Oxide Removal Mechanism and Process Optimization During Integrated Pulsed-Continuous Laser Cleaning of Q235B Carbon Steel
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Pulsed Laser Cleaning
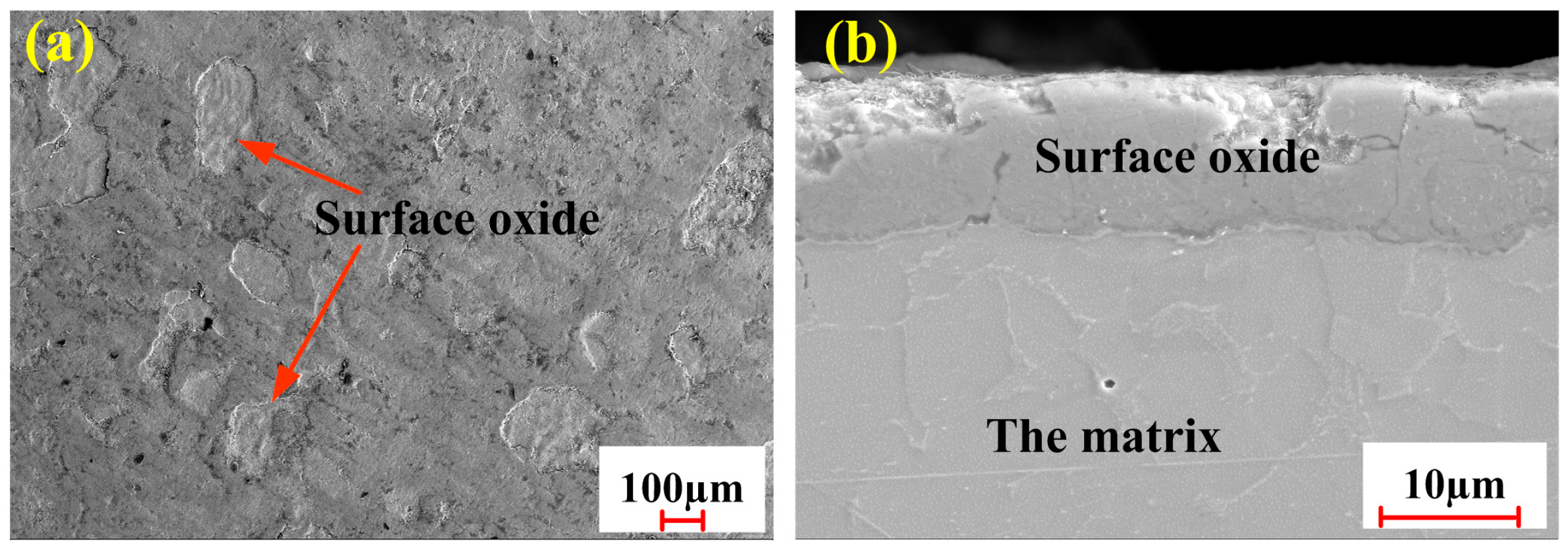
3.1.1. Oxide Removal
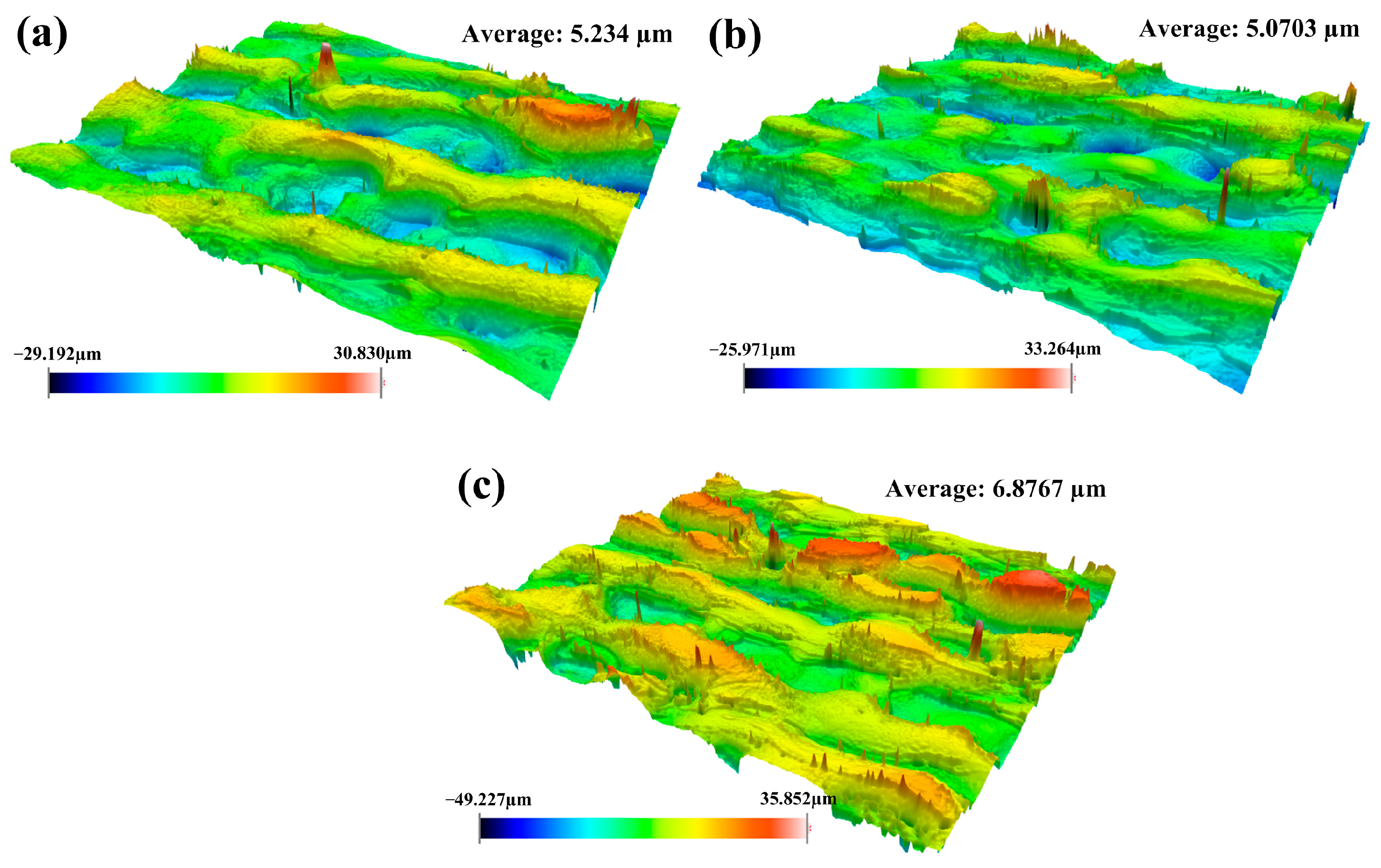
3.1.2. Surface Roughness
3.2. Process Optimization
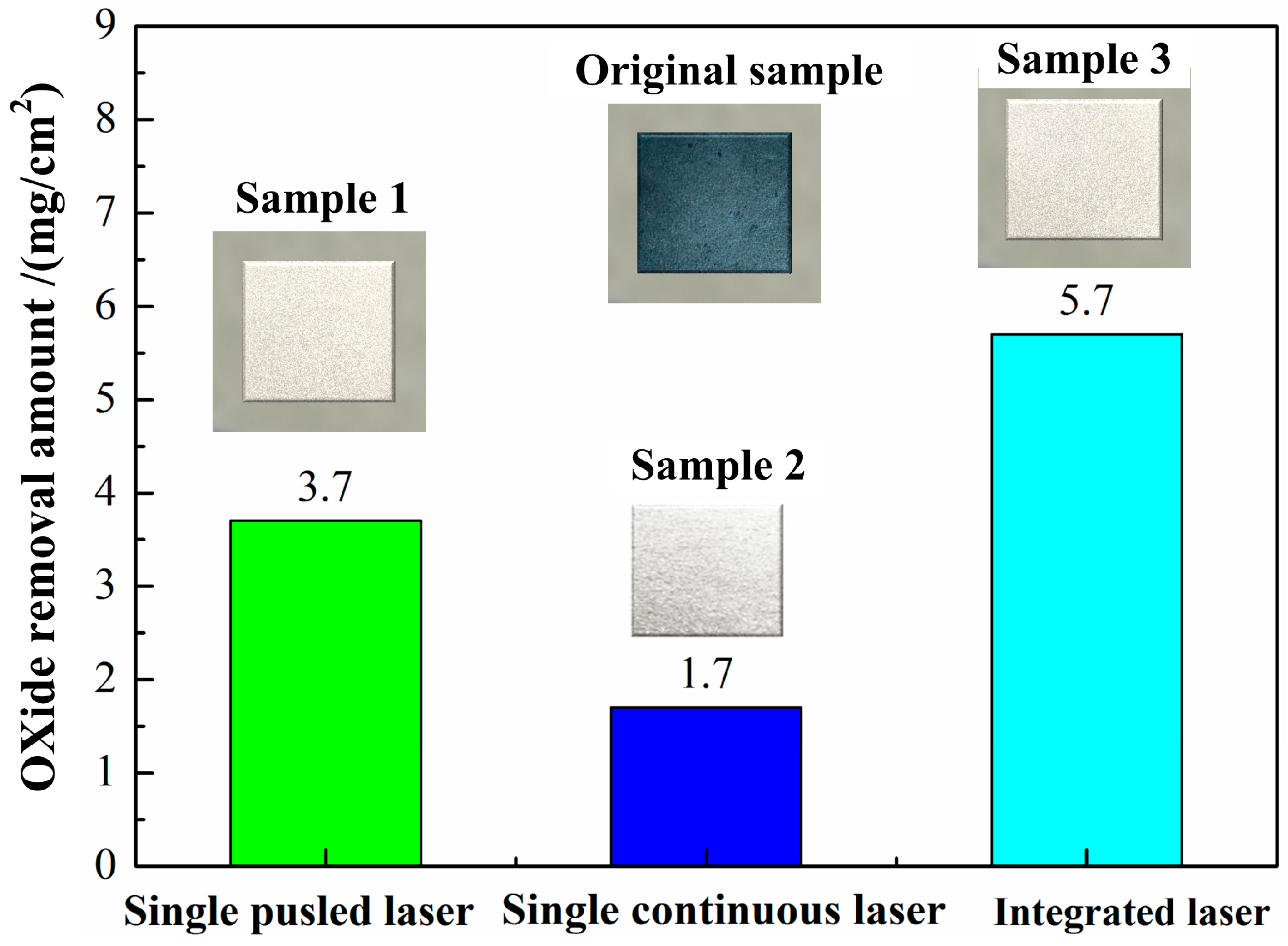
3.2.1. Oxide Removal Under Optimized Process Conditions
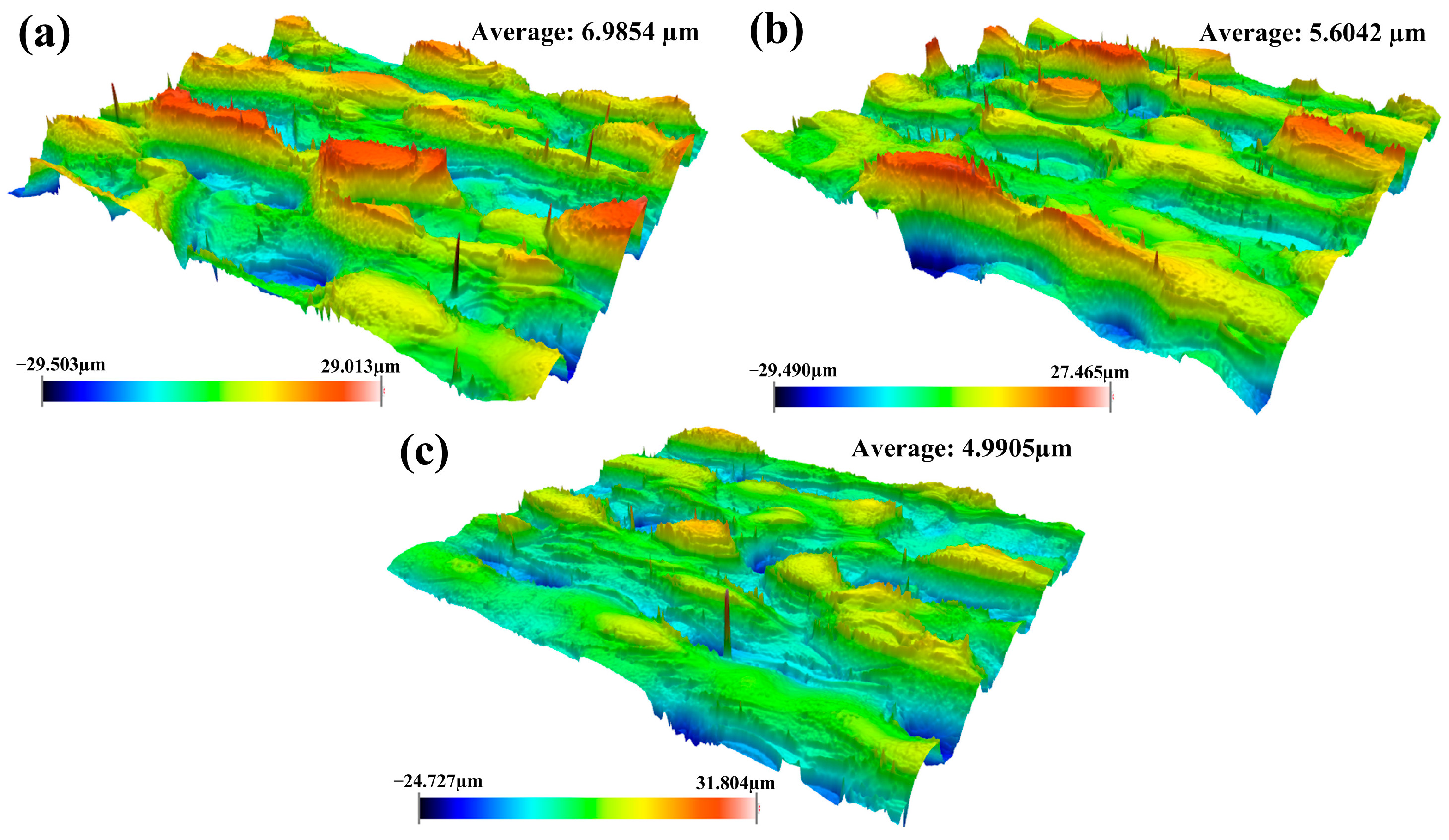
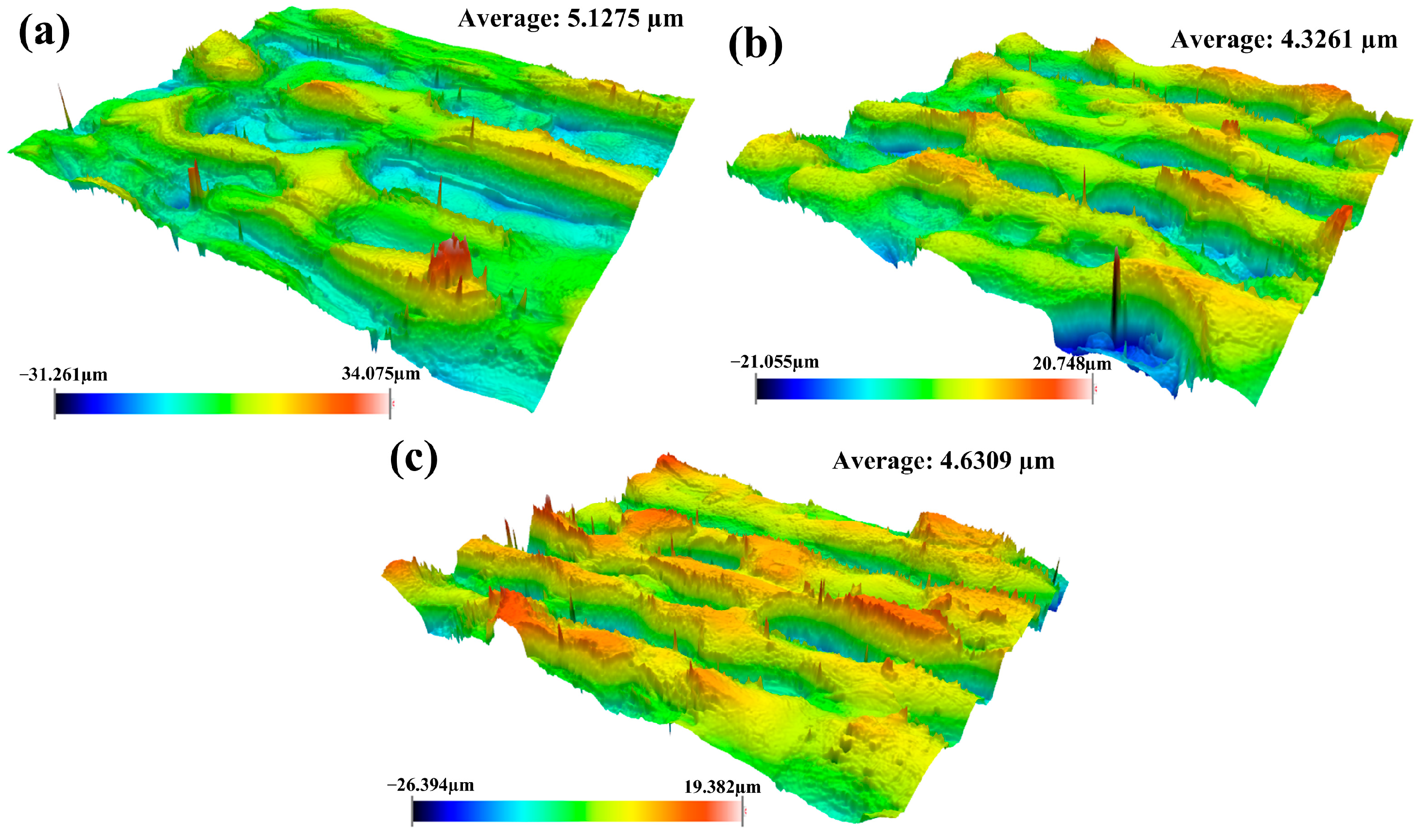
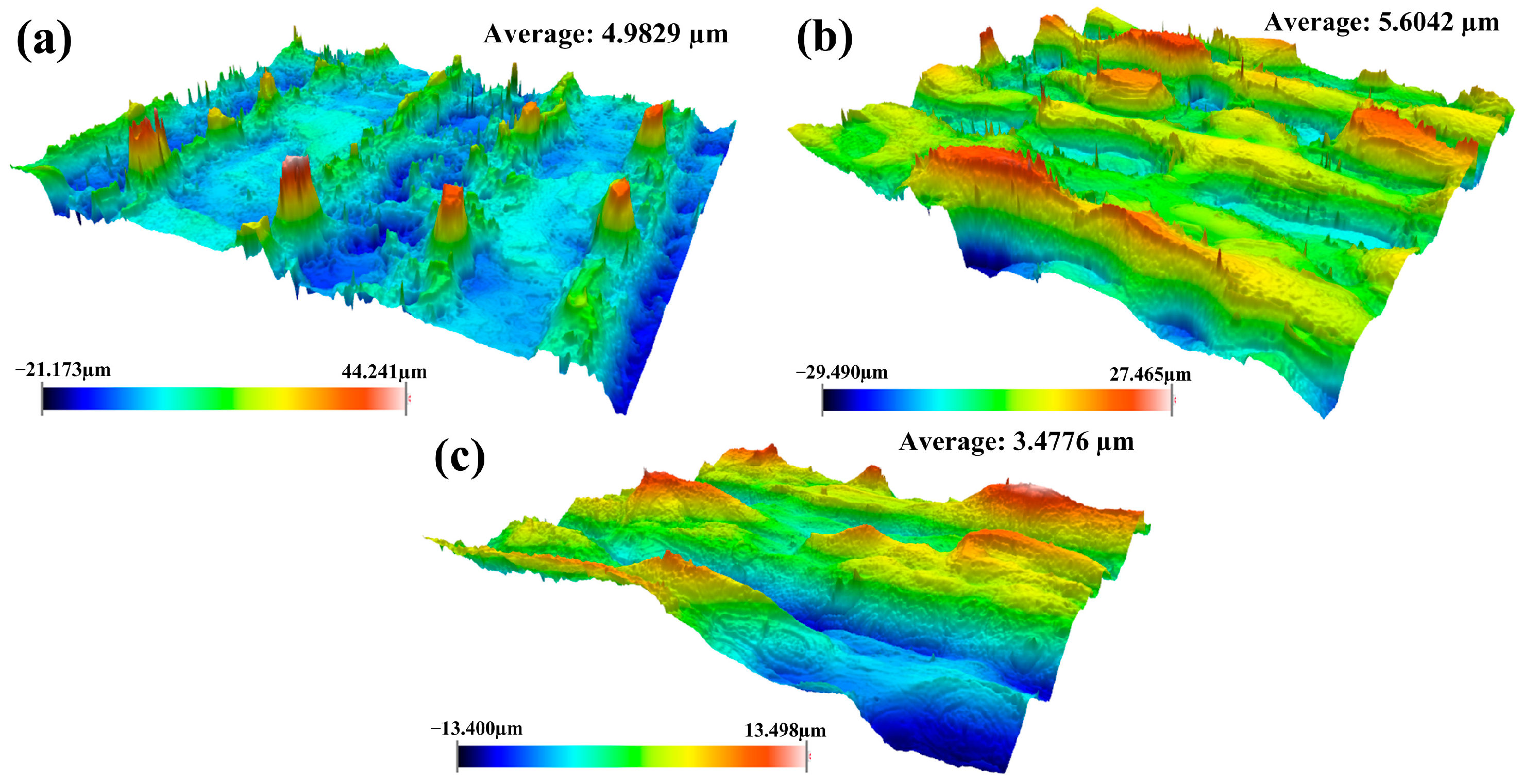
3.2.2. Surface Roughness Under Optimized Process Conditions
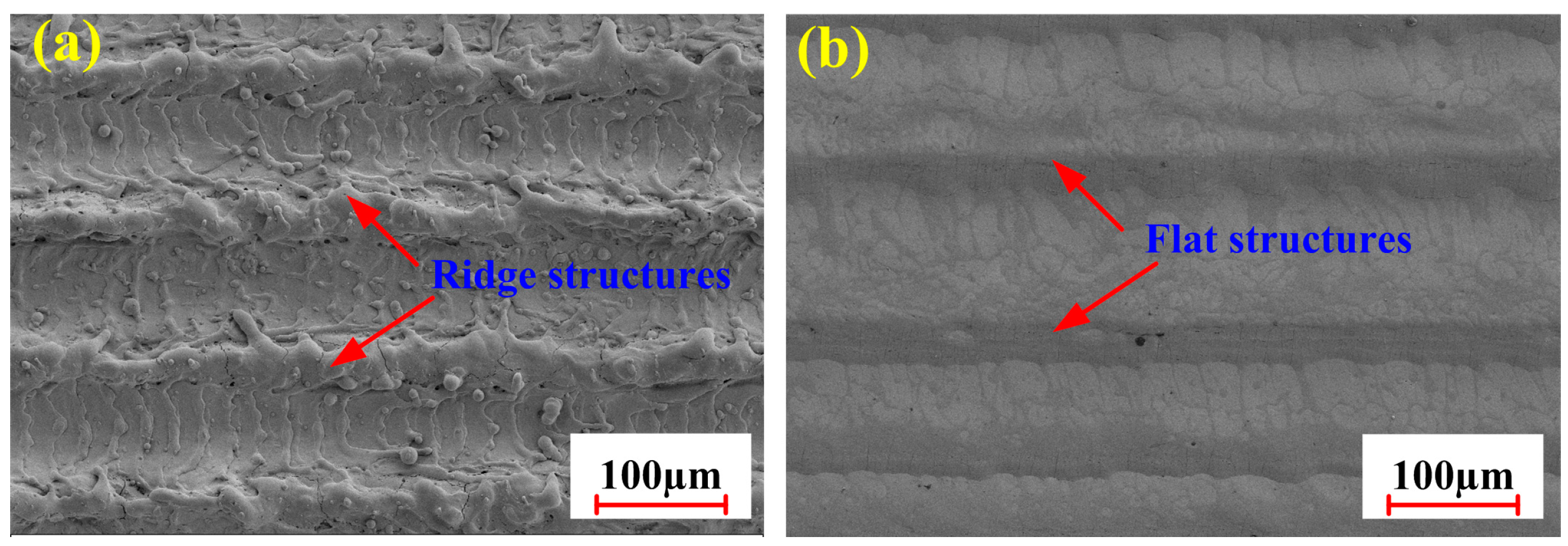
3.2.3. Micro-Morphology Under Optimized Process Conditions
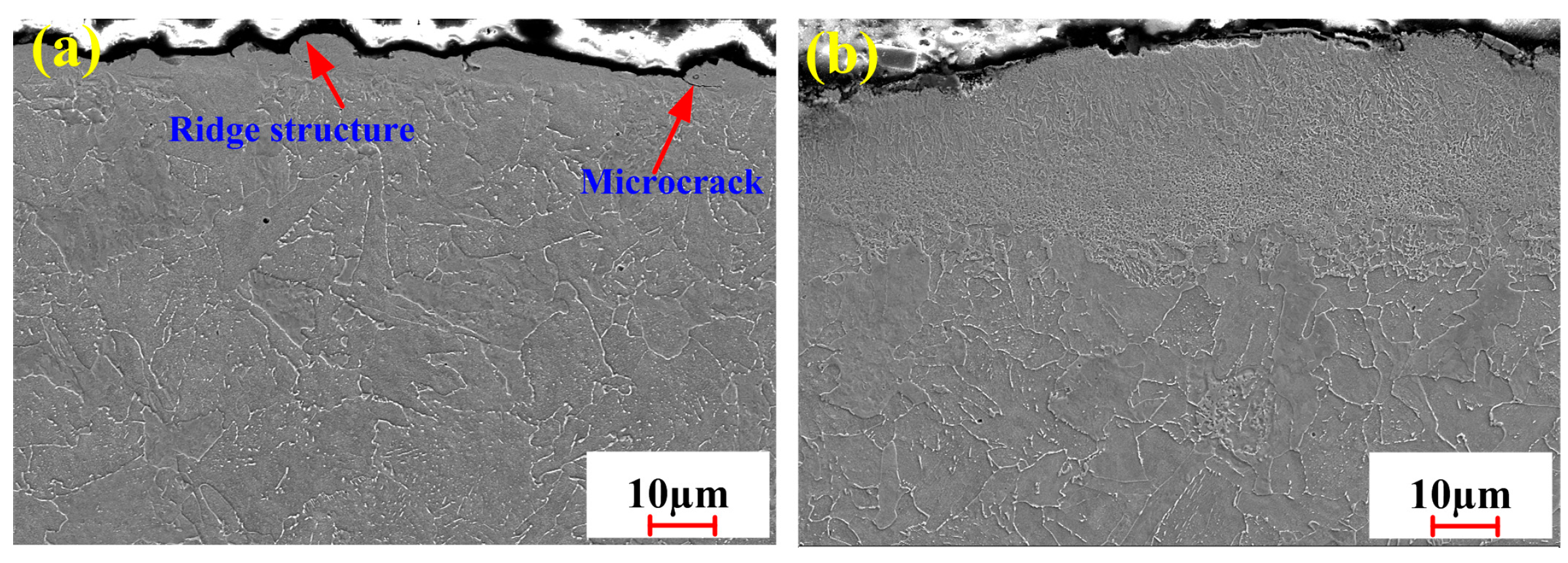
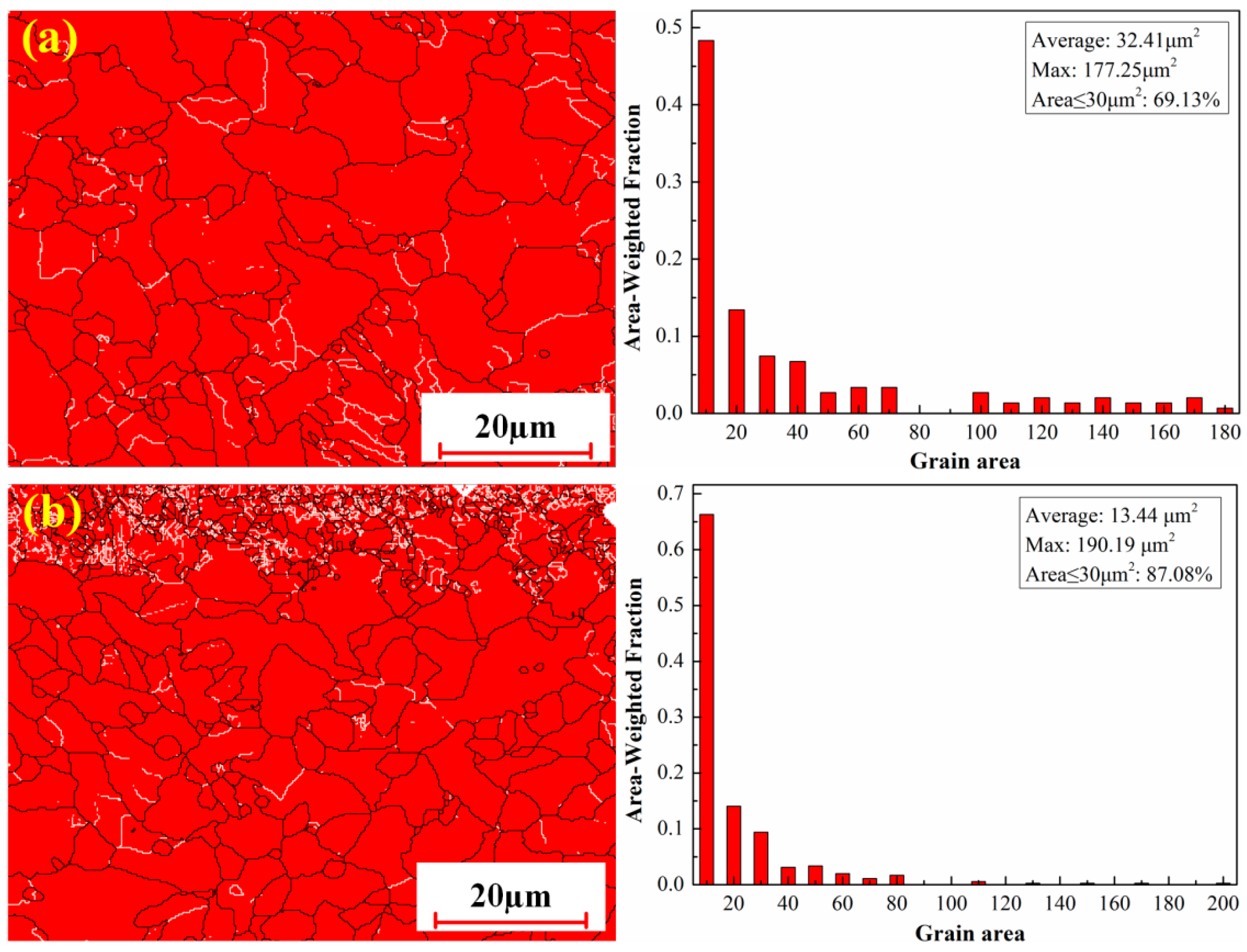
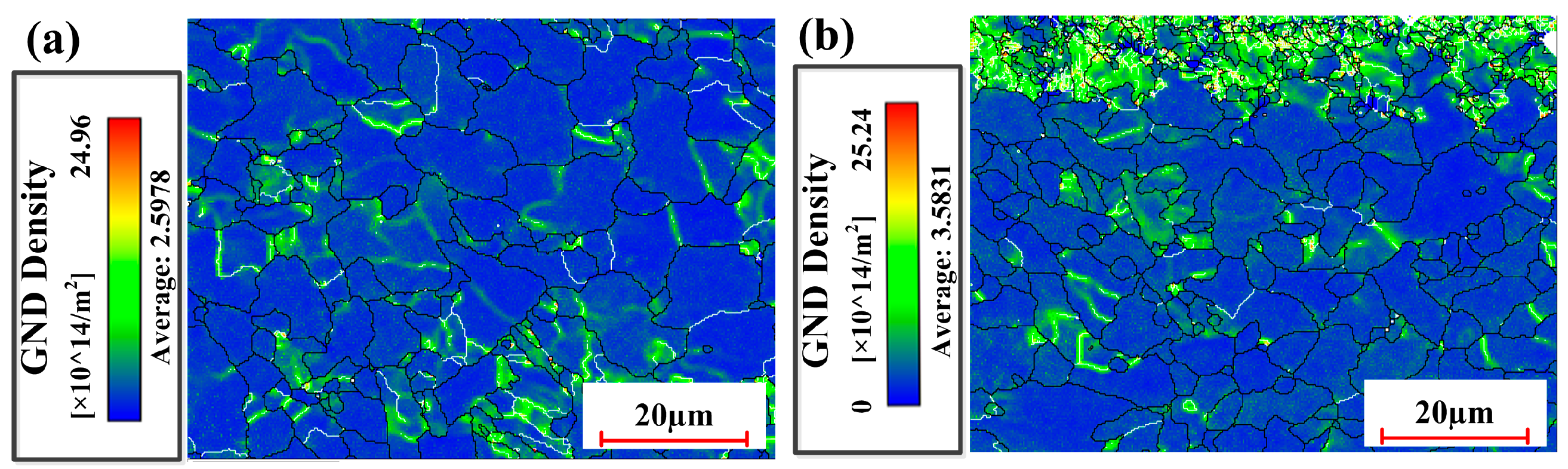
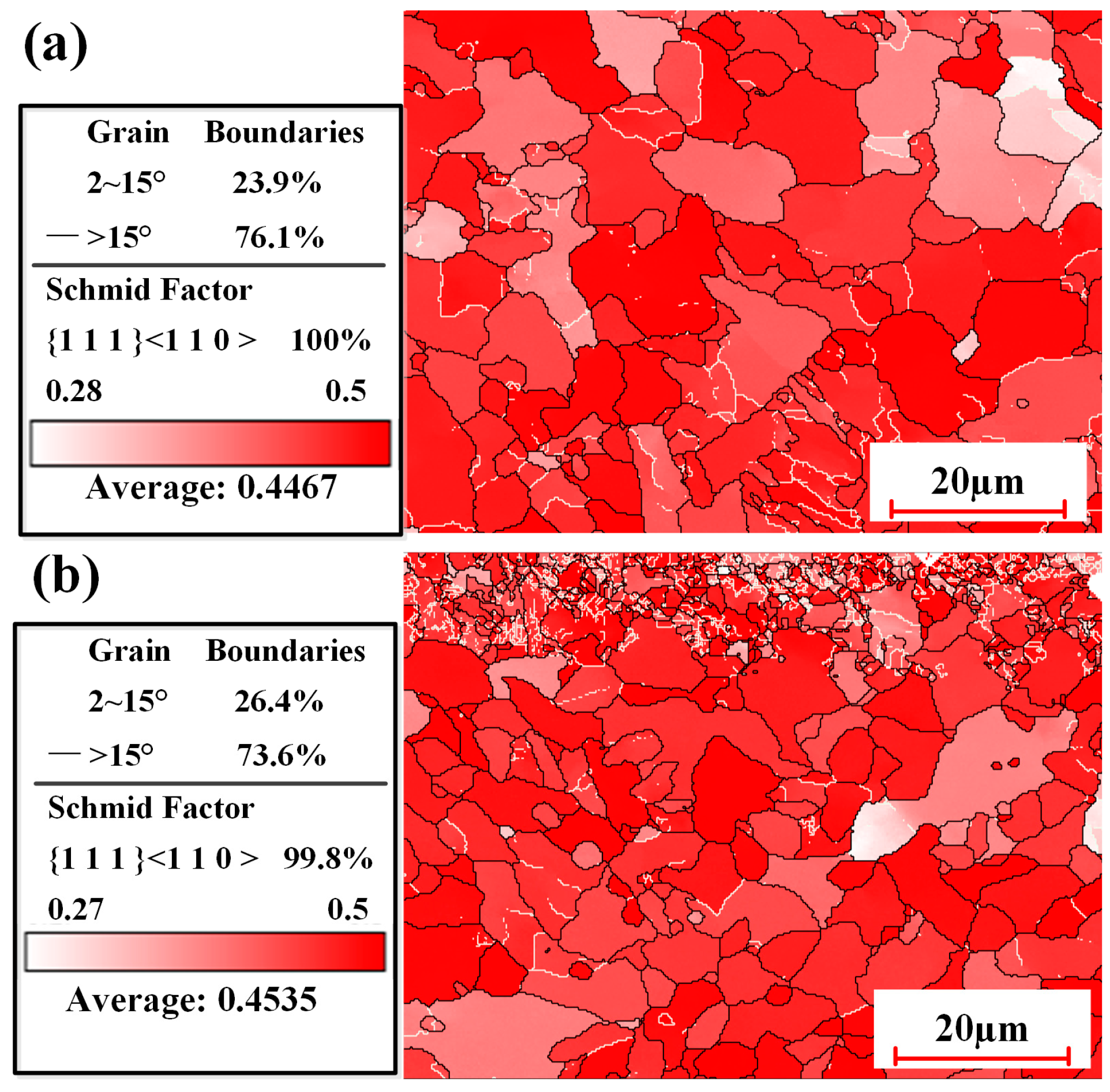
3.2.4. Microstructure Under Optimized Process Conditions
3.3. Micro-Hardness Characterization
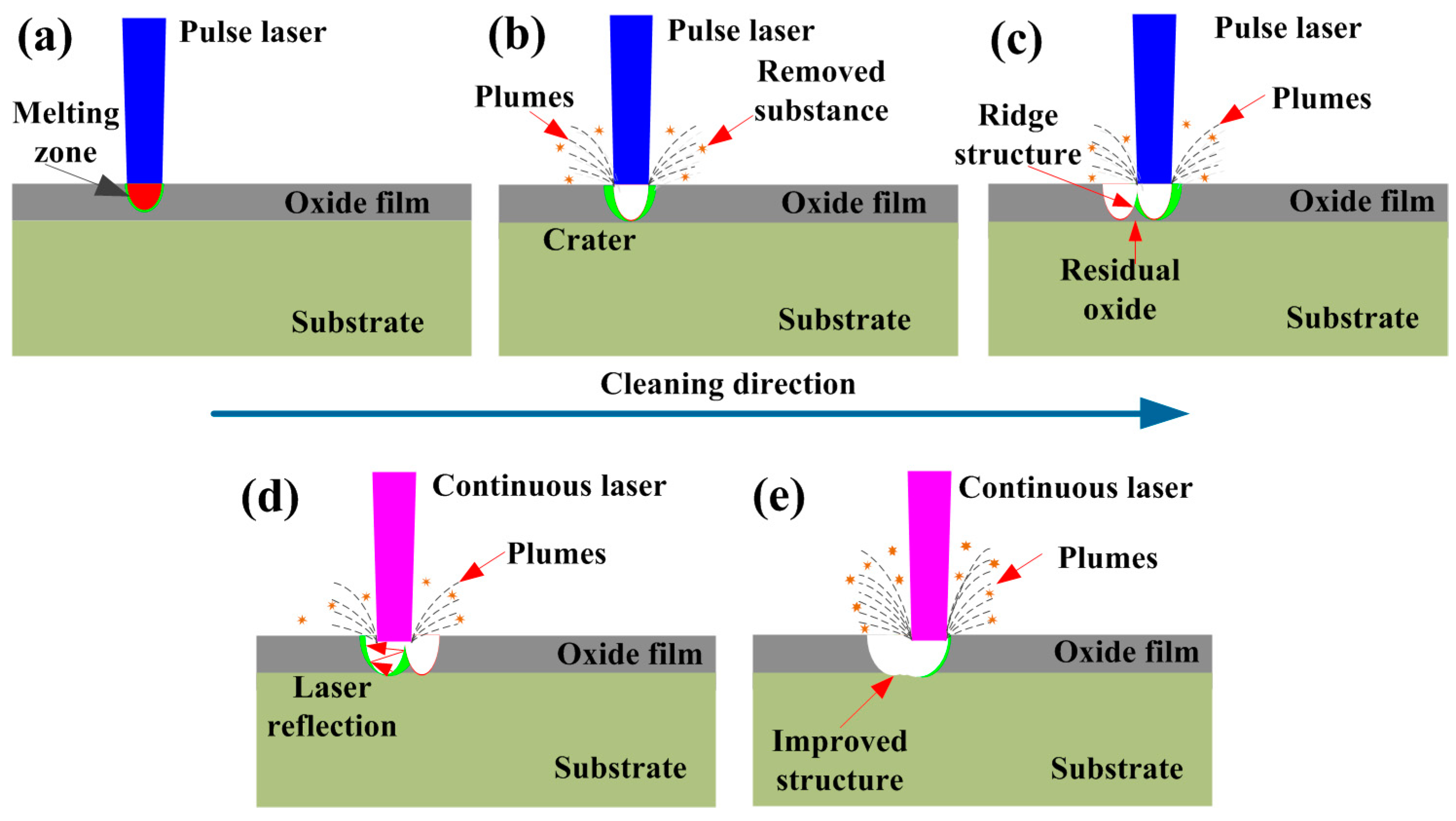
3.4. Oxide Removal Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yilmaz, A.F. Assessment of Combinability of S235JR-S460MC Structural Steels on Fatigue Performance. Trans. Indian Inst. Met. 2024, 77, 323–331. [Google Scholar] [CrossRef]
- Farhan, A.; Fadhilah, T.A. Impact toughness of ASTM A36 low carbon steel by metal active gas (MAG) welding process using different cooling media. Metalurgija 2022, 61, 641–644. [Google Scholar]
- Lim, H.; Kim, D. Laser-assisted chemical cleaning for oxide-scale removal from carbon steel surfaces. J. Laser Appl. 2004, 16, 25–30. [Google Scholar] [CrossRef]
- Javed, M.A.; Rieders, N.; Beech, I.; Avci, R.; Neil, W.C.; Wade, S.A. The influence of chemical cleaning methods on pitting morphology attributed to microbially influenced corrosion of stainless steels. Corrosion 2021, 77, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Gul, A.; Hruza, J.; Yalcinkaya, F. Fouling and chemical cleaning of microfiltration membranes: A mini-review. Polymers 2021, 13, 846. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, X.; Zhang, W.; Wang, X. Enhancing stress corrosion cracking resistance of machined surface via surface mechanical grinding treatment for AISI 316 L stainless steel. Mater. Charact. 2022, 194, 112493. [Google Scholar] [CrossRef]
- Carvalhao, M.; Dionísio, A. Evaluation of mechanical soft-abrasive blasting and chemical cleaning methods on alkyd-paint graffiti made on calcareous stones. J. Cult. Herit. 2015, 16, 579–590. [Google Scholar] [CrossRef]
- Brezinová, J.; Guzanová, A.; Draganovská, D. Abrasive Blast Cleaning and Its Application. Found. Mater. Sci. Eng. 2015, 83, I. [Google Scholar]
- Kishore, P.S.; Rajnish, K.; Vamsi, V.N. Comparative study of mechanical and chemical methods for surface cleaning of a marine shell-and-tube heat exchanger. Heat Transf. Res. 2018, 47, 523–530. [Google Scholar]
- Wang, A.; Feng, A.; Gu, X.; Pan, X.; Yu, J.; Jiang, Z. Effect of picosecond laser cleaning on surface morphology and properties of stainless steel. Opt. Laser Technol. 2023, 159, 109041. [Google Scholar] [CrossRef]
- Jelvani, S.; Maleki, M.H.; Khadir, S.; Darkhal, N.; Ebrahimi, A. Laser cleaning on bronze artefacts by the XeCl laser. Optik 2021, 242, 167316. [Google Scholar] [CrossRef]
- Nie, J.; Zhang, H.; Zhang, D.; Xu, J.; Zhang, J.; Shan, D.; Guo, B. Removal mechanism of laser cleaning for inorganic thermal control coatings on aluminum alloys. Appl. Surf. Sci. 2023, 633, 157578. [Google Scholar] [CrossRef]
- Kumar, A.; Sapp, M.; Vincelli, J.; Gupta, M.C. A study on laser cleaning and pulsed gas tungsten arc welding of Ti–3Al–2.5 V alloy tubes. J. Mater. Process. Technol. 2010, 210, 64–71. [Google Scholar] [CrossRef]
- Guo, H.; Martukanitz, R.; DebRoy, T. Laser assisted cleaning of oxide films on SUS409 stainless steel. J. Laser Appl. 2004, 16, 236–244. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, Q.; Long, W.; Wang, C.; Lin, J.; Liu, Z. Welding defect and mechanical properties of nanosecond laser cleaning 6005A aluminum alloy. Materials 2022, 15, 7841. [Google Scholar] [CrossRef]
- Dong, H.; Li, Y.; Lu, S.; Zhang, W.; Jin, G. Nanosecond Laser Cleaning of Aluminum Alloy Oxide Film. Curr. Opt. Photonics 2023, 7, 714–720. [Google Scholar]
- Daminelli, G.; Meja, P.; Cortona, A.; Krüger, J.; Autric, M.; Kautek, W. Femtosecond and nanosecond laser removal of anodic oxide layers from aluminum. In High-Power Laser Ablation IV; SPIE: Wellingham, WA, USA, 2002; Volume 4760, pp. 239–246. [Google Scholar]
- Li, X.; Guan, Y. Real-time monitoring of laser cleaning for hot-rolled stainless steel by laser-induced breakdown spectroscopy. Metals 2021, 11, 790. [Google Scholar] [CrossRef]
- Zhou, C.; Li, H.; Chen, G.; Wang, G.; Shan, Z. Effect of single pulsed picosecond and 100 nanosecond laser cleaning on surface morphology and welding quality of aluminium alloy. Opt. Laser Technol. 2020, 127, 106197. [Google Scholar] [CrossRef]
- Garbacz, H.; Fortuna, E.; Marczak, J.; Strzelec Rycyk, M.A.; Koss, A.; Mróz, J.; Zatorska, A.; Kurzydlowski, K.J. Laser cleaning of copper roofing sheets subjected to long-lasting environmental corrosion. Appl. Phys. A 2010, 100, 693–701. [Google Scholar] [CrossRef]
- Wang, W.; Shen, J.; Liu, W.; Bian, H.; Li, Q. Effect of laser energy density on surface physical characteristics and corrosion resistance of 7075 aluminum alloy in laser cleaning. Opt. Laser Technol. 2022, 148, 107742. [Google Scholar] [CrossRef]
- Sun, Z.; Xu, J.; Zhou, W. Parameters and mechanism of laser cleaning rust deposit on the steel surface. In Lasers in Material Processing and Manufacturing; SPIE: Wellingham, WA, USA, 2002; Volume 4915, pp. 374–377. [Google Scholar]
- Psyllaki, P.; Oltra, R. Preliminary study on the laser cleaning of stainless steels after high temperature oxidation. Mater. Sci. Eng. A 2000, 282, 145–152. [Google Scholar] [CrossRef]
- Sun, X.; Yu, Q.; Bai, X.; Jin, G.; Cai, J.; Yuan, B. Substrate cleaning threshold for various coated Al alloys using a continuous-wave laser. Photonics 2021, 8, 395. [Google Scholar] [CrossRef]
- Zhuang, S.; Kainuma, S.; Yang, M.; Haraguchi, M.; Sano, T.A. Investigation on the peak temperature and surface defects on the carbon steel treated by rotating CW laser. Opt. Laser Technol. 2021, 135, 106727. [Google Scholar] [CrossRef]
- Barletta, M.; Gisario, A.; Tagliaferri, V. An application of a high power diode laser to remove oxides on AISI 316L stainless steel. Int. J. Mater. Prod. Technol. 2008, 32, 71–91. [Google Scholar] [CrossRef]
- Turner, M.W.; Crouse, P.L.; Li, L. Comparison of mechanisms and effects of Nd: YAG and CO2 laser cleaning of titanium alloys. Appl. Surf. Sci. 2006, 252, 4792–4797. [Google Scholar] [CrossRef]
- Eskandari, M.J.; Karimi, M.; Araghchi, M.; Hadipour, A. Laser cleaning process of high-pressure turbine blade: Characterization and removal of surface contaminants. Surf. Coat. Technol. 2023, 470, 129885. [Google Scholar] [CrossRef]
- Cui, K.; Luo, J.; Xu, K.; Ling, L.; Cheng, R. The Study of Patterns and Mechanisms of Continuous Laser Ablation of Carbon Steel Rust Layers in Multi-Medium Environments. Appl. Sci. 2024, 14, 5052. [Google Scholar] [CrossRef]
- Zhang, G.; Hua, X.; Huang, Y.; Zhang, Y.; Li, F.; Shen, C.; Cheng, J. Investigation on mechanism of oxide removal and plasma behavior during laser cleaning on aluminum alloy. Appl. Surf. Sci. 2020, 506, 144666. [Google Scholar] [CrossRef]
- Hu, C.; Yan, F.; Zhu, Z.; Xu, Y.; Tao, J.; Wang, C. Effects on microstructural refinement of mechanical properties in steel-copper joints laser welded with alternating magnetic field augmentation. Mater. Charact. 2021, 175, 111059. [Google Scholar] [CrossRef]
- Rezayat, M.; Roa, J.J.; Mateo, A. Effect of laser surface texturing on Schmid factor and plastic deformation mechanisms on AISI 301LN steel. Metals 2023, 13, 1789. [Google Scholar] [CrossRef]
- Rezayat, M.; Karamimoghadam, M.; Moradi, M.; Casalino, G.; Rovira, J.J.R.; Mateo, A. Overview of surface modification strategies for improving the properties of metastable austenitic stainless steels. Metals 2023, 13, 1268. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, D.; Su, X.; Yang, S.; Xu, J.; Ma, R.; Shan, D.; Guo, B. Removal mechanism of surface cleaning on TA15 titanium alloy using nanosecond pulsed laser. Opt. Laser Technol. 2021, 139, 106998. [Google Scholar] [CrossRef]
- Ali, N.; Bashir, S.; Umm-i-Kalsoom Akram, M.; Mahmood, K. Effect of dry and wet ambient environment on the pulsed laser ablation of titanium. Appl. Surf. Sci. 2013, 270, 49–57. [Google Scholar] [CrossRef]
- Li, Z.C.; Chen, X.; Yang, S.R.; Zhang, D.H.; Xu, J.; Ma, R.; Shan, D.B.; Guo, B. Removal mechanism of liquid-assisted nanosecond pulsed laser cleaning TA15 titanium alloy oxide film. J. Mater. Res. Technol. 2022, 19, 4986–4997. [Google Scholar] [CrossRef]




| C | S | P | Si | Mn | Fe |
|---|---|---|---|---|---|
| 0.20 | 0.012 | 0.016 | 0.12 | 0.21 | Bal. |
| No. | Laser Linewidth (mm) | Laser Frequency (kHz) | Cleaning Speed (mm/s) | Oxide Removal Amount (mg) |
|---|---|---|---|---|
| 1 | 10 | 500 | 15 | 1.6 |
| 2 | 30 | 500 | 15 | 2.8 |
| 3 | 50 | 500 | 15 | 3.7 |
| 4 | 70 | 500 | 15 | 2.4 |
| 5 | 90 | 500 | 15 | 2.3 |
| 6 | 50 | 100 | 15 | 0.7 |
| 7 | 50 | 300 | 15 | 1.6 |
| 8 | 50 | 700 | 15 | 2.5 |
| 9 | 50 | 900 | 15 | 1.5 |
| 10 | 50 | 500 | 5 | 0.9 |
| 11 | 50 | 500 | 10 | 1.0 |
| 12 | 50 | 500 | 20 | 1.6 |
| 13 | 50 | 500 | 25 | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Wang, C.; Wu, Q.; Yan, F.; Zhu, G.; Wang, J. Oxide Removal Mechanism and Process Optimization During Integrated Pulsed-Continuous Laser Cleaning of Q235B Carbon Steel. Materials 2025, 18, 1247. https://doi.org/10.3390/ma18061247
Zhang W, Wang C, Wu Q, Yan F, Zhu G, Wang J. Oxide Removal Mechanism and Process Optimization During Integrated Pulsed-Continuous Laser Cleaning of Q235B Carbon Steel. Materials. 2025; 18(6):1247. https://doi.org/10.3390/ma18061247
Chicago/Turabian StyleZhang, Wei, Chunming Wang, Qiong Wu, Fei Yan, Guoli Zhu, and Junqiang Wang. 2025. "Oxide Removal Mechanism and Process Optimization During Integrated Pulsed-Continuous Laser Cleaning of Q235B Carbon Steel" Materials 18, no. 6: 1247. https://doi.org/10.3390/ma18061247
APA StyleZhang, W., Wang, C., Wu, Q., Yan, F., Zhu, G., & Wang, J. (2025). Oxide Removal Mechanism and Process Optimization During Integrated Pulsed-Continuous Laser Cleaning of Q235B Carbon Steel. Materials, 18(6), 1247. https://doi.org/10.3390/ma18061247





