Synthesis, Structural and Magnetic Properties of BiFeO3 Substituted with Ag
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Structural Characterization of Bi1−xAgxFeO3 (x = 0.01, 0.02) Nanopowders
2.2. Magnetic Measurements
2.3. Crystal Structure Prediction (CSP) and Bond Valence Calculations (BVC)
2.4. Density Functional Theory (DFT) Calculations
3. Results
3.1. X-Ray Powder Diffraction (XRPD) and Rietveld Refinement
3.2. CSP of Silver-Doped BiFeO3 Perovskites
3.3. Electronic and Magnetic Properties—Density Functional Theory Study
- Doping reduces the magnetization in the FM-ordered systems of all crystals by around 5% except for the R phase, for which magnetization is reduced by 7%.
- In undoped systems, spins are perfectly compensated in AFM magnetic orders, whereas Ag doping induces net spins on Fe and O atoms in AFM magnetic textures.
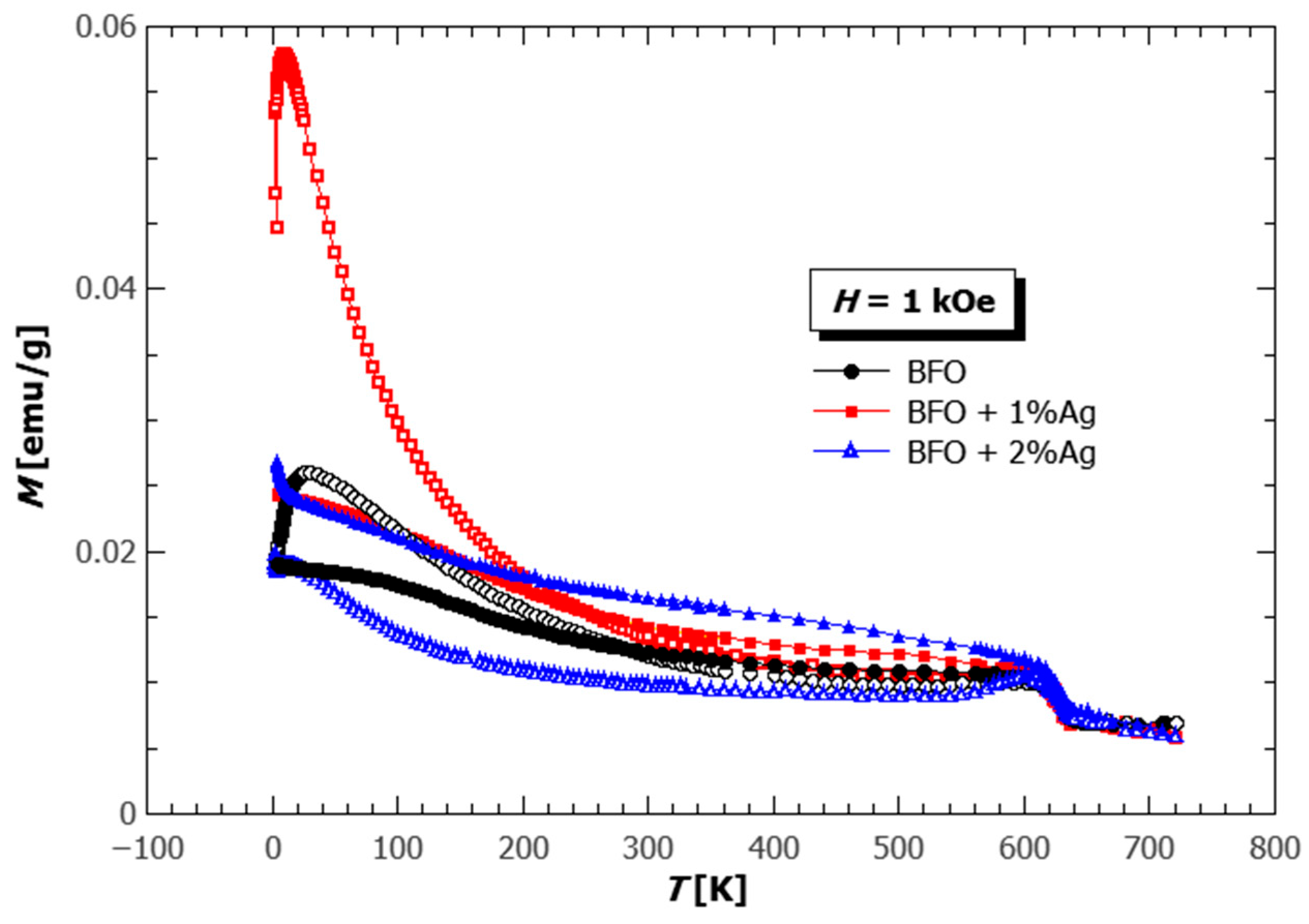
3.4. Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Monem, A.M.A.; Abd-Elmohssen, N.; El-Bahnasawy, H.H.; Makram, N.; Sedeek, K. Comparative studies by X-ray diffraction, Raman, vibrating sample magnetometer and Mössbauer spectroscopy of pure, Sr doped and Sr, Co co-doped BiFeO3 ceramic synthesized via tartaric acid-assisted technique. Ceram. Int. 2023, 49, 15213–15220. [Google Scholar] [CrossRef]
- Jin, C.; Geng, W.; Wang, L.; Han, W.; Zheng, D.; Hu, S.; Ye, M.; Xu, Z.; Ji, Y.; Zhao, J.; et al. Tuning ferroelectricity, and ferromagnetism in BiFeO3/BiMnO3 superlattices. Nanoscale 2020, 12, 9810–9816. [Google Scholar] [CrossRef]
- Cheong, S.W.; Mostovoy, M. Multiferroics: A magnetic twist for ferroelectricity. Nat. Mater. 2007, 6, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Haruna, A.; Abdulkadir, I.; Idris, S.O. Photocatalytic activity and doping effects of BiFeO3 nanoparticles in model organic dyes. Heliyon 2020, 6, e03237. [Google Scholar] [CrossRef]
- Freitas, V.F.; Dias, G.S.; Protzek, O.A.; Montanher, D.Z.; Catellani, I.B.; Silva, D.M.; C’otica, L.F.; dos Santos, I.A. Structural phase relations in perovskite–structured BiFeO3-based multiferroic compounds. J. Adv. Ceram. 2013, 2, 103–111. [Google Scholar] [CrossRef]
- Selbach, S.M.; Tybell, T.; Einarsrudand, T.M.; Grande, T. Size-dependent properties of multiferroic BiFeO3 nanoparticles. Chem. Mater. 2007, 19, 6478–6484. [Google Scholar] [CrossRef]
- Yakout, S.M. Spintronics and innovative memory devices: A review on advances in magnetoelectric BiFeO3. J. Supercond. Nov. Magnetism. 2021, 34, 317–338. [Google Scholar] [CrossRef]
- Rizwan, S.; Awan, S.; Irfan, S. Room-temperature ferromagnetism in Gd and Sn co-doped bismuth ferrite nanoparticles and co-doped BiFeO3/MXene (Ti3C2) nanohybrids of spintronics applications. Ceram. Int. 2020, 46 Pt A, 29011–29021. [Google Scholar] [CrossRef]
- Neogi, S.; Ghosh, R. Behavioral remodelling of perovskite BiFeO3 by Ag-doping strategies for enhanced and ppb level acetone sensing performance: An advanced enlightenment for selectivity and sensing mechanism. Appl Mater Today 2022, 29, 101611. [Google Scholar] [CrossRef]
- Xu, H.; Xu, J.; Li, H.; Zhang, W.; Zhang, Y.; Zhai, Z. Highly sensitive ethanol and acetone gas sensors with reduced working temperature based on Sr-doped BiFeO3 nanomaterial. J. Mater. Res. Technol. 2022, 17, 1955–1963. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Y. Defect chemistry for Mn-doped and Nb-doped BiFeO3-based ceramics. J. Phys. Chem. Solids 2022, 170, 110951. [Google Scholar] [CrossRef]
- Aishwarya, K.; Navamathavan, R. Effect of grain size and orthorhombic phase of La-doped BiFeO3 on thermoelectric properties. J. Alloys Compd. 2023, 947, 169452. [Google Scholar] [CrossRef]
- Nazeer, Z.; Bibi, I.; Majid, F.; Kamal, S.; Ghafoor, A.; Ali, A.; Kausar, A.; Elqahtani, Z.M.; Alwadai, N.; Iqbal, M. Microemulsion synthesis of Ga and Sr doped BiFeO3 nanoparticles and evaluation of their ferroelectric, optical, dielectric and photocatalytic properties. Phys. B 2023, 657, 414788. [Google Scholar] [CrossRef]
- Anwar, A.; Basith, M.A.; Choudhury, S. From bulk to nano: A comparative investigation of structural, ferroelectric and magnetic properties of Sm and Ti co-doped BiFeO3 multiferroics. Mater. Res. Bull. 2019, 111, 93–101. [Google Scholar] [CrossRef]
- Chowdhury, S.S.; Kamal, A.H.M.; Hossain, R.; Hasan, M.; Islam, M.F.; Ahmmad, B.; Basith, M.A. Dy doped BiFeO3: A bulk ceramic with improved multiferroic properties compared to nano counterparts. Ceram. Int. 2017, 43, 9191–9199. [Google Scholar] [CrossRef]
- Moreau, J.M.; Michel, C.; Gerson, R.; James, W.J. Ferroelectric BiFeO3 X-ray and neutron diffraction study. J. Phys. Chem. Solids 1971, 32, 1315–1320. [Google Scholar] [CrossRef]
- Kubel, F.; Schmid, H. Structure of a ferroelectric and ferroelastic monodomain crystal of the perovskite BiFeO3. Acta Crystallog. B 1990, 46, 698–702. [Google Scholar] [CrossRef]
- Wang, J.; Neaton, J.B.; Zheng, H.; Nagarajan, V.; Ogale, S.B.; Liu, B.; Viehland, D.; Vaithyanathan, V.; Schlom, D.G.; Waghmare, U.V.; et al. Epitaxial BiFeO3 Multiferroic Thin Film Heterostructures. Science 2003, 299, 1719–1722. [Google Scholar] [CrossRef] [PubMed]
- Feng, H. Magnetism and electronic properties of BiFeO3 under lower pressure. J. Magn. Magn. Mater. 2010, 322, 3755–3759. [Google Scholar] [CrossRef]
- Catalan, G.; Scott, J.F. Physics and Applications of Bismuth Ferrite. Adv. Mater. 2009, 21, 2463–2485. [Google Scholar] [CrossRef]
- Martin, L.W.; Crane, S.P.; Chu, Y.H.; Holcomb, M.B.; Gajek, M.; Huijben, M.; Yang, C.H.; Balke, N.; Ramesh, R. Multiferroics and magnetoelectrics: Thin films and nanostructures. J. Phys.-Condens. Mat. 2008, 20, 434220. [Google Scholar] [CrossRef]
- Wu, J.; Fan, Z.; Xiao, D.; Zhu, J.; Wang, J. Multiferroic bismuth ferrite-based materials for multifunctional applications: Ceramic bulks, thin films and nanostructures. Prog. Mater. Sci. 2016, 84, 335–402. [Google Scholar] [CrossRef]
- Park, J.-G.; Le, M.D.; Jeong, J.; Lee, S. Structure and spin dynamics of multiferroic BiFeO3. J. Phys.-Condens. Mat. 2014, 26, 433202. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wei, M.; Zhang, J.; Zhang, Y. Role of Ho Doping in Magnetization Mechanism of BiFeO3 Thin Films. J. Supercond. Nov. Magn. 2019, 32, 3495–3501. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Wang, Y.J.; Zhang, J.; Gao, M.; Zhang, Y.J.; Wei, M.B.; Yang, J.H. Effect of Ho substitution on the structure and magnetic property of BiFeO3 prepared by sol-gel method. Mater. Sci. Semicond. Process. 2015, 40, 787–795. [Google Scholar] [CrossRef]
- Sharif, S.; Murtaza, G.; Meydan, T.; Williams, P.I.; Cuenca, J.; Hashimdeen, S.H.; Shaheen, F.; Ahmad, R. Structural, surface morphology, dielectric and magnetic properties of holmium doped BiFeO3 thin films prepared by pulsed laser deposition. Thin Solid Films 2018, 662, 83–89. [Google Scholar] [CrossRef]
- Hou, P.; Liu, B.; Guo, Z.; Zhou, P.; Wang, B.; Zhao, L. Effect of Ho doping on the crystal structure, surface morphology and magnetic property of BiFeO3 thin films prepared via the sol-gel technology. J. Alloys Compd. 2019, 775, 59–62. [Google Scholar] [CrossRef]
- Yun, Q.; Bai, A.; Zhao, S. Lattice distortion of holmium doped bismuth ferrite nanofilms. J. Rare Earth 2014, 32, 884–889. [Google Scholar] [CrossRef]
- Islam, M.R.; Zubair, M.A.; Galib, R.H.; Hoque, M.S.B.; Tomko, J.A.; Aryana, K.; Basak, A.K.; Hopkins, P.E. Vacancy-Induced Temperature-Dependent Thermal and Magnetic Properties of Holmium-Substituted Bismuth Ferrite Nanoparticle Compacts. ACS Appl. Mater. Interfaces 2022, 14, 25886–25897. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Bag, R.; Sathe, V.; Kulkarni, S.; Singh, S. Holmium induced enhanced functionality at room temperature and structural phase transition at high temperature in bismuth ferrite nanoparticles. J. Mater. Chem. C 2016, 4, 780–792. [Google Scholar] [CrossRef]
- Stojadinović, B.; Dohčević-Mitrović, Z.; Stepanenko, D.; Rosić, M.; Petronijević, I.; Tasić, N.; Ilić, N.; Matović, B.; Stojanović, B. Dielectric and ferroelectric properties of Ho-doped BiFeO3 nanopowders across the structural phase transition. Ceram. Int. 2017, 43, 16531–16538. [Google Scholar] [CrossRef]
- Kuang, D.; Yang, F.; Wang, W.; Yang, Z. Effects of Ho3+ and transition metal ion doping on optical and magnetic properties of BiFeO3 nanopowders. J. Mater. Sci.-Mater. Electron. 2018, 29, 4041–4047. [Google Scholar] [CrossRef]
- Thakuria, P.; Joy, P.A. High room temperature ferromagnetic moment of Ho substituted nanocrystalline BiFeO3. Appl. Phys. Lett. 2010, 97, 162504. [Google Scholar] [CrossRef]
- Jeon, N.; Rout, D.; Kim, I.W.; Kang, S.-J.L. Enhanced multiferroic properties of single-phase BiFeO3 bulk ceramics by Ho doping. Appl. Phys. Lett. 2011, 98, 072901. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Das, J.; Rout, P.P.; Mohanta, V.R.; Das, S.K.; Samantray, S.; Sahu, D.R.; Huang, J.L.; Verma, S.; Roul, B.K. Effect of holmium substitution for the improvement of multiferroic properties of BiFeO3. J. Phys. Chem. Solids 2010, 71, 1557–1564. [Google Scholar] [CrossRef]
- Van Minh, N.; Quan, N.G. Structural, optical and electromagnetic properties of Bi1−xHoxFeO3 multiferroic materials. J. Alloys Compd. 2011, 509, 2663–2666. [Google Scholar] [CrossRef]
- Muneeswaran, M.; Lee, S.H.; Kim, D.H.; Jung, B.S.; Chang, S.H.; Jang, J.-W.; Choi, B.C.; Jeong, J.H.; Giridharan, N.V.; Venkateswaran, C. Structural, vibrational, and enhanced magneto-electric coupling in Ho-substituted BiFeO3. J. Alloys Compd. 2018, 750, 276–285. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Kang, W.; Yan, Z.; Shen, Y.; Huo, J. Anti-perovskite nitrides and oxides: Properties and preparation. Comp. Mater. Sci. 2023, 225, 112188. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. Die gesetze der krystallochemie. Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Čebela, M.; Zagorac, D.; Batalović, K.; Radaković, J.; Stojadinović, B.; Spasojević, V.; Hercigonja, R. BiFeO3 perovskites: A multidisciplinary approach to multiferroics. Ceram. Int. 2017, 43, 1256–1264. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Zagorac, D.; Schön, J.C. Energy landscapes of pure and doped ZnO: From bulk crystals to nanostructures. Front. Nanosci. 2022, 21, 151–193. [Google Scholar] [CrossRef]
- Zagorac, J.; Zagorac, D.; Šrot, V.; Ranđelović, M.; Pejić, M.; van Aken, P.A.; Matović, B.; Schön, J.C. Synthesis, Characterization, and Electronic Properties of ZnO/ZnS Core/Shell Nanostructures Investigated Using a Multidisciplinary Approach. Materials 2023, 16, 326. [Google Scholar] [CrossRef]
- Buyer, C.; Grossholz, H.; Wolf, S.; Zagorac, D.; Zagorac, J.; Schön, J.C.; Schleid, T. Crystal-Structure Prediction and Experimental Investigation of the Polymorphic Lanthanum Fluoride Selenides LaFSe and La2F4Se. Cryst. Growth Des. 2022, 22, 7133–7142. [Google Scholar] [CrossRef]
- Pejić, M.; Zagorac, D.; Zagorac, J.; Matović, B.; Schön, J.C. Structure prediction via global energy landscape exploration of the ternary rare-earth compound LaOI. Z.. Anorg. Allg. Chem. 2022, 648, e202200308. [Google Scholar] [CrossRef]
- Zagorac, J.; Zagorac, D.; Zarubica, A.; Schön, J.C.; Djuris, K.; Matovic, B. Prediction of possible CaMnO3 modifications using an ab initio minimization data-mining approach. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2014, 70, 809–819. [Google Scholar] [CrossRef]
- Rosić, M.; Zagorac, D.; Milivojević, D.; Paunović, N.; Zagorac, J.; Dohčević-Mitrović, Z.; Matović, B. Theoretical and experimental study of octahedral tilting of Ca1-xGdxMnO3 (x = 0.05, 0.1, 0.15, 0.2) nanometric powders. J. Alloys Compd. 2016, 678, 219–227. [Google Scholar] [CrossRef]
- Lufaso, M.W.; Woodward, P.M. Prediction of the crystal structures of perovskites using the software program SPuDS. Acta Crystallogr. B 2001, 57, 725–738. [Google Scholar] [CrossRef]
- Lufaso, M.W.; Woodward, P.M. Jahn-Teller distortions, cation ordering and octahedral tilting in perovskites. Acta Crystallogr. B 2004, 60, 10–20. [Google Scholar] [CrossRef]
- Hundt, R.; Schön, J.C.; Hannemann, A.; Jansen, M. Determination of symmetries and idealized cell parameters for simulated structures. J. Appl. Crystallogr. 1999, 32, 413–416. [Google Scholar] [CrossRef]
- Hannemann, A.; Hundt, R.; Schön, J.C.; Jansen, M. A New Algorithm for Space-Group Determination. J. Appl. Crystallogr. 1998, 31, 922–928. [Google Scholar] [CrossRef]
- Hundt, R. KPLOT: A Program for Plotting and Analysing Crystal Structures; Technicum Scientific Publishing: Stuttgart, Germany, 2016. [Google Scholar]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Soler, J.M.; Artacho, E.; Gale, J.D.; Garcia, A.; Junquera, J.; Ordejon, P.; Sanchez-Portal, D. The SIESTA Method for Ab initio Order-N Materials Simulation. J. Phys. Condens. Matter. 2002, 14, 2745. [Google Scholar] [CrossRef]
- Troullier, N.; Martins, J.L. Efficient Psudopotentials for Plane-wave Calculations. Phys. Rev. B 1991, 43, 1993–2006. [Google Scholar] [CrossRef]
- Liechtenstein, A.I.; Anisimov, V.I.; Zaanen, J. Density-functional theory and strong interactions: Orbital ordering in Mott-Hubbard insulators. Phys. Rev. B 1995, 52, R5467–R5470. [Google Scholar] [CrossRef]
- Anisimov, V.I.; Zaanen, J.; Andersen, O.K. Band theory and Mott insulators: Hubbard U instead of Stoner I. Phys. Rev. B 1991, 44, 943–954. [Google Scholar] [CrossRef]
- Vladimir, I.A.; Aryasetiawan, F.; Lichtenstein, A.I. First-principles calculations of the electronic structure and spectra of strongly correlated systems: The LDA+ U method. J. Phys.-Condens. Matter 1997, 9, 767. [Google Scholar] [CrossRef]
- Ivanov, S.A.; Nordblad, P.; Tellgren, R.; Ericsson, T.; Korchagina, S.K.; Rybakova, L.F.; Hewat, A. Influence of PbZrO3 doping on the structural and magnetic properties of BiFeO3. Solid State Sci. 2008, 10, 1875–1885. [Google Scholar] [CrossRef]
- Roisnel, T.; Rodriguez-Carvajal, J. WinPLOTR: A Windows tool for powder diffraction pattern analysis. Mater. Sci. Forum 2001, 378, 118–123. [Google Scholar] [CrossRef]
- Shannon, R. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Sarkar, I.; Shirolkar, M.M.; Jeng, U.-S.; Yeh, Y.-Q.; Rajendra, R.; Ballav, N.; Kulkarni, S. Probing bismuth ferrite nanoparticles by hard x-ray photoemission: Anomalous occurrence of metallic bismuth. Appl. Phys. Lett. 2014, 105, 102910. [Google Scholar] [CrossRef]
- Palai, R.; Katiyar, R.S.; Schmid, H.; Tissot, P.; Clark, S.J.; Robertson, J.; Redfern, S.A.T.; Catalan, G.; Scott, J.F. β phase and γ−β metal-insulator transition in multiferroic BiFeO3. Phys. Rev. B 2008, 77, 014110. [Google Scholar] [CrossRef]
- Haumont, R.; Kreisel, J.; Bouvier, P.; Hippert, F. Phonon anomalies and the ferroelectric phase transition in multiferroic BiFeO3. Phys. Rev. B 2006, 73, 132101. [Google Scholar] [CrossRef]
- Selbach, S.M.; Tybell, T.; Einarsrud, M.-A.; Grande, T. The Ferroic Phase Transitions of BiFeO3. Adv. Mater. 2008, 20, 3692–3696. [Google Scholar] [CrossRef]
- Munro, J.M.; Akamatsu, H.; Padmanabhan, H.; Liu, V.S.; Shi, Y.; Chen, L.-Q.; VanLeeuwen, B.K.; Dabo, I.; Gopalan, V. Discovering minimum energy pathways via distortion symmetry groups. Phys. Rev. B 2018, 98, 085107. [Google Scholar] [CrossRef]
- Zagorac, D.; Zagorac, J.; Schön, J.C.; Stojanović, N.; Matović, B. ZnO/ZnS (hetero)structures: Ab initio investigations of polytypic behaviour of mixed ZnO and ZnS compounds. Acta Crystallogr. B 2018, 74, 628–642. [Google Scholar] [CrossRef]
- Ravindran, P.; Vidya, R.; Kjekshus, A.; Fjellvåg, H.; Eriksson, O. Theoretical investigation of magnetoelectric behaviour in BiFeO3. Phys. Rev. B 2006, 74, 224412. [Google Scholar] [CrossRef]
- Diéguez, O.; González-Vázquez, O.E.; Wojdeł, J.C.; Íñiguez, J. First-principles predictions of low-energy phases of multiferroic BiFeO3. Phys. Rev. B 2011, 83, 094105. [Google Scholar] [CrossRef]
- Lu, J.; Günther, A.; Schrettle, F.; Mayr, F.; Krohns, S.; Lunkenheimer, P.; Pimenov, A.; Travkin, V.D.; Mukhin, A.A.; Loidl, A. On the room temperature multiferroic BiFeO3: Magnetic, dielectric and thermal properties. Eur. Phys. J. B 2010, 75, 451–460. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Wang, D.; Zhang, H.; He, X.; Li, Q. The Study of Magnetic Properties for Non-Magnetic Ions Doped BiFeO3. Materials 2021, 14, 4061. [Google Scholar] [CrossRef]
- Luković Golić, D.; Radojković, A.; Dapčević, A.; Pajić, D.; Dragović, J.; Torić, F.; Ćirković, J.; Branković, G.; Branković, Z. Change in structural, ferroelectric, and magnetic properties of bismuth ferrite induced by doping with gadolinium. Ceram. Int. 2019, 45, 19158–19165. [Google Scholar] [CrossRef]
- Radojković, A.; Luković Golić, D.; Ćirković, J.; Marinković Stanojević, Z.; Pajić, D.; Torić, F.; Dapčević, A.; Vulić, P.; Branković, Z.; Branković, G. Tuning of BiFeO3 multiferroic properties by light doping with Nb. Ceram. Int. 2018, 44, 16739–16744. [Google Scholar] [CrossRef]
- Suchomski, C.; Reitz, C.; Pajic, D.; Jaglicic, Z.; Djerdj, I.; Brezesinski, T. Large-Pore Mesoporous Ho3Fe5O12 Thin Films with a Strong Room-Temperature Perpendicular Magnetic Anisotropy by Sol-Gel Processing. Chem. Mater. 2014, 26, 2337–2343. [Google Scholar] [CrossRef]
- Englich, J.; Lütgemeier, H.; Pieper, M.W.; Jülich, K.F.A.; Germany, W.; Nekvasil, V.; Novák, P. Umbrella structure in Ho substituted YIG–NMR study. Solid State Commun. 1985, 56, 825–828. [Google Scholar] [CrossRef]
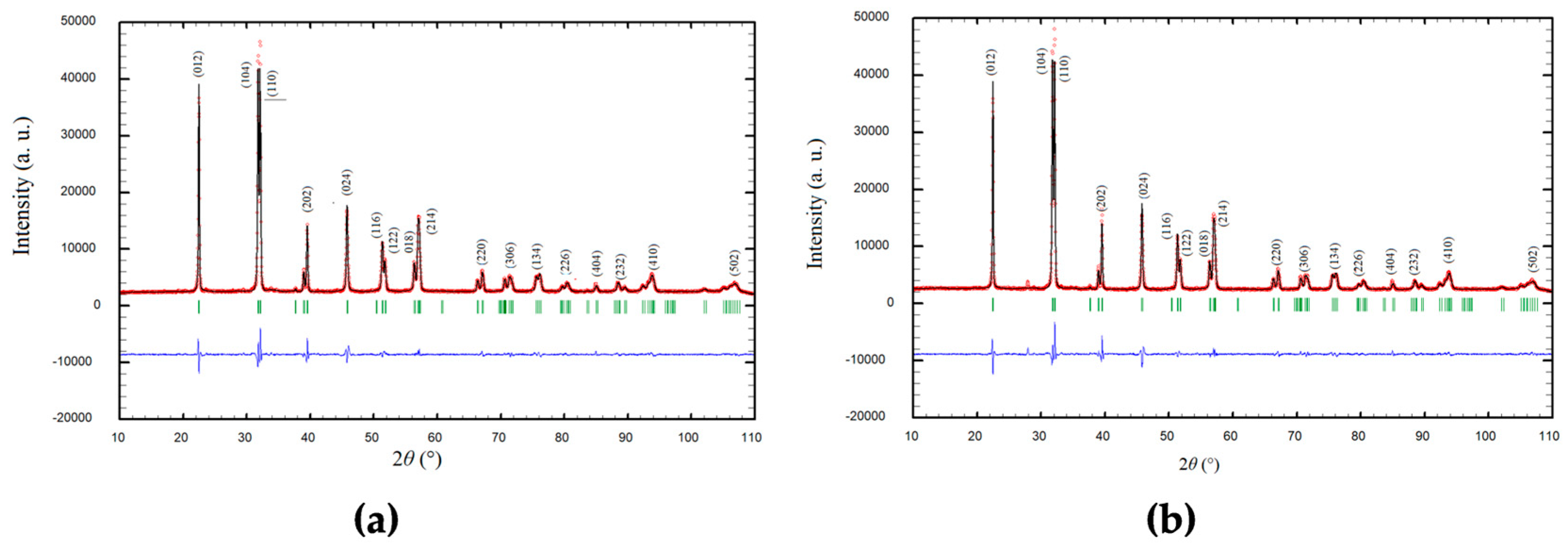
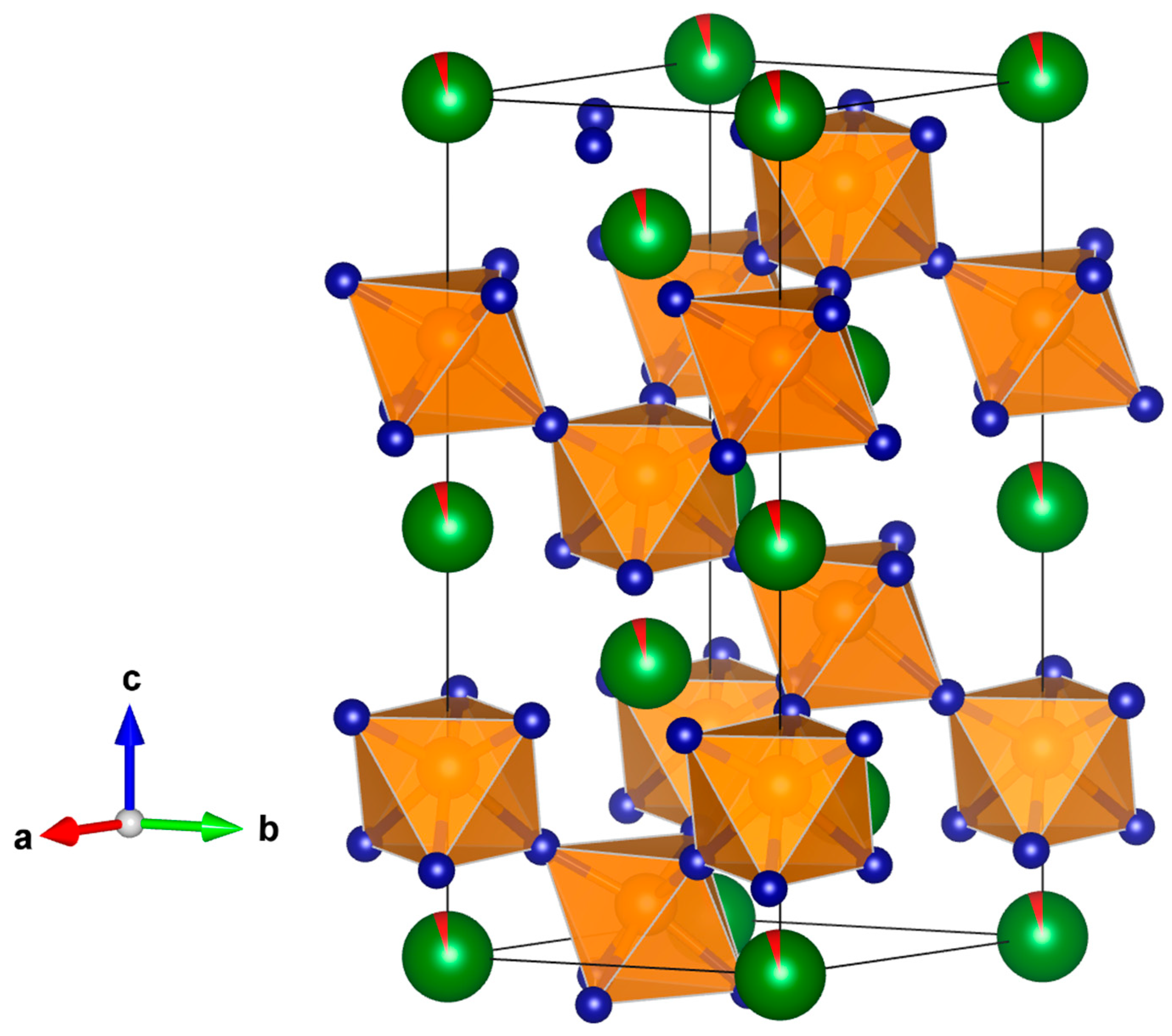
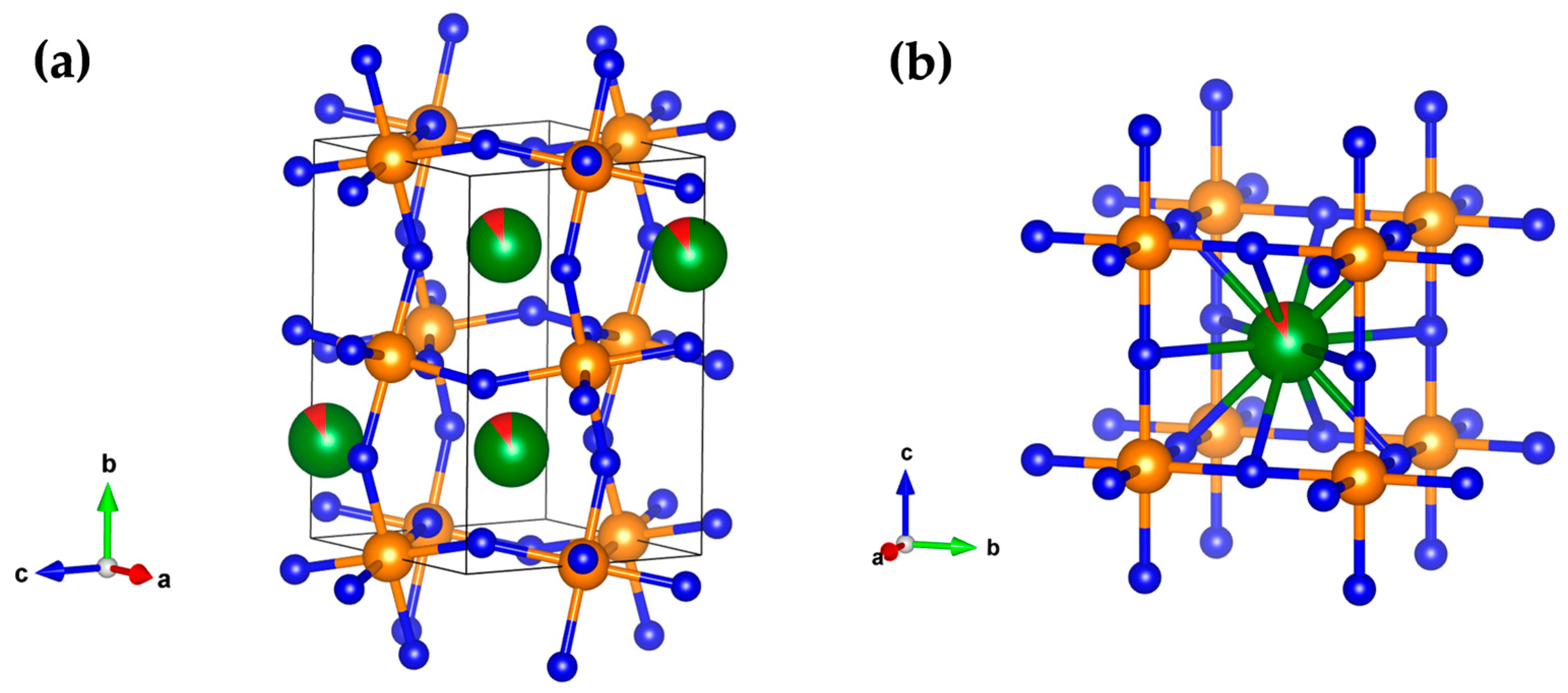

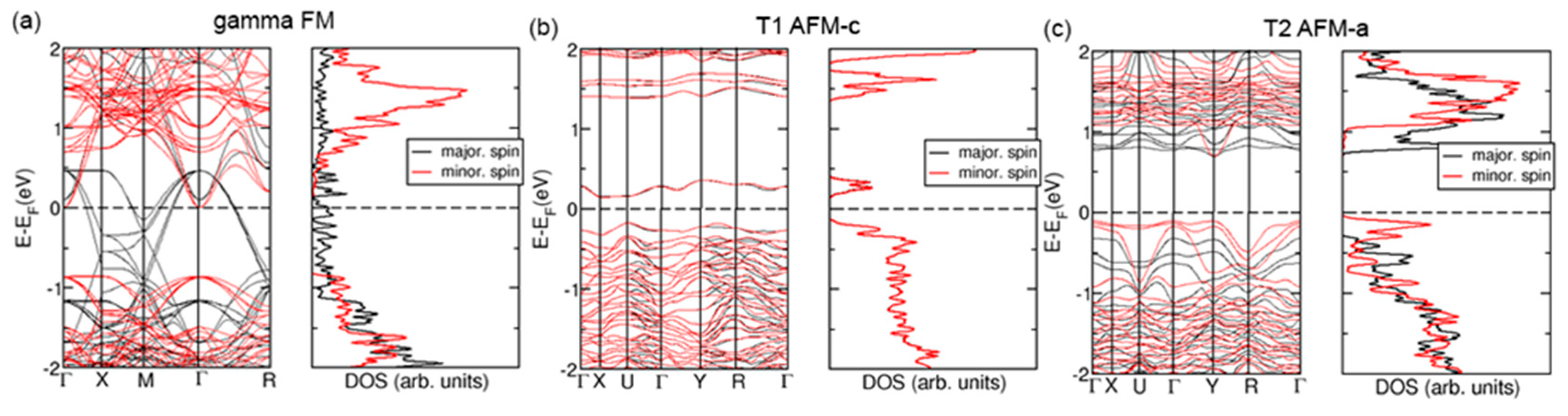

| Sample | Structure Parameters | Distances |
|---|---|---|
| Bi0.99Ag0.01FeO3 | a = 5.57708(6) c = 13.8667(3) V = 373.52 Å3 Bi/Ag 0 0 0 Fe 0 0 0.2170(2) O 0.570(1) 0.027(1) 0.9512(5) | 3 × (Bi-O) = 2.5651(19) Å 3 × (Bi-O) = 2.227(6) Å Average = 2.3963 Å 3 × (Fe-O) = 2.002(4) Å 3 × (Fe-O) = 2.045(5) Å Average = 2.0231 Å |
| Bi0.98Ag0.02FeO3 | a = 5.57765(6) c = 13.8682(3) V = 373.64 Å3 Bi/Ag 0 0 0 Fe 0 0 0.2208(2) O 0.562(2) 0.022(1) 0.9533(6) | 3 × (Bi-O) = 2.586(3) Å 3 × (Bi-O) = 2.262(8) Å Average = 2.4244 Å 3 × (Fe-O) = 1.963(5) Å 3 × (Fe-O) = 2.075(7) Å Average = 2.0191 Å |
| BiFeO3 [1] | a = 5.5767(3) c = 13.8639(8) V = 373.40 Å3 Bi 0 0 0 Fe 0 0 0.2208(2) O 0.4478(4) 0.0196(5) 0.9522(2) | 3 × (Bi-O) = 2.533(3) Å 3 × (Bi-O) = 2.265(3) Å Average = 2.3989 Å 3 × (Fe-O) = 1.957(5) Å 3 × (Fe-O) = 2.101(7) Å Average = 2.0289 Å |
| Composition | Bi0.99Ag0.01FeO3 | ||
|---|---|---|---|
| Modification | Space Group | Tilt System | GII |
| β-type | Pnma | a−b+a− | 0.02601 |
| R-type | R-3c | a−a−a− | 0.13907 |
| T1-type | P4/mbm | a0a0c− | 0.14216 |
| T2-type | I4/mcm | a0a0c− | 0.14216 |
| γ-type | Pm-3m | a0a0a0 | 0.76254 |
| Bi0.98Ag0.02FeO3 | |||
| β-type | Pnma | a−b+a− | 0.03542 |
| R-type | R-3c | a−a−a− | 0.14136 |
| T1-type | P4/mbm | a0a0c− | 0.14433 |
| T2-type | I4/mcm | a0a0c− | 0.14433 |
| γ-type | Pm-3m | a0a0a0 | 0.75986 |
| Phase | FM | AFM-a | AFM-c | AFM-g |
|---|---|---|---|---|
| α | 0.103 | 2.351 | N/A | N/A |
| β | 1.357 | 1.639 | 1.737 | 2.077 |
| γ | −0.350 | 0.127 | 0.704 | 1.531 |
| R | 0.102 | 1.623 | N/A | N/A |
| T1 | −0.383 | −0.294 | −0.609 | −0.133 |
| T2 | −0.068 | −0.353 | 1.456 | −0.073 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čebela, M.; Šenjug, P.; Zagorac, D.; Popov, I.; Zagorac, J.; Rosić, M.; Pajić, D. Synthesis, Structural and Magnetic Properties of BiFeO3 Substituted with Ag. Materials 2025, 18, 1453. https://doi.org/10.3390/ma18071453
Čebela M, Šenjug P, Zagorac D, Popov I, Zagorac J, Rosić M, Pajić D. Synthesis, Structural and Magnetic Properties of BiFeO3 Substituted with Ag. Materials. 2025; 18(7):1453. https://doi.org/10.3390/ma18071453
Chicago/Turabian StyleČebela, Maria, Pavla Šenjug, Dejan Zagorac, Igor Popov, Jelena Zagorac, Milena Rosić, and Damir Pajić. 2025. "Synthesis, Structural and Magnetic Properties of BiFeO3 Substituted with Ag" Materials 18, no. 7: 1453. https://doi.org/10.3390/ma18071453
APA StyleČebela, M., Šenjug, P., Zagorac, D., Popov, I., Zagorac, J., Rosić, M., & Pajić, D. (2025). Synthesis, Structural and Magnetic Properties of BiFeO3 Substituted with Ag. Materials, 18(7), 1453. https://doi.org/10.3390/ma18071453








