Removal of Per- and Polyfluoroalkyl Substances Using Commercially Available Sorbents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
2.2. Sorption and Desorption Experiments
2.3. PFAS Quantification
2.4. Data Analysis
3. Results and Discussion
3.1. Sorbent Characterization
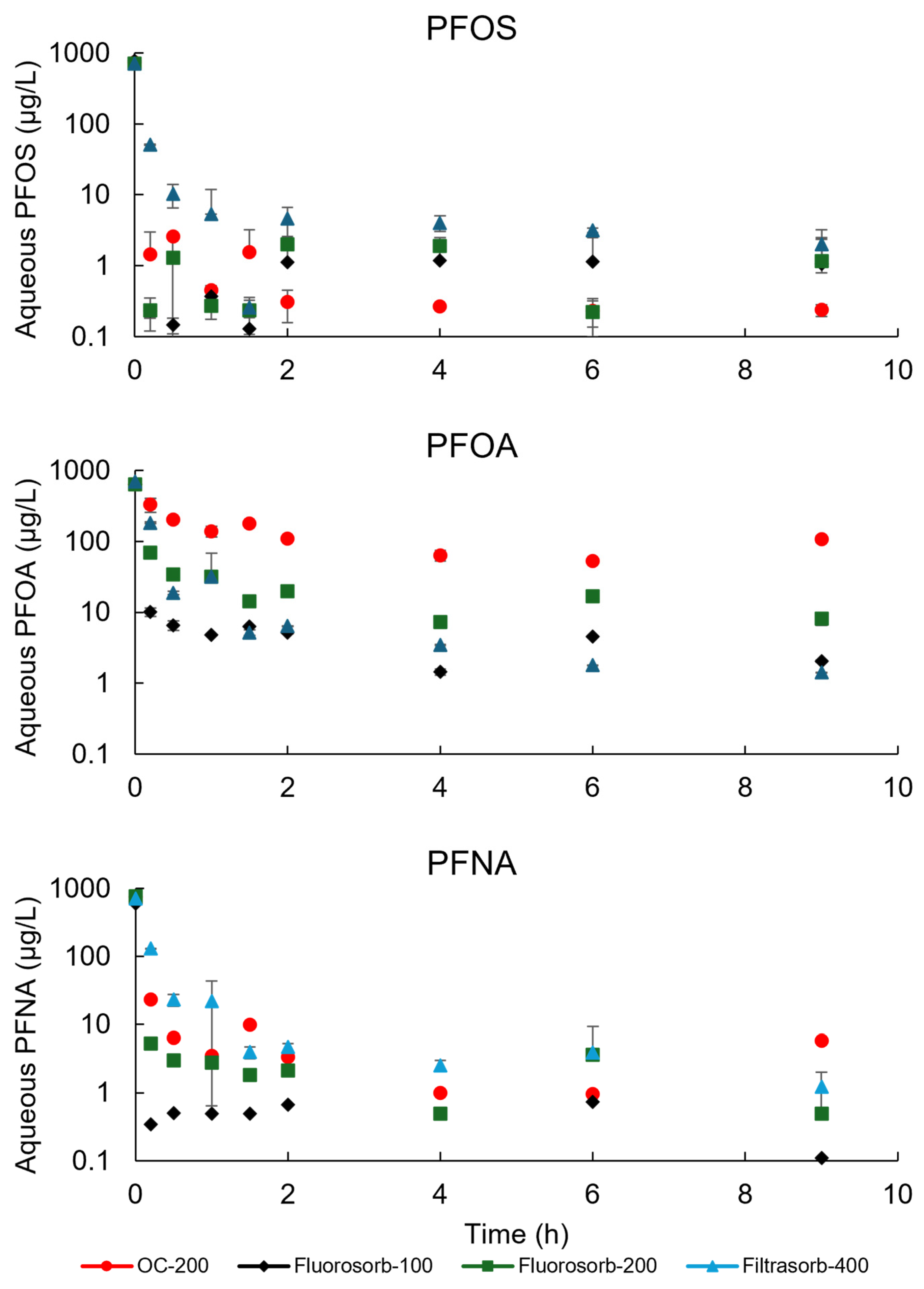
3.2. Sorption Kinetics
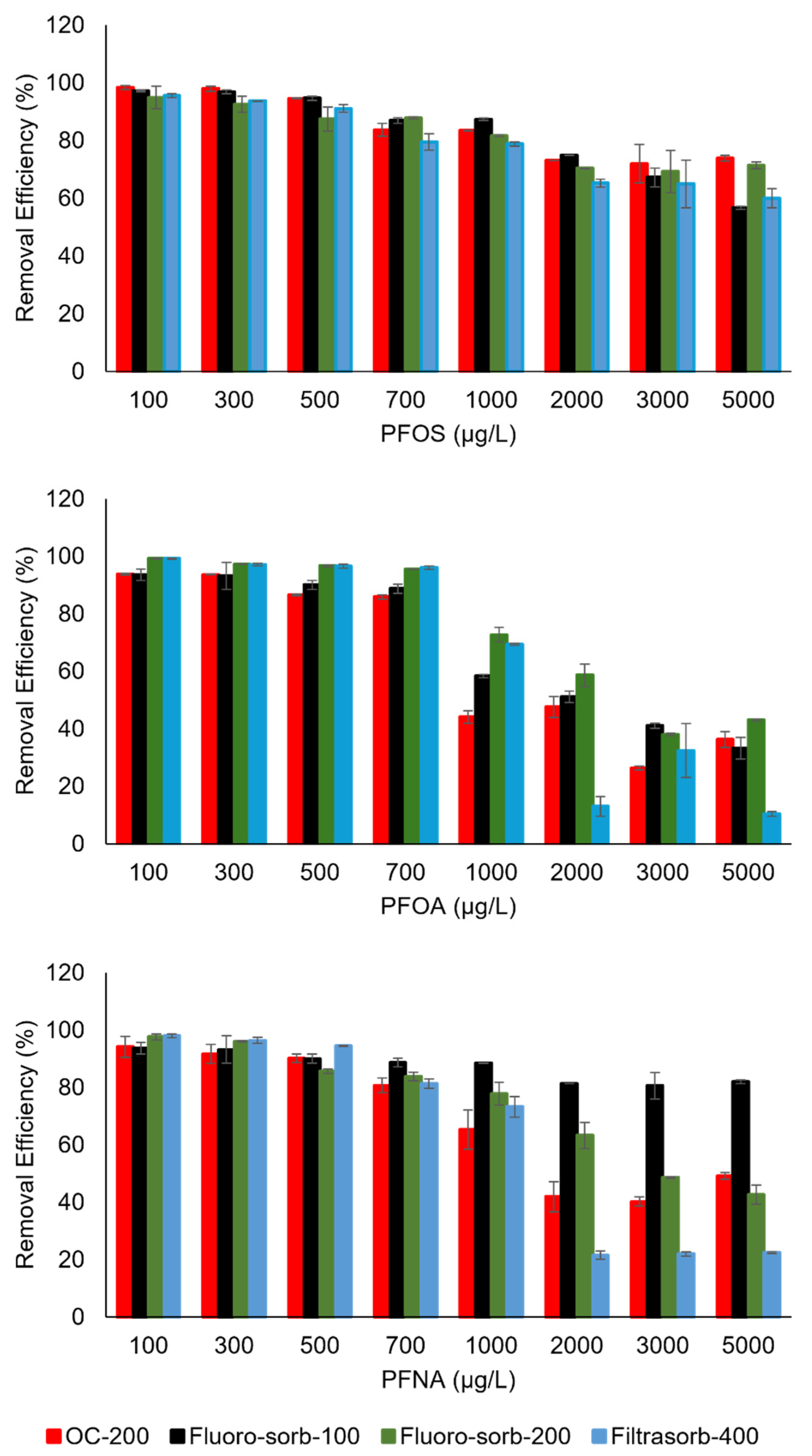
3.3. Sorption Isotherms
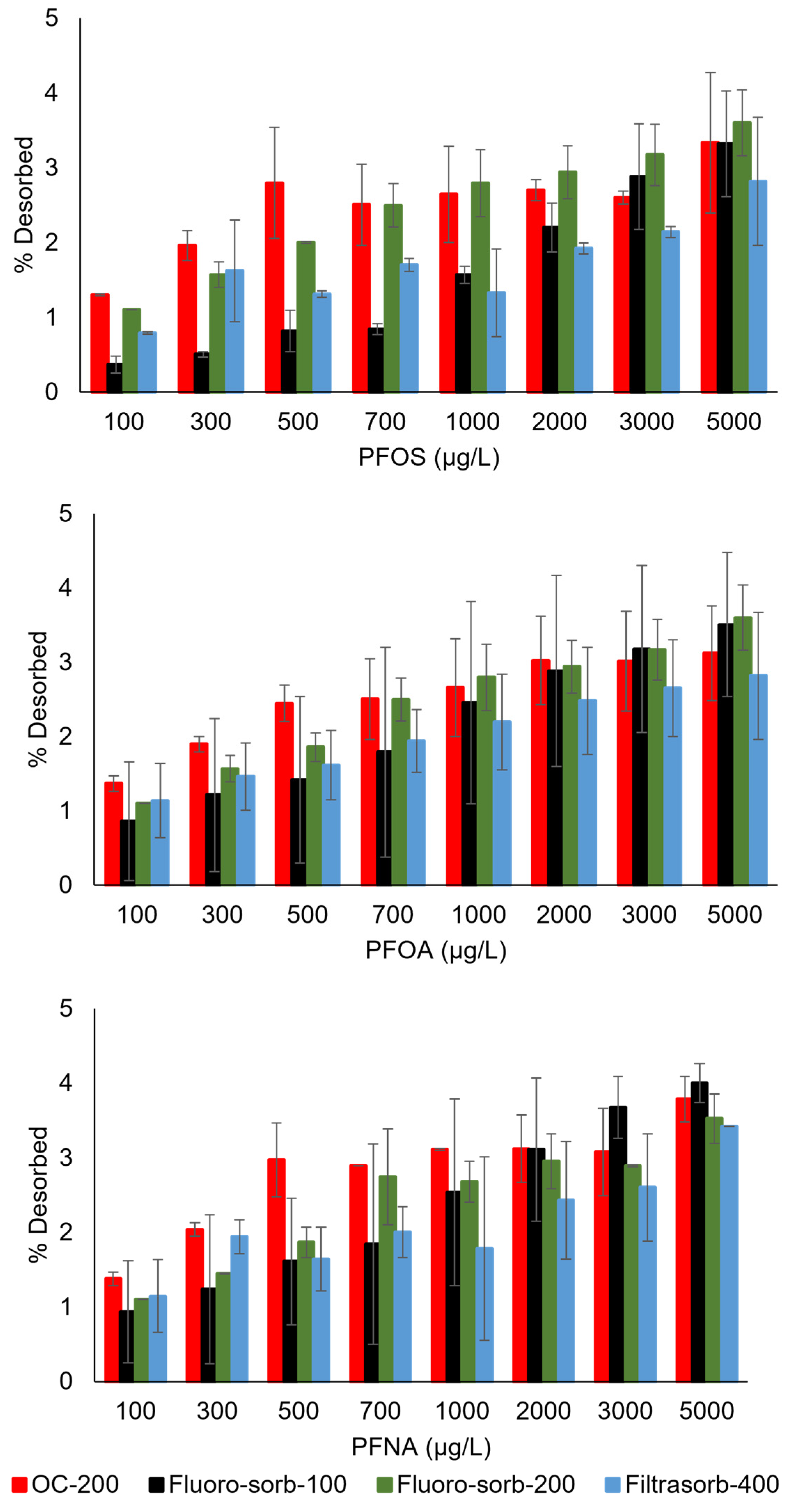
3.4. Potential PFAS Interactions with Sorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EDS | Energy-dispersive spectroscopy |
| FTIR | Fourier transform infrared spectra |
| GAC | Granular activated carbon |
| PFAAs | Perfluoroalkyl acids |
| PFAS | Per- and polyfluoroalkyl substance |
| PFNA | Perfluorononanoic acid |
| PFOA | Polyfluorooctanoic acid |
| PFOS | Polyfluorooctane sulfonic acid |
| PFSAs | Perfluoroalkane sulfonic acids |
| SEM | Scanning electron microscopy |
| XRD | X-ray diffraction |
References
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; De Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef] [PubMed]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per-and polyfluoroalkyl substance toxicity and human health review: Current state of knowledge and strategies for informing future research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.G.; Lim, H.J.; Na, S.H.; Choi, B.I.; Shin, D.S.; Chung, S.Y. Biodegradation of perfluorooctanesulfonate (PFOS) as an emerging contaminant. Chemosphere 2014, 109, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Soriano, A.; Schaefer, C.; Urtiaga, A. Enhanced treatment of perfluoroalkyl acids in groundwater by membrane separation and electrochemical oxidation. Chem. Eng. J. Adv. 2020, 4, 100042. [Google Scholar] [CrossRef]
- Fujioka, T.; Takeuchi, H.; Tahara, H.; Murakami, H.; Boivin, S. Effects of functional groups of polyfluoroalkyl substances on their removal by nanofiltration. Water Res. X 2024, 24, 100233. [Google Scholar] [CrossRef]
- Zhi, Y.; Zhao, X.; Qian, S.; Faria, A.F.; Lu, X.; Wang, X.; Li, W.; Han, L.; Tao, Z.; He, Q. Removing emerging perfluoroalkyl ether acids and fluorotelomer sulfonates from water by nanofiltration membranes: Insights into performance and underlying mechanisms. Sep. Purif. Technol. 2022, 298, 121648. [Google Scholar] [CrossRef]
- Gagliano, E.; Sgroi, M.; Falciglia, P.P.; Vagliasindi, F.G.; Roccaro, P. Removal of poly-and perfluoroalkyl substances (PFAS) from water by adsorption: Role of PFAS chain length, effect of organic matter and challenges in adsorbent regeneration. Water Res. 2020, 171, 115381. [Google Scholar] [CrossRef]
- Zhang, Z.; Sarkar, D.; Datta, R.; Deng, Y. Adsorption of perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) by aluminum-based drinking water treatment residuals. J. Hazard. Mater. Lett. 2021, 2, 100034. [Google Scholar] [CrossRef]
- Kucharzyk, K.H.; Darlington, R.; Benotti, M.; Deeb, R.; Hawley, E. Novel treatment technologies for PFAS compounds: A critical review. J. Environ. Manag. 2017, 204, 757–764. [Google Scholar] [CrossRef]
- Appleman, T.D.; Higgins, C.P.; Quiñones, O.; Vanderford, B.J.; Kolstad, C.; Zeigler-Holady, J.C.; Dickenson, E.R. Treatment of poly-and perfluoroalkyl substances in US full-scale water treatment systems. Water Res. 2014, 51, 246–255. [Google Scholar] [CrossRef]
- Crone, B.C.; Speth, T.F.; Wahman, D.G.; Smith, S.J.; Abulikemu, G.; Kleiner, E.J.; Pressman, J.G. Occurrence of per-and polyfluoroalkyl substances (PFAS) in source water and their treatment in drinking water. Crit. Rev. Environ. Sci. Technol. 2019, 49, 2359–2396. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D. Permeable reactive barriers: A sustainable technology for cleaning contaminated groundwater in developing countries. Desalination 2009, 248, 352–359. [Google Scholar] [CrossRef]
- Naidu, R.; Bekele, D.N.; Birke, V. Permeable reactive barriers: Cost-effective and sustainable remediation of groundwater. In Permeable Reactive Barrier: Sustainable Groundwater Remediation; CRC Press: Boca Raton, FL, USA, 2014; pp. 1–23. [Google Scholar]
- Chen, L.; Wang, C.; Zhang, C.; Zhang, X.; Liu, F. Eight-year performance evaluation of a field-scale zeolite permeable reactive barrier for the remediation of ammonium-contaminated groundwater. Appl. Geochem. 2022, 143, 105372. [Google Scholar] [CrossRef]
- Sakr, M.; El Agamawi, H.; Klammler, H.; Mohamed, M.M. A review on the use of permeable reactive barriers as an effective technique for groundwater remediation. Groundw. Sustain. Dev. 2023, 21, 100914. [Google Scholar] [CrossRef]
- McCleaf, P.; Englund, S.; Östlund, A.; Lindegren, K.; Wiberg, K.; Ahrens, L. Removal efficiency of multiple poly-and perfluoroalkyl substances (PFASs) in drinking water using granular activated carbon (GAC) and anion exchange (AE) column tests. Water Res. 2017, 120, 77–87. [Google Scholar] [CrossRef]
- Zeng, C.; Atkinson, A.; Sharma, N.; Ashani, H.; Hjelmstad, A.; Venkatesh, K.; Westerhoff, P. Removing per-and polyfluoroalkyl substances from groundwaters using activated carbon and ion exchange resin packed columns. AWWA Water Sci. 2020, 2, e1172. [Google Scholar] [CrossRef]
- Cantoni, B.; Turolla, A.; Wellmitz, J.; Ruhl, A.S.; Antonelli, M. Perfluoroalkyl substances (PFAS) adsorption in drinking water by granular activated carbon: Influence of activated carbon and PFAS characteristics. Sci. Total Environ. 2021, 795, 148821. [Google Scholar] [CrossRef]
- Mao, S.; Gao, M. Functional organoclays for removal of heavy metal ions from water: A review. J. Mol. Liq. 2021, 334, 116143. [Google Scholar] [CrossRef]
- McNamara, J.D.; Franco, R.; Mimna, R.; Zappa, L. Comparison of activated carbons for removal of perfluorinated compounds from drinking water. J.-Am. Water Work. Assoc. 2018, 110, E2–E14. [Google Scholar] [CrossRef]
- Benstoem, F.; Nahrstedt, A.; Boehler, M.; Knopp, G.; Montag, D.; Siegrist, H.; Pinnekamp, J. Performance of granular activated carbon to remove micropollutants from municipal wastewater—A meta-analysis of pilot-and large-scale studies. Chemosphere 2017, 185, 105–118. [Google Scholar] [CrossRef]
- Park, M.; Wu, S.; Lopez, I.J.; Chang, J.Y.; Karanfil, T.; Snyder, S.A. Adsorption of perfluoroalkyl substances (PFAS) in groundwater by granular activated carbons: Roles of hydrophobicity of PFAS and carbon characteristics. Water Res. 2020, 170, 115364. [Google Scholar] [CrossRef] [PubMed]
- Militao, I.M.; Roddick, F.A.; Bergamasco, R.; Fan, L. Removing PFAS from aquatic systems using natural and renewable material-based adsorbents: A review. J. Environ. Chem. Eng. 2021, 9, 105271. [Google Scholar] [CrossRef]
- Márquez, P.; Benítez, A.; Chica, A.; Martín, M.; Caballero, A. Evaluating the thermal regeneration process of massively generated granular activated carbons for their reuse in wastewater treatments plants. J. Clean. Prod. 2022, 366, 132685. [Google Scholar] [CrossRef]
- Nafees, M.; Waseem, A. Organoclays as sorbent material for phenolic compounds: A review. CLEAN–Soil Air Water 2014, 42, 1500–1508. [Google Scholar] [CrossRef]
- Guégan, R. Organoclay applications and limits in the environment. C. R. Chim. 2019, 22, 132–141. [Google Scholar] [CrossRef]
- Xi, Y.; Ding, Z.; He, H.; Frost, R.L. Structure of organoclays—An X-ray diffraction and thermogravimetric analysis study. J. Colloid Interface Sci. 2004, 277, 116–120. [Google Scholar] [CrossRef]
- Ateia, M.; Alsbaiee, A.; Karanfil, T.; Dichtel, W. Efficient PFAS removal by amine-functionalized sorbents: Critical review of the current literature. Environ. Sci. Technol. Lett. 2019, 6, 688–695. [Google Scholar] [CrossRef]
- Ji, W.; Xiao, L.; Ling, Y.; Ching, C.; Matsumoto, M.; Bisbey, R.P.; Helbling, D.E.; Dichtel, W.R. Removal of GenX and perfluorinated alkyl substances from water by amine-functionalized covalent organic frameworks. J. Am. Chem. Soc. 2018, 140, 12677–12681. [Google Scholar] [CrossRef]
- Ochoa-Herrera, V.; Sierra-Alvarez, R. Removal of perfluorinated surfactants by sorption onto granular activated carbon, zeolite and sludge. Chemosphere 2008, 72, 1588–1593. [Google Scholar] [CrossRef]
- Ho, Y.-S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Keshmiri-Naqab, R.; Taghavijeloudar, M. Could organoclay be used as a promising natural adsorbent for efficient and cost-effective dye wastewater treatment? J. Environ. Manag. 2023, 342, 118322. [Google Scholar] [CrossRef] [PubMed]
- Rajab-Qurchi, M.; Toiserkani, H. Fabrication and evaluation of organoclay-reinforced epoxidized natural rubber nanocomposites: A comprehensive study. Polymer 2024, 299, 126976. [Google Scholar] [CrossRef]
- Yan, B.; Munoz, G.; Sauvé, S.; Liu, J. Molecular mechanisms of per-and polyfluoroalkyl substances on a modified clay: A combined experimental and molecular simulation study. Water Res. 2020, 184, 116166. [Google Scholar] [CrossRef] [PubMed]
- Mirzaee, E.; Sartaj, M. Synthesis and evaluation of recoverable activated carbon/Fe3O4 composites for removal of polycyclic aromatic hydrocarbons from aqueous solution. Environ. Technol. Innov. 2022, 25, 102174. [Google Scholar] [CrossRef]
- Abulikemu, G.; Wahman, D.G.; Sorial, G.A.; Nadagouda, M.; Stebel, E.K.; Womack, E.A.; Smith, S.J.; Kleiner, E.J.; Gray, B.N.; Taylor, R.D. Role of grinding method on granular activated carbon characteristics. Carbon Trends 2023, 11, 100261. [Google Scholar] [CrossRef]
- Umeh, A.C.; Hassan, M.; Egbuatu, M.; Zeng, Z.; Al Amin, M.; Samarasinghe, C.; Naidu, R. Multicomponent PFAS sorption and desorption in common commercial adsorbents: Kinetics, isotherm, adsorbent dose, pH, and index ion and ionic strength effects. Sci. Total Environ. 2023, 904, 166568. [Google Scholar] [CrossRef]
- Fabregat-Palau, J.; Vidal, M.; Rigol, A. Examining sorption of perfluoroalkyl substances (PFAS) in biochars and other carbon-rich materials. Chemosphere 2022, 302, 134733. [Google Scholar] [CrossRef]
- Zhou, Q.; Deng, S.; Yu, Q.; Zhang, Q.; Yu, G.; Huang, J.; He, H. Sorption of perfluorooctane sulfonate on organo-montmorillonites. Chemosphere 2010, 78, 688–694. [Google Scholar] [CrossRef]
- Das, P.; Arias, E.V.A.; Kambala, V.; Mallavarapu, M.; Naidu, R. Remediation of perfluorooctane sulfonate in contaminated soils by modified clay adsorbent—A risk-based approach. Water Air Soil Pollut. 2013, 224, 1714. [Google Scholar] [CrossRef]
- Zhao, L.; Bian, J.; Zhang, Y.; Zhu, L.; Liu, Z. Comparison of the sorption behaviors and mechanisms of perfluorosulfonates and perfluorocarboxylic acids on three kinds of clay minerals. Chemosphere 2014, 114, 51–58. [Google Scholar] [CrossRef]
- Lei, X.; Lian, Q.; Zhang, X.; Karsili, T.K.; Holmes, W.; Chen, Y.; Zappi, M.E.; Gang, D.D. A review of PFAS adsorption from aqueous solutions: Current approaches, engineering applications, challenges, and opportunities. Environ. Pollut. 2023, 321, 121138. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.R.; Brusseau, M.L.; Carroll, K.C.; Tick, G.R.; Duncan, C.M. Global distributions, source-type dependencies, and concentration ranges of per-and polyfluoroalkyl substances in groundwater. Sci. Total Environ. 2022, 841, 156602. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.C.; Safulko, A.; Vatankhah, H.; Liu, C.J.; Tajdini, B.; Marshall, R.E.; Bellona, C. PFAS adsorbent selection: The role of adsorbent use rate, water quality, and cost. J. Hazard. Mater. 2023, 454, 131481. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Kannan, K.; Lim, B.J.; An, K.G.; Kim, S.D. Effects of salinity and organic matter on the partitioning of perfluoroalkyl acid (PFAs) to clay particles. J. Environ. Monit. 2011, 13, 1803–1810. [Google Scholar] [CrossRef]
- Kothawala, D.N.; Köhler, S.J.; Östlund, A.; Wiberg, K.; Ahrens, L. Influence of dissolved organic matter concentration and composition on the removal efficiency of perfluoroalkyl substances (PFASs) during drinking water treatment. Water Res. 2017, 121, 320–328. [Google Scholar] [CrossRef]
- Qi, Y.; Cao, H.; Pan, W.; Wang, C.; Liang, Y. The role of dissolved organic matter during Per-and Polyfluorinated Substance (PFAS) adsorption, degradation, and plant uptake: A review. J. Hazard. Mater. 2022, 436, 129139. [Google Scholar] [CrossRef]
- Ünlü, N.; Ersoz, M. Adsorption characteristics of heavy metal ions onto a low cost biopolymeric sorbent from aqueous solutions. J. Hazard. Mater. 2006, 136, 272–280. [Google Scholar] [CrossRef]
- Wang, F.; Liu, C.; Shih, K. Adsorption behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) on boehmite. Chemosphere 2012, 89, 1009–1014. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, W.; Ilango, A.K.; Feldblyum, J.I.; Wei, Z.; Efstathiadis, H.; Yigit, M.V.; Liang, Y. Surfactant-modified clay for adsorption of mixtures of per-and polyfluoroalkyl substances (PFAS) in aqueous solutions. ACS Appl. Eng. Mater. 2022, 1, 394–407. [Google Scholar] [CrossRef]
- Xiao, F.; Bedane, A.H.; Mallula, S.; Sasi, P.C.; Alinezhad, A.; Soli, D.; Hagen, Z.M.; Mann, M.D. Production of granular activated carbon by thermal air oxidation of biomass charcoal/biochar for water treatment in rural communities: A mechanistic investigation. Chem. Eng. J. Adv. 2020, 4, 100035. [Google Scholar] [CrossRef]
- Wang, F.; Shih, K. Adsorption of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) on alumina: Influence of solution pH and cations. Water Res. 2011, 45, 2925–2930. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Deng, S.; Wang, B.; Huang, J.; Wang, Y.; Yu, G. Adsorption behavior and mechanism of perfluorooctane sulfonate on nanosized inorganic oxides. J. Colloid Interface Sci. 2016, 474, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Wołowicz, A.; Wawrzkiewicz, M. Screening of ion exchange resins for hazardous Ni (II) removal from aqueous solutions: Kinetic and equilibrium batch adsorption method. Processes 2021, 9, 285. [Google Scholar] [CrossRef]
- Pradhan, B.K.; Sandle, N. Effect of different oxidizing agent treatments on the surface properties of activated carbons. Carbon 1999, 37, 1323–1332. [Google Scholar] [CrossRef]
- Guo, X.; Li, M.; Liu, A.; Jiang, M.; Niu, X.; Liu, X. Adsorption mechanisms and characteristics of Hg2+ removal by different fractions of biochar. Water 2020, 12, 2105. [Google Scholar] [CrossRef]
- Tadić, T.T.; Momčilović, M.Z.; Nastasović, A.B.; Marković, B.M.; Nešić, A.; Bojić, A.L.; Onjia, A.E. Novel eco-friendly sorbent derived from Acer pseudoplatanus seed for atenolol removal from pharmaceutical wastewater. J. Water Process Eng. 2024, 64, 105564. [Google Scholar] [CrossRef]
- El-Hendawy, A.-N.A. Influence of HNO3 oxidation on the structure and adsorptive properties of corncob-based activated carbon. Carbon 2003, 41, 713–722. [Google Scholar] [CrossRef]
- Shao, Y.; Li, J.; Fang, X.; Yang, Z.; Qu, Y.; Yang, M.; Tan, W.; Li, G.; Wang, H. Chemical modification of bamboo activated carbon surface and its adsorption property of simultaneous removal of phosphate and nitrate. Chemosphere 2022, 287, 132118. [Google Scholar] [CrossRef]
- Li, C.; Zhao, S.; Li, M.; Yao, Z.; Li, Y.; Zhu, C.; Xu, S.-M.; Li, J.; Yu, J. The effect of the active carbonyl groups and residual acid on the ammonia adsorption over the acid-modified activated carbon. Front. Environ. Sci. 2023, 11, 976113. [Google Scholar] [CrossRef]

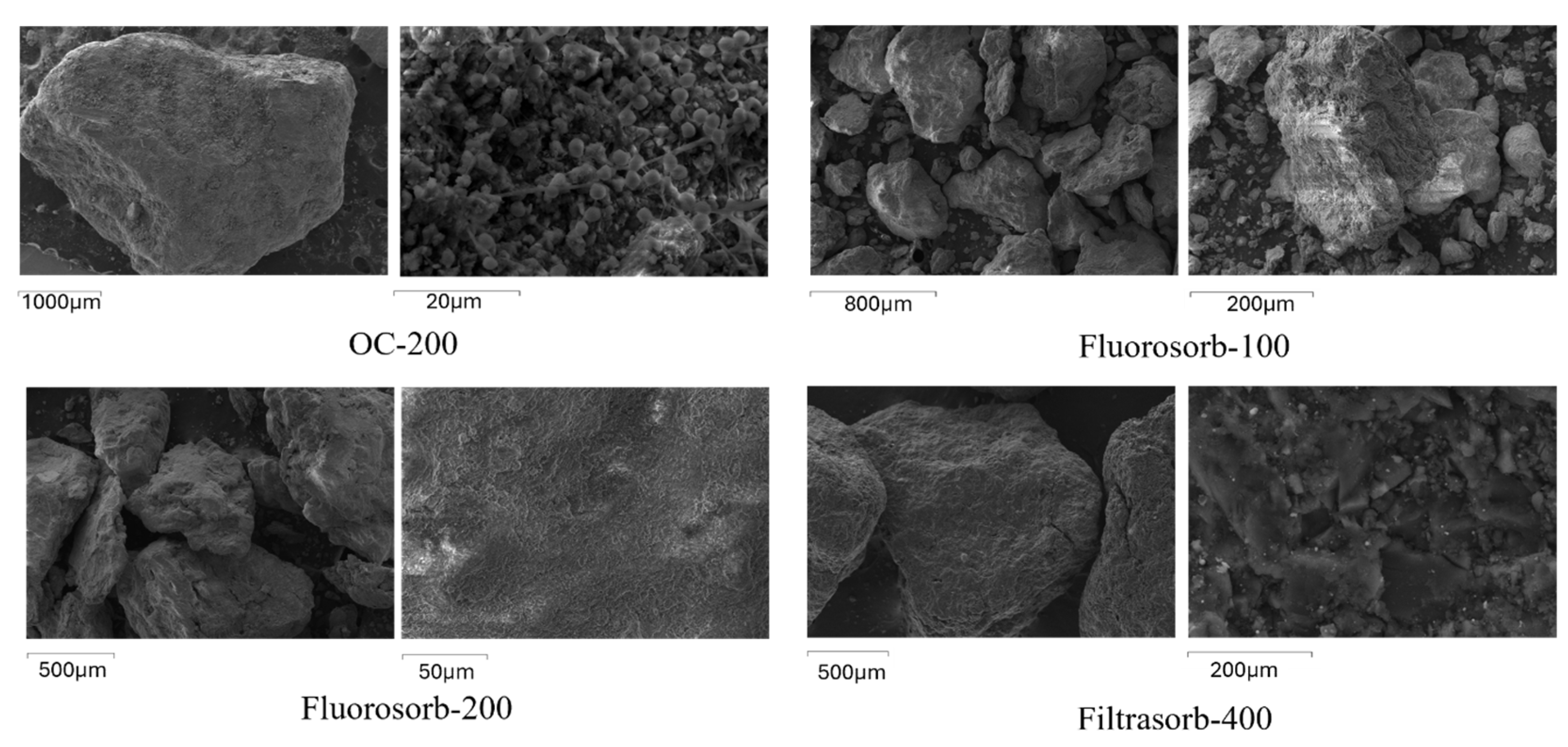
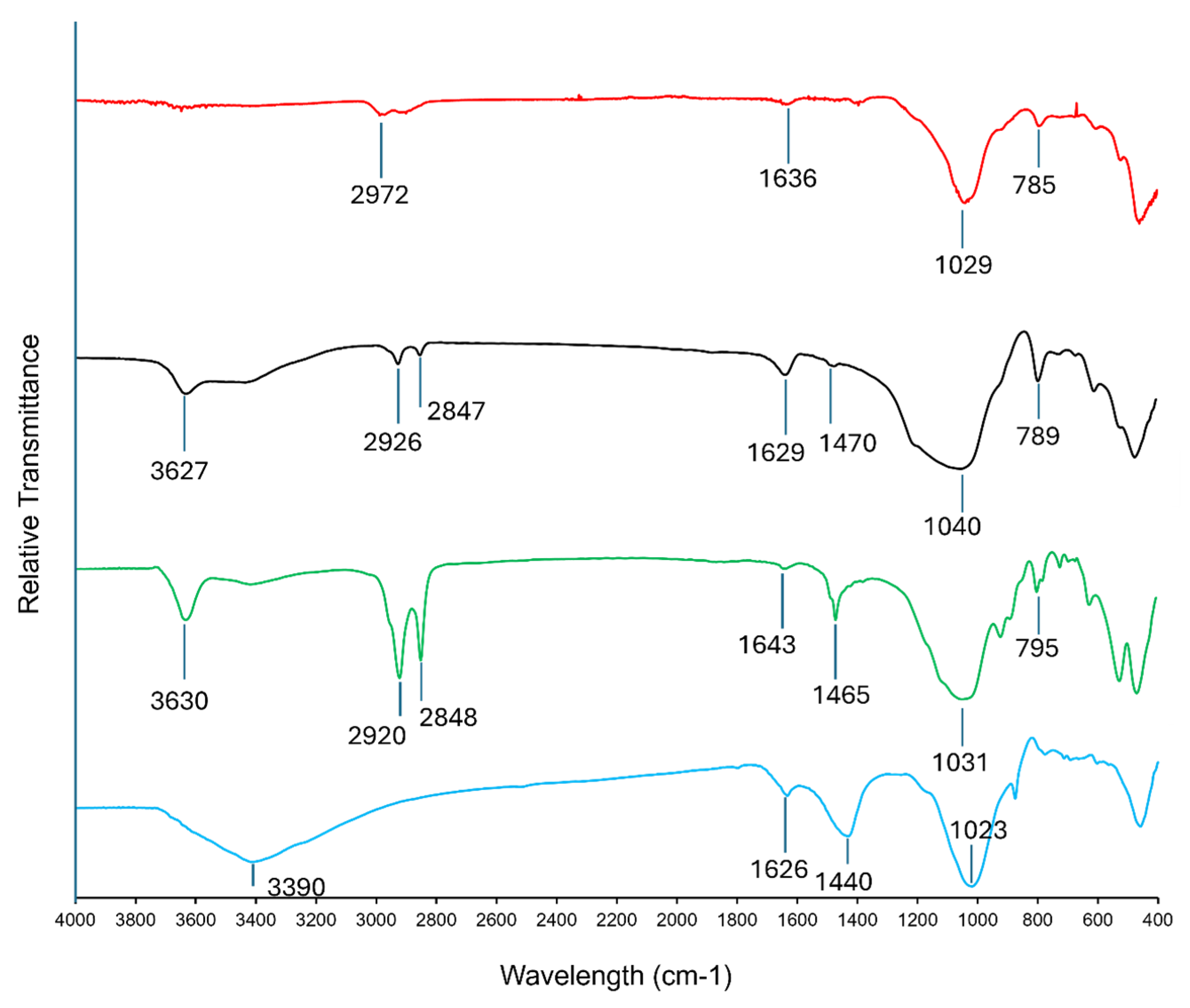
| Sorbent | Specific Surface Area (m2/g) | Micropore Volume (cm3/g) | Total Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|---|
| OC-200 | 106 | <LOD | 0.20 | 6.69 |
| Fluoro-sorb-100 | 92 | <LOD | 0.08 | 4.54 |
| Fluoro-sorb-200 | 113 | <LOD | 0.10 | 4.45 |
| Filtrasorb-400 | 827 | 0.23 | 0.56 | 4.53 |
| Sorbent | Sorbate | Pseudo-First Order | Pseudo-Second Order | |||
|---|---|---|---|---|---|---|
| R2 | k1 (hr−1) | R2 | qe | k2 | ||
| OC-200 | PFOA | 0.46 | 0.17 | 0.99 | 27.7 | 0.43 |
| PFOS | 0.31 | 0.46 | 1.00 | 36.5 | 25.0 | |
| PFNA | 0.24 | 0.32 | 1.00 | 38.0 | 34.5 | |
| Fluoro-sorb-100 | PFOA | 0.29 | 0.31 | 1.00 | 31.6 | 9.99 |
| PFOS | 0.02 | 0.11 | 1.00 | 38.5 | 67.6 | |
| PFNA | 0.20 | 0.38 | 1.00 | 30.4 | 54.1 | |
| Fluoro-sorb-200 | PFOA | 0.42 | 0.28 | 0.99 | 31.1 | 1.47 |
| PFOS | 0.06 | 0.19 | 1.00 | 35.1 | 40.6 | |
| PFNA | 0.29 | 0.38 | 1.00 | 38.2 | 13.7 | |
| Filtrasorb-400 | PFOA | 0.60 | 0.53 | 1.00 | 34.8 | 1.03 |
| PFOS | 0.21 | 0.33 | 1.00 | 35.2 | 4.03 | |
| PFNA | 0.55 | 0.50 | 1.00 | 35.7 | 1.31 | |
| Sorbent | Sorbate | Langmuir Constants | Freundlich Constants | ||||
|---|---|---|---|---|---|---|---|
| R2 | R2 | n | |||||
| OC-200 | PFOS | 0.979 | 55.56 | 0.056 | 0.952 | 2.116 | 4.399 |
| PFOA | 0.973 | 47.17 | 0.087 | 0.818 | 2.846 | 3.872 | |
| PFNA | 0.990 | 47.62 | 0.018 | 0.912 | 2.344 | 3.020 | |
| Fluoro-sorb-100 | PFOS | 0.990 | 65.79 | 0.027 | 0.974 | 2.110 | 3.983 |
| PFOA | 0.990 | 22.12 | 0.16 | 0.924 | 2.561 | 3.557 | |
| PFNA | 0.995 | 99.01 | 0.0080 | 0.991 | 1.399 | 1.386 | |
| Fluoro-sorb-200 | PFOS | 0.984 | 58.82 | 0.017 | 0.987 | 1.679 | 1.927 |
| PFOA | 0.961 | 10.81 | 0.52 | 0.913 | 3.202 | 7.533 | |
| PFNA | 0.973 | 43.29 | 0.052 | 0.983 | 2.448 | 4.093 | |
| Filtrasorb-400 | PFOS | 0.985 | 54.95 | 0.021 | 0.979 | 1.916 | 2.515 |
| PFOA | 0.906 | 25.45 | 0.29 | 0.369 | 6.998 | 10.62 | |
| PFNA | 0.982 | 32.15 | 0.089 | 0.703 | 4.149 | 7.272 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Joudiazar, S.; Satpathy, A.; Fernando, E.; Rahmati, R.; Kim, J.; de Falco, G.; Datta, R.; Sarkar, D. Removal of Per- and Polyfluoroalkyl Substances Using Commercially Available Sorbents. Materials 2025, 18, 1299. https://doi.org/10.3390/ma18061299
Zhang Z, Joudiazar S, Satpathy A, Fernando E, Rahmati R, Kim J, de Falco G, Datta R, Sarkar D. Removal of Per- and Polyfluoroalkyl Substances Using Commercially Available Sorbents. Materials. 2025; 18(6):1299. https://doi.org/10.3390/ma18061299
Chicago/Turabian StyleZhang, Zhiming, Sevda Joudiazar, Anshuman Satpathy, Eustace Fernando, Roxana Rahmati, Junchul Kim, Giacomo de Falco, Rupali Datta, and Dibyendu Sarkar. 2025. "Removal of Per- and Polyfluoroalkyl Substances Using Commercially Available Sorbents" Materials 18, no. 6: 1299. https://doi.org/10.3390/ma18061299
APA StyleZhang, Z., Joudiazar, S., Satpathy, A., Fernando, E., Rahmati, R., Kim, J., de Falco, G., Datta, R., & Sarkar, D. (2025). Removal of Per- and Polyfluoroalkyl Substances Using Commercially Available Sorbents. Materials, 18(6), 1299. https://doi.org/10.3390/ma18061299












