Review of Layered Double Hydroxide (LDH) Nanosheets in Corrosion Mitigation: Recent Developments, Challenges, and Prospects
Abstract
1. Introduction
2. Physicochemical Characteristics
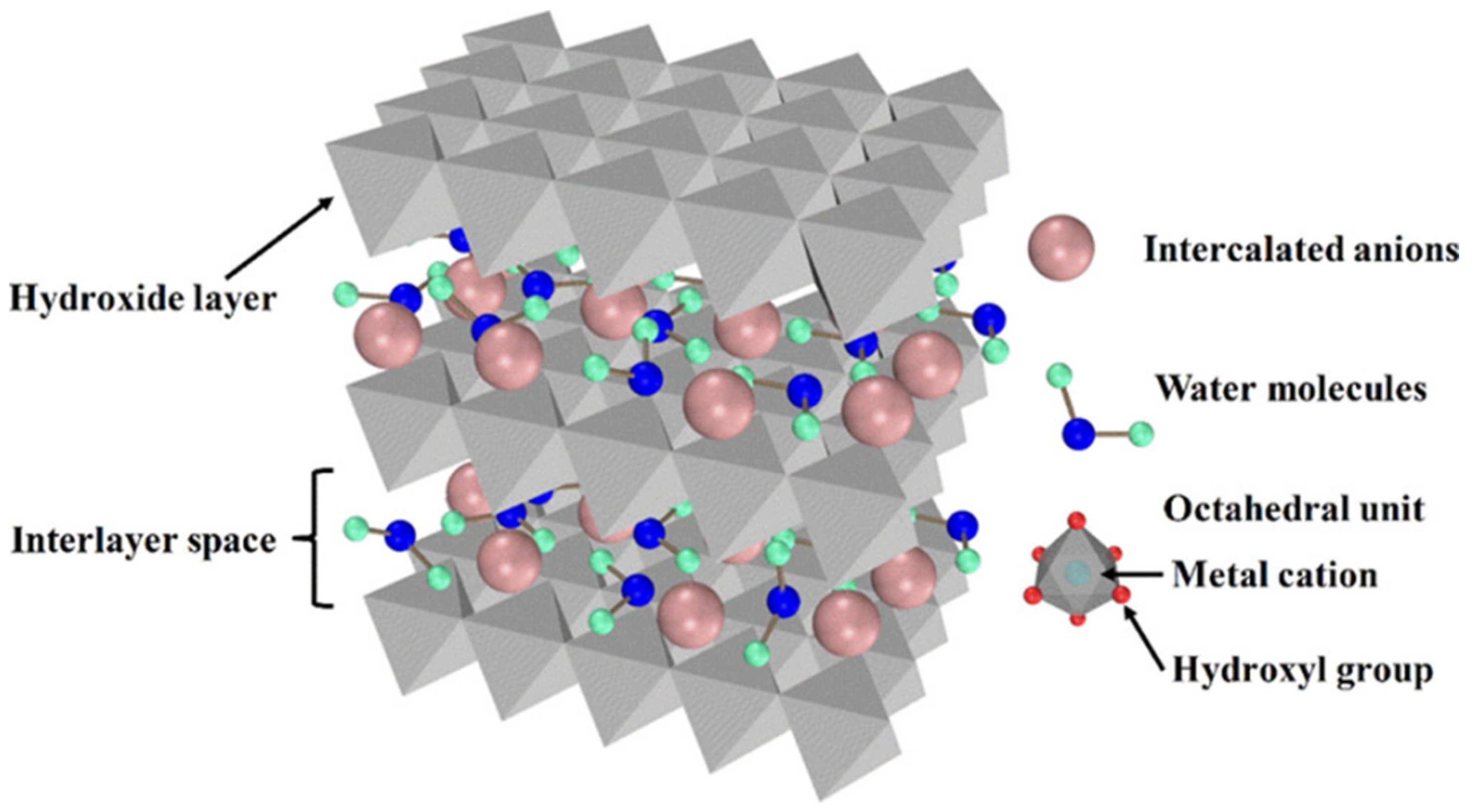
2.1. Structure and Properties
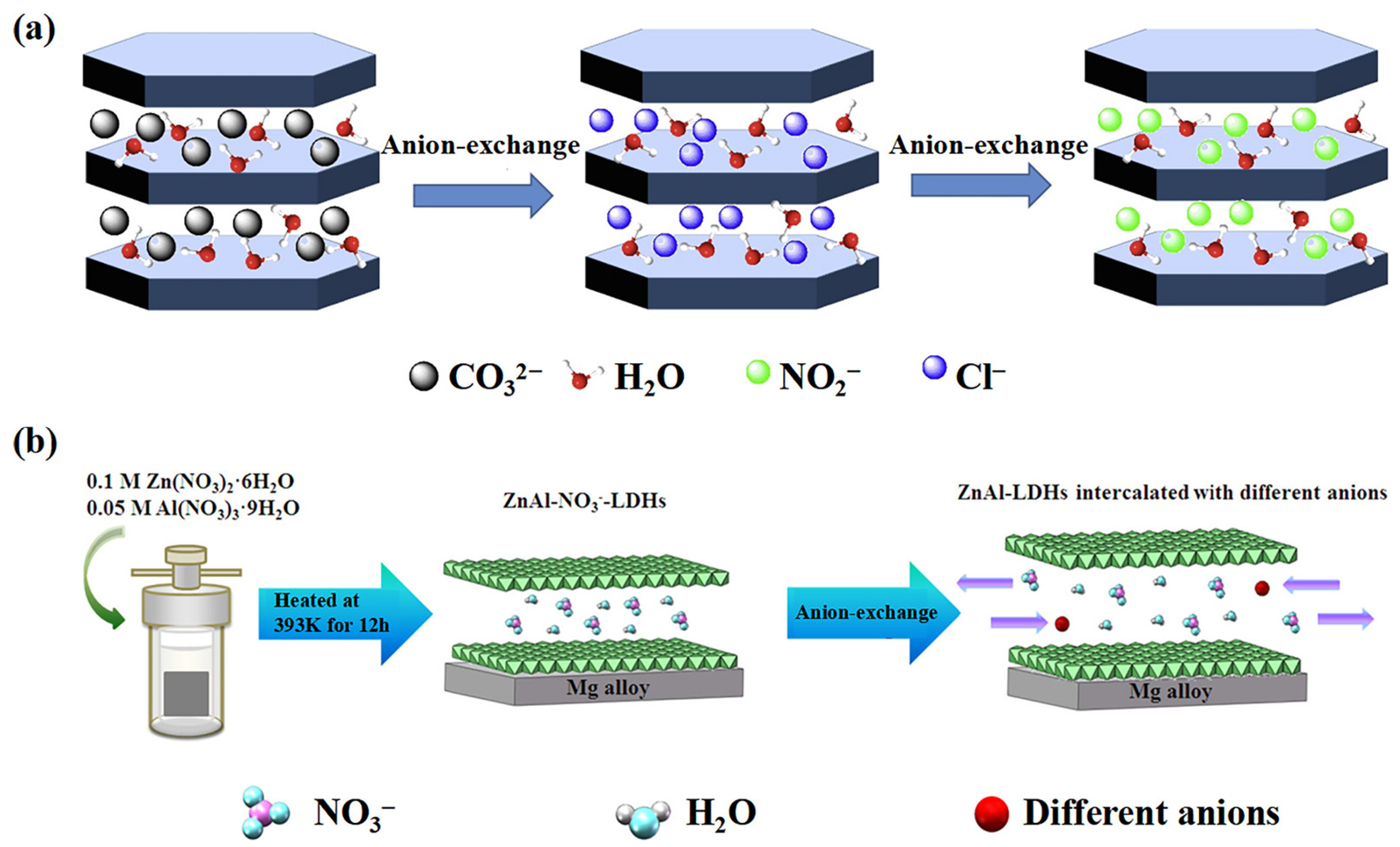
2.2. Preparation
3. Anti-Corrosion Mechanisms
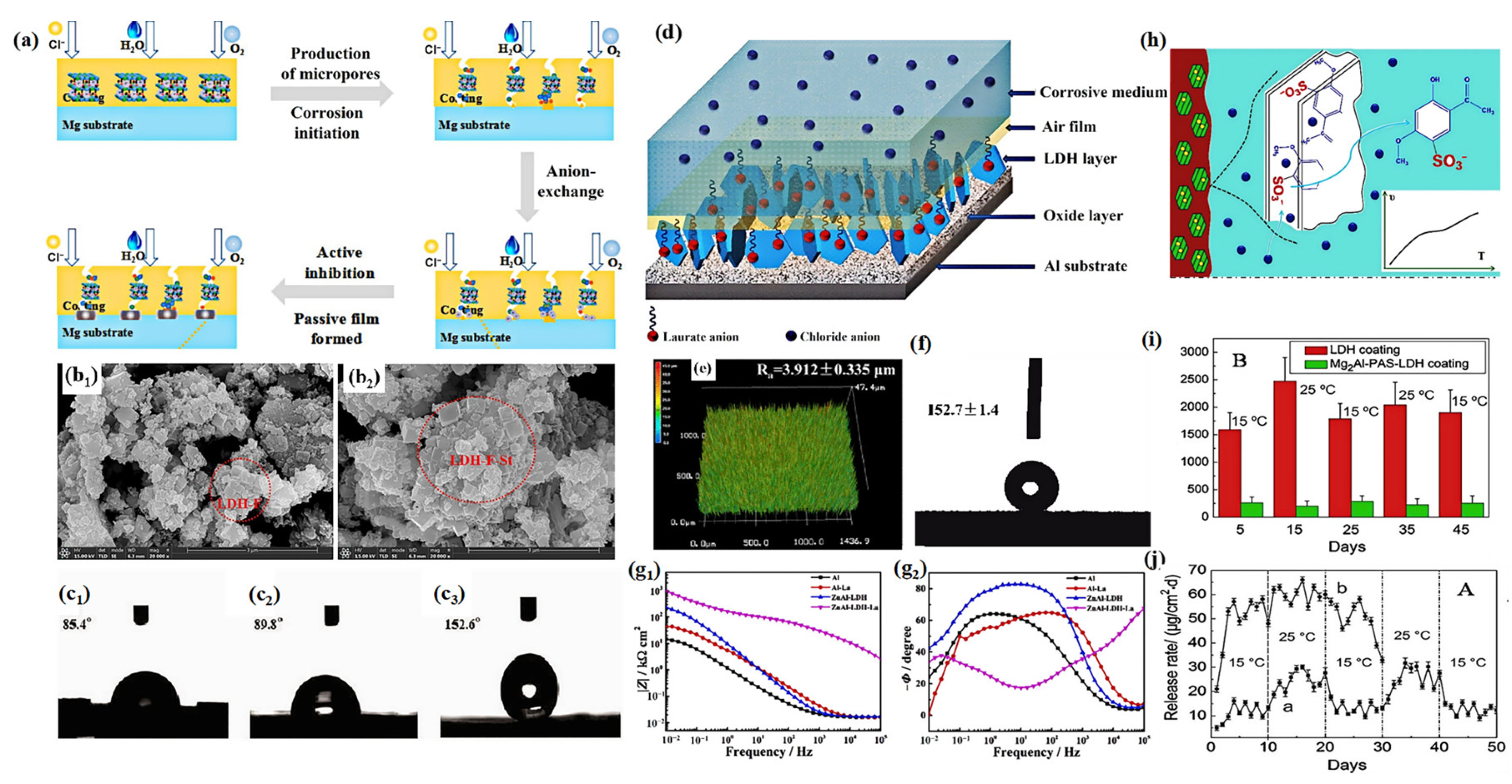
3.1. Physical Barrier
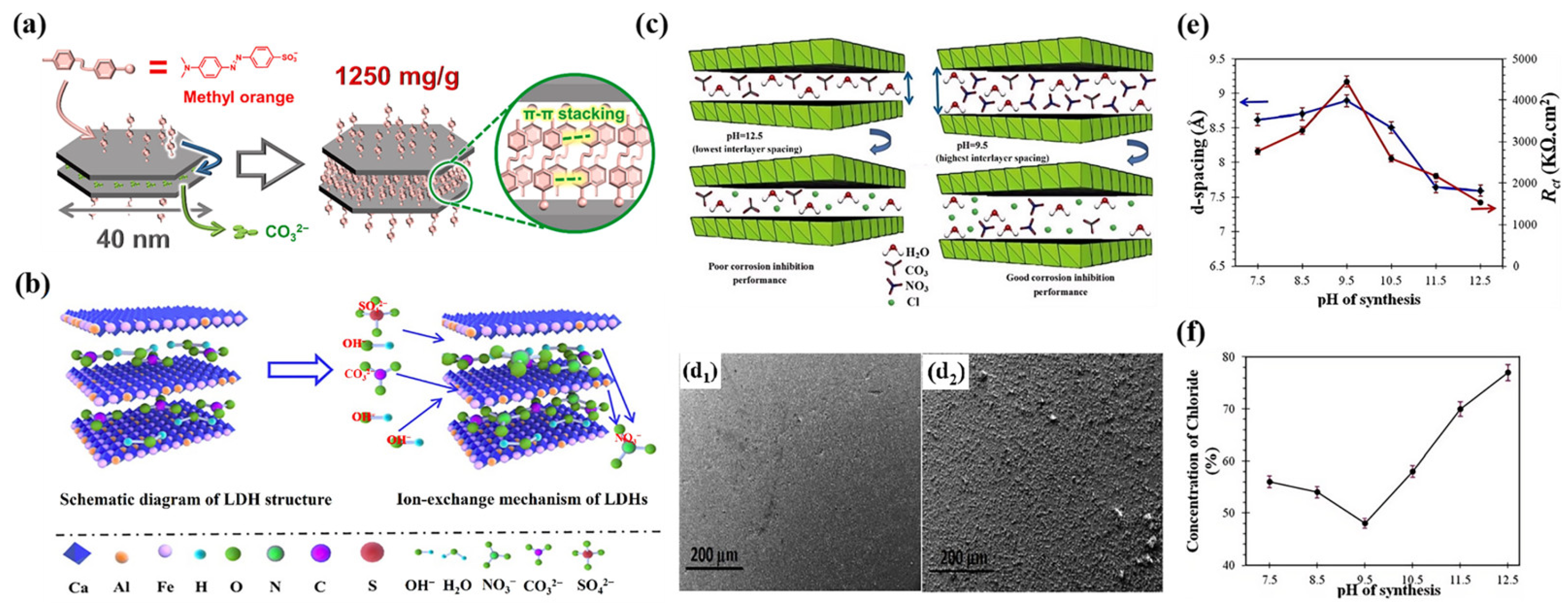
3.2. Chloride Trapping Effect

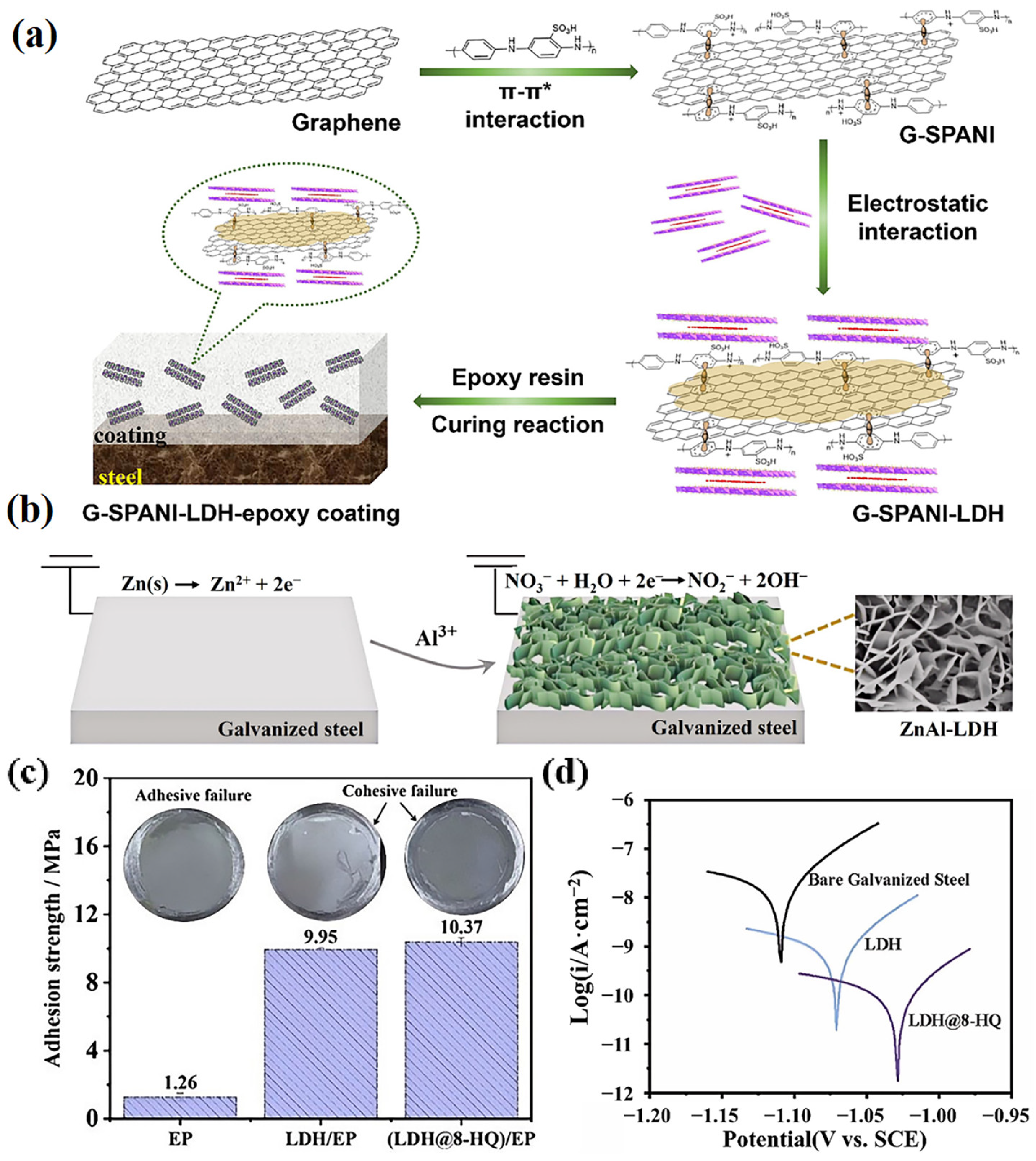
3.3. Self-Healing
3.3.1. Inorganic Anions
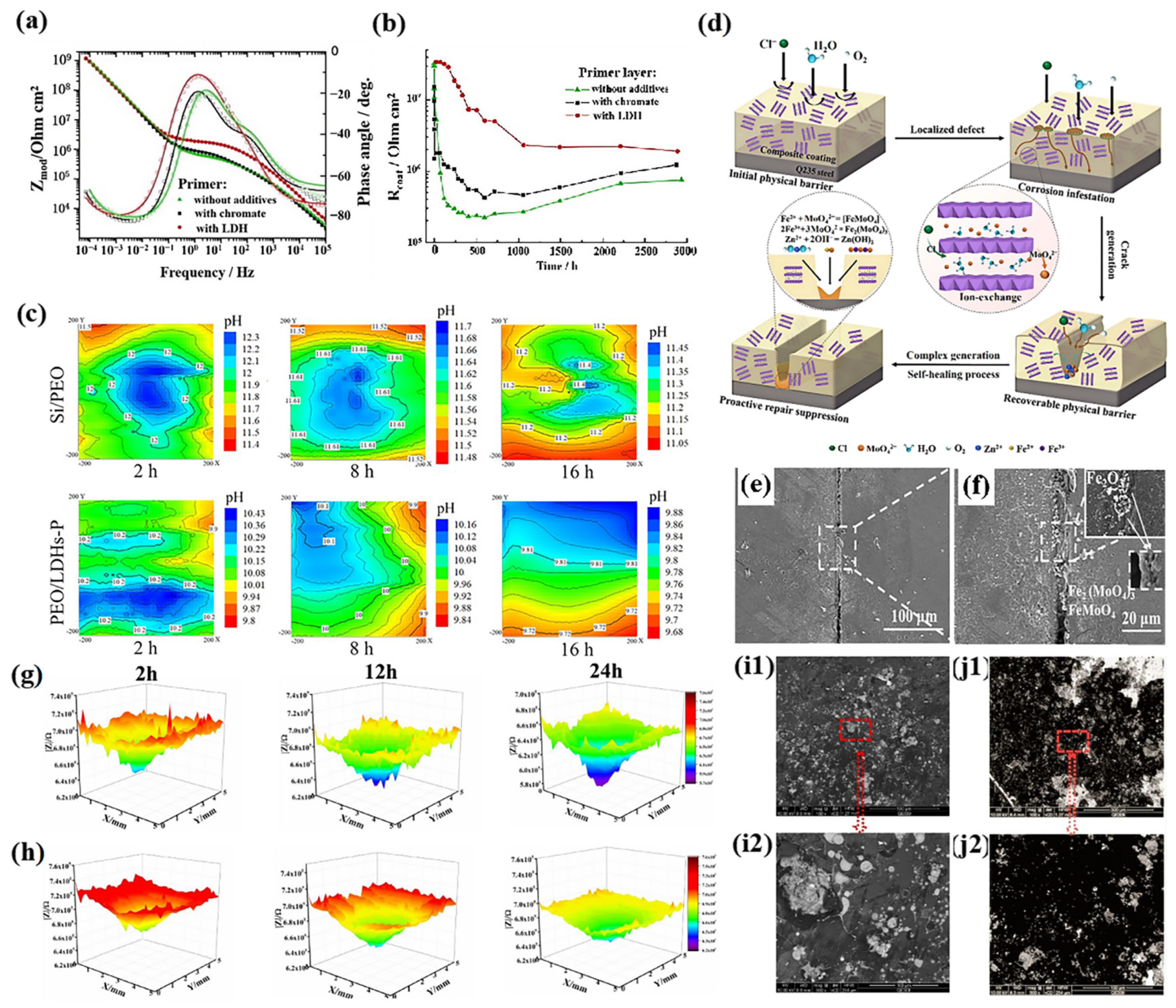
3.3.2. Organic Anions
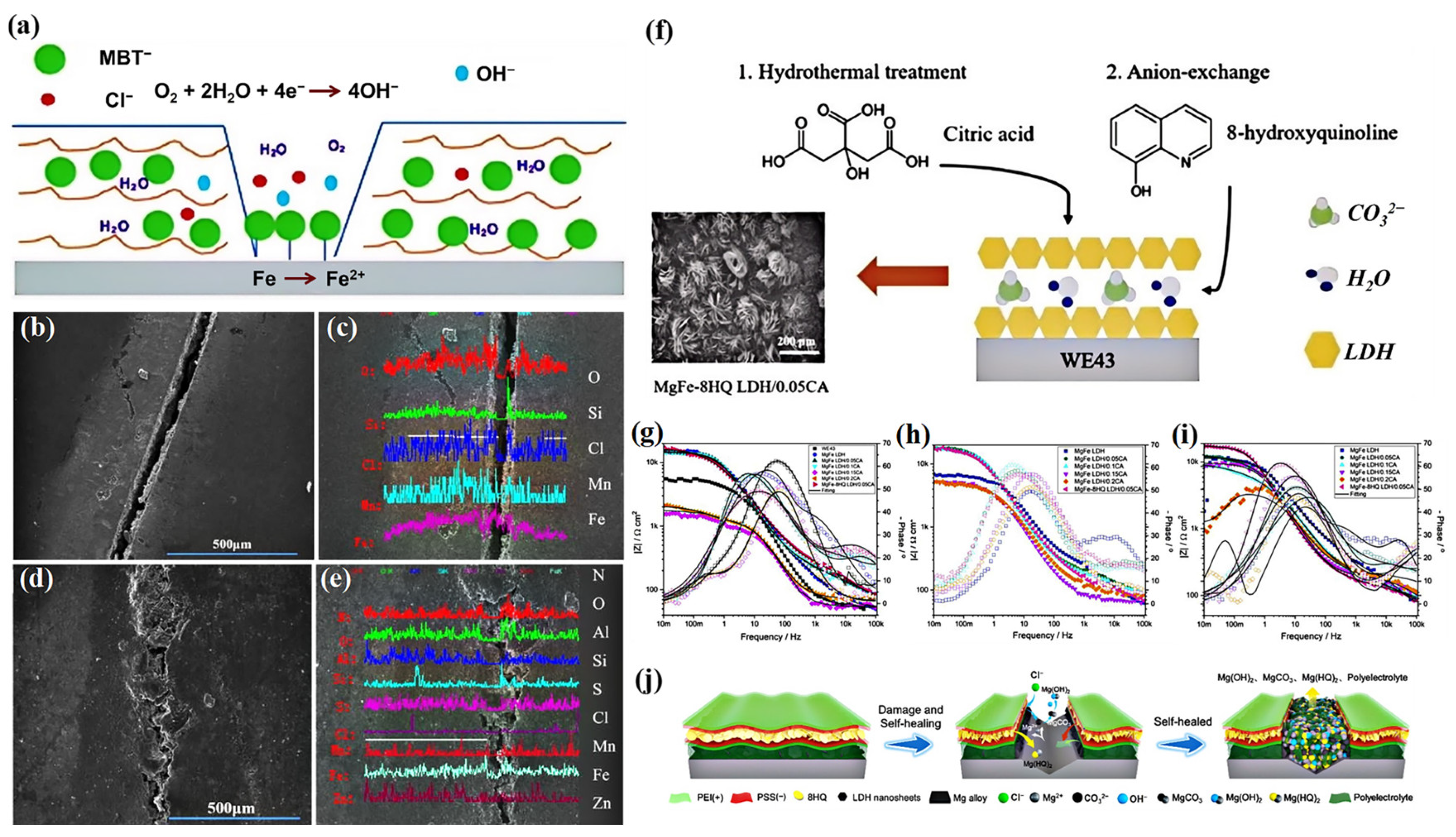
3.4. Hydrophobic Effect
4. Main Factors Affecting Anti-Corrosion Properties of LDHs
4.1. Driving Force
4.2. Layer Spacing
4.3. External Environmental Factors
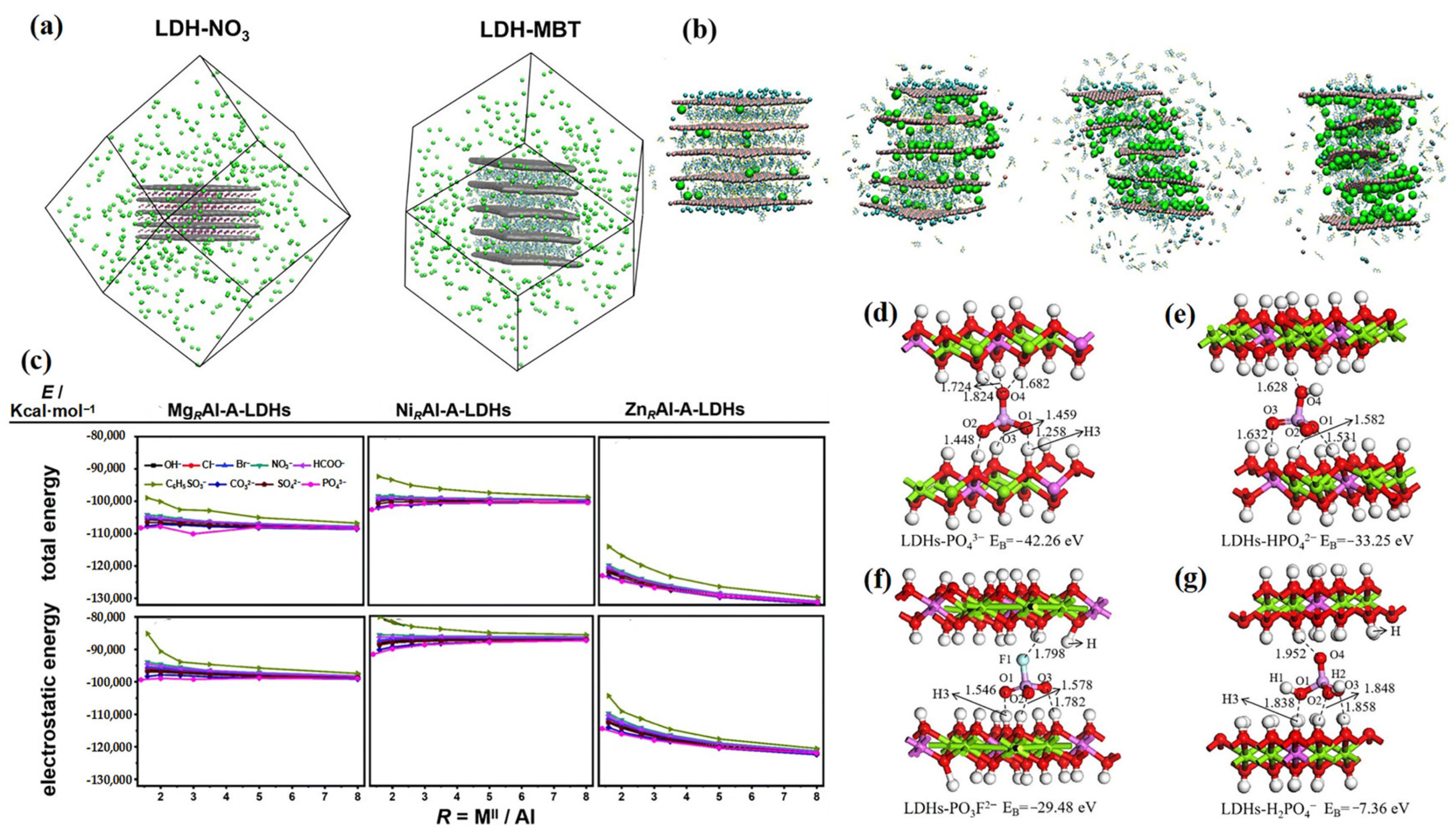
5. Computational Simulation
6. Conclusions and Future Perspectives
6.1. Synthesis of LDHs for More Effective Response to Various Environments
6.2. Exploring New Insert Materials
6.3. Exploring Multifunctional Integrated LDH-Reinforced Composite Coatings
6.4. Development of Advanced Multifunctional Integrated Coatings Represents Urgent Requirement for Advancing LDH-Reinforced Coating Engineering
6.5. Machine Learning-Assisted Preparation of High-Performance LDH-Reinforced Composite Coatings
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCartney, B. Engineering the oceans. Nature 1995, 377, 268. [Google Scholar] [CrossRef]
- Niu, G.; Yuan, R.; Wang, E.; Yang, X.; Liu, Z.; Li, Z.; Zhang, Z.; Gong, N.; Li, K.; Su, B.; et al. Unraveling the influence of Mo on the corrosion mechanism of Ni-advanced weathering steel in harsh marine atmospheric environments. J. Mater. Sci. Technol. 2024, 195, 41–62. [Google Scholar] [CrossRef]
- Tian, H.; Cui, Z.; Ma, H.; Zhao, P.; Yan, M.; Wang, X.; Cui, H. Corrosion evolution and stress corrosion cracking behavior of a low carbon bainite steel in the marine environments: Effect of the marine zones. Corros. Sci. 2022, 206, 110490. [Google Scholar] [CrossRef]
- Cao, X.; Huang, F.; Huang, C.; Liu, J.; Cheng, Y.F. Preparation of graphene nanoplate added zinc-rich epoxy coatings for enhanced sacrificial anode-based corrosion protection. Corros. Sci. 2019, 159, 108120. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, W.; Wang, L. State of the art and current trends on the metal corrosion and protection strategies in deep sea. J. Mater. Sci. Technol. 2025, 215, 192–213. [Google Scholar] [CrossRef]
- Zhu, Y.; Free, M.L.; Woollam, R.; Durnie, W. A review of surfactants as corrosion inhibitors and associated modeling. Prog. Mater. Sci. 2017, 90, 159–223. [Google Scholar] [CrossRef]
- Yimyai, T.; Crespy, D.; Rohwerder, M. Corrosion-Responsive Self-Healing Coatings. Adv. Mater. 2023, 35, 2300101. [Google Scholar] [CrossRef]
- Tran, T.H.; Vimalanandan, A.; Genchev, G.; Fickert, J.; Landfester, K.; Crespy, D.; Rohwerder, M. Regenerative Nano-Hybrid Coating Tailored for Autonomous Corrosion Protection. Adv. Mater. 2015, 27, 3825–3830. [Google Scholar] [CrossRef]
- Li, T.; Ma, Y.; Liu, J.-K.; Liu, J.-C.; Liu, Z.-X.; Ma, Y.-S.; Liu, P.-P. In-situ construction of lamellar stacked zinc molybdate-zinc/aluminium layered double hydroxides heterojunction material with synergistic corrosion protection. Prog. Org. Coat. 2024, 189, 108294. [Google Scholar] [CrossRef]
- Wang, F.; Guo, Z. Facile synthesis of superhydrophobic three-metal-component layered double hydroxide films on aluminum foils for highly improved corrosion inhibition. New J. Chem. 2019, 43, 2289–2298. [Google Scholar] [CrossRef]
- Cao, Y.; Zheng, D.; Zhang, F.; Pan, J.; Lin, C. Layered double hydroxide (LDH) for multi-functionalized corrosion protection of metals: A review. J. Mater. Sci. Technol. 2022, 102, 232–263. [Google Scholar] [CrossRef]
- Tabish, M.; Yasin, G.; Anjum, M.J.; Malik, M.U.; Zhao, J.; Yang, Q.; Manzoor, S.; Murtaza, H.; Khan, W.Q. Reviewing the current status of layered double hydroxide-based smart nanocontainers for corrosion inhibiting applications. J. Mater. Res. Technol. 2021, 10, 390–421. [Google Scholar] [CrossRef]
- Bouali, A.C.; Serdechnova, M.; Blawert, C.; Tedim, J.; Ferreira, M.G.S.; Zheludkevich, M.L. Layered double hydroxides (LDHs) as functional materials for the corrosion protection of aluminum alloys: A review. Appl. Mater. Today 2020, 21, 100857. [Google Scholar] [CrossRef]
- Cao, Y.; Zheng, D.; Li, X.; Lin, J.; Wang, C.; Dong, S.; Lin, C. Enhanced Corrosion Resistance of Superhydrophobic Layered Double Hydroxide Films with Long-Term Stability on Al Substrate. ACS Appl. Mater. Interfaces 2018, 10, 15150–15162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Hu, J.; Jia, Y.B.; Liu, R.T.; Cai, T.; Xu, Z.P. Two-dimensional layered double hydroxide nanoadjuvant: Recent progress and future direction. Nanoscale 2021, 13, 7533–7549. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Dong, S.; Zheng, D.; Wang, J.; Zhang, X.; Du, R.; Song, G.; Lin, C. Multifunctional inhibition based on layered double hydroxides to comprehensively control corrosion of carbon steel in concrete. Corros. Sci. 2017, 126, 166–179. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Q.; O’Hare, D.; Sun, L. Preparation of two dimensional layered double hydroxide nanosheets and their applications. Chem. Soc. Rev. 2017, 46, 5950–5974. [Google Scholar] [CrossRef]
- Duan, M.; Liu, S.; Jiang, Q.; Guo, X.; Zhang, J.; Xiong, S. Recent progress on preparation and applications of layered double hydroxides. Chin. Chem. Lett. 2022, 33, 4428–4436. [Google Scholar] [CrossRef]
- Jung, K.-W.; Lee, S.Y.; Choi, J.-W.; Hwang, M.-J.; Shim, W.G. Synthesis of Mg–Al layered double hydroxides-functionalized hydrochar composite via an in situ one-pot hydrothermal method for arsenate and phosphate removal: Structural characterization and adsorption performance. Chem. Eng. J. 2021, 420, 129775. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Ghasemi, E.; Mahdavian, M.; Ramezanzadeh, B.; Mana, y. The effect of interlayer spacing on the inhibitor release capability of layered double hydroxide based nanocontainers. J. Clean. Prod. 2020, 251, 119676. [Google Scholar] [CrossRef]
- Ma, G.; Xu, J.; Wang, Z. Inhibitive effect of sepiolite-supported MgAl-LDH on chloride-induced corrosion of steel in simulated concrete pore solution. Corros. Sci. 2023, 225, 111571. [Google Scholar] [CrossRef]
- Xu, J.; Song, Y.; Zhao, Y.; Jiang, L.; Mei, Y.; Chen, P. Chloride removal and corrosion inhibitions of nitrate, nitrite-intercalated MgAl layered double hydroxides on steel in saturated calcium hydroxide solution. Appl. Clay Sci. 2018, 163, 129–136. [Google Scholar] [CrossRef]
- Dai, X.; Wu, L.; Ci, W.; Yao, W.; Yuan, Y.; Xie, Z.; Jiang, B.; Wang, J.; Andrej, A.; Pan, F. Dual self-healing effects of salicylate intercalated MgAlY-LDHs film in-situ grown on the micro-arc oxidation coating on AZ31 alloys. Corros. Sci. 2023, 220, 111285. [Google Scholar] [CrossRef]
- Pan, S.-Q.; Zhang, F.; Wen, C.; Zeng, R.-C. Advances in Mg–Al-layered double hydroxide steam coatings on Mg alloys: A review. J. Magnes. Alloys 2023, 11, 1505–1518. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.-H.; Meng, X.-Z.; Liu, P.; Wu, L.-K.; Cao, F.-H. A novel cerium organic network modified graphene oxide prepared multifunctional waterborne epoxy-based coating with excellent mechanical and passive/active anti-corrosion properties. Chem. Eng. J. 2023, 465, 142997. [Google Scholar] [CrossRef]
- Ye, H.; Liu, S.; Yu, D.; Zhou, X.; Qin, L.; Lai, C.; Qin, F.; Zhang, M.; Chen, W.; Chen, W.; et al. Regeneration mechanism, modification strategy, and environment application of layered double hydroxides: Insights based on memory effect. Coord. Chem. Rev. 2022, 450, 214253. [Google Scholar] [CrossRef]
- Chubar, N.; Gilmour, R.; Gerda, V.; Mičušík, M.; Omastova, M.; Heister, K.; Man, P.; Fraissard, J.; Zaitsev, V. Layered double hydroxides as the next generation inorganic anion exchangers: Synthetic methods versus applicability. Adv. Colloid. Interface Sci. 2017, 245, 62–80. [Google Scholar] [CrossRef]
- Fang, D.; Huang, L.; Fan, J.; Xiao, H.; Wu, G.; Wang, Y.; Zeng, Z.; Shen, F.; Deng, S.; Ji, F. New insights into the arrangement pattern of layered double hydroxide nanosheets and their ion-exchange behavior with phosphate. Chem. Eng. J. 2022, 441, 136057. [Google Scholar] [CrossRef]
- Su, Y.; Qiu, S.; Yang, D.; Liu, S.; Zhao, H.; Wang, L.; Xue, Q. Active anti-corrosion of epoxy coating by nitrite ions intercalated MgAl LDH. J. Hazard. Mater. 2020, 391, 122215. [Google Scholar] [CrossRef]
- Yu, L.; Lin, Y.; Li, L.; Zong, H.; Zhou, Y.; Zhao, S.; Zhang, Z.; Grobert, N.; Maciejewska, B.M.; Qin, L. Understanding interfacial dynamics: Hydrostatic pressure-induced sono-dispersion of carbon nanotubes. Surf. Interfaces 2024, 51, 104740. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, F.; Fang, L.; Guan, T.; Hu, J.; Zhang, S. A comparative study and optimization of corrosion resistance of ZnAl layered double hydroxides films intercalated with different anions on AZ31 Mg alloys. Surf. Coat. Technol. 2019, 358, 594–603. [Google Scholar] [CrossRef]
- Anjum, M.J.; Zhao, J.; Zahedi Asl, V.; Yasin, G.; Wang, W.; Wei, S.; Zhao, Z.; Qamar Khan, W. In-situ intercalation of 8-hydroxyquinoline in Mg-Al LDH coating to improve the corrosion resistance of AZ31. Corros. Sci. 2019, 157, 1–10. [Google Scholar] [CrossRef]
- Anjum, M.J.; Zhao, J.-M.; Asl, V.Z.; Malik, M.U.; Yasin, G.; Khan, W.Q. Green corrosion inhibitors intercalated Mg:Al layered double hydroxide coatings to protect Mg alloy. Rare Met. 2021, 40, 2254–2265. [Google Scholar] [CrossRef]
- Cao, Y.; Zheng, D.; Lin, C. Effect of physical barrier and anion-exchange process of nitrate-intercalated ZnAl layered double hydroxide films grown on Al on corrosion protection. Surf. Coat. Technol. 2021, 421, 127436. [Google Scholar] [CrossRef]
- Mei, Y.; Xu, J.; Jiang, L.; Tan, Q. Enhancing corrosion resistance of epoxy coating on steel reinforcement by aminobenzoate intercalated layered double hydroxides. Prog. Org. Coat. 2019, 134, 288–296. [Google Scholar] [CrossRef]
- Yan, H.; Wang, J.; Zhang, Y.; Hu, W. Preparation and inhibition properties of molybdate intercalated ZnAlCe layered double hydroxide. J. Alloys Compd. 2016, 678, 171–178. [Google Scholar] [CrossRef]
- Tavares, S.R.; Haddad, J.F.S.; Ivo, R.; Moraes, P.; Leitão, A.A. Computational exploration of the anion exchange on the basal surface of layered double hydroxides by molecular dynamics. Appl. Surf. Sci. 2020, 513, 145743. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, Y.; Zhang, Z.; Wang, Y.; Hu, J.; Ma, Y.; Zhang, Z.; Huang, H.; Wei, J.; Shi, C.; et al. Insight into ion exchange behavior of LDHs: Asynchronous chloride adsorption and intercalated ions release processes. Cem. Concr. Compos. 2024, 147, 105433. [Google Scholar] [CrossRef]
- Santos, F.d.S.; Binder, L.; Scharnagl, N.; da Conceição, T.F. Sustainable smart coatings of chitosan and LDH loaded with natural inhibitors for corrosion protection of Mg AZ31 alloy. Colloids Surf. A Physicochem. Eng. Asp. 2024, 688, 133639. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, L.; Tang, A.; Ma, Y.; Song, G.-L.; Zheng, D.; Jiang, B.; Atrens, A.; Pan, F. Active corrosion protection by a smart coating based on a MgAl-layered double hydroxide on a cerium-modified plasma electrolytic oxidation coating on Mg alloy AZ31. Corros. Sci. 2018, 139, 370–382. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Y.; Sun, Y.; Zhao, W.; Wang, L. 2D Nanomaterials Reinforced Organic Coatings for Marine Corrosion Protection: State of the Art, Challenges, and Future Prospectives. Adv. Mater. 2024, 36, e2312460. [Google Scholar] [CrossRef] [PubMed]
- Kuang, J.; Ba, Z.; Li, Z.; Jia, Y.; Wang, Z. Fabrication of a superhydrophobic Mg-Mn layered double hydroxides coating on pure magnesium and its corrosion resistance. Surf. Coat. Technol. 2019, 361, 75–82. [Google Scholar] [CrossRef]
- Wu, L.; Wu, J.; Zhang, Z.; Zhang, C.; Zhang, Y.; Tang, A.; Li, L.; Zhang, G.; Zheng, Z.; Atrens, A.; et al. Corrosion resistance of fatty acid and fluoroalkylsilane-modified hydrophobic Mg-Al LDH films on anodized magnesium alloy. Appl. Surf. Sci. 2019, 487, 569–580. [Google Scholar] [CrossRef]
- Bai, W.; Zhang, X.; He, Z.; Qian, Y.; Jian, R.; Lin, Y.; Xu, Y. Intelligent anti-corrosion coating with multiple protections using active nanocontainers of Zn Al LDH equipped with ZIF-8 encapsulated environment-friendly corrosion inhibitors. Prog. Org. Coat. 2023, 185, 107940. [Google Scholar] [CrossRef]
- Su, Y.; Qiu, S.; Wei, J.; Zhu, X.; Zhao, H.; Xue, Q. Sulfonated polyaniline assisted hierarchical assembly of graphene-LDH nanohybrid for enhanced anticorrosion performance of waterborne epoxy coatings. Chem. Eng. J. 2021, 426, 131269. [Google Scholar] [CrossRef]
- Cai, M.; Fan, X.; Yan, H.; Li, Y.; Song, S.; Li, W.; Li, H.; Lu, Z.; Zhu, M. In situ assemble Ti3C2Tx MXene@MgAl-LDH heterostructure towards anticorrosion and antiwear application. Chem. Eng. J. 2021, 419, 130050. [Google Scholar] [CrossRef]
- Xu, T.; Yu, L.; Hu, J.-M. In-situ Zn-Al layered double hydroxide conversion coatings prepared on galvanized steels by a two-step electrochemical method. Corros. Sci. 2024, 233, 112057. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Q.-Y.; Zhang, J.-T.; Hu, J.-M. Electrodeposited graphene/layered double hydroxides micro/nanocontainers for both passive and active corrosion protection. NPJ Mater. Degrad. 2024, 8, 22. [Google Scholar] [CrossRef]
- Poznyak, S.K.; Tedim, J.; Rodrigues, L.M.; Salak, A.N.; Zheludkevich, M.L.; Dick, L.F.P.; Ferreira, M.G.S. Novel Inorganic Host Layered Double Hydroxides Intercalated with Guest Organic Inhibitors for Anticorrosion Applications. ACS Appl. Mater. Interfaces 2009, 1, 2353–2362. [Google Scholar] [CrossRef]
- Tedim, J.; Kuznetsova, A.; Salak, A.N.; Montemor, F.; Snihirova, D.; Pilz, M.; Zheludkevich, M.L.; Ferreira, M.G.S. Zn–Al layered double hydroxides as chloride nanotraps in active protective coatings. Corros. Sci. 2012, 55, 1–4. [Google Scholar] [CrossRef]
- Miyata, S. Anion-Exchange Properties of Hydrotalcite-Like Compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Zuo, J.; Wu, B.; Luo, C.; Dong, B.; Xing, F. Preparation of MgAl layered double hydroxides intercalated with nitrite ions and corrosion protection of steel bars in simulated carbonated concrete pore solution. Corros. Sci. 2019, 152, 120–129. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Chen, K.; Zhang, X.; Zhang, B.; Fang, S.; Liang, Y.; Huang, C.; Wang, X. A Comparative Study of Chloride Adsorption Ability and Corrosion Protection Effect in Epoxy Coatings of Various Layered Double Hydroxides. Coatings 2022, 12, 1631. [Google Scholar] [CrossRef]
- Farshbaf, P.; Ahmadi, N.P.; Yazdani, S. Self-healing vanadate-doped NiAl-layered double hydroxide (LDH) coatings synthesized for active corrosion protection of 2024 aluminum alloy. Mater. Today Commun. 2024, 39, 108795. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Kuznetsova, A.; Maltanava, H.M.; Poznyak, S.K.; Ferreira, M.G.S.; Zheludkevich, M.L. Corrosion protection of zinc by LDH conversion coatings. Corros. Sci. 2024, 229, 111889. [Google Scholar] [CrossRef]
- Abrantes Leal, D.; Wypych, F.; Bruno Marino, C.E. Zinc-Layered Hydroxide Salt Intercalated with Molybdate Anions as a New Smart Nanocontainer for Active Corrosion Protection of Carbon Steel. ACS Appl. Mater. Interfaces 2020, 12, 19823–19833. [Google Scholar] [CrossRef]
- Abrantes Leal, D.; Sousa, I.; Bastos, A.C.; Tedim, J.; Wypych, F.; Bruno Marino, C.E. Combination of layered-based materials as an innovative strategy for improving active corrosion protection of carbon steel. Surf. Coat. Technol. 2023, 473, 129972. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, L.; Tang, A.; Chen, X.-B.; Ma, Y.; Long, Y.; Peng, P.; Ding, X.; Pan, H.; Pan, F. Growth behavior of MgAl-layered double hydroxide films by conversion of anodic films on magnesium alloy AZ31 and their corrosion protection. Appl. Surf. Sci. 2018, 456, 419–429. [Google Scholar] [CrossRef]
- Nie, G.; Wu, L.; Qiu, S.; Xu, Z.; Wang, H. Preferable phosphate sequestration using polymer-supported Mg/Al layered double hydroxide nanosheets. J. Colloid. Interface Sci. 2022, 614, 583–592. [Google Scholar] [CrossRef]
- Hu, T.; Ouyang, Y.; Xie, Z.-H.; Wu, L. One-pot scalable in situ growth of highly corrosion-resistant MgAl-LDH/MBT composite coating on magnesium alloy under mild conditions. J. Mater. Sci. Technol. 2021, 92, 225–235. [Google Scholar] [CrossRef]
- Wang, J.; Cao, Y.; Xue, J.; Zhang, X.; Liang, Y.; Chen, K.; Huang, C.; Zheng, D. A comparative experimental and theoretical calculation study of CaAl-LDH modified with various aromatic inhibitors for corrosion protection study in epoxy coatings. Corros. Sci. 2024, 231, 111994. [Google Scholar] [CrossRef]
- Yao, W.; Chen, Y.; Wu, L.; Jiang, B.; Pan, F. Preparation of slippery liquid-infused porous surface based on MgAlLa-layered double hydroxide for effective corrosion protection on AZ31 Mg alloy. J. Taiwan Inst. Chem. Eng. 2022, 131, 104176. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.; Yao, W.; Chen, Y.; Zhong, Z.; Ci, W.; Wu, J.; Xie, Z.; Yuan, Y.; Pan, F. A self-healing corrosion protection coating with graphene oxide carrying 8-hydroxyquinoline doped in layered double hydroxide on a micro-arc oxidation coating. Corros. Sci. 2022, 194, 109941. [Google Scholar] [CrossRef]
- Tan, J.K.E.; Birbilis, N.; Choudhary, S.; Thomas, S.; Balan, P. Corrosion protection enhancement of Mg alloy WE43 by in-situ synthesis of MgFe LDH/citric acid composite coating intercalated with 8HQ. Corros. Sci. 2022, 205, 110444. [Google Scholar] [CrossRef]
- Chen, J.; Fang, L.; Wu, F.; Xie, J.; Hu, J.; Jiang, B.; Luo, H. Corrosion resistance of a self-healing rose-like MgAl-LDH coating intercalated with aspartic acid on AZ31 Mg alloy. Prog. Org. Coat. 2019, 136, 105234. [Google Scholar] [CrossRef]
- Lin, K.; Luo, X.; Pan, X.; Zhang, C.; Liu, Y. Enhanced corrosion resistance of LiAl-layered double hydroxide (LDH) coating modified with a Schiff base salt on aluminum alloy by one step in-situ synthesis at low temperature. Appl. Surf. Sci. 2019, 463, 1085–1096. [Google Scholar] [CrossRef]
- Zhong, F.; He, Y.; Wang, P.; Chen, C.; Xie, P.; Li, H.; Chen, J. One-step hydrothermal synthesis of reduced graphene oxide/aspartic acid intercalated layered double hydroxide for enhancing barrier and self-healing properties of epoxy coating. React. Funct. Polym. 2019, 145, 104380. [Google Scholar] [CrossRef]
- Buchheit, R.G.; Guan, H.; Mahajanam, S.; Wong, F. Active corrosion protection and corrosion sensing in chromate-free organic coatings. Prog. Org. Coat. 2003, 47, 174–182. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Poznyak, S.K.; Rodrigues, L.M.; Raps, D.; Hack, T.; Dick, L.F.; Nunes, T.; Ferreira, M.G.S. Active protection coatings with layered double hydroxide nanocontainers of corrosion inhibitor. Corros. Sci. 2010, 52, 602–611. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Kuznetsova, A.; Kallip, S.; Starykevich, M.; Tedim, J.; Ferreira, M.G.S.; Zheludkevich, M.L. A novel bilayer system comprising LDH conversion layer and sol-gel coating for active corrosion protection of AA2024. Corros. Sci. 2018, 143, 299–313. [Google Scholar] [CrossRef]
- Ahmad, S.; Nawaz, M.; Mohammad, S.; Shakoor, R.A.; Kahraman, R.; Al Tahtamouni, T.M. Modified Ni-Al layer double hydroxides as nanoparticles for self-healing anti-corrosion composite coating. Surf. Coat. Technol. 2024, 476, 130172. [Google Scholar] [CrossRef]
- Zhang, G.; Jiang, E.; Wu, L.; Tang, A.; Atrens, A.; Pan, F. Active corrosion protection of phosphate loaded PEO/LDHs composite coatings: SIET study. J. Magnes. Alloys 2022, 10, 1351–1357. [Google Scholar] [CrossRef]
- Li, H.; Cui, H.; Li, L.; Zhang, Y.; Wang, Q.; Wei, N.; Song, X.; Shao, S.; Mao, N. Incorporation of Nanometer-Sized Layered Double Hydroxide with Anions that Improve the Corrosion Resistance of Epoxy Polymer. ACS Appl. Nano Mater. 2023, 6, 20419–20430. [Google Scholar] [CrossRef]
- Hayatdavoudi, H.; Rahsepar, M. Smart inhibition action of layered double hydroxide nanocontainers in zinc-rich epoxy coating for active corrosion protection of carbon steel substrate. J. Alloys Compd. 2017, 711, 560–567. [Google Scholar] [CrossRef]
- Carneiro, J.; Caetano, A.F.; Kuznetsova, A.; Maia, F.; Salak, A.N.; Tedim, J.; Scharnagl, N.; Zheludkevich, M.L.; Ferreira, M.G.S. Polyelectrolyte-modified layered double hydroxide nanocontainers as vehicles for combined inhibitors. RSC Adv. 2015, 5, 39916–39929. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, F.; Zhou, Q. Protective behaviors of 2-mercaptobenzothiazole intercalated Zn–Al-layered double hydroxide coating. J. Coat. Technol. Res. 2014, 11, 793–803. [Google Scholar] [CrossRef]
- Xie, Z.-H.; Zhang, W.; Li, Y.; Yong, Q.; Wu, L.; Fan, X.; Zhong, C.-J. Inhibitor-Sandwiched Polyelectrolyte Film for Micro/Nanopore Sealing to Enable Corrosion-Resistant Self-Healing Capability. ACS Appl. Polym. Mater. 2024, 6, 4037–4049. [Google Scholar] [CrossRef]
- Cao, Y.; Zheng, D.; Luo, J.; Zhang, F.; Wang, C.; Dong, S.; Ma, Y.; Liang, Z.; Lin, C. Enhanced corrosion protection by Al surface immobilization of in-situ grown layered double hydroxide films co-intercalated with inhibitors and low surface energy species. Corros. Sci. 2020, 164, 108340. [Google Scholar] [CrossRef]
- Xie, P.; He, Y.; Zhong, F.; Zhang, C.; Chen, C.; Li, H.; Liu, Y.; Bai, Y.; Chen, J. Cu-BTA complexes coated layered double hydroxide for controlled release of corrosion inhibitors in dual self-healing waterborne epoxy coatings. Prog. Org. Coat. 2021, 153, 106164. [Google Scholar] [CrossRef]
- Cao, K.; Yu, Z.; Zhu, L.; Yin, D.; Chen, L.; Jiang, Y.; Wang, J. Fabrication of superhydrophobic layered double hydroxide composites to enhance the corrosion-resistant performances of epoxy coatings on Mg alloy. Surf. Coat. Technol. 2021, 407, 126763. [Google Scholar] [CrossRef]
- Yang, M.; Gu, L.; Yang, B.; Wang, L.; Sun, Z.; Zheng, J.; Zhang, J.; Hou, J.; Lin, C. Antifouling composites with self-adaptive controlled release based on an active compound intercalated into layered double hydroxides. Appl. Surf. Sci. 2017, 426, 185–193. [Google Scholar] [CrossRef]
- Guo, X.; Xu, S.; Zhao, L.; Lu, W.; Zhang, F.; Evans, D.G.; Duan, X. One-Step Hydrothermal Crystallization of a Layered Double Hydroxide/Alumina Bilayer Film on Aluminum and Its Corrosion Resistance Properties. Langmuir 2009, 25, 9894–9897. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D. Synthesis, characterization, and controlled release anticorrosion behavior of benzoate intercalated Zn–Al layered double hydroxides. Mater. Res. Bull. 2011, 46, 1963–1968. [Google Scholar] [CrossRef]
- Yan, D.; Wang, Y.; Liu, J.; Song, D.; Zhang, T.; Liu, J.; He, F.; Zhang, M.; Wang, J. Self-healing system adapted to different pH environments for active corrosion protection of magnesium alloy. J. Alloys Compd. 2020, 824, 153918. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, Z.; Zhang, Z.; Xin, Y.; Fujita, T.; Wei, Y. In situ one-step fabrication of superhydrophobic layered double hydroxide on Al alloys for anti-corrosion. Appl. Surf. Sci. 2022, 593, 153400. [Google Scholar] [CrossRef]
- Mohammadi, I.; Shahrabi, T.; Mahdavian, M.; Izadi, M. Construction of an epoxy coating with excellent protection performance on the AA 2024-T3 using ion-exchange materials loaded with eco-friendly corrosion inhibitors. Prog. Org. Coat. 2022, 166, 106786. [Google Scholar] [CrossRef]
- Li, Y.; Lu, X.; Serdechnova, M.; Blawert, C.; Zheludkevich, M.L.; Qian, K.; Zhang, T.; Wang, F. Incorporation of LDH nanocontainers into plasma electrolytic oxidation coatings on Mg alloy. J. Magnes. Alloys 2023, 11, 1236–1246. [Google Scholar] [CrossRef]
- Ding, C.; Tai, Y.; Wang, D.; Tan, L.; Fu, J. Superhydrophobic composite coating with active corrosion resistance for AZ31B magnesium alloy protection. Chem. Eng. J. 2019, 357, 518–532. [Google Scholar] [CrossRef]
- Ishizaki, T.; Chiba, S.; Watanabe, K.; Suzuki, H. Corrosion resistance of Mg–Al layered double hydroxide container-containing magnesium hydroxide films formed directly on magnesium alloy by chemical-free steam coating. J. Mater. Chem. A 2013, 1, 8968–8977. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.-G.; Zeng, R.-C.; Li, S.-Q.; Cui, H.-Z.; Song, L.; Han, E.-H. Corrosion resistance of Mg–Al-LDH coating on magnesium alloy AZ31. Surf. Coat. Technol. 2014, 258, 1152–1158. [Google Scholar] [CrossRef]
- Peng, F.; Li, H.; Wang, D.; Tian, P.; Tian, Y.; Yuan, G.; Xu, D.; Liu, X. Enhanced Corrosion Resistance and Biocompatibility of Magnesium Alloy by Mg–Al-Layered Double Hydroxide. ACS Appl. Mater. Interfaces 2016, 8, 35033–35044. [Google Scholar] [CrossRef] [PubMed]
- Alibakhshi, E.; Ghasemi, E.; Mahdavian, M.; Ramezanzadeh, B.; Farashi, S. Active corrosion protection of Mg-Al-PO43− LDH nanoparticle in silane primer coated with epoxy on mild steel. J. Taiwan Inst. Chem. Eng. 2017, 75, 248–262. [Google Scholar] [CrossRef]
- Huang, M.; Lu, G.; Pu, J.; Qiang, Y. Superhydrophobic and smart MgAl-LDH anti-corrosion coating on AZ31 Mg surface. J. Ind. Eng. Chem. 2021, 103, 154–164. [Google Scholar] [CrossRef]
- Shulha, T.; Serdechnova, M.; Lamaka, S.V.; Lu, X.; Feiler, C.; Blawert, C.; Zheludkevich, M.L. Corrosion Inhibitors Intercalated into Layered Double Hydroxides Prepared In Situ on AZ91 Magnesium Alloys: Structure and Protection Ability. ACS Appl. Mater. Interfaces 2023, 15, 6098–6112. [Google Scholar] [CrossRef]
- Wen, T.; Yan, R.; Wang, N.; Li, Y.; Chen, T.; Ma, H. PPA-containing layered double hydroxide (LDH) films for corrosion protection of a magnesium alloy. Surf. Coat. Technol. 2020, 383, 125255. [Google Scholar] [CrossRef]
- Pancrecious, J.K.; Vineetha, S.V.; Bill, U.S.; Gowd, E.B.; Rajan, T.P.D. Ni-Al polyvanadate layered double hydroxide with nanoceria decoration for enhanced corrosion protection of aluminium alloy. Appl. Clay Sci. 2021, 211, 106199. [Google Scholar] [CrossRef]
- del Olmo, R.; Mohedano, M.; Matykina, E.; Arrabal, R. Permanganate loaded Ca-Al-LDH coating for active corrosion protection of 2024-T3 alloy. Corros. Sci. 2022, 198, 110144. [Google Scholar] [CrossRef]
- Chen, M.; Wu, F.; Yu, L.; Cai, Y.; Chen, H.; Zhang, M. Chloride binding capacity of LDHs with various divalent cations and divalent to trivalent cation ratios in different solutions. CrystEngComm 2019, 21, 6790–6800. [Google Scholar] [CrossRef]
- Ko, S.-J.; Yamaguchi, T.; Salles, F.; Oh, J.-M. Systematic utilization of layered double hydroxide nanosheets for effective removal of methyl orange from an aqueous system by π-π stacking-induced nanoconfinement. J. Environ. Manag. 2021, 277, 111455. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, P.; Liang, C.; Chen, M.; Niu, L.; Xu, J.; Sun, D.; Lu, L. Characterization and adaptability of layered double hydroxides in cement paste. Appl. Clay Sci. 2021, 211, 106197. [Google Scholar] [CrossRef]
- Shkirskiy, V.; Keil, P.; Hintze-Bruening, H.; Leroux, F.; Vialat, P.; Lefèvre, G.; Ogle, K.; Volovitch, P. Factors Affecting MoO42– Inhibitor Release from Zn2Al Based Layered Double Hydroxide and Their Implication in Protecting Hot Dip Galvanized Steel by Means of Organic Coatings. ACS Appl. Mater. Interfaces 2015, 7, 25180–25192. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-J.; Zhu, Y.-Q.; Xu, S.-M.; Liu, H.-M.; Yin, P.; Feng, Y.-L.; Yan, H. Anion exchange behavior of MIIAl layered double hydroxides: A molecular dynamics and DFT study. Phys. Chem. Chem. Phys. 2020, 22, 19758–19768. [Google Scholar] [CrossRef] [PubMed]
- Novell Leruth, G.; Kuznetsova, A.; Tedim, J.; Gomes, J.R.B.; Galvão, T.L.P. Molecular Dynamics Model to Explore the Initial Stages of Anion Exchange involving Layered Double Hydroxide Particles. Nanomaterials 2022, 12, 4039. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, E.; Li, J.; Chen, Z.; Yu, B.; Nong, Y. A novel LDHs-VB3- inhibitor for rebar corrosion in simulated concrete pore solution: Synthesis, performance and mechanism. Compos. Part B Eng. 2024, 277, 111414. [Google Scholar] [CrossRef]
- Pérez-Sánchez, G.; Galvão, T.L.P.; Tedim, J.; Gomes, J.R.B. A molecular dynamics framework to explore the structure and dynamics of layered double hydroxides. Appl. Clay Sci. 2018, 163, 164–177. [Google Scholar] [CrossRef]
- Li, J.; Luo, M.; Chen, Z.; Zhuang, E.; Yu, B.; Chen, Y.; Nong, Y. Anti-corrosion mechanism of MgAl-LDHs inhibitors with varying anionic charge on reinforcing steel in simulated concrete pore solutions. Constr. Build. Mater. 2023, 363, 129882. [Google Scholar] [CrossRef]
| LDH Type | Interlayer Anion | Morphometric | Solution | Corrosion Current Density A/cm2 | Immersion Time | |Z|0.01 Hz Ω cm2 | Refs. |
|---|---|---|---|---|---|---|---|
| ZnAl-LDH | NO3− | Film | 3.5 wt% NaCl | 10−8 | 14 days | 1.6 × 107 | [82] |
| ZnAl-LDH /EP | VO3− | Powder | 0.05 M NaCl | N/A | 4 months | >106 | [69] |
| ZnAl-LDH /EP | C6H5COO− | Powder | 3.5 wt% NaCl | 4.9 × 10−6 | 24 h | >105 | [83] |
| ZnAl-LDH /EP | PO43− | Powder | 3.5 wt% NaCl | 4.23 × 10−9 | 100 days | ≈107 | [73] |
| ZnAl-LDH /EP | MoO42− | Powder | 3.5 wt% NaCl | 2.94 × 10−9 | 100 days | ≈107 | [73] |
| ZnAl-LDH /sol–gel | V2O74− | Powder | 3.5 wt% NaCl | 1.71 × 10−6 | 14 days | >106 | [70] |
| ZnAl-LDH /EP | Methionine | Powder | 3.5 wt% NaCl | 9.98 × 10−6 | 85 days | >108 | [84] |
| ZnAl-LDH | VOx and La | Film | 3.5 wt% NaCl | 5.19 × 10−9 | 24 h | >106 | [78] |
| ZnAl-LDH | SDS | Film | 3.5 wt% NaCl | 2.01 × 10−8 | 12 h | 7 × 104 | [85] |
| ZnAl-LDH /EP | DEDTC | Film | 3.5 wt% NaCl | N/A | 24 h | ≈106 | [86] |
| ZnAl-LDH | MBT | Powder | 0.05 M NaCl | N/A | 7 days | >105 | [49] |
| ZnAl-LDH /ZRE | MBT | Powder | 3.5 wt% NaCl | N/A | 25 days | ≈104 | [74] |
| ZnAl-LDH /PEO | Fumarate | Film | 3.5 wt% NaCl | 1.5 × 107 | 10 days | ≈105 | [87] |
| MgAl-LDH | MBT | Powder | 0.05 M NaCl | N/A | 7 days | >104 | [49] |
| MgAl-LDH /EP | F− | Powder | 3.5 wt% NaCl | N/A | 30 days | >108 | [80] |
| MgAl-LDH | WO42− | Film | 3.5 wt% NaCl | 7.44 × 10−6 | 7 days | >104 | [88] |
| MgAl-LDH | CO32− | Film | 5 wt% NaCl | 4.82 × 10−11 | 10 days | N/A | [89] |
| MgAl-LDH /EP | CO32− | Powder | 3.5 wt% NaCl | 6.52 × 10−8 | 48 h | >105 | [90] |
| MgAl-LDH | NO3− | Film | 3.5 wt% NaCl | 3.626 × 10−7 | 21 days | 4.77 × 107 | [91] |
| MgAl-LDH /EPSP | PO43− | Powder | 3.5 wt% NaCl | 4.8 × 10−6 | 24 h | ≈104 (artificial defect) | [92] |
| MgAl-LDH /EP | NO2− | Powder | 3.5 wt% NaCl | N/A | 25 days | 3.52 × 107 | [29] |
| MgAl-LDH | Sodium laurate | Film | 3.5 wt% NaCl | 4.33 × 10−9 | 7 days | ≈107 | [93] |
| MgAl-LDH | Oxalate | Film | 3.5 wt% NaCl | N/A | 48 h | >104 | [94] |
| MgAl-LDH | PPA | Film | 3.5 wt% NaCl | 2.47 × 10−9 | 36 h | ≈106 | [95] |
| MgAl-LDH /EP | Methionine | Powder | 3.5 wt% NaCl | 5.8 × 10−6 | 60 h | ≈104 | [84] |
| MgAl-LDH /EP | MoO42− and BTA | Powder | 3.5 wt% NaCl | N/A | 120 days | >107 | [6] |
| LiAl-LDH | Asp | Film | 3.5 wt% NaCl | 10−8 | 20 days | 4.41 × 105 | [79] |
| LiAl-LDH | L-aspartic | Film | 3.5 wt% NaCl | 3 × 10−8 | 5 days | 3.6 × 106 | [66] |
| MgFe-LDH | 8HQ | Film | 3.5 wt% NaCl | N/A | 7 days | >1.5 × 104 | [64] |
| NiAl-LDH /PU | PO43− | Powder | 3.5 wt% NaCl | N/A | 14 days | ≈1011 | [71] |
| NiAl-LDH | VOx | Powder | 3.5 wt% NaCl | 1.13 × 10−6 | 24 h | 2.54 × 105 | [96] |
| CaAl-LDH | MnO4− | Film | 3.5 wt% NaCl | N/A | 28 days | ≈105 | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Wu, Y.; Zhao, W. Review of Layered Double Hydroxide (LDH) Nanosheets in Corrosion Mitigation: Recent Developments, Challenges, and Prospects. Materials 2025, 18, 1190. https://doi.org/10.3390/ma18061190
Cao J, Wu Y, Zhao W. Review of Layered Double Hydroxide (LDH) Nanosheets in Corrosion Mitigation: Recent Developments, Challenges, and Prospects. Materials. 2025; 18(6):1190. https://doi.org/10.3390/ma18061190
Chicago/Turabian StyleCao, Jintao, Yangmin Wu, and Wenjie Zhao. 2025. "Review of Layered Double Hydroxide (LDH) Nanosheets in Corrosion Mitigation: Recent Developments, Challenges, and Prospects" Materials 18, no. 6: 1190. https://doi.org/10.3390/ma18061190
APA StyleCao, J., Wu, Y., & Zhao, W. (2025). Review of Layered Double Hydroxide (LDH) Nanosheets in Corrosion Mitigation: Recent Developments, Challenges, and Prospects. Materials, 18(6), 1190. https://doi.org/10.3390/ma18061190








