The Effect of B2O3 Doping on the Properties of Electrical and Thermal Conductivity for SnO2 Varistors
Abstract
:1. Introduction
2. Experimental
3. Sample Testing
4. Experimental Results
4.1. Microstructure
4.2. Electrical Characteristics
4.3. Thermal Conductivity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Yu, Z.; He, J.; Peng, X.; Li, Z. Analysis of Lightning Protection Performance Improvement for Multi-Circuit Lines on the Same Tower Using Transmission Line Arresters. High Volt. Eng. 2011, 37, 3120–3128. [Google Scholar]
- Dong, C.; Jianhua, L.; Chenxi, J. Study on lightning resistance level based on ATP-EMTP. Electr. Porcelain Surge Arrester 2011, 53, 47–50. [Google Scholar]
- Shahraki, M.M.; Mahmoudi, P.; Abdollahi, M.; Ebadzadeh, T. Fne-grained SnO2 varistors prepared by microwave sintering for ultra- high voltage applications. Mater. Lett. 2018, 230, 9–11. [Google Scholar] [CrossRef]
- Meng, P.; Hu, J.; Wu, J.; He, J. Multi-element doping comprehensive regulation of comprehensive performance of zinc oxide varistor. High Volt. Technol. 2018, 44, 241–247. [Google Scholar]
- Ferreira, T.V.; Bolacell, G.S.; da Rosa, M.A.; da Costa, E.G.; Lira, G.R.; Pissolato, J.F.; Andrade, F.L.; Ferreira, R.S.; Neto, E.T.W.; Francisco, R.P. Technological Development of a Composite Insulator for High Voltage Transmission Line Monitoring. IEEE Electr. Insul. Mag. 2023, 39, 7–16. [Google Scholar]
- Jinhua, Z. Optimization study on voltage level and transmission capacity. IEEE Trans. Power Syst. 2009, 24, 193–197. [Google Scholar]
- Bueno, P.R.; Varela, J.A.; Barrado, C.M.; Longo, E.; Leite, E.R. A Comparative Study of Thermal Conductivity in ZnO- and SnO2-Based Varistor Systems. J. Am. Ceram. Soc. 2005, 88, 2629–2631. [Google Scholar]
- Wei, Q.; He, J.; Hu, J.; Wang, Y. Influence of Cr2O3 on the residual voltage ratio of SnO2-based varistor. J. Am. Ceram. Soc. 2011, 94, 1999–2002. [Google Scholar] [CrossRef]
- Mahmoudi, P.; Nemati, A.; Shahraki, M.M. Gra in growth kinetics and electrical properties of CuO doped SnO2 -based varistors. J. Alloys Compd. 2019, 770, 784–791. [Google Scholar]
- Cheng, K.; Zhao, H.; Zhou, Y. The Influence of Co doping of Y2O3, Ga2O3, and B2O3 on the Microstructure and Electrical Properties of ZnO Varistors. High Volt. Technol. 2023, 49, 4707–4716. [Google Scholar]
- Pianaro, S.A.; Bueno, P.R.; Olivi, P.; Longo, E.; Varela, J.A. Electrical properties of the SnO2-based varistor. J. Mater. Sci. Lett. 1997, 16, 634–638. [Google Scholar]
- Pianaro, S.A.; Bueno, P.R.; Longo, E.; Varela, J.A. A new SnO2-based varistor system. J. Mater. Sci. Lett. 1995, 14, 692–694. [Google Scholar] [CrossRef]
- Xin, L. Simulation of the Conductive Process of Nano ZnO Varistors Based on Animation Plane Form. J. Chem. 2020, 2020, 9726173. [Google Scholar]
- Hembram, K.; Rao, T.N.; Ramakrishana, M.; Srinivasa, R.S.; Kulkarni, A.R. Influence of Cao Doping on Phase, Microstructure, Electrical and Dielectric Properties of Zno Varistors. J. Technol. 2020, 817, 152700. [Google Scholar] [CrossRef]
- Hembram, K.; Rao, T.N.; Ramakrishna, M.; Srinivasa, R.S.; Kulkarni, A.R. A Novel Economical Grain Boundary Engineered Ultra-high Performance Zno Varistor with Lesser Dopants. J. Technol. Sci. 2019, 38, 5021–5029. [Google Scholar]
- Bueno, P.R.; Leite, E.R.; Oliveira, M.M.; Orlandi, M.O.; Longo, E. Role of oxygen at the grain boundary of metal oxide varistors: A potential barrier formation mechanism. Appl. Phys. Lett. 2001, 79, 48. [Google Scholar] [CrossRef]
- Tsuboi, T.; Takami, J.; Okabe, S.; Kido, T.; Maekawa, T. Energy absorption capacity of a 500 kV surge arrester for direct and multiple lightning strokes. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 916–924. [Google Scholar] [CrossRef]
- Tominc, S.; Rečnik, A.; Samardžija, Z.; Dražić, G.; Podlogar, M.; Bernik, S.; Daneu, N. Twinning and charge compensation in Nb2O5-doped SnO2-CoO ceramics exhibiting promising varistor characteristics. Ceram. Int. 2018, 44, 1603–1613. [Google Scholar]
- Wurst, J.C.; Nelson, J.A. Lineal Intercept Technique for Measuring Grain Size in Two-Phase Polycrystalline Ceramics. J. Am. Ceram. Soc. 1972, 55, 109. [Google Scholar]
- Mukae, K.; Tsuda, K.; Nagasawa, I. Capacitance-vs-voltage characteristics of ZnO varistors. J. Appl. Phys. 1979, 50, 4475–4476. [Google Scholar]
- Zhao, H.; He, J.; Hu, J.; Chen, S.; Xie, Q. High nonlinearity and low residual-voltage ZnO varistor ceramics by synchronously doping Ga2O3 and Al2O3. Mater. Lett. 2016, 164, 80–83. [Google Scholar] [CrossRef]
- Paul, G.; Chopkar, M.; Manna, I.; Das, P.K. Techniques for measuring the thermal conductivity of nanofluids: A review. Renew. Sustain. Energy Rev. 2010, 14, 1913–1924. [Google Scholar]
- Wu, J.W.; Sung, W.F.; Chu, H.S. Thermal conductivity of polyurethane foams. Int. J. Heat Mass Tran. 1999, 42, 2211–2217. [Google Scholar]
- Knacke, O.; Kubaschewski, O.; Hesselmann, K. (Eds.) Thermochemical Properties of Inorganic Substances, 2nd ed.; Springer: Berlin, Germany, 1976. [Google Scholar]
- Yang, X.; Zhao, H.; Wu, Y.; Zhao, Q. Effect of sintering temperature on microstructure and electrical properties of SnO2 varistor doped with B2O3. Electr. Porcelain Light. Arrester 2023, 5, 72–76. [Google Scholar] [CrossRef]
- Miranda-López, M.I.; Padilla-Zarate, E.A.; Hernández, M.B.; Falcón-Franco, L.A.; García-Villarreal, S.; García-Quiñonez, L.V.; Zambrano-Robledo, P.; Toxqui-Terán, A.; Aguilar-Martínez, J.A. Comparison between the use of Co3O4 or CoO on microstructure and electrical properties in a varistor system based on SnO2. J. Alloys Compd. 2020, 824, 153952. [Google Scholar]
- Goh, W.F.; Yoon, T.L.; Khan, S.A. Molecular dynamics simulation of thermodynamic and thermal transport properties of strontium titanate with improved potential parameters. Comput. Mater. Sci. 2012, 60, 123–129. [Google Scholar]
- Clarke, D.R. Materials selection guidelines for low thermal conductivity thermal barrier coatings. Surf. Coat. Technol. 2003, 163, 67–74. [Google Scholar]
- Chen, Y.; Jiang, P.; Zhang, R.; Sun, J.; Zhang, B.; Zhang, Y. Effect of porosity on thermal conductivity of five-component ceramic system. J. Aeronaut. Mater. 2023, 543, 66–74. [Google Scholar]

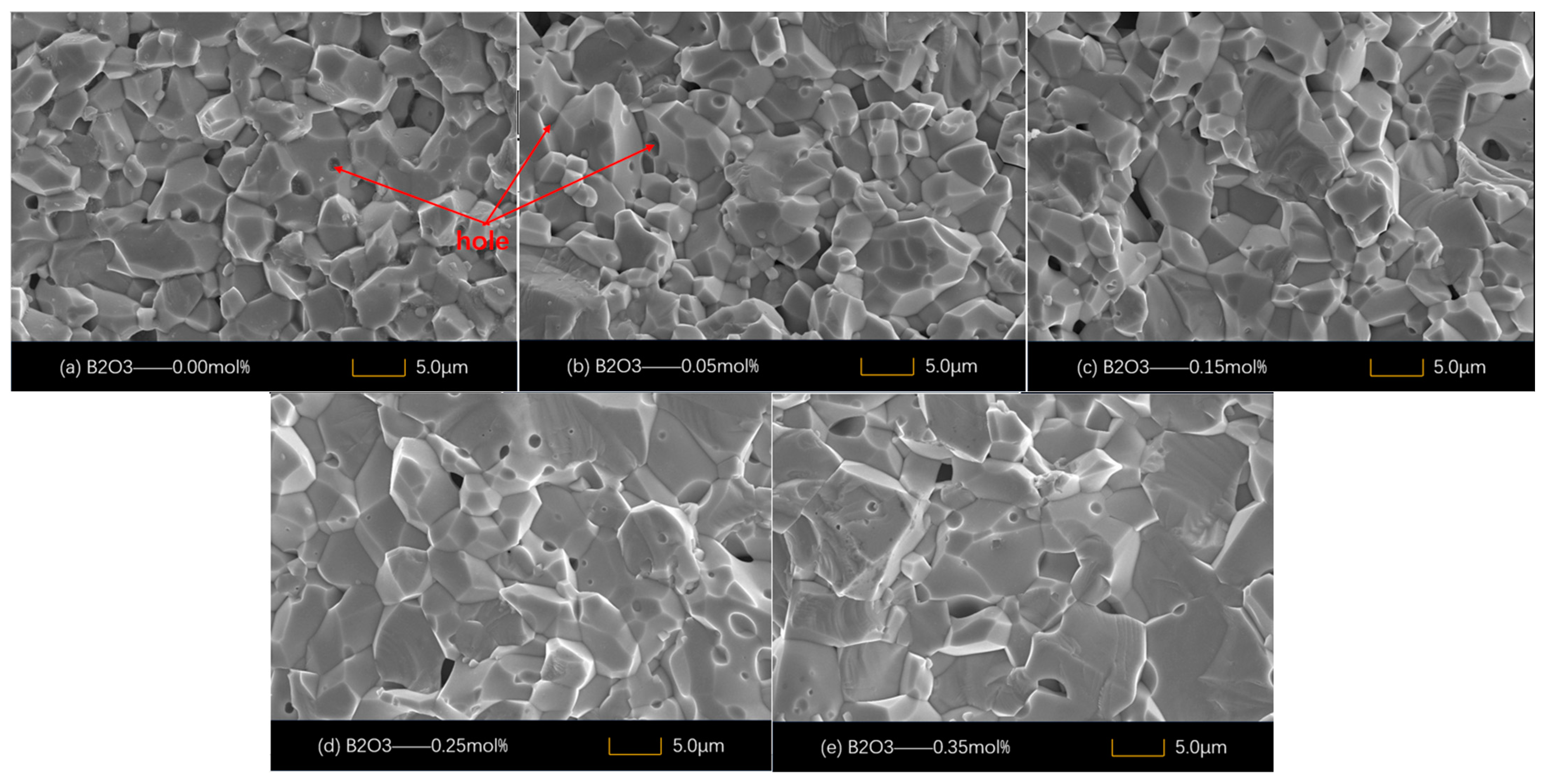
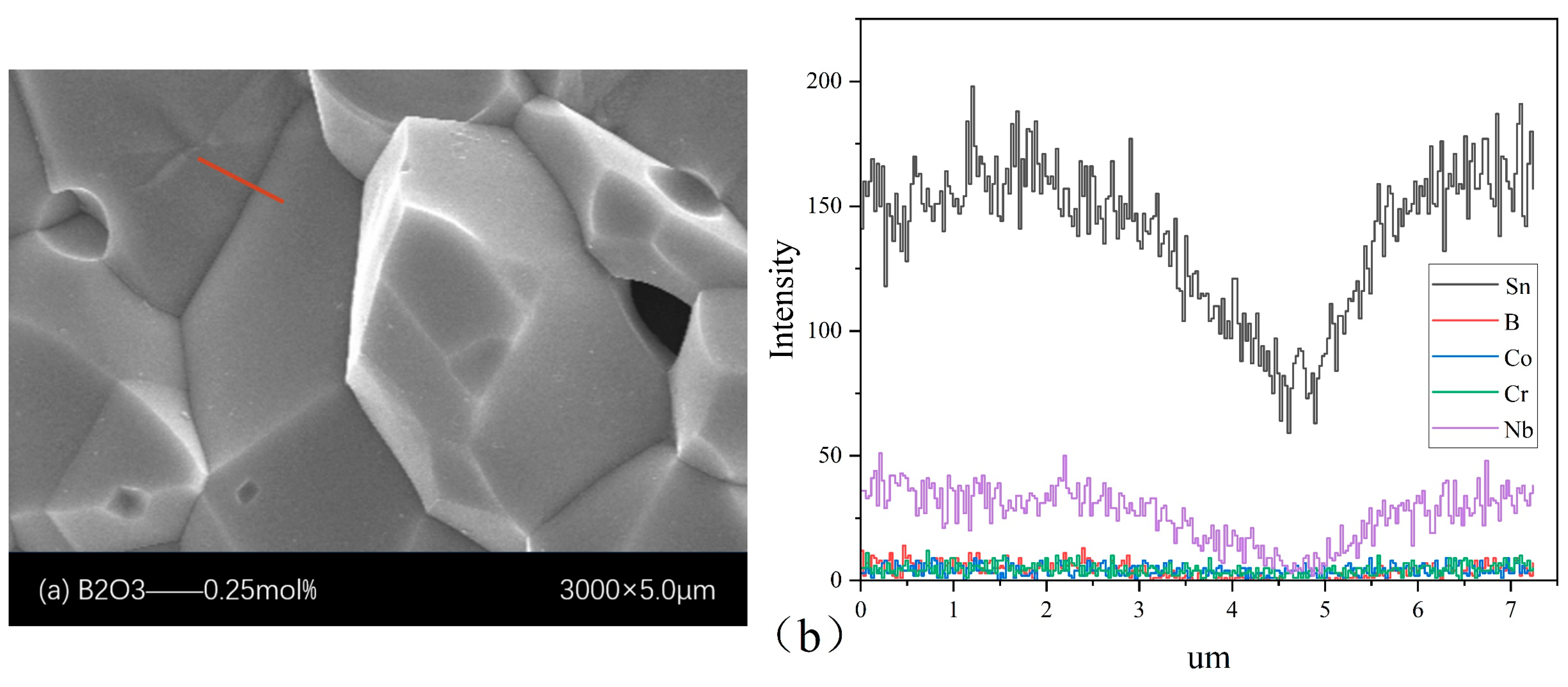
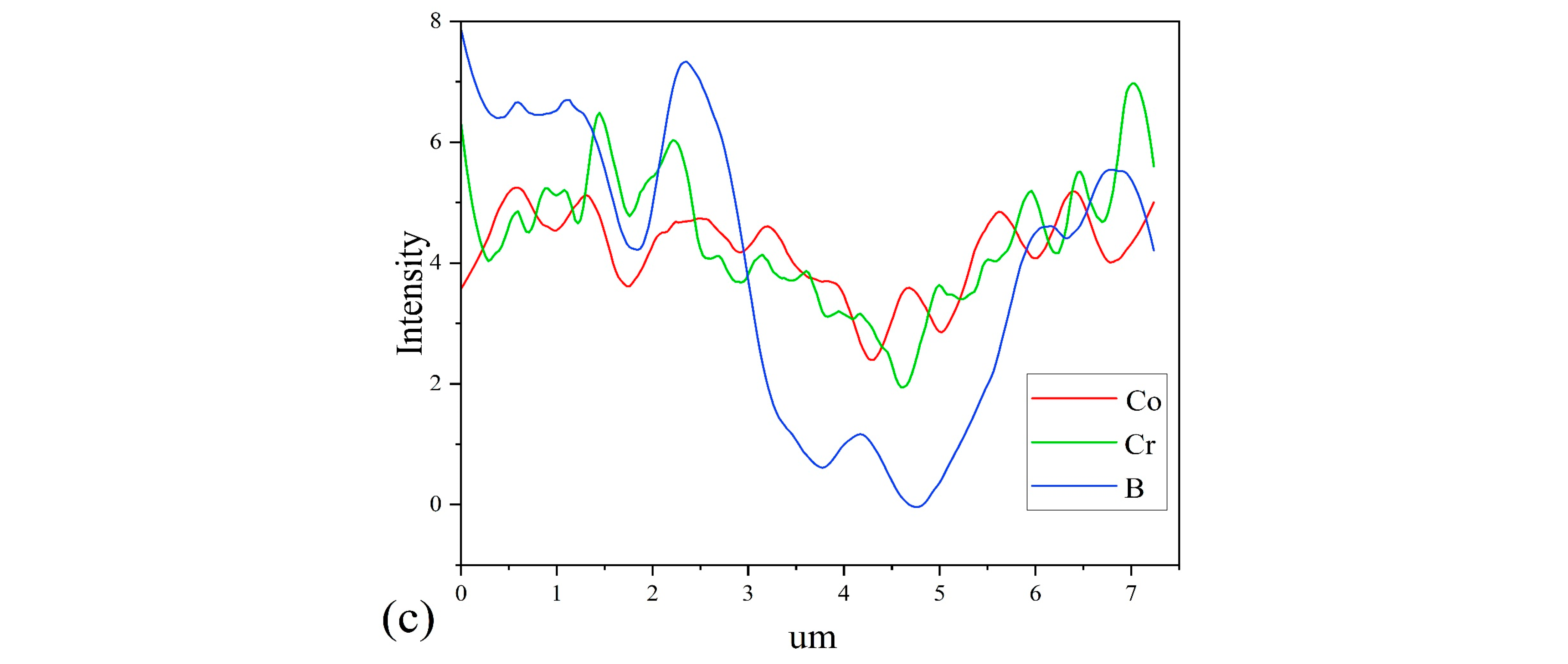
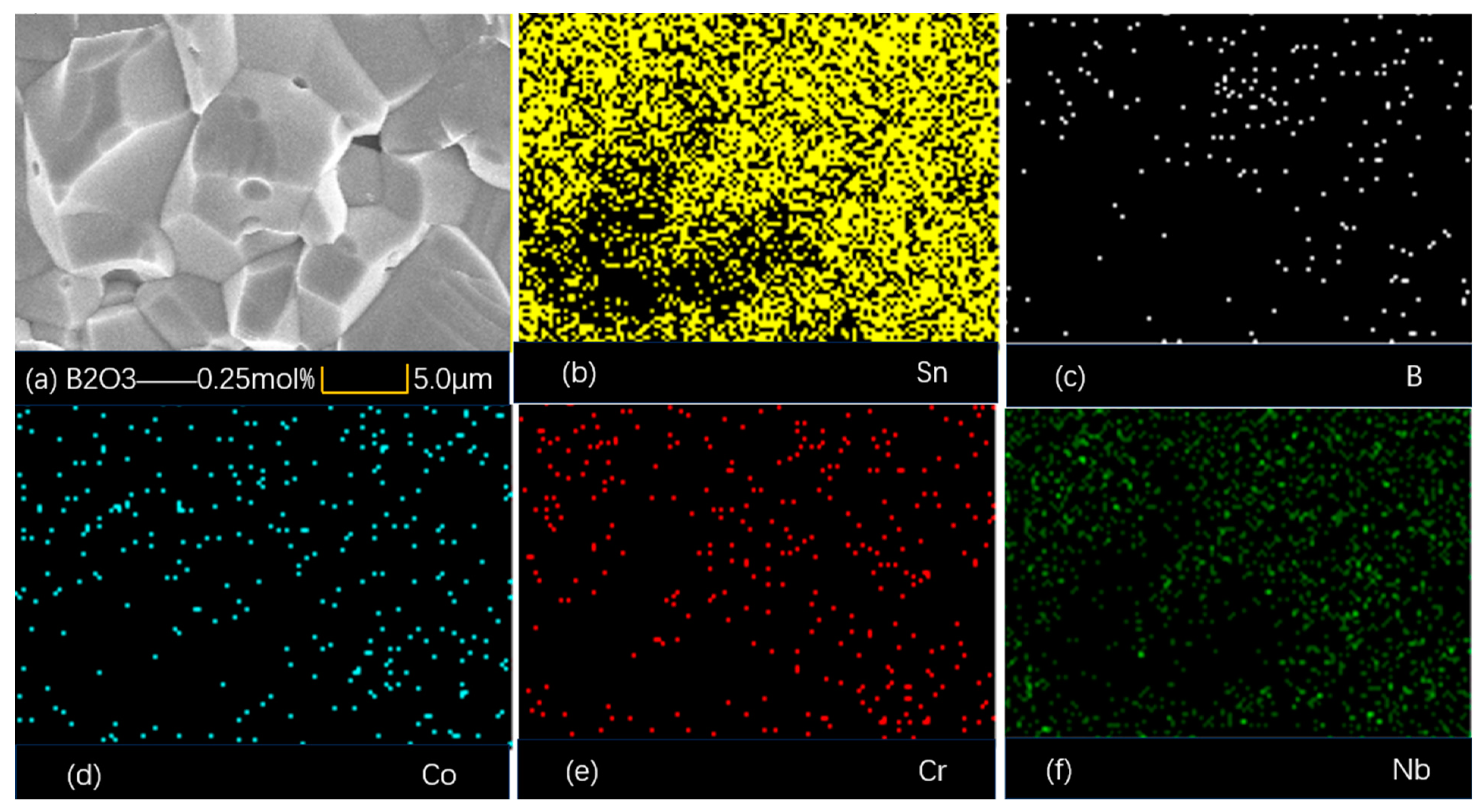
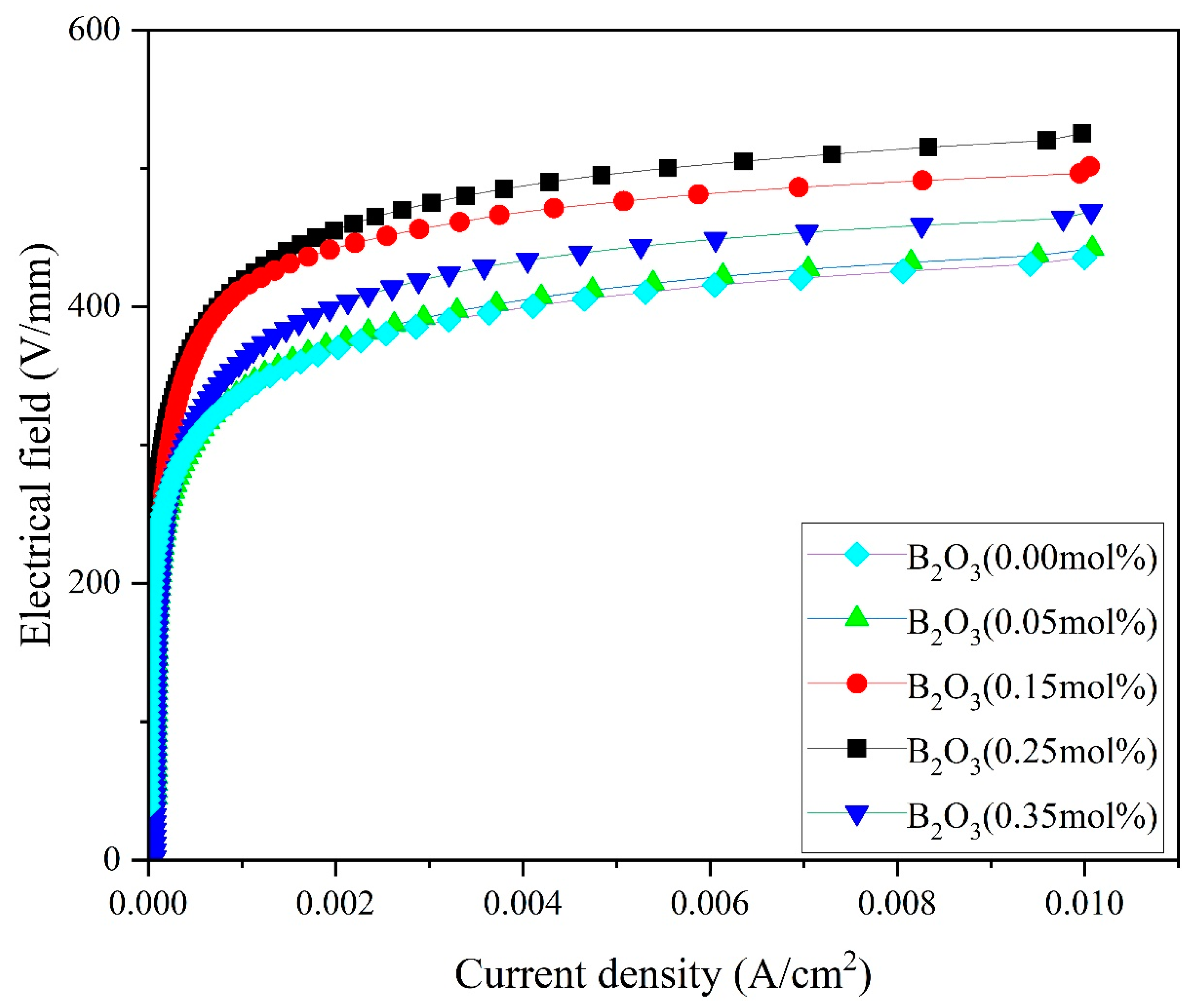
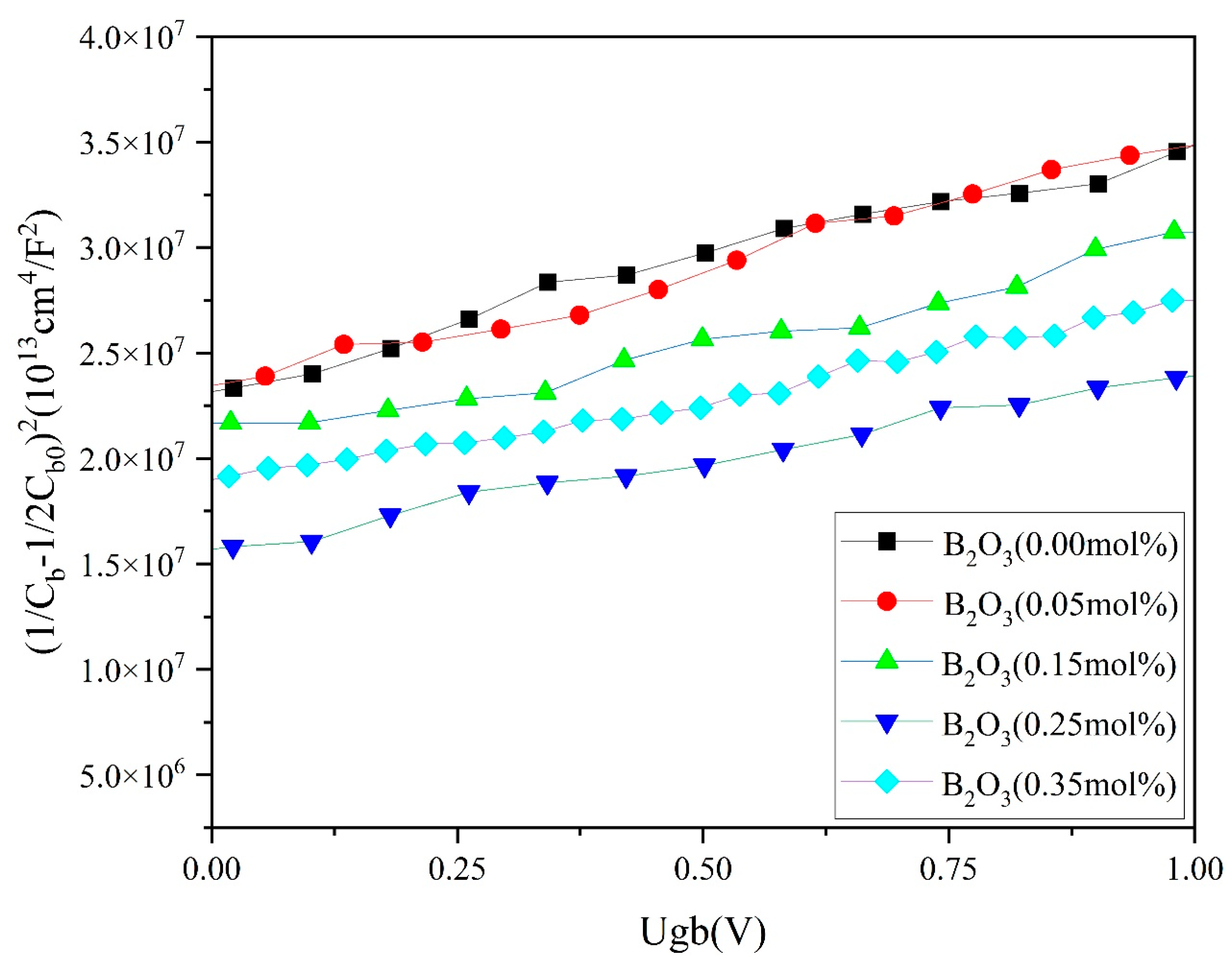
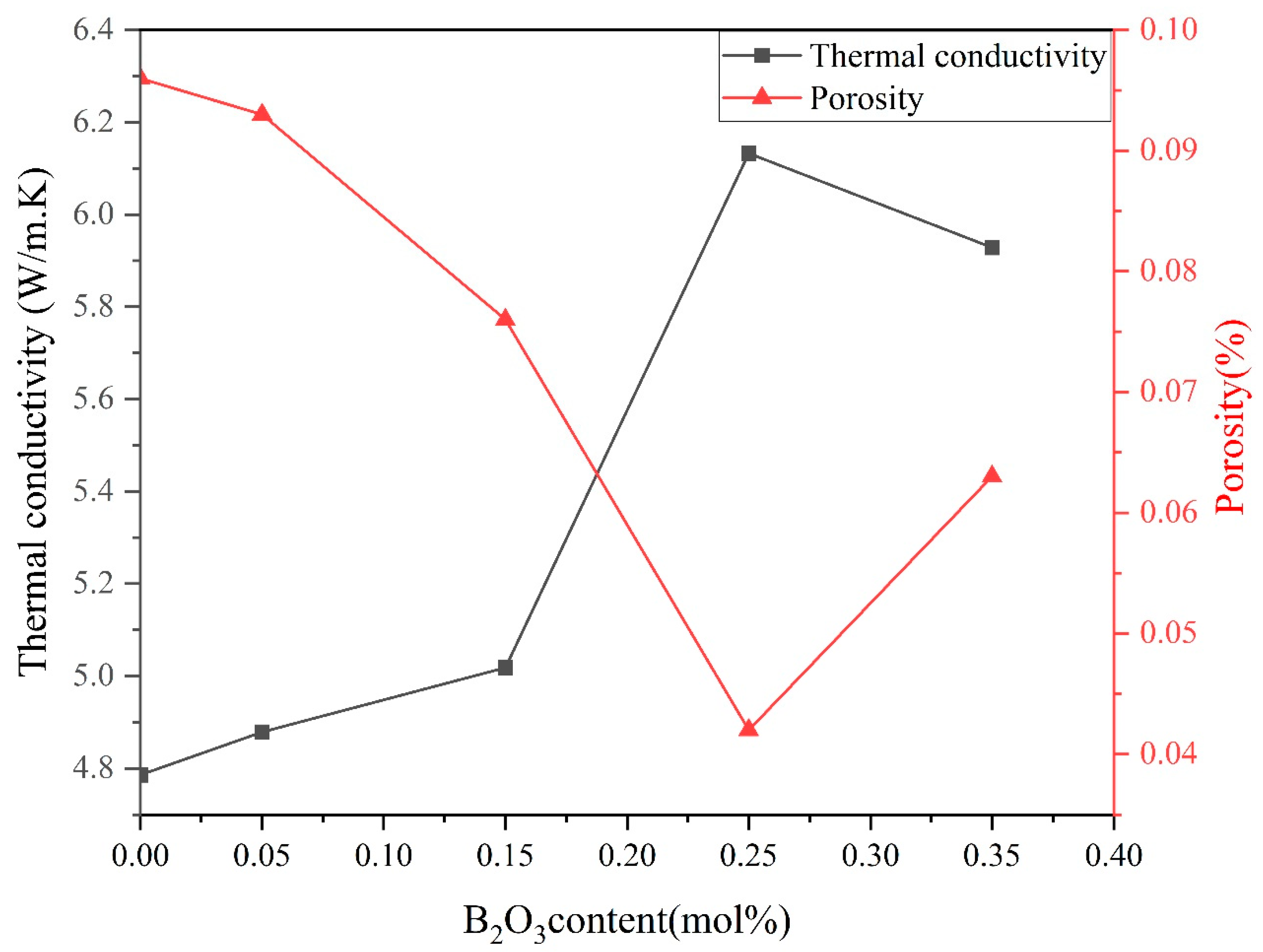
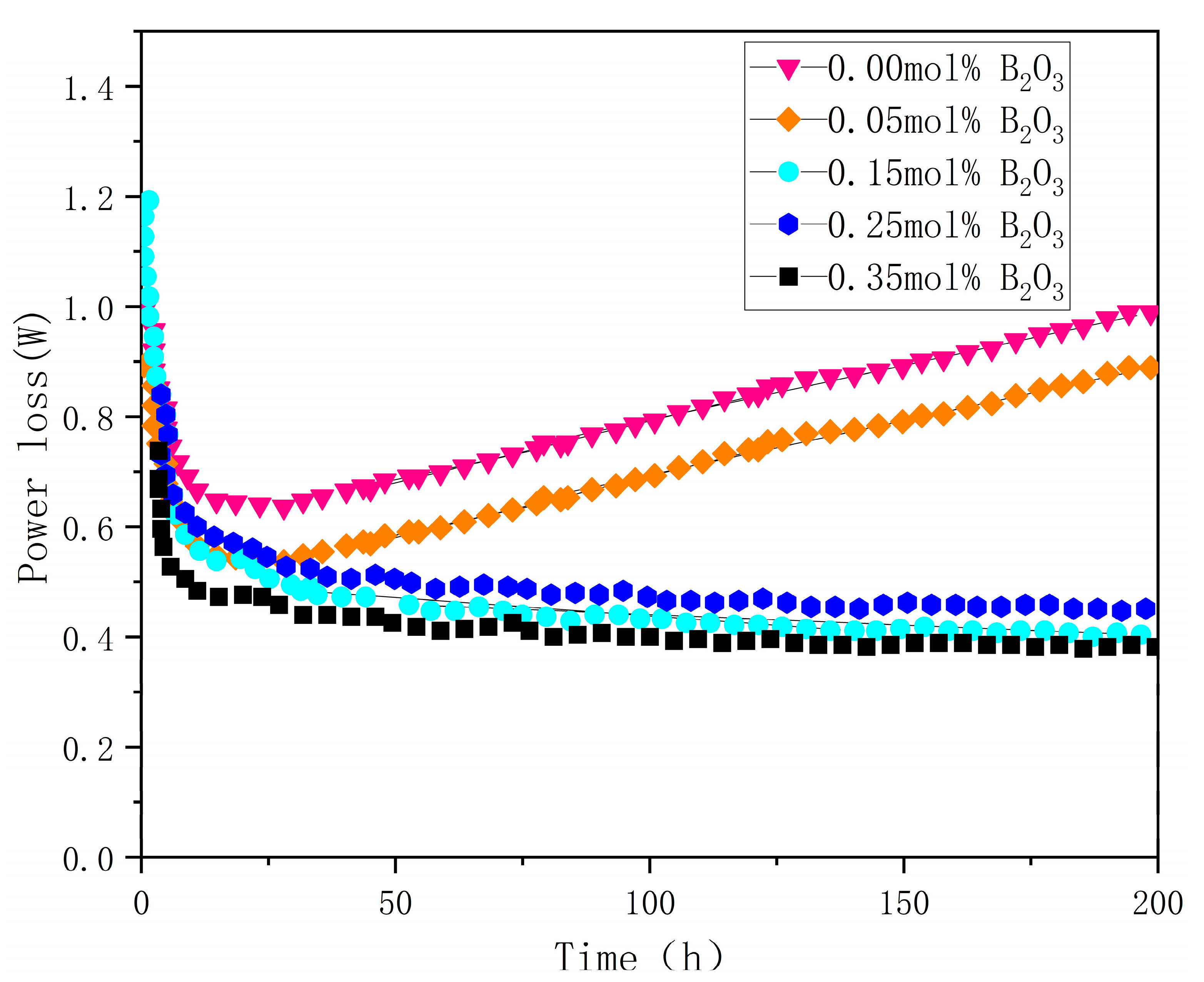
| B2O3 (mol%) | Porosity % | d (μm) | E1 mA (V/mm) | JL (μA/cm2) | α | Nd (1022 m−2) | Ni (1015 m−2) | Φb (eV) |
|---|---|---|---|---|---|---|---|---|
| 0.00 | 9.4% | 6.7 | 421 | 25 | 26 | 1.8 | 3.0 | 0.82 |
| 0.05 | 9.3% | 6.8 | 448 | 23 | 29 | 1.9 | 3.2 | 0.85 |
| 0.15 | 7.6% | 7.2 | 501 | 18.3 | 31 | 2.6 | 4.4 | 0.98 |
| 0.25 | 4.2% | 7.4 | 525 | 11.2 | 36 | 4.7 | 6.8 | 1.24 |
| 0.35 | 6.3% | 8.6 | 428 | 14.6 | 33 | 3.6 | 5.4 | 1.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, S.; Zhao, H. The Effect of B2O3 Doping on the Properties of Electrical and Thermal Conductivity for SnO2 Varistors. Materials 2025, 18, 1399. https://doi.org/10.3390/ma18071399
Gong S, Zhao H. The Effect of B2O3 Doping on the Properties of Electrical and Thermal Conductivity for SnO2 Varistors. Materials. 2025; 18(7):1399. https://doi.org/10.3390/ma18071399
Chicago/Turabian StyleGong, Siqiao, and Hongfeng Zhao. 2025. "The Effect of B2O3 Doping on the Properties of Electrical and Thermal Conductivity for SnO2 Varistors" Materials 18, no. 7: 1399. https://doi.org/10.3390/ma18071399
APA StyleGong, S., & Zhao, H. (2025). The Effect of B2O3 Doping on the Properties of Electrical and Thermal Conductivity for SnO2 Varistors. Materials, 18(7), 1399. https://doi.org/10.3390/ma18071399





