Oxygen Vacancy Modification MIL-125(Ti) Promotes CO2 Photoreduction to CO with Near 100% Selectivity
Abstract
1. Introduction
2. Experimental Section
2.1. Chemical Reagent
2.2. Preparation of MIL-125(Ti)
2.3. Preparation of MIL-125-xH
2.4. Photocatalytic Measurements
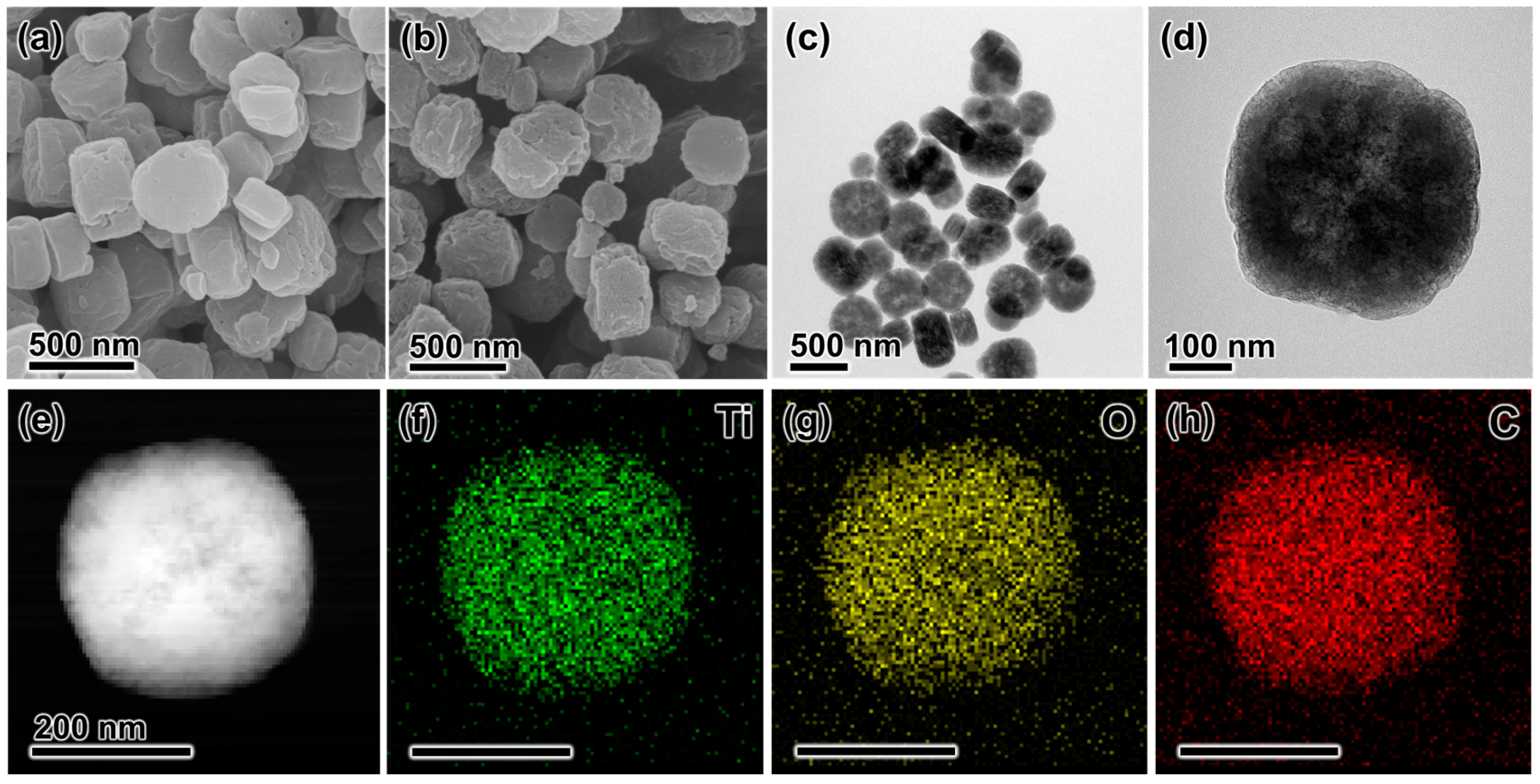
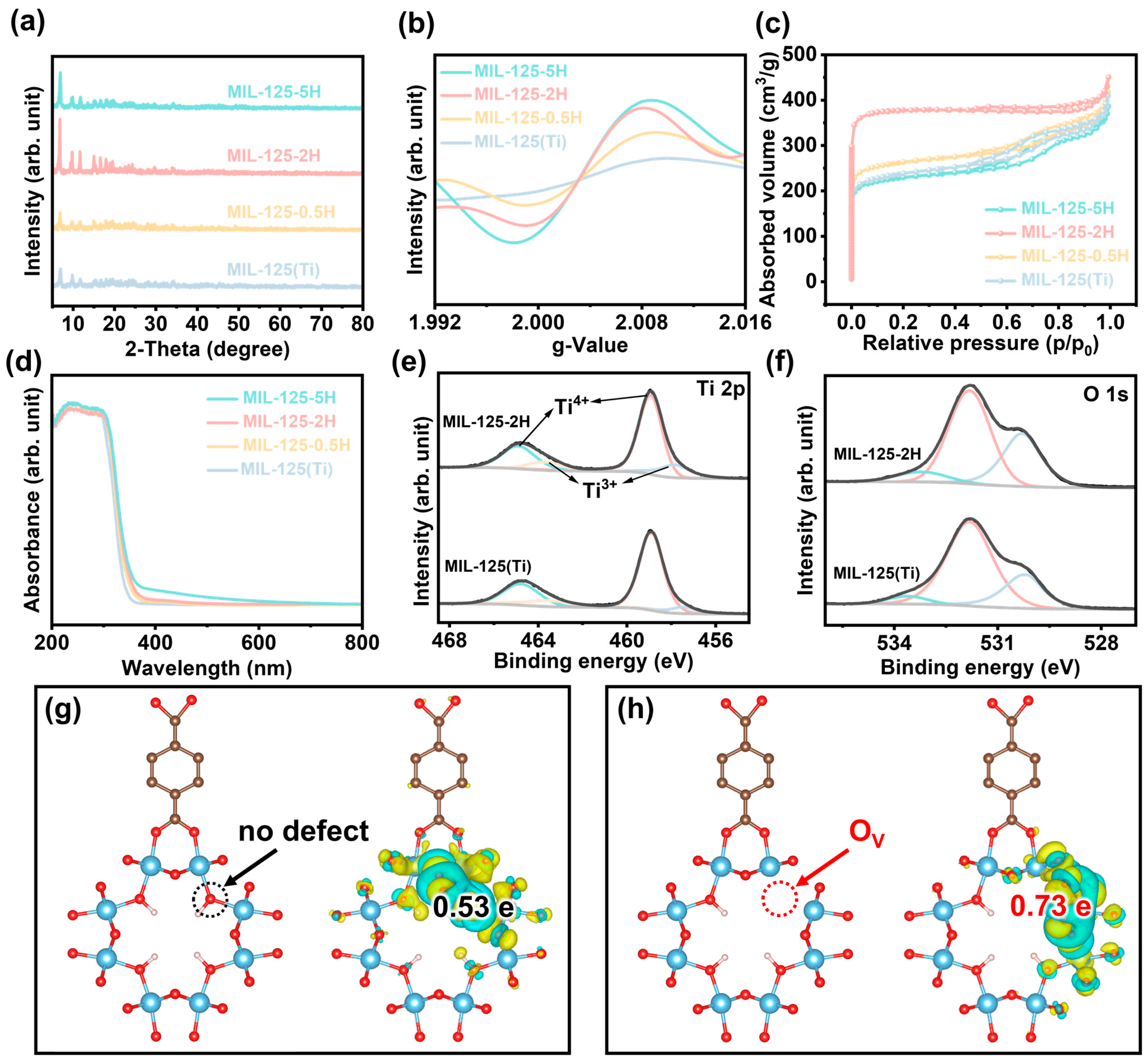
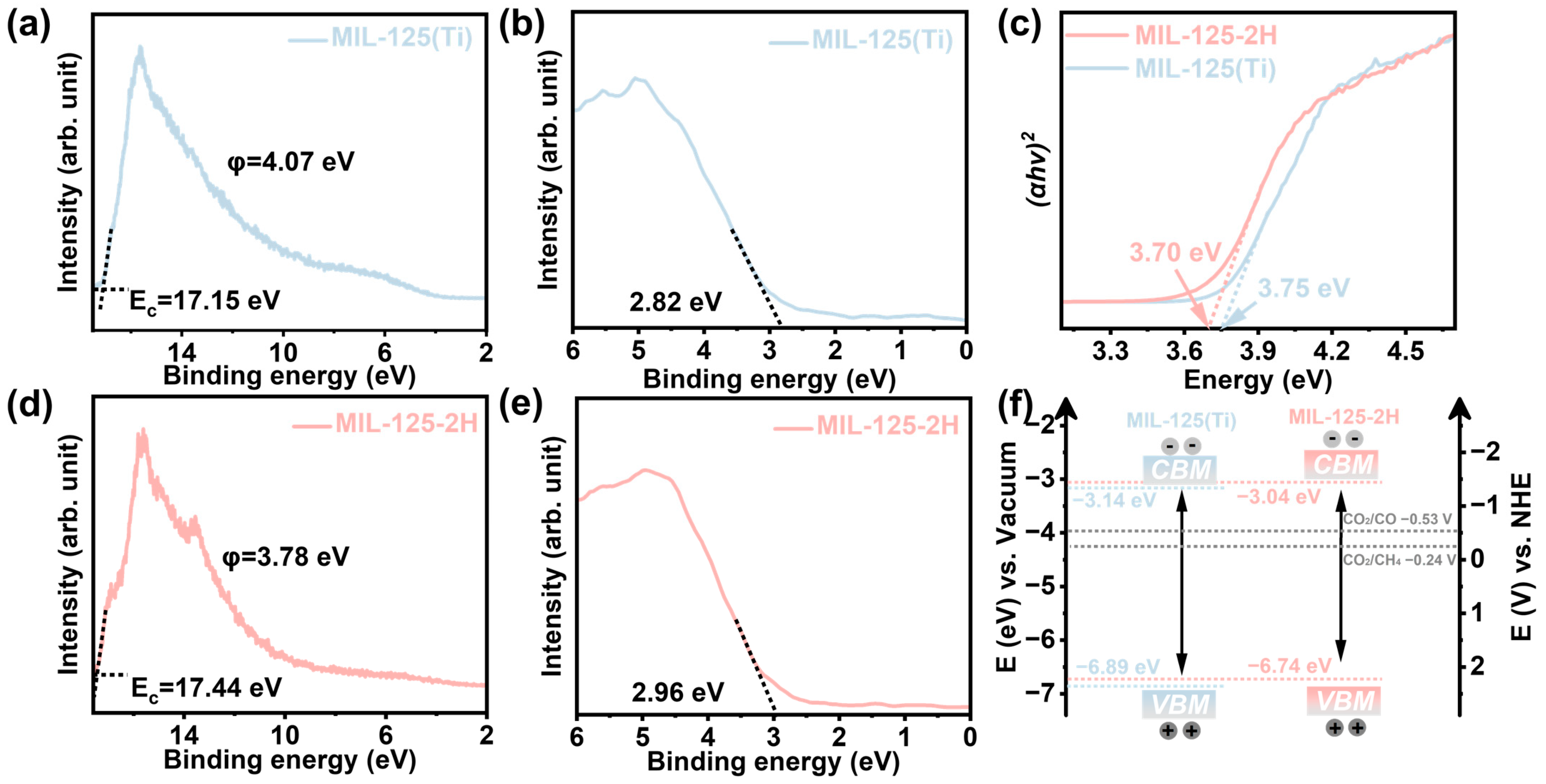
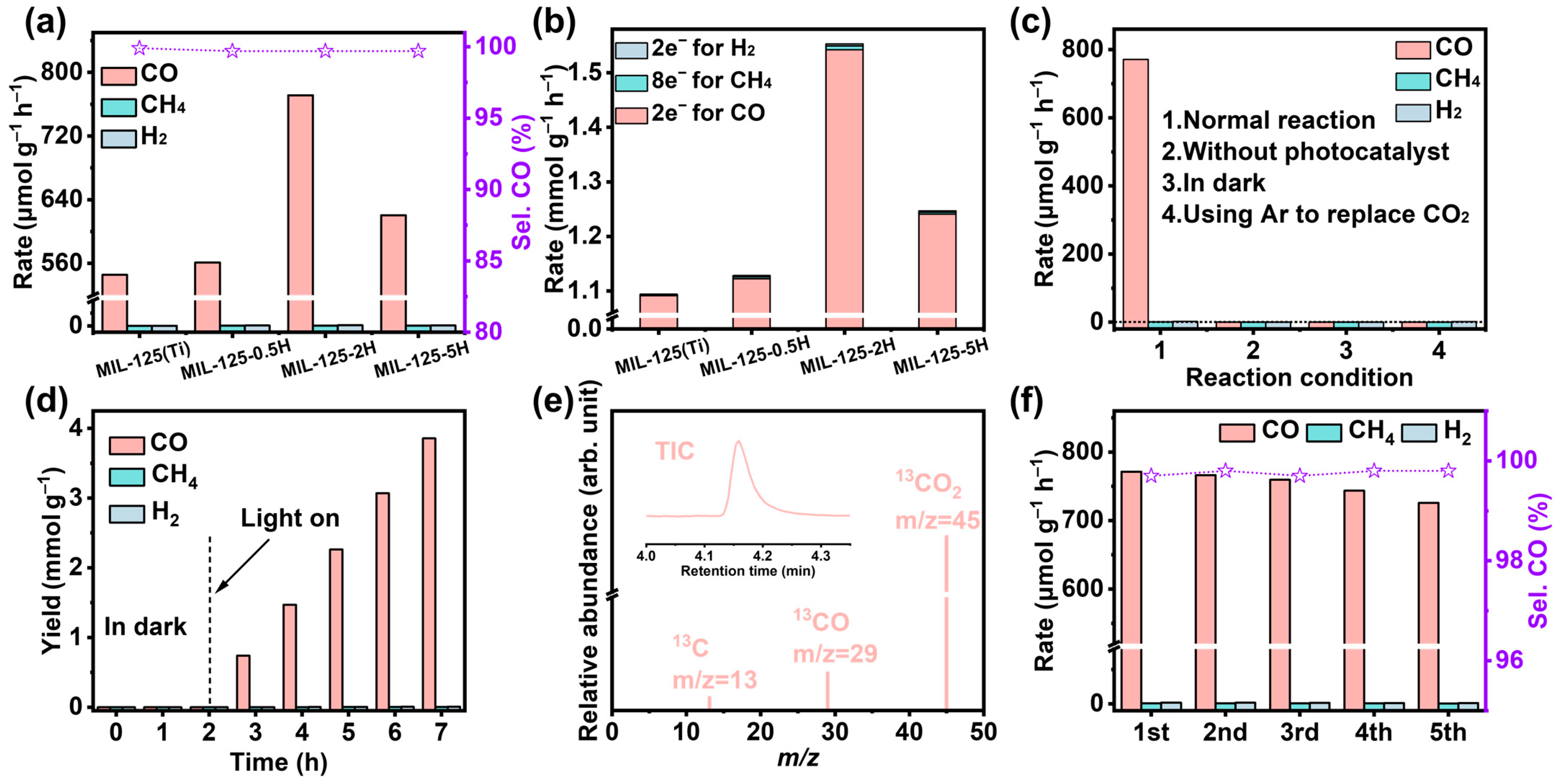
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, X.; Wang, T.; Gong, J. CO2 photo-reduction: Insights into CO2 activation and reaction on surfaces of photocatalysts. Energy Environ. Sci. 2016, 9, 2177–2196. [Google Scholar] [CrossRef]
- Müller, L.J.; Kätelhön, A.; Bringezu, S.; McCoy, S.; Suh, S.; Edwards, R.; Sick, V.; Kaiser, S.; Cuéllar-Franca, R.; El Khamlichi, A.; et al. The carbon footprint of the carbon feedstock CO2. Energy Environ. Sci. 2020, 13, 2979–2992. [Google Scholar] [CrossRef]
- Sullivan, I.; Goryachev, A.; Digdaya, I.A.; Li, X.; Atwater, H.A.; Vermaas, D.A.; Xiang, C. Coupling electrochemical CO2 conversion with CO2 capture. Nat. Catal. 2021, 4, 952–958. [Google Scholar] [CrossRef]
- Fang, S.; Rahaman, M.; Bharti, J.; Reisner, E.; Robert, M.; Ozin, G.A.; Hu, Y.H. Photocatalytic CO2 reduction. Nat. Rev. Methods Primers 2023, 3, 61. [Google Scholar] [CrossRef]
- Ran, J.; Jaroniec, M.; Qiao, S.Z. Cocatalysts in Semiconductor-Based Photocatalytic CO2 Reduction: Achievements, Challenges, and Opportunities. Adv. Mater. 2018, 30, 1704649. [Google Scholar] [CrossRef]
- Zhou, H.-C.J.; Kitagawa, S. Metal–Organic Frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar] [CrossRef]
- Liang, J.; Gvilava, V.; Jansen, C.; Öztürk, S.; Spieß, A.; Lin, J.; Xing, S.; Sun, Y.; Wang, H.; Janiak, C. Cucurbituril-Encapsulating Metal–Organic Framework via Mechanochemistry: Adsorbents with Enhanced Performance. Angew. Chem. Int. Ed. 2021, 60, 15365–15370. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, G.; Zhou, H.; Cao, S.; Zhang, Y.; Wang, S.; Pang, H. Enhanced Active Sites and Stability in Nano-MOFs for Electrochemical Energy Storage Through Dual Regulation by Tannic Acid. Angew. Chem. Int. Ed. 2023, 62, e202311075. [Google Scholar] [CrossRef]
- Wen, L.; Sun, K.; Liu, X.; Yang, W.; Li, L.; Jiang, H.L. Electronic State and Microenvironment Modulation of Metal Nanoparticles Stabilized by MOFs for Boosting Electrocatalytic Nitrogen Reduction. Adv. Mater. 2023, 35, 2210669. [Google Scholar] [CrossRef]
- Cano, F.J.; Coste, S.; Reyes-Vallejo, O.; Makowska-Janusik, M.; Velumani, S.; Olvera, M.d.l.L.; Kassiba, A. Influence of GO oxidation degrees on the organization and physical features of TiO2–GO-based nanocomposites for water dye removal. Surf. Interfaces 2024, 46, 104004. [Google Scholar] [CrossRef]
- Wu, S.; Xing, X.; Wang, D.; Zhang, J.; Chu, J.; Yu, C.; Wei, Z.; Hu, M.; Zhang, X.; Li, Z. Highly Ordered Hierarchically Macroporous MIL-125 with High Specific Surface Area for Photocatalytic CO2 Fixation. ACS Sustain. Chem. Eng. 2019, 8, 148–153. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Y.; Zhang, J.; Zeng, F.; Bao, J.; Li, W.; Tian, G. Engineering defect-rich H-W18O49 nanowire/MIL-125(Ti) nanosheet S-scheme heterostructure for remarkable CO2 photoconversion. Chem. Eng. J. 2024, 501, 157310. [Google Scholar] [CrossRef]
- Hussain, M.B.; Kang, B.; Cheng, X.; Ma, C.; Wang, X.; Mehmood, R.; Iqbal, S. Oxygen vacancy induced Pt-decorated MOF photocatalyst for hydrogen production. Int. J. Hydrog. Energy 2023, 48, 13780–13790. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, C.; Chen, L.; Fan, J.; Liu, C.; Xue, L.; Sun, J.; Zhang, W.; Wang, X.; Xiong, P.; et al. High Entropy-Driven Role of Oxygen Vacancies for Water Oxidation. Adv. Funct. Mater. 2024, 34, 2314820. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Zhai, W.; Liu, H.; Sakthivel, T.; Guo, S.; Dai, Z. Metastabilizing the Ruthenium Clusters by Interfacial Oxygen Vacancies for Boosted Water Splitting Electrocatalysis. Adv. Energy Mater. 2024, 14, 2400059. [Google Scholar] [CrossRef]
- Xin, Y.; Tian, J.; Xiong, X.; Wu, C.; Carabineiro, S.A.C.; Yang, X.; Chen, Z.; Xia, Y.; Jin, Y. Enhanced Photocatalytic Efficiency Through Oxygen Vacancy-Driven Molecular Epitaxial Growth of Metal–Organic Frameworks on BiVO4. Adv. Mater. 2025, 37, 2417589. [Google Scholar] [CrossRef]
- Sun, Y.; Ji, H.; Sun, Y.; Zhang, G.; Zhou, H.; Cao, S.; Liu, S.; Zhang, L.; Li, W.; Zhu, X.; et al. Synergistic Effect of Oxygen Vacancy and High Porosity of Nano MIL-125(Ti) for Enhanced Photocatalytic Nitrogen Fixation. Angew. Chem. Int. Ed. 2023, 63, e202316973. [Google Scholar] [CrossRef]
- Song, Q.; Sun, C.; Wang, Z.; Bai, X.; Wu, K.; Li, Q.; Zhang, H.; Zhou, L.; Pang, H.; Liang, Y.; et al. Directed charge transfer in all solid state heterojunction of Fe doped MoS2 and C–TiO2 nanosheet for enhanced nitrogen photofixation. Mater. Today Phys. 2021, 21, 100563. [Google Scholar] [CrossRef]
- Han, Y.; Huang, W.; He, M.; An, B.; Chen, Y.; Han, X.; An, L.; Kippax-Jones, M.; Li, J.; Yang, Y.; et al. Trace benzene capture by decoration of structural defects in metal–organic framework materials. Nat. Mater. 2024, 23, 1531–1538. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, D.; Chen, Y.; Huang, R.; Ding, Z.; Fu, X.; Li, Z. An amine-functionalized titanium metal-organic framework photocatalyst with visible-light-induced activity for CO2 reduction. Angew. Chem. Int. Ed. 2012, 51, 3364–3367. [Google Scholar] [CrossRef]
- Dai, Q.; Gao, G.; Tang, J.; Jiang, R.; Sun, S.; Ye, Y.; Li, S.; Xie, R.; Zhang, J. The MIL-125(Ti)/Co3O4 towards efficiently removing tetracycline by synergistic adsorption-photocatalysis roles. Mater. Des. 2025, 250, 113608. [Google Scholar] [CrossRef]
- Wang, Z.; Teramura, K.; Hosokawa, S.; Tanaka, T. Photocatalytic conversion of CO2 in water over Ag-modified La2Ti2O7. Appl. Catal. B Environ. 2015, 163, 241–247. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, X.; Xu, Q.; Mei, Z.; Duan, L.; Guo, H. Understanding dual-vacancy heterojunction for boosting photocatalytic CO2 reduction with highly selective conversion to CH4. Appl. Catal. B Environ. 2022, 316, 121679. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, H.; Bi, C.; Song, H.; Zhou, G.; Zhong, K.; Yang, J.; Yi, J.; Xu, H.; Wang, X. Piezo-photocatalysis for efficient charge separation to promote CO2 photoreduction in nanoclusters. Ultrason. Sonochem. 2023, 101, 106653. [Google Scholar] [CrossRef]
- Xu, H.; Song, H.; Bi, C.; Zhou, G.; Liu, X.; Zhong, K.; Jiang, W.; Yang, J.; Shen, W.; Hao, N.; et al. Breaking the intrinsic activity barriers of bilayer metal oxides for catalytic CO2 reduction. J. Colloid Interface Sci. 2024, 675, 419–428. [Google Scholar] [CrossRef]
- Wu, T.; Zhao, H.; Zhu, X.; Xing, Z.; Liu, Q.; Liu, T.; Gao, S.; Lu, S.; Chen, G.; Asiri, A.M.; et al. Identifying the Origin of Ti3+ Activity Toward Enhanced Electrocatalytic N2 Reduction over TiO2 Nanoparticles Modulated by Mixed-Valent Copper. Adv. Mater. 2020, 32, 2000299. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, H.; Sun, Z.; Wang, J.; Wang, H.; Xue, J.; Shen, Q.; Li, Q. Creation of robust oxygen vacancies in 2D ultrathin BiOBr nanosheets by irradiation through photocatalytic memory effect for enhanced CO2 reduction. Chem. Eng. J. 2023, 477, 146892. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, H.; Liu, J.; Bi, C.; Tian, J.; Zhong, K.; Wang, B.; Ding, P.; Wang, X.; Chu, P.K.; et al. Stacking Engineering of Heterojunctions in Half-Metallic Carbon Nitride for Efficient CO2 Photoreduction. Adv. Sci. 2023, 10, 2307192. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, M.; Chen, H.; Weng, Y.-X.; Zhong, J.; Li, Y.; Wang, S.; Chen, Z.; Xia, J.; Li, H. Interfacial chemical bond modulated Bi19S27Br3/g-C3N4 Z-scheme heterojunction for enhanced photocatalytic CO2 conversion. Appl. Catal. B Environ. 2022, 307, 121162. [Google Scholar] [CrossRef]
- Liu, Q.; Cheng, H.; Chen, T.; Lo, T.W.B.; Ma, J.; Ling, A.; Wang, F. Boosted CO desorption behaviors induced by spatial dyadic heterostructure in polymeric carbon nitride for efficient photocatalytic CO2 conversion. Appl. Catal. B Environ. 2021, 295, 120289. [Google Scholar] [CrossRef]
- Ning, C.; Yang, J.; Bai, S.; Chen, G.; Liu, G.; Shen, T.; Zheng, L.; Xu, S.M.; Kong, X.; Liu, B.; et al. An Efficient Intercalation Supramolecular Structure for Photocatalytic CO2 Reduction to Ethylene Under Visible Light. Adv. Funct. Mater. 2023, 33, 2300365. [Google Scholar] [CrossRef]
- Wang, J.; Yang, C.; Mao, L.; Cai, X.; Geng, Z.; Zhang, H.; Zhang, J.; Tan, X.; Ye, J.; Yu, T. Regulating the Metallic Cu–Ga Bond by S Vacancy for Improved Photocatalytic CO2 Reduction to C2H4. Adv. Funct. Mater. 2023, 33, 2213901. [Google Scholar] [CrossRef]
- Zhou, G.; Chen, Y.; Chen, G.; Xu, H.; Yin, W.; Wang, B.; Zhu, X.; Ning, X.; Chu, P.K.; Wang, X.; et al. Enhanced CO2 photoreduction in pure water systems by surface-reconstructed asymmetric Mn–Cu sites. Appl. Catal. B Environ. Energy 2025, 361, 124617. [Google Scholar] [CrossRef]
- Ma, M.; Huang, Z.; Doronkin, D.E.; Fa, W.; Rao, Z.; Zou, Y.; Wang, R.; Zhong, Y.; Cao, Y.; Zhang, R.; et al. Ultrahigh surface density of Co-N2C single-atom-sites for boosting photocatalytic CO2 reduction to methanol. Appl. Catal. B Environ. 2022, 300, 120695. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, T.; Zhou, X.; Yang, Y. Oxygen-vacancy-boosted visible light driven photocatalytic oxidative dehydrogenation of saturated N-heterocycles over Nb2O5 nanorods. Appl. Catal. B Environ. 2022, 316, 121622. [Google Scholar] [CrossRef]
- Miao, Z.; Wang, Q.; Zhang, Y.; Meng, L.; Wang, X. In situ construction of S-scheme AgBr/BiOBr heterojunction with surface oxygen vacancy for boosting photocatalytic CO2 reduction with H2O. Appl. Catal. B Environ. 2022, 301, 120802. [Google Scholar] [CrossRef]
- Wu, J.; Xiong, L.; Hu, Y.; Yang, Y.; Zhang, X.; Wang, T.; Tang, Z.; Sun, A.; Zhou, Y.; Shen, J.; et al. Organic half-metal derived erythroid-like BiVO4/hm-C4N3 Z-Scheme photocatalyst: Reduction sites upgrading and rate-determining step modulation for overall CO2 and H2O conversion. Appl. Catal. B Environ. 2021, 295, 120277. [Google Scholar] [CrossRef]
- Yang, H.; Hou, H.; Yang, M.; Zhu, Z.; Fu, H.; Zhang, D.; Luo, Y.; Yang, W. Engineering the S-scheme heterojunction between NiO microrods and MgAl-LDH nanoplates for efficient and selective photoreduction of CO2 to CH4. Chem. Eng. J. 2023, 474, 145813. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, X.; Yu, Q.; He, M.; Zhang, W.; Mo, Z.; Yuan, J.; She, Y.; Xu, H.; Li, H. Multidimensional In2O3/In2S3 heterojunction with lattice distortion for CO2 photoconversion. Chin. J. Catal. 2022, 43, 1286. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, G.; Wang, Z.; Zhong, K.; Ding, P.; Song, Y.; Yuan, J.; She, Y.; Li, H.; Xu, H. Nanostructure and functional group engineering of black phosphorus via plasma treatment for CO2 photoreduction. J. CO2 Util. 2021, 54, 101745. [Google Scholar] [CrossRef]
- Liang, J.; Yu, H.; Shi, J.; Li, B.; Wu, L.; Wang, M. Dislocated Bilayer MOF Enables High-Selectivity Photocatalytic Reduction of CO2 to CO. Adv. Mater. 2023, 35, e2209814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, G.; Chen, G.; Wang, H.; Zhao, Q.; Yin, W.; Yi, J.; Zhu, X.; Wang, X.; Ning, X. Bimetallic NiCu catalyst derived from spent MOF adsorbent for efficient photocatalytic CO2 reduction. Chem. Eng. J. 2024, 497, 154701. [Google Scholar] [CrossRef]
- Gao, X.; Guo, B.; Guo, C.; Meng, Q.; Liang, J.; Liu, J. Zirconium-Based Metal-Organic Framework for Efficient Photocatalytic Reduction of CO2 to CO: The Influence of Doped Metal Ions. ACS Appl. Mater. Interfaces 2020, 12, 24059–24065. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, L.; Jin, X.; Xu, M.; Yin, S.; Li, J.; Li, X.; Shen, D.; Yan, Y.; Huo, P. Cu media constructed Z-scheme heterojunction of UiO-66-NH2/Cu2O/Cu for enhanced photocatalytic induction of CO2. Appl. Surf. Sci. 2021, 545, 148967. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, L.; Yu, X.; Sun, L.; Li, J.; Yang, J.; Liu, Q. Precise regulation of built-in electric field over NH2-MIL-125-Ti/WO3-x S-scheme heterojunction for achieving simultaneous formation of CO and H2O2 from CO2 and H2O. Chem. Eng. J. 2023, 466, 143129. [Google Scholar] [CrossRef]
- Li, Z.; Bai, W.; Liu, D.; Han, B.; Liang, Y.; Qi, J. Preloaded oxygen vacancy conditioning Ni/TiO2 to enhance photocatalytic CO2 reduction. Sep. Purif. Technol. 2024, 330, 125250. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Hu, Z.; Huang, J.; Yang, S.; Li, H. High-efficiency photocatalytic CO2 reduction enabled by interfacial Ov and isolated Ti3+ of g-C3N4/TiO2 Z-scheme heterojunction. J. Colloid Interface Sci. 2024, 663, 891–901. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Song, H.; Wang, X.; Zhu, X. Oxygen Vacancy Modification MIL-125(Ti) Promotes CO2 Photoreduction to CO with Near 100% Selectivity. Materials 2025, 18, 1343. https://doi.org/10.3390/ma18061343
Xu H, Song H, Wang X, Zhu X. Oxygen Vacancy Modification MIL-125(Ti) Promotes CO2 Photoreduction to CO with Near 100% Selectivity. Materials. 2025; 18(6):1343. https://doi.org/10.3390/ma18061343
Chicago/Turabian StyleXu, Hangmin, Hao Song, Xiaozhi Wang, and Xingwang Zhu. 2025. "Oxygen Vacancy Modification MIL-125(Ti) Promotes CO2 Photoreduction to CO with Near 100% Selectivity" Materials 18, no. 6: 1343. https://doi.org/10.3390/ma18061343
APA StyleXu, H., Song, H., Wang, X., & Zhu, X. (2025). Oxygen Vacancy Modification MIL-125(Ti) Promotes CO2 Photoreduction to CO with Near 100% Selectivity. Materials, 18(6), 1343. https://doi.org/10.3390/ma18061343





