Recovery of Valuable Materials Based on Pb and Zn in the Hydrometallurgical Processing of Copper Shaft Furnace Dust
Abstract
:1. Introduction
| Reference | Material * | Qualitative Analysis | Quantitative Analysis | Leaching | |
|---|---|---|---|---|---|
| Medium | Conditions | ||||
| [16] | EAF dust | ZnO, ZnFe2O4, PbO, Fe2O3. | 7–40% Zn, 4–9% Pb, 24–27% Fe. | 10 M NaOH. | T = 95.8 °C, t = 120 min, L/S = 7:0.1. |
| [17] | SZL residue | PbSO4 PbO, Fe2O3, ZnFe2O4. | 7.98% Zn, 19.02% Pb. | 11% NaOH. | p = 1 bar, T = 100 °C, t = 60 min, 700 rpm. |
| [18] | EAF dust | ZnFe2O4, Fe3O4, ZnO, PbO. | 33.16%, Zn, 1.64% Pb. | 0.8 M Citric acid and O2. | T = 40 °C, t = 60 min, 500 rpm, O2 = 2000 mL/min. |
| [5] | CA dust | ZnO, Zn2SnO4, SnO2, PbCl2, SnCl2. | 28.35% Zn, 10.28% Pb, 0.67% Fe, 1.5% Sn. | 1 M H2SO4. | L/S = 10, T = 25 °C, t = 10 min, 300 rpm. |
| [9] | CC dust | ZnO, PbO, SnO2. | 9.3% Pb, 29.90% Zn, 0.52% Fe. | Acetic acid. | T = 25 °C, 400 rpm, L/S = 40, t = 60 min. |
| [19] | BF dust | ZnO, ZnSO4, ZnS, ZnFe2O4, Fe2O4, Fe3O4 | 36.16% Fe, 51.27% Fe2O3, 11.84% Zn. | 3 M NH4Cl. | t = 90 min, T = 70 °C, L/S = 10 mL/g, A = 400 rpm. |
| [20] | LSF dust | ZnO, PbSO4. Pb4(SO4)(CO3)2(OH)2. | 44.27% Zn, 27.92% Pb. | 7 M NH4Cl. | T = 100 °C, L/S 10 mL/g, 450 rpm, t = 60 min. |
| [21] | SZL residue | PbSO4, SnO2. | 0,46% Sn, 54% Pb, 4.28% Zn. | 0.5 M H2SO4 24 g/L oxalic acid. | T = 60 °C, t = 30 min, L/S 10 mL/g, 400 rpm. |
| [22] | CCM dust | PbSO4, ZnSO4.H2O. | 5.91%, 3.04% Cu, 25.06% Pb, 13% As. | H2SO4. | S:L = 1.5; t = 60 °C; τ = 60 min, pH = 0.8–1.0, 300 rpm. |
2. Materials and Methods
2.1. Experimental Samples and Reagents
2.2. Analytical and Experimental Methods
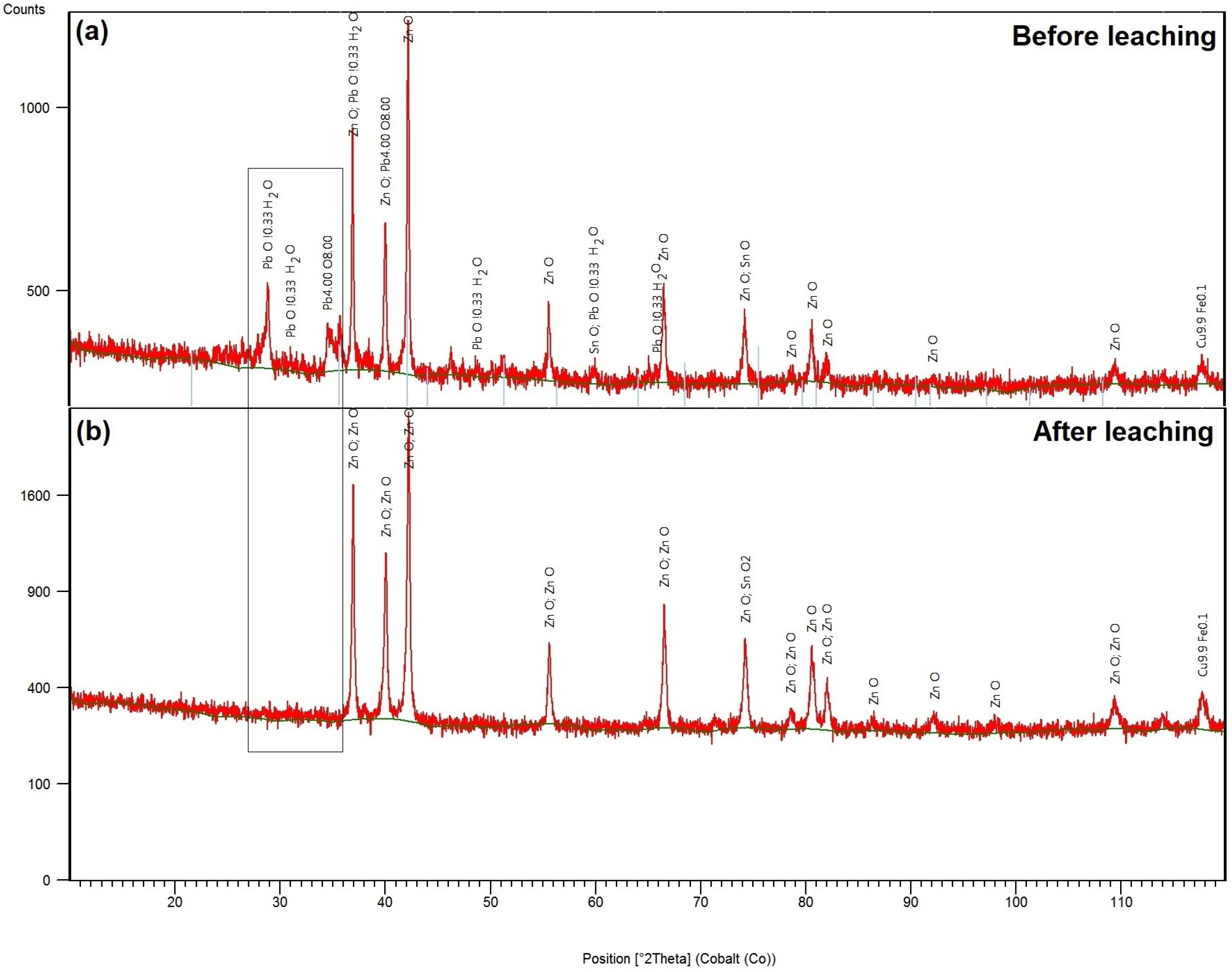
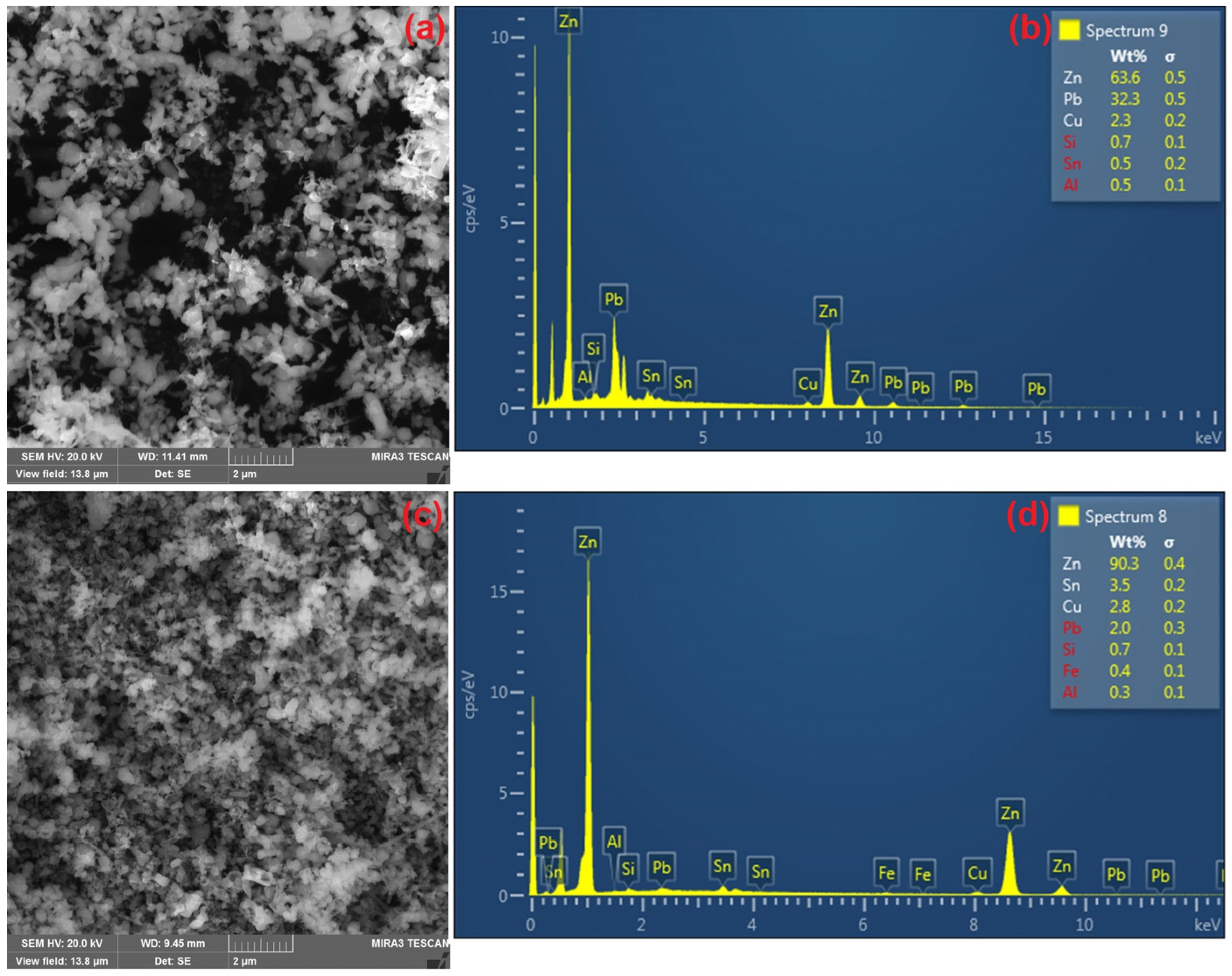
3. Results and Discussion
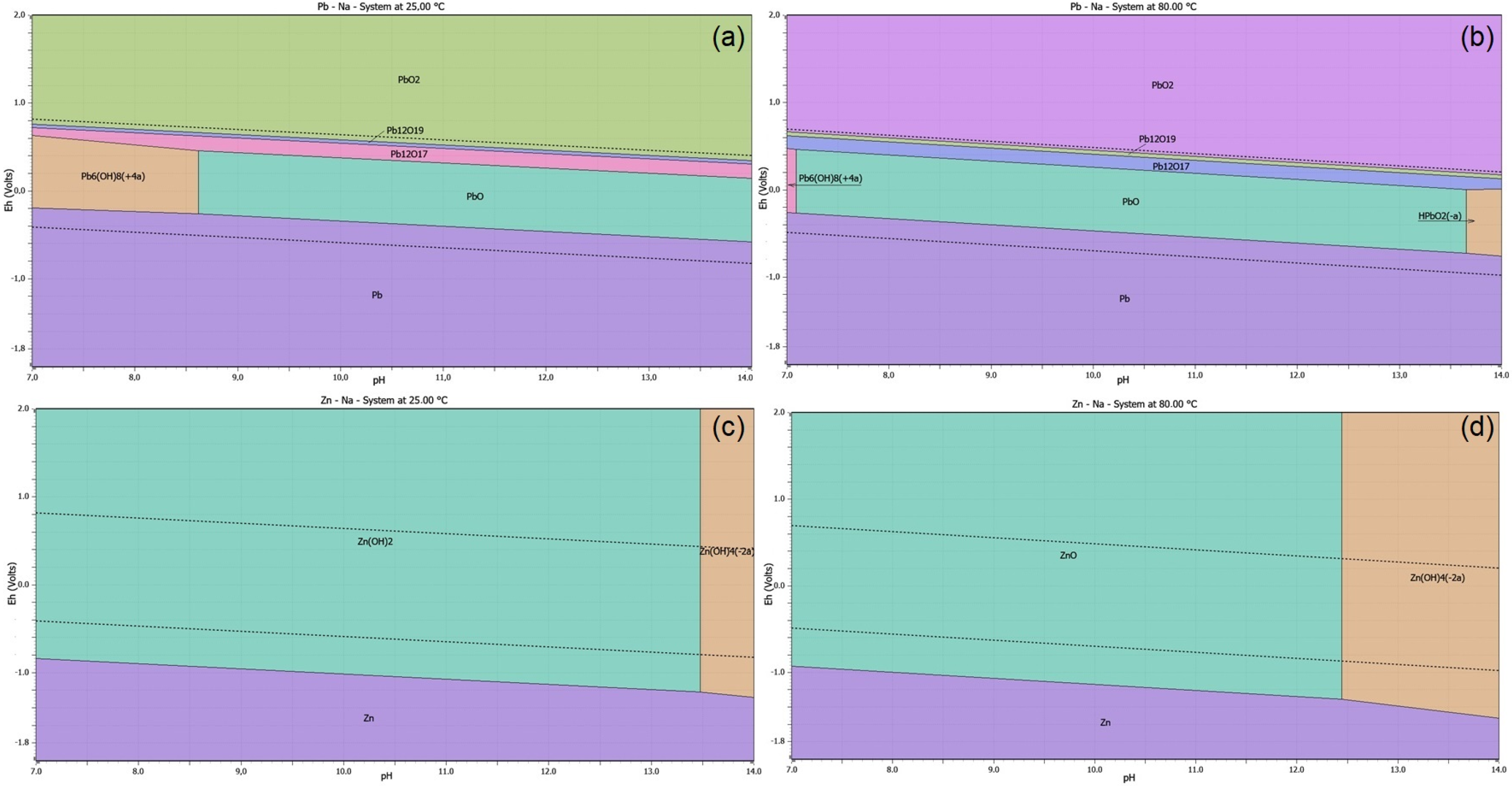
3.1. Thermodynamic Study of Alkaline Leaching of CSF Dust
3.2. Thermodynamic Study of Precipitation Pb from Alkaline Solution
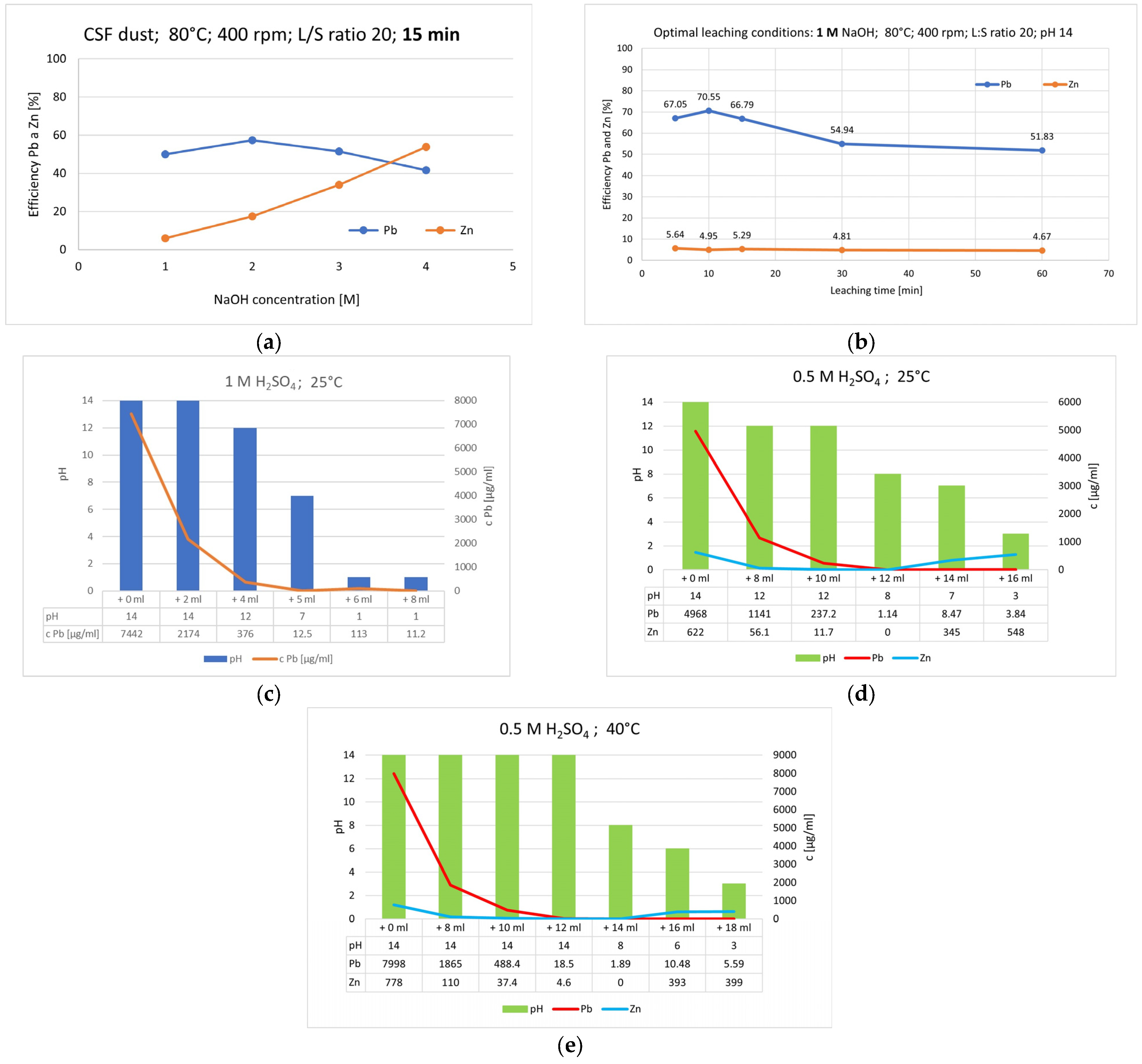
3.3. Alkaline Leaching of CSF Dust and Precipitation of Lead
4. Conclusions
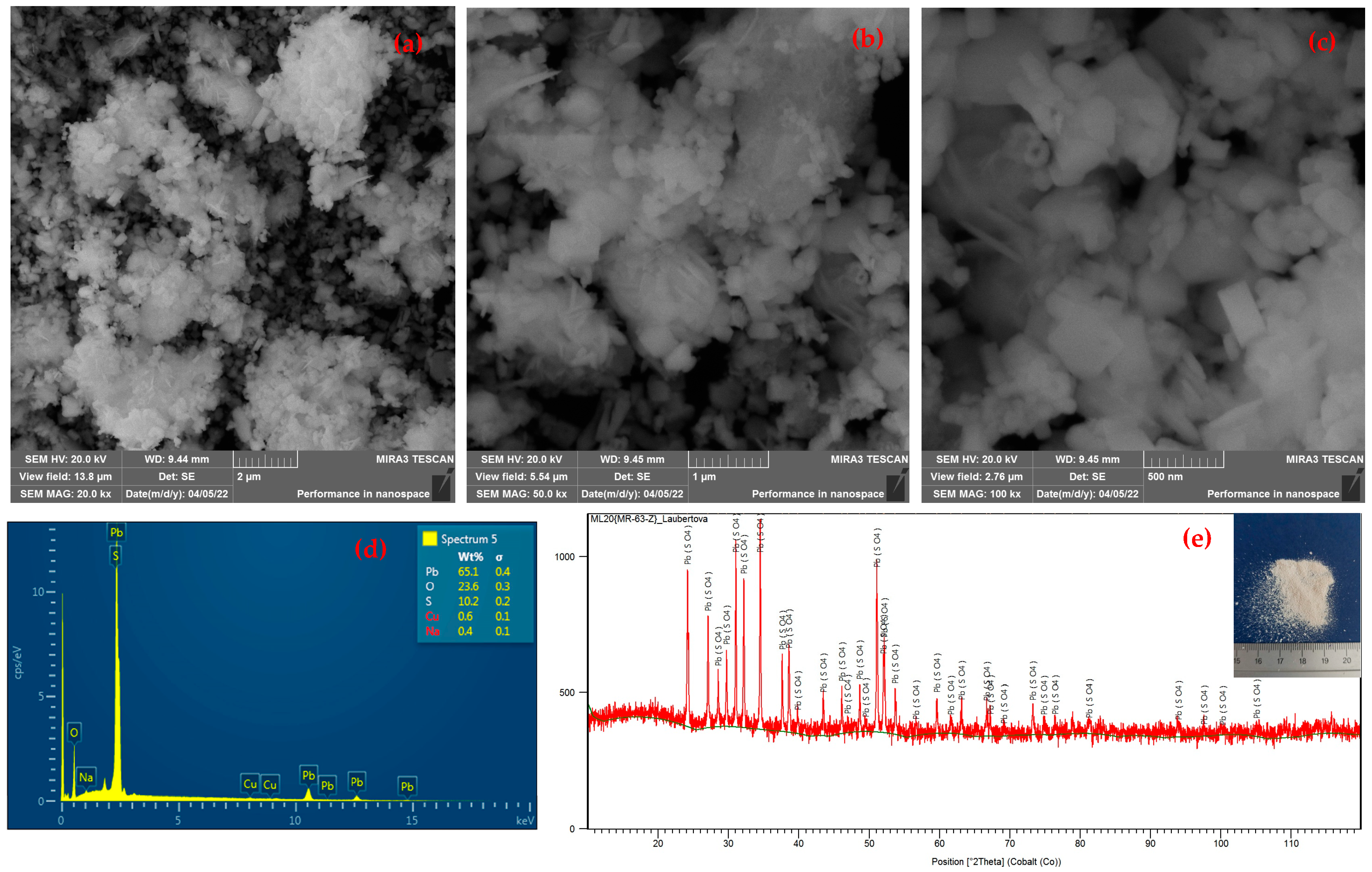
- The optimal conditions for the leaching of CSF dust were 1 M NaOH at 80 °C, a liquid-to-solid ratio of 20, and a leaching time of 10 min, resulting in 70.55% lead recovery efficiency.
- It was confirmed that a suitable precipitating agent is 0.5 M H2SO4 at pH 3.1 and Eh 0.22 V at 25 °C. Although increasing the temperature reduced the time required for the precipitate to age, it did not affect the amount of lead precipitated from the alkaline solution.
- The newly recovered material (PbSO4), produced from industrial hazardous waste as an unconventional raw material, can be used as a by-product in lead-acid battery paste production.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iloeje, C.O.; Tesfaye, F.; Anderson, A.E.; Hamuyuni, J. Recovery of Rare Earth and Critical Metals from Unconventional Sources. J. Miner. Met. Mater. Soc. 2022, 74, 990–992. [Google Scholar] [CrossRef]
- Rudnik, E. Recovery of zinc from steelmaking flue dust by hydrometallurgical route. Arch. Metall. Mater. 2020, 65, 601–608. [Google Scholar] [CrossRef]
- European Waste Catalogue and Hazardous Waste List. Available online: https://www.umweltbundesamt.de/sites/default/files/medien/2503/dokumente/2014-955-eg-en.pdf (accessed on 3 February 2025).
- Orac, D.; Laubertova, M.; Piroskova, J.; Klein, D.; Bures, R.; Klimko, J. Characterization of dusts from secondary copper production. J. Min. Metall. Sect. B Metall. 2020, 56, 221–228. [Google Scholar] [CrossRef]
- Oráč, D.; Klimko, J.; Klein, D.; Pirošková, J.; Liptai, P.; Vindt, T.; Miškufová, A. Hydrometallurgical recycling of copper anode furnace dust for a complete recovery of metal values. Metals 2021, 12, 36. [Google Scholar] [CrossRef]
- Oráč, D.; Hlucháňová, B.; Havlík, T.; Miškufová, A.; Petrániková, M. Leaching of zinc and copper from blast furnace dust of copper production of secondary raw materials. Acta Metall. Slovaca 2009, 3, 147–153. [Google Scholar]
- Balladares, E.; Kelm, U.; Helle, S.; Parra, R.; Araneda, E. Chemical-mineralogical characterization of copper smelting flue dust. Dyna 2014, 81, 11. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Z.; Taskinen, P.; Liang, Y.; Ouyang, H.; Peng, B.; Jokilaakso, A.; Zhou, S.; Chen, T.; Peng, N.; et al. Characterization of Copper Smelting Flue Dusts from a Bottom-Blowing Bath Smelting Furnace and a Flash Smelting Furnace. Metall. Mater. Trans. B 2020, 51, 2596–2608. [Google Scholar] [CrossRef]
- Laubertová, M.; Kollová, A.; Trpčevská, J.; Plešingerová, B.; Briančin, J. Hydrometallurgical treatment of converter dust from secondary copper production: A study of the lead cementation from acetate solution. Minerals 2021, 11, 1326. [Google Scholar] [CrossRef]
- Kukurugya, F.; Vindt, T.; Havlík, T. Behavior of zinc, iron and calcium from electric arc furnace (EAF) dust in hydrometallurgical processing in sulfuric acid solutions: Thermodynamic and kinetic aspects. Hydrometallurgy 2015, 154, 20–32. [Google Scholar] [CrossRef]
- Bhargava, S.K.; Pownceby, M.I.; Ram, R. Hydrometallurgy. Metals 2016, 6, 122. [Google Scholar] [CrossRef]
- Havlik, T. Hydrometallurgy; Woodhead Publishing: Cambridge, UK, 2008; ISBN 9781845694074. [Google Scholar]
- Guo, L.; Lan, J.; Du, Y.; Zhang, T.C.; Du, D. Microwave-enhanced selective leaching of arsenic from copper smelting flue dusts. J. Hazard. Mater. 2020, 386, 121964. [Google Scholar] [CrossRef] [PubMed]
- Halli, P.; Hamuyuni, J.; Revitzer, H.; Lundström, M. Selection of leaching media for metal dissolution from electric arc furnace dust. J. Clean. Prod. 2017, 164, 265–276. [Google Scholar] [CrossRef]
- Halli, P.; Hamuyuni, J.; Leikola, M.; Lundström, M. Developing a sustainable solution for recycling electric arc furnace dust via organic acid leaching. Miner. Eng. 2018, 124, 1–9. [Google Scholar] [CrossRef]
- Orhan, G. Leaching and cementation of heavy metals from electric arc furnace dust in alkaline medium. Hydrometallurgy 2005, 78, 236–245. [Google Scholar] [CrossRef]
- Şahin, M.; Erdem, M. Cleaning of high lead-bearing zinc leaching residue by recovery of lead with alkaline leaching. Hydrometallurgy 2015, 153, 170–178. [Google Scholar] [CrossRef]
- Halli, P.; Agarwal, V.; Partinen, J.; Lundström, M. Recovery of Pb and Zn from a citrate leach liquor of a roasted EAF dust using precipitation and solvent extraction. Sep. Purif. Technol. 2020, 236, 116264. [Google Scholar] [CrossRef]
- Ye, F.; Li, M.; Su, S.; Xia, H.; Wei, C.; Li, X.; Deng, Z. Separation and recovery of zinc from blast furnace dust via coordination leaching of Cl− and hydrolysis of NH4+. Sep. Purif. Technol. 2024, 330, 125361. [Google Scholar] [CrossRef]
- Li, Z.-B.; Xia, Z.-M.; Liu, S.-F.; Ye, L.-G.; Qi, J.-H.; Li, X.-M. Recovery of zinc and lead by simultaneously leaching from lead slag fuming dust with ammonium chloride solution. Trans. Nonferrous Met. Soc. China 2024, 34, 4075–4084. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Sun, H.; Huang, Y.; Han, G. Selective extraction and recovery of tin from hazardous zinc-leaching residue by oxalic acid/sulfuric acid mixture leaching and hydrolytic precipitation. J. Clean. Prod. 2022, 342, 130955. [Google Scholar] [CrossRef]
- Dosmukhamedov, N.; Zholdasbay, E.; Argyn, A. Extraction of Pb, Cu, Zn and As from Fine Dust of Copper Smelting Industry via Leaching with Sulfuric Acid. Sustainability 2023, 15, 15881. [Google Scholar] [CrossRef]
- Ruzickova, M.; Laubertova, M.; Trpcevska, J.; Kollova, A.; Takacova, Z.; Vindt, T. Extraction of Lead from Hydrometallurgical Processing of Copper Shaft Flue Dust. Eng. Proc. 2024, 64, 1. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, P.; Yang, J.; Li, M.; Hu, Y.; Liang, S.; Wang, J.; Li, S.; Xiao, K.; Hou, H.; et al. A low-emission strategy to recover lead compound products directly from spent lead-acid battery paste: Key issue of impurities removal. J. Clean. Prod. 2019, 210, 1534–1544. [Google Scholar] [CrossRef]
- Pirošková, J.; Laubertová, M.; Miškufová, A.; Oráč, D. Hydrometallurgical treatment of copper shaft furnace dust for lead recovery. World Metall. Erzmetall 2018, 71, 37–42. [Google Scholar]

| No | Name | Content | Purity | Usage | Purpose | Producer | City/State |
|---|---|---|---|---|---|---|---|
| 1 | NaOH | 98 (%) | Analytical | 1 M aq. solution | Leaching medium | Centralchem | Bratislava/Slovakia |
| 2 | H2SO4 | 96 (%) | Analytical | 0.5 M aq. solution | Precipitant | Penta Chemicals Unlimited | Prague/Czech Republic |
| Content [wt. %] | Zn | Pb | Sn | Fe | Cu | Ni | Cl− | L.O.I. 1 |
|---|---|---|---|---|---|---|---|---|
| Average | 44.02 | 14.57 | 1.59 | 0.232 | 1.2 | 0.02 | 30.58 | 7.788 |
| Standard Deviation | 5.642 | 1.791 | 0.04 | 0.021 | 0.081 | 4 × 10−3 | 3.51 | - |
| Variance | 31.83 | 3.207 | 0.002 | 0.021 | 0.007 | 2 × 10−3 | 12.37 | - |
| Chemical Reaction | ΔG°T (kJ) | ||
|---|---|---|---|
| 20 °C | 80 °C | ||
| −32.569 | −31.128 | (1) | |
| −86.151 | −78.120 | (2) | |
| −407.395 | −440.306 | (3) | |
| −231.576 | −246.813 | (4) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laubertová, M.; Sisol, M.; Briančin, J.; Trpčevská, J.; Ružičková, M. Recovery of Valuable Materials Based on Pb and Zn in the Hydrometallurgical Processing of Copper Shaft Furnace Dust. Materials 2025, 18, 1935. https://doi.org/10.3390/ma18091935
Laubertová M, Sisol M, Briančin J, Trpčevská J, Ružičková M. Recovery of Valuable Materials Based on Pb and Zn in the Hydrometallurgical Processing of Copper Shaft Furnace Dust. Materials. 2025; 18(9):1935. https://doi.org/10.3390/ma18091935
Chicago/Turabian StyleLaubertová, Martina, Martin Sisol, Jaroslav Briančin, Jarmila Trpčevská, and Michaela Ružičková. 2025. "Recovery of Valuable Materials Based on Pb and Zn in the Hydrometallurgical Processing of Copper Shaft Furnace Dust" Materials 18, no. 9: 1935. https://doi.org/10.3390/ma18091935
APA StyleLaubertová, M., Sisol, M., Briančin, J., Trpčevská, J., & Ružičková, M. (2025). Recovery of Valuable Materials Based on Pb and Zn in the Hydrometallurgical Processing of Copper Shaft Furnace Dust. Materials, 18(9), 1935. https://doi.org/10.3390/ma18091935







