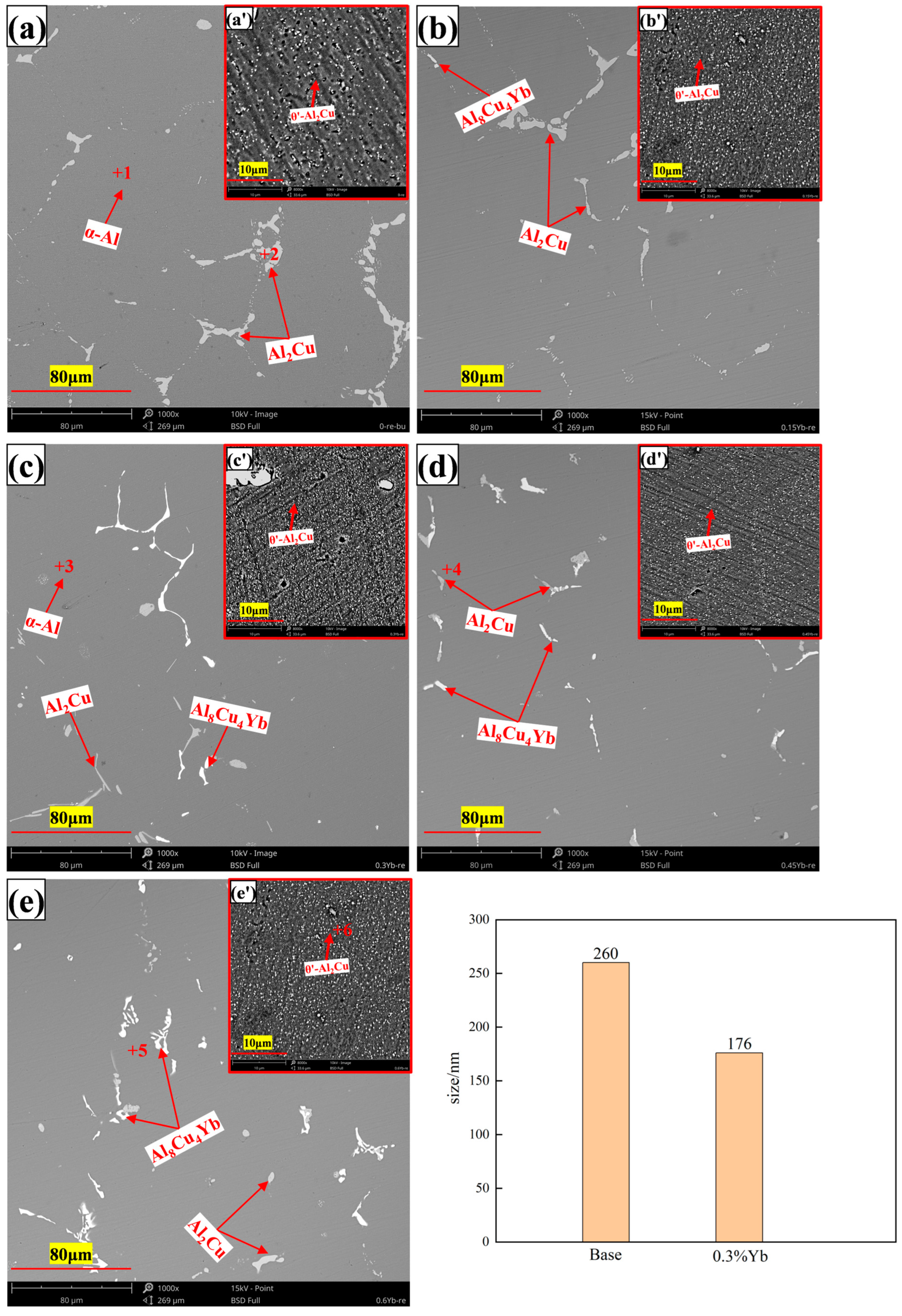
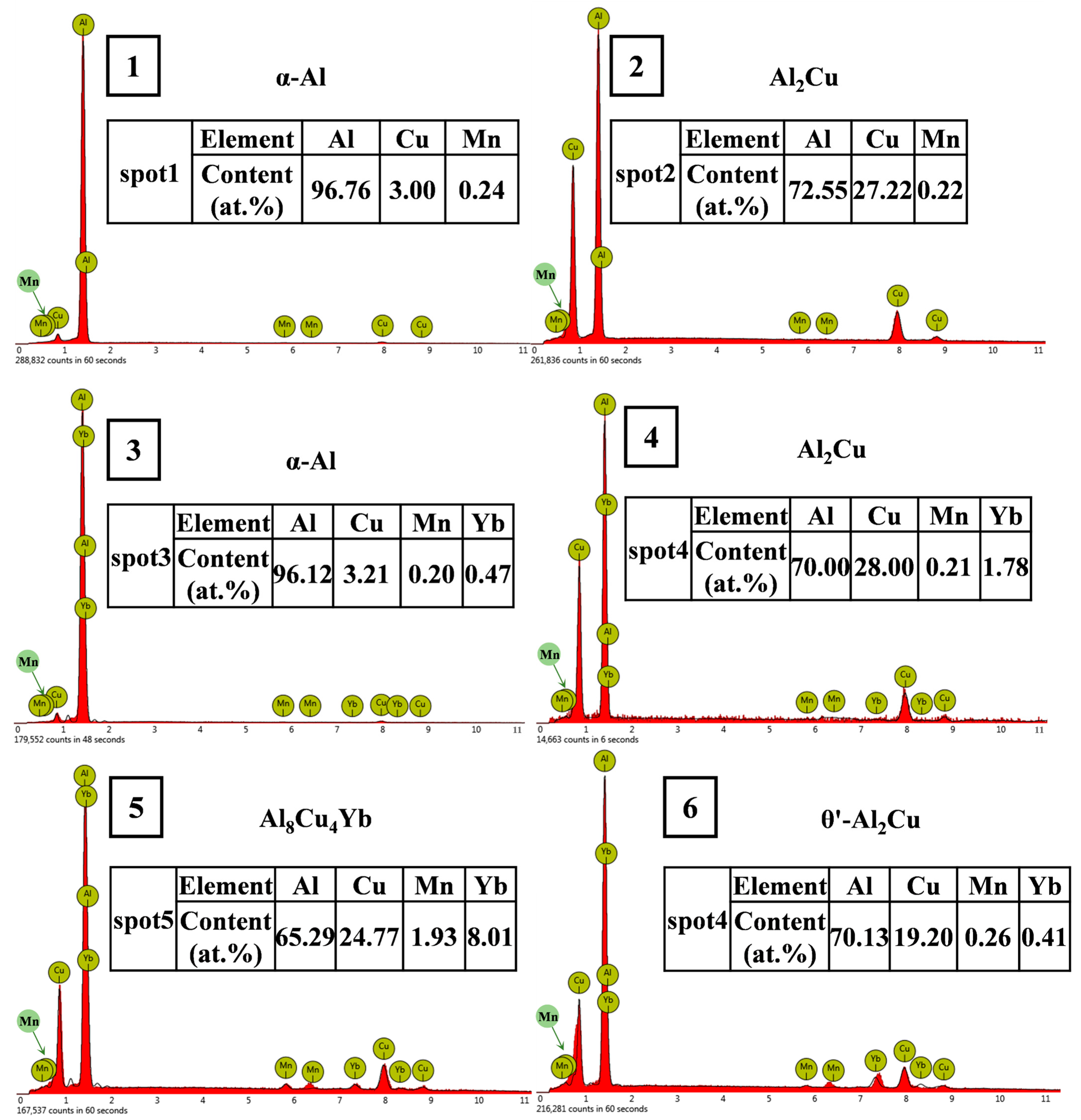
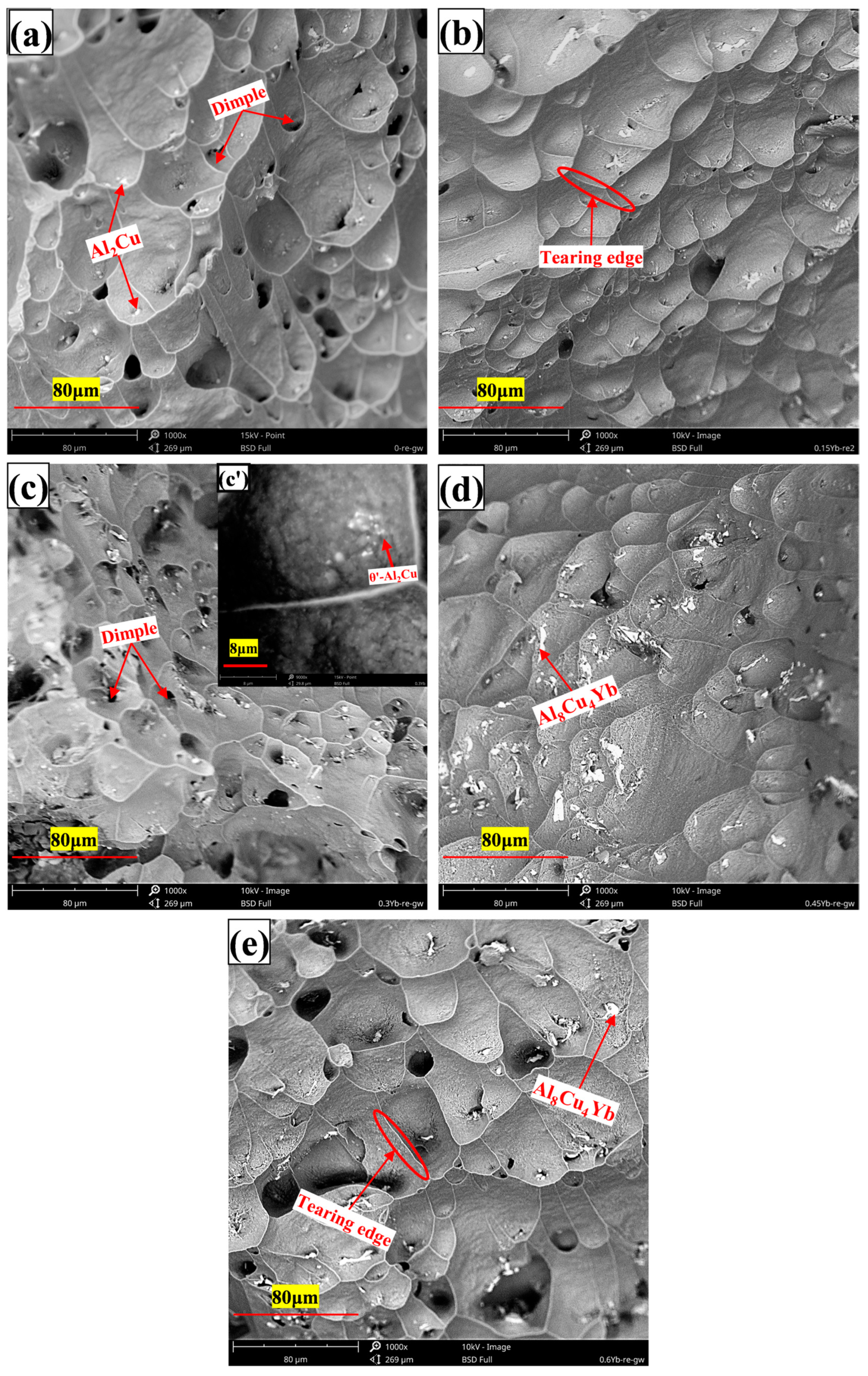
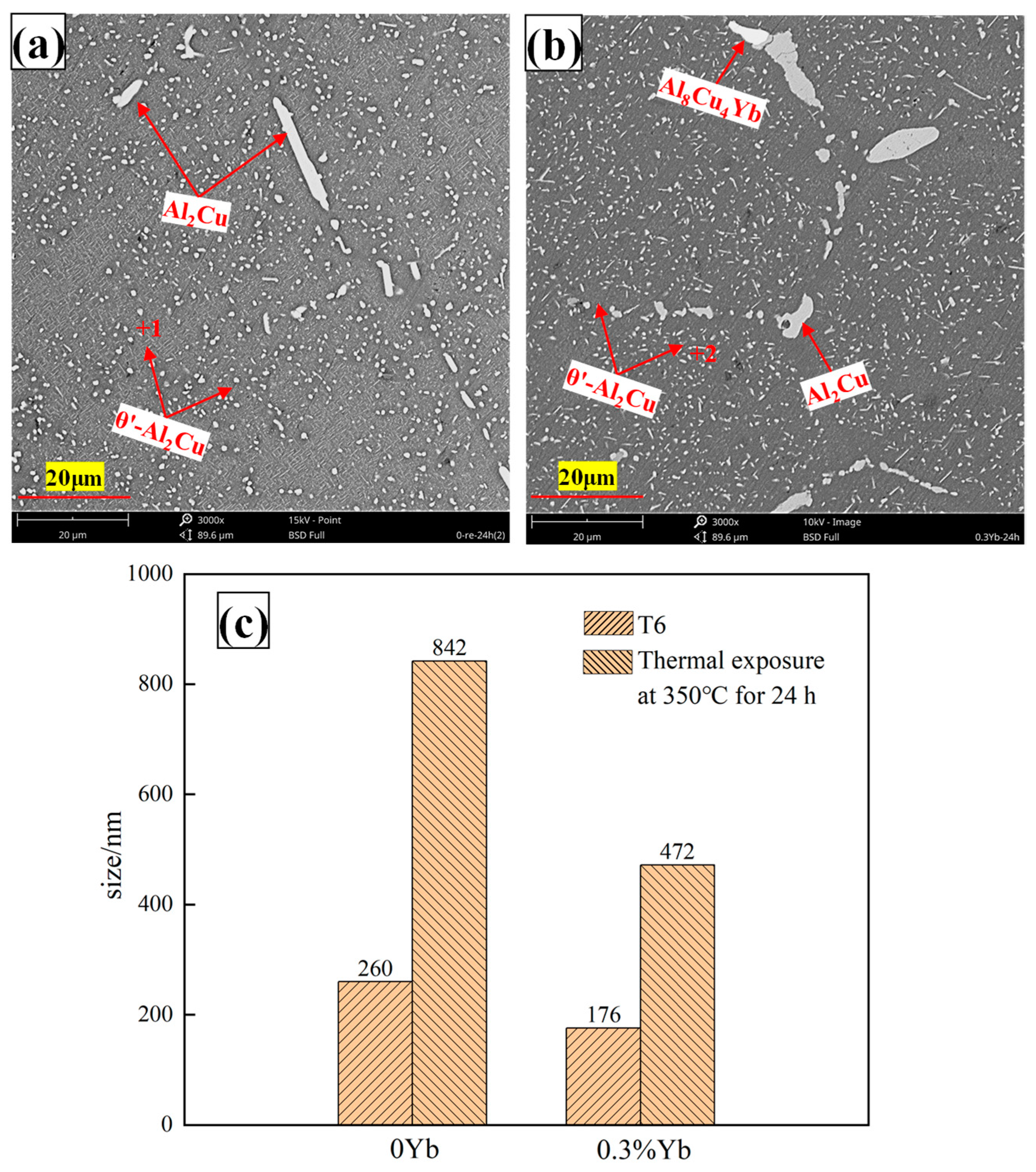
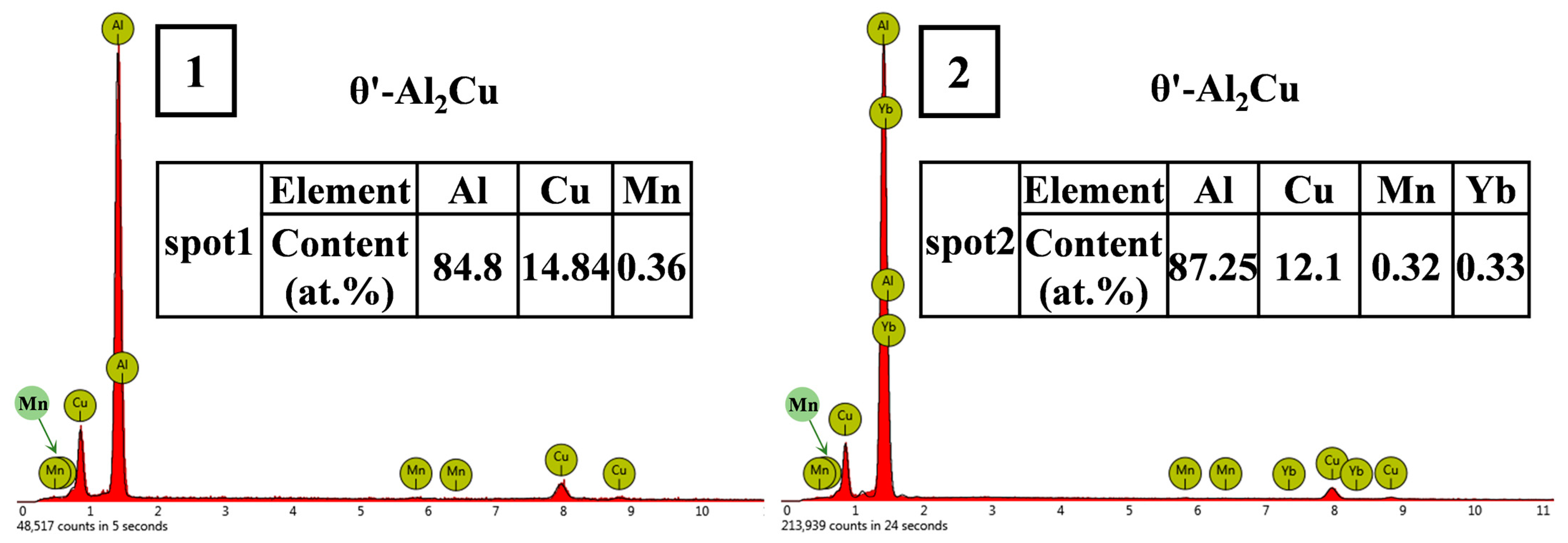
3.3. Microstructure of Al-6Cu-0.4Mn-xYb Alloy After T6 Heat Treatment
3.4. Tensile Properties of Al-6Cu-0.4Mn-xYb Alloy After T6 Heat Treatment
4. Conclusions
- (1)
- The as-cast Al-6Cu-0.4Mn-xYb alloy mainly consists of α-Al, θ-Al2Cu, and Al8Cu4Yb phases. Yb can refine the α-Al grain and α-Al + θ-Al2Cu eutectic structure in the as-cast alloy. The refining effect of α-Al grain and eutectic structure increased at first and then decreased with the increase of Yb addition, and the refining effect was the best when the Yb addition was 0.3 wt.%. When the addition of Yb reaches 0.45 wt.%, the grain refinement effect begins to weaken, and the coarse Al8Cu4Yb phase will be precipitated. After T6 heat treatment, there is still a small amount of undissolved θ-Al2Cu phase in the matrix. Adding Yb can promote the precipitation and refinement of the θ′-Al2Cu phase when the content of Yb is 0.3 wt.%, θ′-Al2Cu particles have the smallest size and the largest number, and the average particle size is about 176 nm. The size and quantity of the Al8Cu4Yb phase have hardly changed after solution aging treatment, indicating the Al8Cu4Yb phase has good thermal stability up to 540 °C.
- (2)
- The addition of the Yb element can improve the mechanical properties of as-cast and T6 heat-treated alloys at room temperature and 350 °C. The mechanical properties of as-cast and heat-treated alloys at room and high temperatures generally increased first and then decreased with Yb content. The ultimate tensile strength of the as-cast alloy containing 0.3 wt.%Yb is the highest at both room temperature and high temperature, which are 174.85 MPa and 95.46 MPa, respectively, which are 14.21% and 13.65% higher than that of the base alloy, respectively. The ultimate tensile strength of the heat-treated alloy containing 0.3 wt.%Yb also reached the highest at room temperature and high temperature, which were 328.98 MPa and 142.26 MPa, respectively, and increased by 21.63% and 17.71% compared with the base alloy, respectively.
- (3)
- The main reason for improving mechanical properties at room temperature and high temperature of the as-cast alloy is that adding Yb promotes the refining of α-Al grains. A small amount of Al8Cu4Yb phase can also play a role in the second phase strengthening, and excessive coarse Al8Cu4Yb has adverse mechanical properties. The main reason for improving the mechanical properties of heat-treated alloys at room and high temperatures is that Yb promotes the precipitation and refinement of the θ′-Al2Cu phase and enhances the dispersion strengthening effect. However, Yb with a low thermal diffusion coefficient can promote the nucleation and precipitation of the θ′-Al2Cu phase through the “congestion” effect during aging. Heat exposure experiments show that Yb can improve the coarsening resistance of the θ′-Al2Cu phase at 350 °C. It is suggested that in the presence of Mn, the addition of Yb may induce co-segregation at the Al/θ′-Al2Cu semi-coherent interface to form a new interface stacking mode, which is conducive to reducing the interface energy, thus inhibiting the coarsening of θ′-Al2Cu and improving the thermal stability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boyer, R.R.; Briggs, R.D. The use of β titanium alloys in the aerospace industry. J. Mater. Eng. Perform. 2005, 14, 681–685. [Google Scholar] [CrossRef]
- Shyam, A.; Bahl, S. Heat-resistant aluminium alloys. Nat. Mater. 2023, 22, 425–426. [Google Scholar] [CrossRef]
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. (1980–2015) 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Williams, J.C.; Starke, E.A. Progress in structural materials for aerospace systems. Acta Mater. 2003, 51, 5775–5799. [Google Scholar] [CrossRef]
- Gariboldi, E.; Casaro, F. Intermediate temperature creep behaviour of a forged Al-Cu-Mg-Si-Mn alloy. Mater. Sci. Eng. A 2007, 462, 384–388. [Google Scholar] [CrossRef]
- Michi, R.A.; Plotkowski, A.J.; Shyam, A.; Dehoff, R.R.; Babu, S.S. Towards high-temperature applications of aluminium alloys enabled by additive manufacturing. Int. Mater. Rev. 2021, 67, 298–345. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, E.; Miao, J.; Lin, S.; Yu, S. Arc additive remanufacturing of a new type of Al-Cu-Ni aviation case: Microstructure and high-temperature properties under T6 heat treatment. Sci. Technol. Weld. Join. 2023, 28, 506–513. [Google Scholar] [CrossRef]
- Hernandez-Sandoval, J.; Garza-Elizondo, G.; Samuel, A.; Valtiierra, S.; Samuel, F.H. The ambient and high temperature deformation behavior of Al-Si-Cu-Mg alloy with minor Ti, Zr, Ni additions. Mater. Des. 2014, 58, 89–101. [Google Scholar] [CrossRef]
- Zamani, M.; Morini, L.; Ceschini, L.; Seifeddine, S. The role of transition metal additions on the ambient and elevated temperature properties of Al-Si alloys. Mater. Sci. Eng. A 2017, 693, 42–50. [Google Scholar] [CrossRef]
- Choi, S.W.; Cho, H.S.; Kumai, S. Effect of the precipitation of secondary phases on the thermal diffusivity and thermal conductivity of Al-4.5Cu alloy. J. Alloys Compd. 2016, 688, 897–902. [Google Scholar] [CrossRef]
- Sepehrband, P.; Mahmudi, R.; Khomamizadeh, F. Effect of Zr addition on the aging behavior of A319 aluminum cast alloy. Scr. Mater. 2005, 52, 253–257. [Google Scholar] [CrossRef]
- Abdulwahab, M.; Madugu, I.A.; Yaro, S.A.; Hassan, S.; Popoola, A. Effects of multiple-step thermal ageing treatment on the hardness characteristics of A356.0-type Al-Si-Mg alloy. Mater. Des. 2011, 32, 1159–1166. [Google Scholar] [CrossRef]
- Dar, S.M.; Liao, H. Creep behavior of heat resistant Al-Cu-Mn alloys strengthened by fine (θ′) and coarse (Al20Cu2Mn3) second phase particles. Mater. Sci. Eng. A 2019, 763, 138062. [Google Scholar] [CrossRef]
- Shyam, A.; Roy, S.; Shin, D.; Poplawsky, J.; Allard, L.; Yamamoto, Y.; Morris, J.; Mazumder, B.; Idrobo, J.; Rodriguez, A.; et al. Elevated temperature microstructural stability in cast AlCuMnZr alloys through solute segregation. Mater. Sci. Eng. A 2019, 765, 138279. [Google Scholar] [CrossRef]
- Poplawsky, J.D.; Milligan, B.K.; Allard, L.F.; Shin, D.; Shower, P.; Chisholm, M.F.; Shyam, A. The synergistic role of Mn and Zr/Ti in producing θ′/L12 co-precipitates in Al-Cu alloys. Acta Mater. 2020, 194, 577–586. [Google Scholar] [CrossRef]
- Biswas, A.; Siegel, D.J.; Wolverton, C.; Seidman, D.N. Precipitates in Al-Cu alloys revisited: Atom-probe tomographic experiments and first-principles calculations of compositional evolution and interfacial segregation. Acta Mater. 2011, 59, 6187–6204. [Google Scholar] [CrossRef]
- Rosalie, J.M.; Bourgeois, L. Silver segregation to θ′ (Al2Cu)-Al interfaces in Al-Cu-Ag alloys. Acta Mater. 2012, 60, 6033–6041. [Google Scholar] [CrossRef]
- Bourgeois, L.; Dwyer, C.; Weyland, M.; Nie, J.F.; Muddle, B.C. The magic thicknesses of θ′ precipitates in Sn-microalloyed Al–Cu. Acta Mater. 2012, 60, 633–644. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, P.; Shao, D.; Wang, R.; Cao, L.; Zhang, J.; Liu, G.; Chen, B.; Sun, J. The influence of Sc solute partitioning on the microalloying effect and mechanical properties of Al-Cu alloys with minor Sc addition. Acta Mater. 2016, 119, 68–79. [Google Scholar] [CrossRef]
- Dorin, T.; Ramajayam, M.; Lamb, J.; Langan, T. Effect of Sc and Zr additions on the microstructure/strength of Al-Cu binary alloys. Mater. Sci. Eng. A 2017, 707, 58–64. [Google Scholar] [CrossRef]
- Marquis, E.A.; Seidman, D.N.; Asta, M.; Woodward, C.; Ozoliņš, V. Mg segregation at Al/Al3Sc heterophase interfaces on an atomic scale: Experiments and computations. Phys. Rev. Lett. 2003, 91, 036101. [Google Scholar] [CrossRef]
- Shin, D.; Shyam, A.; Lee, S.; Yamamoto, Y.; Haynes, J.A. Solute segregation at the Al/θ′-Al2Cu interface in Al-Cu alloys. Acta Mater. 2017, 141, 327–340. [Google Scholar] [CrossRef]
- Jiang, L.; Rouxel, B.; Langan, T.; Dorin, T. Coupled segregation mechanisms of Sc, Zr and Mn at θ′ interfaces enhances the strength and thermal stability of Al-Cu alloys. Acta Mater. 2021, 206, 116634. [Google Scholar] [CrossRef]
- Lamb, J.; Rouxel, B.; Langan, T.; Dorin, T. Novel Al-Cu-Mn-Zr-Sc Compositions Exhibiting Increased Mechanical Performance after a High-Temperature Thermal Exposure. J. Mater. Eng. Perform. 2020, 29, 5672–5684. [Google Scholar] [CrossRef]
- Fu, X.; Yang, H.; Wang, H.; Huang, C.; Chen, Y.; Huang, Q.; Chen, Y.; Huang, Q.; Li, A.; Pan, L. Effect of Mn/Ag Ratio on Microstructure and Mechanical Properties of Heat-Resistant Al-Cu Alloys. Materials 2024, 17, 1371. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.; Barkov, R.; Pozdniakov, A. Microstructure and Mechanical Properties of Novel Quasibinary Al-Cu-Yb and Al-Cu-Gd Alloys. Materials 2021, 11, 476. [Google Scholar] [CrossRef]
- Xiao, D.-H.; Huang, B.-Y. Effect of Yb addition on precipitation and microstructure of Al-Cu-Mg-Ag alloys. Trans. Nonferrous Met. Soc. China 2007, 17, 1181–1185. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Wang, W.-T.; Chen, M.-A.; Gao, Z.-G.; Jia, Y.-Z.; Ye, L.-Y.; Zheng, D.-W.; Liu, L.; Kuang, X.-Y. Effects of Yb addition on microstructures and mechanical properties of 2519A aluminum alloy plate. Trans. Nonferrous Met. Soc. China 2010, 20, 727–731. [Google Scholar] [CrossRef]
- Vaithyanathan, V.; Wolverton, C.; Chen, L.Q. Multiscale modeling of θ′ precipitation in Al-Cu binary alloys. Acta Mater. 2004, 52, 2973–2987. [Google Scholar] [CrossRef]
- Hume-Rothery, W.; Raynor, G.V., XI. Atomic and ionic radii.-II. Application to the theory of solid solubility in alloys. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1938, 26, 143–152. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, K.; Wen, S.; Huang, H.; Wang, W.; Zhu, Z.; Nie, Z.; Zhou, D. Determination of Er and Yb solvuses and trialuminide nucleation in Al-Er and Al-Yb alloys. J. Alloys Compd. 2014, 590, 526–534. [Google Scholar] [CrossRef]
- Martorano, M.A.; Biscuola, V.B. Predicting the columnar-to-equiaxed transition for a distribution of nucleation undercoolings. Acta Mater. 2009, 57, 607–615. [Google Scholar] [CrossRef]
- Amer, S.M.; Mamzurina, O.I.; Loginova, I.S.; Glavatskikh, M.V.; Barkov, R.Y.; Pozdniakov, A.V. Effect of Mn Addition on the Phase Composition and Strengthening Behavior of AlCuYbZr and AlCuGdZr Alloys. JOM 2022, 74, 3646–3654. [Google Scholar] [CrossRef]
- Van Dalen, M.E.; Karnesky, R.A.; Cabotaje, J.R.; Dunand, D.C.; Seidman, D.N. Erbium and ytterbium solubilities and diffusivities in aluminum as determined by nanoscale characterization of precipitates. Acta Mater. 2009, 57, 4081–4089. [Google Scholar] [CrossRef]
- Sanaty-Zadeh, A. Comparison between current models for the strength of particulate-reinforced metal matrix nanocomposites with emphasis on consideration of Hall-Petch effect. Mater. Sci. Eng. A 2012, 531, 112–118. [Google Scholar] [CrossRef]
- Chokshi, A.H.; Rosen, A.; Karch, J.; Gleiter, H. On the validity of the hall-petch relationship in nanocrystalline materials. Scr. Metall. 1989, 23, 1679–1683. [Google Scholar] [CrossRef]
- Czerwinski, F. Thermal Stability of Aluminum Alloys. Materials 2020, 13, 3441. [Google Scholar] [CrossRef]
- Zou, L.; Yang, C.; Lei, Y.; Zakharov, D.; Wiezorek, J.M.; Su, D.; Yin, Q.; Li, J.; Liu, Z.; Zhou, G.; et al. Dislocation nucleation facilitated by atomic segregation. Nat. Mater. 2018, 17, 56–63. [Google Scholar] [CrossRef]
- Cui, J.G.; Chen, Y.B.; Fu, X.Z.; Yang, H.L.; Wang, H.Z.; Li, A.M.; Pan, L.W. Effect of V on high-temperature mechanical properties of Al-5Cu-1.5Ni alloy. J. Iron Steel Res. Int. 2024, 31, 1792–1810. [Google Scholar] [CrossRef]
- Zan, Y.N.; Zhang, Q.; Zhou, Y.T.; Liu, Z.Y.; Wang, Q.Z.; Wang, D.; Xiao, B.L.; Ma, Z.Y. Introducing graphene (reduced graphene oxide) into Al matrix composites for enhanced high-temperature strength. Compos. Part B Eng. 2020, 195, 108095. [Google Scholar] [CrossRef]
- Hu, K.; Xu, Q.; Ma, X.; Sun, Q.; Gao, T.; Liu, X. A novel heat-resistant Al–Si–Cu–Ni–Mg base material synergistically strengthened by Ni-rich intermetallics and nano-AlNp microskeletons. J. Mater. Sci. Technol. 2019, 35, 306–312. [Google Scholar] [CrossRef]
- Dai, H.; Wang, L.; Dong, B.; Miao, J.; Lin, S.; Chen, H. Microstructure and high-temperature mechanical properties of new-type heat-resisting aluminum alloy Al6.5Cu2Ni0.5Zr0.3Ti0.25V under the T7 condition. Mater. Lett. 2023, 332, 133503. [Google Scholar] [CrossRef]
- Chankitmunkong, S.; Eskin, D.G.; Patakham, U.; Limmaneevichitr, C. Microstructure and elevated temperature mechanical properties of a direct-chill cast AA4032 alloy with copper and erbium additions. J. Alloys Compd. 2019, 782, 865–874. [Google Scholar] [CrossRef]
- Sun, T.-T.; Geng, J.-W.; Bian, Z.-Y.; Wu, Y.; Wang, M.-L.; Chen, D.; Ma, N.-H.; Wang, H.-W. Enhanced thermal stability and mechanical properties of high-temperature resistant Al−Cu alloy with Zr and Mn micro-alloying. Trans. Nonferrous Met. Soc. China 2022, 32, 64–78. [Google Scholar] [CrossRef]
- Shaha, S.K.; Czerwinski, F.; Kasprzak, W.; Friedman, J.; Chen, D.L. Ageing characteristics and high-temperature tensile properties of Al-Si-Cu-Mg alloys with micro-additions of Cr, Ti, V and Zr. Mater. Sci. Eng. A 2016, 652, 353–364. [Google Scholar] [CrossRef]
- Xue, H.; Yang, C.; De Geuser, F.; Zhang, P.; Zhang, J.; Chen, B.; Liu, F.; Peng, Y.; Bian, J.; Liu, G.; et al. Highly stable coherent nanoprecipitates via diffusion-dominated solute uptake and interstitial ordering. Nat. Mater. 2023, 22, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Rahimian, M.; Amirkhanlou, S.; Blake, P.; Ji, S. Nanoscale Zr-containing precipitates; a solution for significant improvement of high-temperature strength in Al-Si-Cu-Mg alloys. Mater. Sci. Eng. A 2018, 721, 328–338. [Google Scholar] [CrossRef]
- Colombo, M.; Gariboldi, E.; Morri, A. Er addition to Al-Si-Mg-based casting alloy: Effects on microstructure, room and high temperature mechanical properties. J. Alloys Compd. 2017, 708, 1234–1244. [Google Scholar] [CrossRef]
- Shin, D.; Poplawsky, J.D.; Chisholm, M.F.; Allard, L.F.; Haynes, J.A.; Shyam, A. The many faces of θ’-Al2Cu precipitates: Energetics of pristine and solute segregated Al/θ’ semi-coherent interfaces. Acta Mater. 2024, 281, 120444. [Google Scholar] [CrossRef]
- Ma, K.; Wen, H.; Hu, T.; Topping, T.D.; Isheim, D.; Seidman, D.N.; Lavernia, E.J.; Schoenung, J.M. Mechanical behavior and strengthening mechanisms in ultrafine grain precipitation-strengthened aluminum alloy. Acta Mater. 2014, 62, 141–155. [Google Scholar] [CrossRef]
- Nembach, E. Particle Strengthening of Metals and Alloys; Wiley: Hoboken, NJ, USA, 1997. [Google Scholar]
- Fuller, C.B.; Seidman, D.N. Temporal evolution of the nanostructure of Al(Sc,Zr) alloys: Part II-coarsening of Al3(Sc1-xZrx) precipitates. Acta Mater. 2005, 53, 5415–5428. [Google Scholar] [CrossRef]
- Knipling, K.E.; Dunand, D.C.; Seidman, D.N. Criteria for developing castable, creep-resistant aluminum-based alloys—A review. Int. J. Mater. Res. 2006, 97, 246–265. [Google Scholar] [CrossRef]
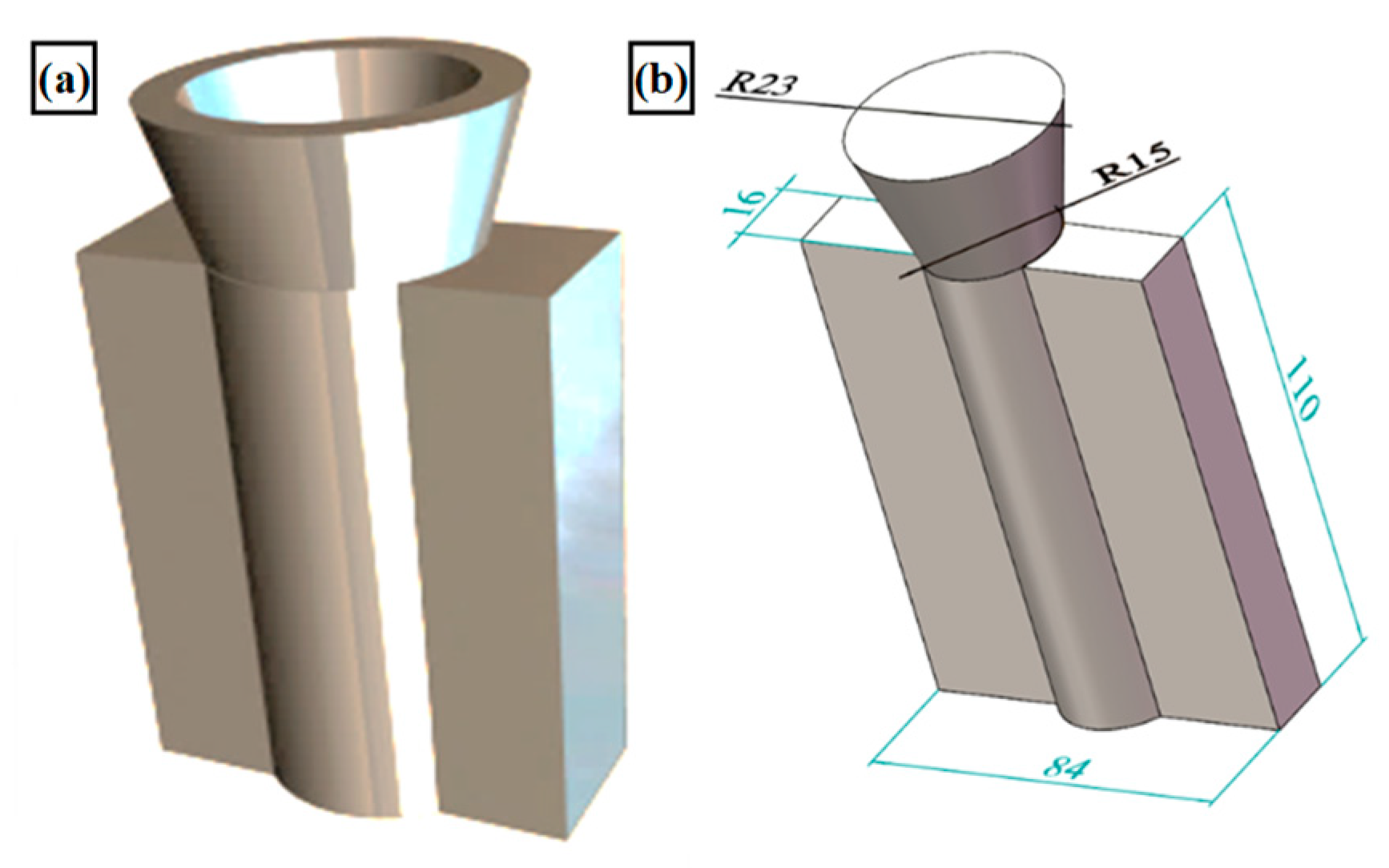
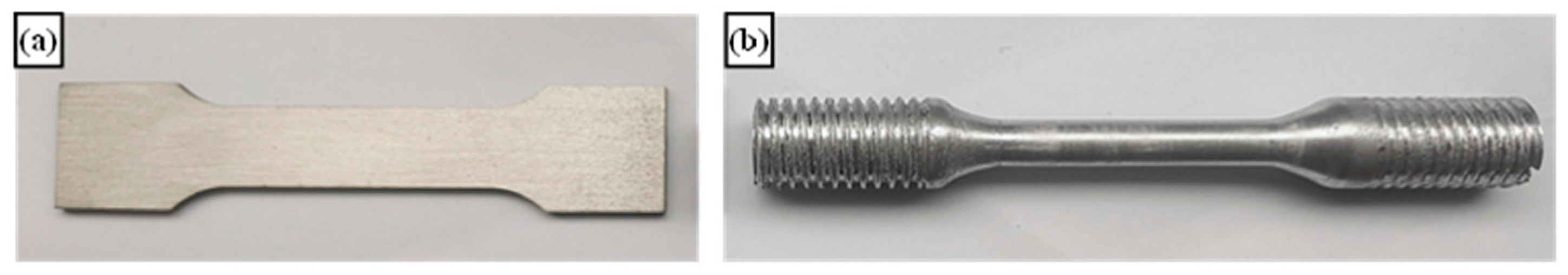

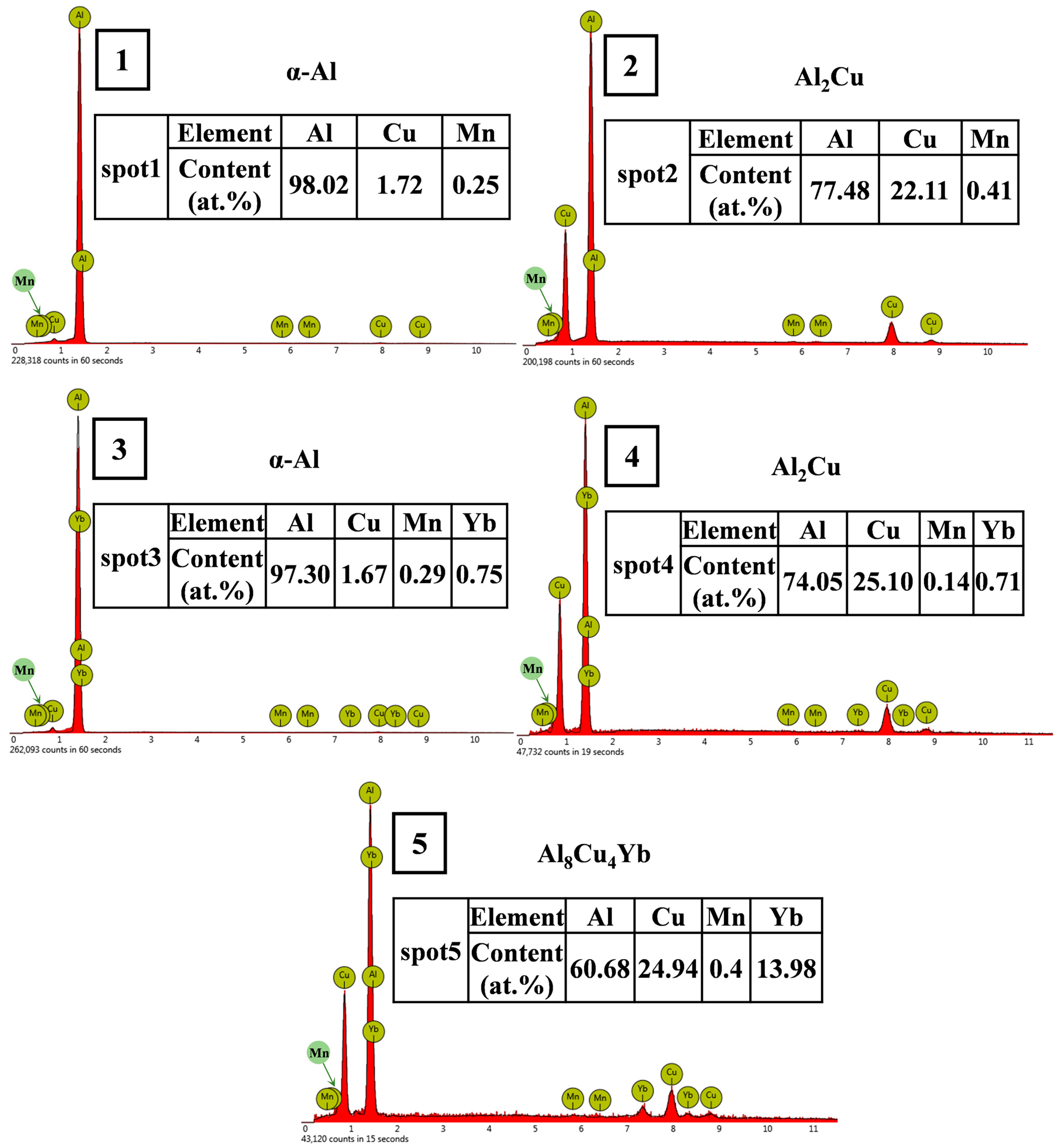

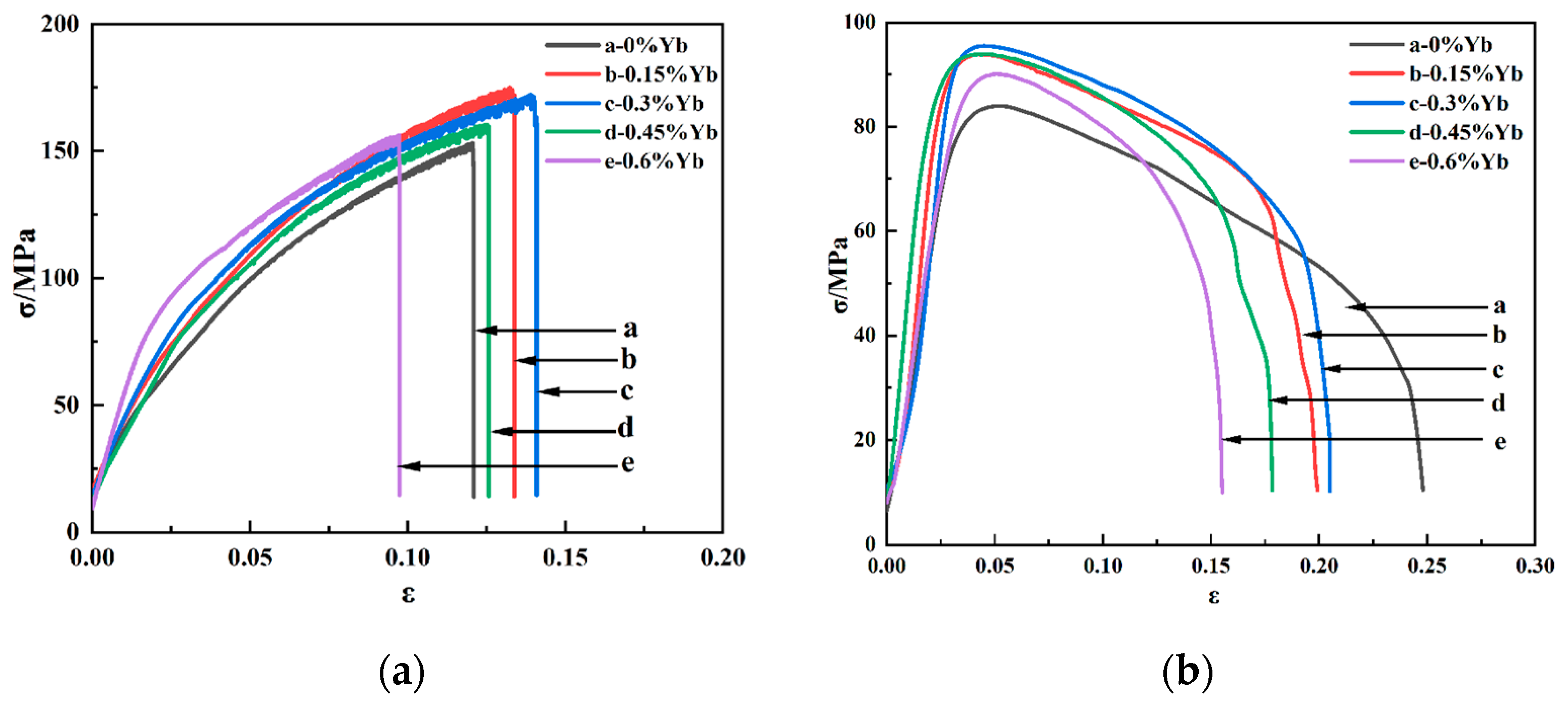



| Alloys Number | Al | Cu | Mn | Yb |
|---|---|---|---|---|
| 1 | Bal. | 6 | 0.4 | 0.00 |
| 2 | Bal. | 6 | 0.4 | 0.15 |
| 3 | Bal. | 6 | 0.4 | 0.30 |
| 4 | Bal. | 6 | 0.4 | 0.45 |
| 5 | Bal. | 6 | 0.4 | 0.60 |
| Yb/wt.% | UTS/MPa | YS/MPa | FS/% | El/% |
|---|---|---|---|---|
| 0 | 153.09 ± 7.0 | 96.19 ± 5.6 | 12.10 ± 2.1 | 4.69 ± 1.4 |
| 0.15 | 173.15 ± 9.3 | 104.97 ± 7.6 | 13.38 ± 3.4 | 5.78 ± 2.5 |
| 0.3 | 174.85 ± 10.5 | 113.84 ± 9.8 | 14.09 ± 4.0 | 6.35 ± 2.3 |
| 0.45 | 160.44 ± 10.1 | 103.70 ± 8.4 | 12.57 ± 2.6 | 4.88 ± 1.9 |
| 0.6 | 156.11 ± 8.4 | 93.76 ± 7.1 | 9.73 ± 1.9 | 3.18 ± 1.2 |
| Yb/wt.% | UTS/MPa | YS/MPa | FS/% | El/% |
|---|---|---|---|---|
| 0 | 83.99 ± 4.5 | 71.54 ± 5.5 | 24.81 ± 4.2 | 20.22 ± 3.0 |
| 0.15 | 93.86 ± 6.9 | 82.85 ± 8.3 | 19.92 ± 3.8 | 16.91 ± 2.1 |
| 0.3 | 95.46 ± 8.4 | 85.21 ± 7.8 | 20.49 ± 3.8 | 17.52 ± 3.1 |
| 0.45 | 93.93 ± 7.9 | 82.99 ± 8.0 | 17.83 ± 2.9 | 15.24 ± 2.5 |
| 0.6 | 90.06 ± 5.1 | 78.65 ± 7.4 | 15.54 ± 2.5 | 11.21 ± 2.2 |
| Yb/wt.% | UTS/MPa | YS/MPa | FS/% | El/% |
|---|---|---|---|---|
| 0 | 270.48 ± 8.5 | 203.26 ± 8.4 | 8.82 ± 1.6 | 3.42 ± 1.2 |
| 0.15 | 302.29 ± 10.3 | 248.12 ± 9.5 | 11.94 ± 2.4 | 5.52 ± 2.3 |
| 0.3 | 328.98 ± 11.4 | 272.86 ± 10.9 | 11.65 ± 2.7 | 5.36 ± 2.4 |
| 0.45 | 300.18 ± 10.2 | 239.12 ± 11.4 | 11.34 ± 2.1 | 4.84 ± 2.2 |
| 0.6 | 289.06 ± 9.4 | 218.51 ± 10.3 | 10.74 ± 1.9 | 3.99 ± 1.8 |
| Yb/wt.% | UTS/MPa | YS/MPa | FS/% | El/% |
|---|---|---|---|---|
| 0 | 120.86 ± 5.7 | 101.61 ± 6.1 | 18.67 ± 4.3 | 16.22 ± 3.0 |
| 0.15 | 131.76 ± 6.4 | 114.74 ± 7.4 | 16.67 ± 4.1 | 14.82 ± 3.2 |
| 0.3 | 142.26 ± 7.5 | 124.54 ± 8.6 | 15.25 ± 3.4 | 13.94 ± 2.8 |
| 0.45 | 137.07 ± 7.4 | 120.12 ± 9.1 | 13.14 ± 2.2 | 11.14 ± 2.4 |
| 0.6 | 123.26 ± 5.6 | 102.68 ± 5.0 | 12.08 ± 1.8 | 10.23 ± 1.9 |
| Materials (wt.%) | Temperature (°C) | UTS (MPa) | Year | Ref. |
|---|---|---|---|---|
| Al-6Cu-0.4Mn-0.3Yb | 350 | 142.3 | 2025 | Present work |
| Al-5Cu-1.5Ni-0.3V | 350 | 111.8 | 2024 | [39] |
| Al-6Cu-0.4Mn-0.4Ag | 350 | 135.9 | 2024 | [25] |
| rGO/Al | 350 | 128.0 | 2020 | [40] |
| Al-12Si-4Cu-2Ni-1Mg-AlNp | 350 | 106.0 | 2019 | [41] |
| Al-6.5Cu-2Ni-0.5Zr-0.3Ti-0.25V | 350 | 127.5 | 2023 | [42] |
| Al-12.95Si-3.57Cu-0.72Mg-0.91Ni-0.53Fe-0.4Er | 350 | 117.0 | 2019 | [43] |
| Al-5Cu-0.2Mn-0.2Zr | 300 | 124.9 | 2022 | [44] |
| Al-7Si-Cu-0.5Mg-0.5Cr-0.4Ti-0.4V-0.25Zr | 300 | 197.4 | 2016 | [45] |
| Al-Cu-Mg-Ag-Sc | 400 | >100 | 2023 | [46] |
| Al-7Si-2Cu-0.2Zr | 200 | 246.0 | 2018 | [47] |
| Al-7.38Si-0.36Mg-0.14Ti-0.22Er | 200 | 220.6 | 2017 | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Yang, H.; Xiong, Y.; Jia, N.; Nong, J.; Pan, L. Effect of Yb on Microstructure and Mechanical Properties of Al-Cu-Mn Heat-Resistant Aluminum Alloys. Materials 2025, 18, 958. https://doi.org/10.3390/ma18050958
Huang C, Yang H, Xiong Y, Jia N, Nong J, Pan L. Effect of Yb on Microstructure and Mechanical Properties of Al-Cu-Mn Heat-Resistant Aluminum Alloys. Materials. 2025; 18(5):958. https://doi.org/10.3390/ma18050958
Chicago/Turabian StyleHuang, Chifu, Hailong Yang, Yu Xiong, Nannan Jia, Junhong Nong, and Liwen Pan. 2025. "Effect of Yb on Microstructure and Mechanical Properties of Al-Cu-Mn Heat-Resistant Aluminum Alloys" Materials 18, no. 5: 958. https://doi.org/10.3390/ma18050958
APA StyleHuang, C., Yang, H., Xiong, Y., Jia, N., Nong, J., & Pan, L. (2025). Effect of Yb on Microstructure and Mechanical Properties of Al-Cu-Mn Heat-Resistant Aluminum Alloys. Materials, 18(5), 958. https://doi.org/10.3390/ma18050958




