Advancements in Biochar for Soil Remediation of Heavy Metals and/or Organic Pollutants
Abstract
1. Introduction
2. Preparation of Biochar and Its Physicochemical Properties
2.1. Biochar Preparation
2.2. Physicochemical Properties of Biochar
2.2.1. pH
2.2.2. Specific Surface Area and Porosity
2.2.3. Surface Functional Groups
2.2.4. Stability
2.3. Adsorption Performance of Biochar
3. Biochar Used in Soils Polluted with Organic Contaminants
3.1. Biochar in Pesticide-Contaminated Soils
3.2. Biochar in Petroleum Hydrocarbon-Contaminated Soil
3.3. Exploring the Use of Biochar in the Remediation of Soils Polluted by Polycyclic Aromatic Hydrocarbons (PAHs)
3.4. Biochar in Polychlorinated Biphenyl-Contaminated Soil
4. Application of Biochar in Heavy Metal-Contaminated Soils
4.1. Immobilization of Oxygen-Containing Anionic Heavy Metals by Biochar and Modified Biochar
| Biochar and Modified Biochar | Preparation Conditions | Modification Agent | Soil | Addition Amount (wt. %) | Research Results | References | |
|---|---|---|---|---|---|---|---|
| pH | As (mg·kg−1) | ||||||
| Rice straw biochar | 400 °C, 2 h | Fe2+/CaCO3 | 4.65 | 46.89 | 1–3% | Ca-MBC reduced the bioavailability of As. | [62] |
| Oil palm fiber biochar | 700 °C, 4 h | Nano zero-valent iron (nZVI) | - | - | 1% | The As content in rice was reduced by 61%. | [63] |
| Iron-modified biochar derived from Platycladus orientalis | 650 °C, 2 h | FeCl3·6H2O | 5.8 | 141.3 | 3% | The concentration of potentially available As in the soil decreased by 41.7%, while the As content in rice straw and brown rice decreased by 61.5% and 80.1%, respectively. | [64] |
| Iron-rich corn cobs and eggshells biochar (FCEB) | 450 °C, 1 h | FeSO4·7H2O | 5.56 | 90.5 | 2% | The application of FCEB reduced the As content in brown rice by 29% to 60% and lowered the average As mobility in rhizosphere pore water by 56%. | [65] |
| Wheat straw biochar | 600 °C, 1 h, 5 °C·min−1 | Goethite | 5.11 ± 0.1 | 2.42 ± 0.32 | 1.5% | The As content in the rice roots and shoots decreased by 32.2% and 46.6%, respectively. | [73] |
| Corn stover biochar | 600 °C, 2 h | Fe-Mn-Ce | 6.08–7.81 | 33–138 | 0.5–2% | Biochar reduced the bioavailable forms of As and facilitated the transformation of As from specific or non-specific binding forms into amorphous hydrated oxide forms and crystalline hydrated oxide forms. | [74] |
| Cedar sawdust biochar | 300 °C, 2 h | Fe3O4 | 5.4 | 47.3 ± 6.7 | 9% | Biochar removed 20% to 30% of As within 24 h. | [75] |
| Wood sawdust biochar | 800 °C, 1 h | Fe2O3 | 7.91–8.19 | 1900–3020 | 2–5% | The concentration of As in the (NH4)2SO4 extract decreased by 93.7% to 97.7%. | [76] |
| Wood sawdust biochar | 800 °C, 1 h | - | 7.91–8.19 | 1900–3020 | 3% | The concentration of As in the (NH4)2SO4 extract decreased by 31% to 56.5%. | [77] |
| Pig manure and rice straw biochar | 600 °C, 6 h, 10 °C·min−1 | FeCl3/FeCl2 | 6.3 | 280 | 2% | The application of magnetic biochar as an amendment for 7 days increased the As leaching rate by 34%. | [78] |
4.2. The Role of Biochar and Modified Biochar in the Fixation of Heavy Metal Cations
| Biochar/Modified Biochar | Preparation Conditions | Modification Agent | Soil | Addition Amount (wt. %) | Research Results | References | |
|---|---|---|---|---|---|---|---|
| pH | Pb (mg·kg−1) | ||||||
| Cedar sawdust biochar | 300 °C, 2 h | Fe3O4 | 5.4 | 12.1 ± 4.4 | 9% | The bioavailable Pb content was reduced by 32%. | [75] |
| Kitchen waste, corn stover, and peanut shell biochar | 400 °C, 2 h | - | 6.77 | 1343.8 | 0.6% | The extractable Pb concentrations in the soil decreased by 56.42–71.01% (KWB), 48.24–64.35% (CSB), and 43.74–60.25% (PHB). | [94] |
| Bamboo-based biochar | 500 °C, 30 min, 10 °C·min−1 | - | 6.23 ± 1.56 | 25.87 ± 2.28 | 2% | The extractable Pb concentration in the soil decreased from 47% to 18%. | [80] |
| Crayfish shell biochar | 300–700 °C, 2 h, 15 °C·min−1 | - | 4.50–7.85 | - | 5% | The bioavailable Pb content in acidic soils decreased by 1.87–16.48%, while that in alkaline soils decreased by 1.00–11.09%. | [81] |
| Biochar from discarded branches of bougainvillea | 450 °C | Magnesium potassium phosphate cement | 7.1 | 600 | 3% | The Pb concentrations in the shoots (85%) and roots (78%), as well as the extractable Pb concentration (73%), were significantly reduced. | [82] |
| Pistachio shell biochar | 350 °C | Dicalcium phosphate | 5.73 | 600 | 2% | The maximum reduction rate of DTPA-extractable Pb, as well as the Pb concentrations in the shoots and roots, reached 58%,66%, and 53%, respectively. | [83] |
| Coconut shell biochar | 350 °C, 4 h | - | 6.72 | 47.35 | 1% | The bioavailable Pb content in the soil decreased by 45.3%. | [84] |
| Fe3+ | The bioavailable Pb content in the soil decreased by 21.8%. | ||||||
| Green tea biochar | 400 °C, 2 h, 3 °C·min−1 | Nanoscale zero-valent iron | 6.8 ± 0.5 | 386 | 1% | The Pb immobilization efficiency increased by 57.14%. | [85] |
| Palm fiber biochar | 550 °C | Alkali residue | 4.73 | 621.7 | 10 t ha−1 | The bioavailable Pb content decreased by 28.3–40.8%. | [86] |
| Green waste biochar (GWB) | 650 °C, 2 h | FeCl3·6H2O | 5.8 | 736.2 | 3% | The concentration of DTPA-extractable Pb decreased by 20.6%. | [95] |
| Biochar/Modified Biochar | Preparation Conditions | Modification Agent | Soil | Addition Amount (wt. %) | Research Results | References | |
|---|---|---|---|---|---|---|---|
| pH | Cd (mg·kg−1) | ||||||
| Iron-modified biochar derived from sycamore branches | 650 °C, 2 h | FeCl3·6H2O | 5.8 | 0.5 | 3% | The application of FeBC increased the Cd concentration in rice straw by 169.0% to 390.1% and the Cd concentration in brown rice by 263.6% to 268.8% (p < 0.05). | [64] |
| Wheat straw biochar | 600 °C, 1 h, 5 °C·min−1 | Goethite | 5.11 ± 0.1 | 2.42 ± 0.32 | 1.5% | The Cd content in the rice roots and shoots decreased by 42.9% and 56.7%, respectively. | [73] |
| Corn stalk biochar | 500 °C, 4 h, 4 °C·min−1 | - | 7.90 ± 0.12 | 3.73 ± 0.16 | 5% | Cd accumulation in beetroot increased by 206%, while Cd concentrations in the leaves and roots rose by 36% and 52%, respectively. | [88] |
| Palm fiber biochar | 550 °C | Soda water | 4.73 | 7.49 | 10 t ha−1 | The bioavailable Cd was reduced by 52.4% to 68.6%. | [89] |
| Rice husk-based biochar | 450 °C, 2 h | Sodium dimethyldithiocarbamate (SDD) | 8.56 | 8.78 | 3% | The Cd extracted by DTPA decreased by 92.02%. | [90] |
| Rice husk-based biochar | 600 °C | - | 7.67 | 41.02 ± 5.47 | 0.8% | The Cd concentration in brown rice was reduced by 74%. | [92] |
| Zero-valent iron | 0.8%, BC 5%, ZVI | The Cd concentration in brown rice decreased by 83%. | |||||
| Fe3+ | The bioavailable Cd in the soil was reduced by 38.1%. | ||||||
| Wheat straw biochar (WB) | 600 °C, 1 h, 10 °C·min−1 | - | 8.55 | 1.26 | 15 t ha−1 | The Cd concentration extractable by CaCl2 decreased by 32.8% to 60.5%. | [96] |
| Rice husk-based biochar | 400 °C | Acid treatment (HCl, HNO3, H3PO4) | 7.52 | 30 | 2% | The bioavailable Cd content in the soil decreased by 87%, while Cd accumulation in the stems and rice decreased by 83.4% and 95.7%, respectively. | [97] |
4.3. The Impact of Biochar/Modified Biochar on the Immobilization of Other Heavy Metals (Nickel, Zinc, and Copper)
| Biochar | Operational Conditions | Modifiers | Soil | Application Rate (wt. %) | Effects | References | |||
|---|---|---|---|---|---|---|---|---|---|
| pH | Heavy Metal (mg·kg−1) | Concomitant HMs | |||||||
| Rice hull | 450 °C, 2 h | Dimethyl dithio carbamate sodium | 8.56 | 60.31 | Cd | 3% | Decreased DTPA-extractable Cu by 100.00%. | [93] | |
| Imperata cylindrica | 400 °C, 0.5 h, 5 °C·min−1 | - | 5.62 ± 0.24 | 56 | - | 0.75% | The biochar application significantly (p < 0.05) decreased exchangeable and acid-soluble Ni (58%). | [106] | |
| Rice straw | 500 °C, 2 h | - | 5.3 | 35 | - | 2% | Reduced Ni mobility (DTPA) by 88.9%. | [101] | |
| Maize straw | 500 °C | - | 7.9 ± 0.08 | 0.2 ± 0.01 | - | 1–5% | The application of 1–5% rates of maize straw biochar reduced the DTPA-extractable Ni by 56%. | [102] | |
| Sewage sludge/cotton stalks (SCB) | 650 °C, 1.5 h | - | 7.1 ± 0.3 | Zn | 203.5 ± 10.2 | Pb, Cu | 7.5 t/ha | SCB amendment decreased the bioavailable forms of Zn in the soil by 18.2%. | [107] |
| Sewage sludge/cotton stalks (SCB) | 650 °C, 1.5 h | - | 7.1 ± 0.3 | Cu | 45.7 ± 3.0 | Pb, Zn | 7.5 t/ha | SCB amendment decreased the bioavailable forms of Cu in the soil by 34.9%. | [107] |
| Shell and apple tree | 500 °C | - | 8.4 | 1860 | Cd | 10% | Biochar reduces the concentration of Zn in roots (36–41%) and shoots (25–31%). | [103] | |
| Sheep bone | 500–800 °C, 2 h, 10 °C·min−1 | - | 8.2 | 981 | Cd | 10% | The content of Zn in maize roots (57%) and shoots (42%) was reduced. | [104] | |
| Cow bone | 500–800 °C, 2 h, 10 °C·min−1 | - | 8.46 | 474 ± 15 | Cd | 10% | Reduced the content of Zn (55% and 40%) in the maize roots and shoots. | [105] | |
| Maize straw | 400 °C, 8 h | - | 7.85 | 247 | Cd | 5% | DTPA-Cu was reduced by up to 42.3%. | [108] | |
| Rice straw | - | - | 6.87 | 222,115 | - | 10% | Bioavailable Cu reduced by 96%. | [109] | |
| Rape straw | 500 °C, 2 h | Ca (H2PO4)2·H2O and KH2PO4 | 5.1 | 218.1 | Pb, Cu | 3% | The additions of BC, BC-Ca, and BC-K reduced Cu concentrations in TCLP extracts by 19.2%,10.7%, and 31.0%, respectively. | [110] | |
| De-inking paper sludge | 300 °C, 500 °C | - | 7.80 | Ni | 35.5, 36.8 | - | 5% | Biochar significantly decreased the leached, mobile, and plant bioavailable forms of Ni. | [111] |
| Rice straw | 500 °C, 2 h | - | 5.3 | 35 | - | 1–2% | Reduced the exchangeable fraction of Ni by 59–71% when biochar is applied at 1% and 2% rates. | [110] | |
| Digestate, cow manure, oak | 500 °C, 10 min | - | 298 | Cd | 2% | Reduced the leached Zn with 65–73% compared with the control. | [107] | ||
5. The Role of Biochar in Soils Contaminated with Organic Pollutants and Heavy Metals
6. Mechanisms of Biochar for Remediation of Contaminated Soils
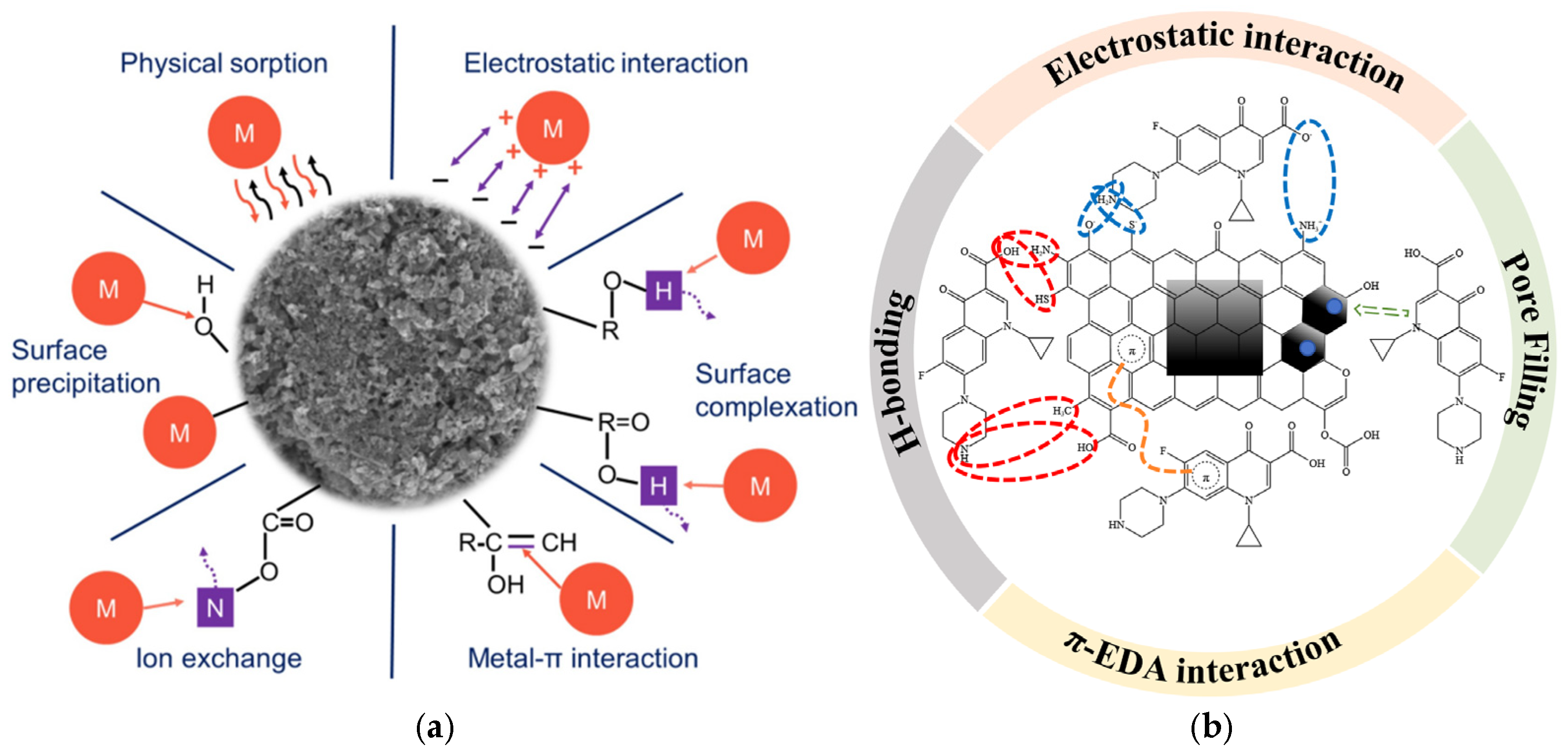
6.1. Van Der Waals Force and Hydrogen Bonding
6.2. Electrostatic Interactions
6.3. Surface Complexation
6.4. Ion Exchange
6.5. π–π Stacking and Other π Interactions
6.6. Surface Coprecipitation
6.7. Distribution Mechanism
6.8. Pore Filling
6.9. Adsorption Enthalpy and Partition Coefficient
7. Biotoxicity Assessment of Biochar in Soil Remediation Processes
8. Conclusions and Prospects
Funding
Conflicts of Interest
References
- Deng, X.; Teng, F.; Chen, M.; Du, Z.; Wang, B.; Li, R.; Wang, P. Exploring negative emission potential of biochar to achieve carbon neutrality goal in China. Nat. Commun. 2024, 15, 1085. [Google Scholar] [PubMed]
- Kuzyakov, Y.; Subbotina, I.; Chen, H.; Bogomolova, I.; Xu, X. Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol. Biochem. 2009, 41, 210–219. [Google Scholar]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [PubMed]
- Zhang, X.; Wang, H.; He, L.; Lu, K.; Sarmah, A.; Li, J.; Bolan, N.S.; Pei, J.; Huang, H. Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ. Sci. Pollut. Res. 2013, 20, 8472–8483. [Google Scholar]
- Hou, R.; Zhang, J.; Fu, Q.; Li, T.; Gao, S.; Wang, R.; Zhao, S.; Zhu, B. The boom era of emerging contaminants: A review of remediating agricultural soils by biochar. Sci. Total Environ. 2024, 931, 172899. [Google Scholar]
- Ji, M.; Wang, X.; Usman, M.; Liu, F.; Dan, Y.; Zhou, L.; Campanaro, S.; Luo, G.; Sang, W. Effects of different feedstocks-based biochar on soil remediation: A review. Environ. Pollut. 2022, 294, 118655. [Google Scholar]
- Qiu, B.; Yang, C.; Shao, Q.; Liu, Y.; Chu, H. Recent advances on industrial solid waste catalysts for improving the quality of bio-oil from biomass catalytic cracking: A review. Fuel 2022, 315, 123218. [Google Scholar]
- Liu, J.; Yang, X.; Liu, H.; Jia, X.; Bao, Y. Mixed biochar obtained by the co-pyrolysis of shrimp shell with corn straw: Co-pyrolysis characteristics and its adsorption capability. Chemosphere 2021, 294, 118655. [Google Scholar]
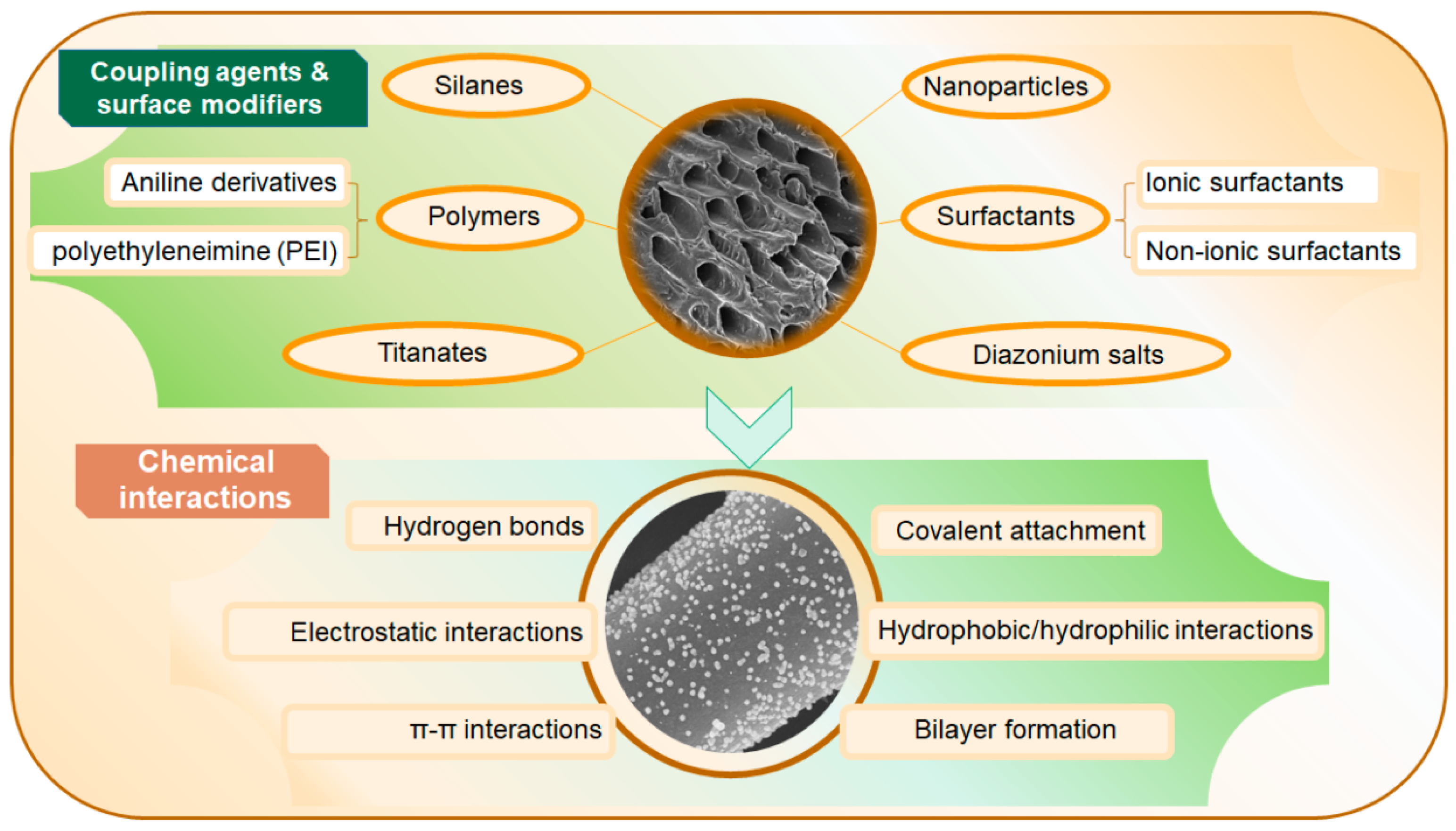
- Gęca, M.; Khalil, A.M.; Tang, M.; Bhakta, A.K.; Snoussi, Y.; Nowicki, P.; Wiśniewska, M.; Chehimi, M.M. Surface Treatment of Biochar—Methods, Surface Analysis and Potential Applications: A Comprehensive Review. Surfaces 2023, 6, 179–213. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, T.; Bai, L.; Han, C.; Sun, X. Application of biochar-based materials for remediation of arsenic contaminated soil and water: Preparation, modification, and mechanisms. J. Environ. Chem. Eng. 2022, 10, 108292. [Google Scholar]
- Qiu, B.; Shao, Q.; Shi, J.; Yang, C.; Chu, H. Application of biochar for the adsorption of organic pollutants from wastewater: Modification strategies, mechanisms and challenges. Sep. Purif. Technol. 2022, 300, 121925. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameed, B.H. Insight into the co-pyrolysis of different blended feedstocks to biochar for the adsorption of organic and inorganic pollutants: A review. J. Clean. Prod. 2020, 265, 121762. [Google Scholar]
- Zhao, B.; O’Connor, D.; Zhang, J.; Peng, T.; Shen, Z.; Tsang, D.C.W.; Hou, D. Effect of pyrolysis temperature, heating rate, and residence time on rapeseed stem derived biochar. J. Clean. Prod. 2018, 174, 977–987. [Google Scholar] [CrossRef]
- Kwon, G.; Bhatnagar, A.; Wang, H.; Kwon, E.E.; Song, H. A review of recent advancements in utilization of biomass and industrial wastes into engineered biochar. J. Hazard. Mater. 2020, 400, 123242. [Google Scholar] [CrossRef]
- Zhang, X.; Chu, Y.; Zhang, H.; Hu, J.; Wu, F.; Wu, X.; Shen, G.; Yang, Y.; Wang, B.; Wang, X. A mechanistic study on removal efficiency of four antibiotics by animal and plant origin precursors-derived biochars. Sci. Total Environ. 2021, 772, 145468. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, L.; Li, Z.; Zhou, X.; Cheng, S.; Zhang, L.; Zhang, Q. Effects of various pyrolysis conditions and feedstock compositions on the physicochemical characteristics of cow manure-derived biochar. J. Clean. Prod. 2021, 311, 127458. [Google Scholar] [CrossRef]
- Lee, S.; Han, J.; Ro, H.-M. Interactive effect of pH and cation valence in background electrolyte solutions on simazine sorption to Miscanthus biochar produced at two different pyrolysis temperatures. Korean J. Chem. Eng. 2020, 37, 456–465. [Google Scholar]
- Pan, X.; Gu, Z.; Chen, W.; Li, Q. Preparation of biochar and biochar composites and their application in a Fenton-like process for wastewater decontamination: A review. Sci. Total Environ. 2021, 754, 142104. [Google Scholar]
- Li, H.; Hu, J.; Yao, L.; Shen, Q.; An, L.; Wang, X. Ultrahigh adsorbability towards different antibiotic residues on fore-modified self-functionalized biochar: Competitive adsorption and mechanism studies. J. Hazard. Mater. 2020, 390, 122127. [Google Scholar] [CrossRef]
- Li, L.; Zou, D.; Xiao, Z.; Zeng, X.; Zhang, L.; Jiang, L.; Wang, A.; Ge, D.; Zhang, G.; Liu, F. Biochar as a sorbent for emerging contaminants enables improvements in waste management and sustainable resource use. J. Clean. Prod. 2019, 210, 1324–1342. [Google Scholar]
- Amalina, F.; Krishnan, S.; Zularisam, A.W.; Nasrullah, M. Pristine and modified biochar applications as multifunctional component towards sustainable future: Recent advances and new insights. Sci. Total Environ. 2024, 914, 169608. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Huang, H.; Li, H.; Li, J.; Zhou, W. Biochar stability assessment methods: A review. Sci. Total Environ. 2019, 647, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Głąb, T.; Gondek, K.; Mierzwa–Hersztek, M. Biological effects of biochar and zeolite used for remediation of soil contaminated with toxic heavy metals. Sci. Rep. 2021, 11, 6998. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Yao, B.; Yang, X.; Wang, L.; Xu, Z.; Yan, X.; Tian, L.; Zhou, H.; Zhou, Y. Novel insights into the adsorption of organic contaminants by biochar: A review. Chemosphere 2022, 287, 132113. [Google Scholar] [CrossRef]
- Haider, F.U.; Wang, X.; Zulfiqar, U.; Farooq, M.; Hussain, S.; Mehmood, T.; Naveed, M.; Li, Y.; Liqun, C.; Saeed, Q.; et al. Biochar application for remediation of organic toxic pollutants in contaminated soils; An update. Ecotoxicol. Environ. Saf. 2022, 248, 114322. [Google Scholar] [CrossRef]
- Qiu, M.; Liu, L.; Ling, Q.; Cai, Y.; Yu, S.; Wang, S.; Fu, D.; Hu, B.; Wang, X. Biochar for the removal of contaminants from soil and water: A review. Biochar 2022, 4, 19. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Ying, G.-G.; Kookana, R.S. Reduced plant uptake of pesticides with biochar additions to soil. Chemosphere 2009, 76, 665–671. [Google Scholar] [CrossRef]
- Twagirayezu, G.; Cheng, H.; Wu, Y.; Lu, H.; Huang, S.; Fang, X.; Irumva, O. Insights into the influences of biochar on the fate and transport of pesticides in the soil environment: A critical review. Biochar 2024, 6, 9. [Google Scholar] [CrossRef]
- Dong, X.; Chu, Y.; Tong, Z.; Sun, M.; Meng, D.; Yi, X.; Gao, T.; Wang, M.; Duan, J. Mechanisms of adsorption and functionalization of biochar for pesticides: A review. Ecotoxicol. Environ. Saf. 2024, 272, 116019. [Google Scholar] [CrossRef]
- Lin, Q.; Tan, X.; Almatrafi, E.; Yang, Y.; Wang, W.; Luo, H.; Qin, F.; Zhou, C.; Zeng, G.; Zhang, C. Effects of biochar-based materials on the bioavailability of soil organic pollutants and their biological impacts. Sci. Total Environ. 2022, 826, 153956. [Google Scholar] [CrossRef]
- Zhang, X.; Sarmah, A.K.; Bolan, N.S.; He, L.; Lin, X.; Che, L.; Tang, C.; Wang, H. Effect of aging process on adsorption of diethyl phthalate in soils amended with bamboo biochar. Chemosphere 2016, 142, 28–34. [Google Scholar] [PubMed]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar]
- Liu, Y.; Lonappan, L.; Brar, S.K.; Yang, S. Impact of biochar amendment in agricultural soils on the sorption, desorption, and degradation of pesticides: A review. Sci. Total Environ. 2018, 645, 60–70. [Google Scholar] [PubMed]
- Yang, Y.; Sheng, G.; Huang, M. Bioavailability of diuron in soil containing wheat-straw-derived char. Sci. Total Environ. 2006, 354, 170–178. [Google Scholar]
- Tan, G.; Sun, W.; Xu, Y.; Wang, H.; Xu, N. Sorption of mercury (II) and atrazine by biochar, modified biochars and biochar based activated carbon in aqueous solution. Bioresour. Technol. 2016, 211, 727–735. [Google Scholar]
- Wei, Z.; Wei, Y.; Liu, Y.; Niu, S.; Xu, Y.; Park, J.-H.; Wang, J.J. Biochar-based materials as remediation strategy in petroleum hydrocarbon-contaminated soil and water: Performances, mechanisms, and environmental impact. J. Environ. Sci. 2024, 138, 350–372. [Google Scholar]
- Wang, H.; Lv, Y.; Bao, J.; Chen, Y.; Zhu, L. Petroleum-contaminated soil bioremediation and microbial community succession induced by application of co-pyrolysis biochar amendment: An investigation of performances and mechanisms. J. Hazard. Mater. 2024, 466, 133600. [Google Scholar]
- Qin, G.; Gong, D.; Fan, M.-Y. Bioremediation of petroleum-contaminated soil by biostimulation amended with biochar. Int. Biodeterior. Biodegrad. 2013, 85, 150–155. [Google Scholar]
- Bushnaf, K.M.; Puricelli, S.; Saponaro, S.; Werner, D. Effect of biochar on the fate of volatile petroleum hydrocarbons in an aerobic sandy soil. J. Contam. Hydrol. 2011, 126, 208–215. [Google Scholar]
- Guo, S.; Liu, X.; Tang, J. Enhanced degradation of petroleum hydrocarbons by immobilizing multiple bacteria on wheat bran biochar and its effect on greenhouse gas emission in saline-alkali soil. Chemosphere 2022, 286, 131663. [Google Scholar]
- Saeed, M.; Ilyas, N.; Bibi, F.; Shabir, S.; Jayachandran, K.; Sayyed, R.Z.; Shati, A.A.; Alfaifi, M.Y.; Show, P.L.; Rizvi, Z.F. Development of novel kinetic model based on microbiome and biochar for in-situ remediation of total petroleum hydrocarbons (TPHs) contaminated soil. Chemosphere 2023, 324, 138311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, M.; Zhang, T.; Gao, H.; Ou, Y.; Li, M. Effects of biochar immobilization of Serratia sp. F4 OR414381 on bioremediation of petroleum contamination and bacterial community composition in loess soil. J. Hazard. Mater. 2024, 470, 134137. [Google Scholar] [PubMed]
- Yousaf, U.; Ali Khan, A.H.; Farooqi, A.; Muhammad, Y.S.; Barros, R.; Tamayo-Ramos, J.A.; Iqbal, M.; Yousaf, S. Interactive effect of biochar and compost with Poaceae and Fabaceae plants on remediation of total petroleum hydrocarbons in crude oil contaminated soil. Chemosphere 2022, 286, 131782. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Zhang, F.; Gong, H.; Sun, N.; Huang, J.; Chi, J. Responses of phenanthrene degradation to the changes in bioavailability and microbial community structure in soils amended with biochars pyrolyzed at low and high temperatures. J. Hazard. Mater. 2021, 410, 124584. [Google Scholar]
- Liu, L.; Chen, P.; Sun, M.; Shen, G.; Shang, G. Effect of biochar amendment on PAH dissipation and indigenous degradation bacteria in contaminated soil. J. Soils Sediments 2014, 15, 313–322. [Google Scholar]
- Li, Q.; Chen, R.; Xu, Y.; Chen, C.; Xiong, J.; Tan, W.; Fang, L. Examining diverse remediation mechanisms of biochar in soil contaminated with polycyclic aromatic hydrocarbon (PAH) of various ring structures: A global meta-analysis. Sci. Total Environ. 2024, 921, 171178. [Google Scholar] [CrossRef]
- Gomez-Eyles, J.L.; Sizmur, T.; Collins, C.D.; Hodson, M.E. Effects of biochar and the earthworm Eisenia fetida on the bioavailability of polycyclic aromatic hydrocarbons and potentially toxic elements. Environ. Pollut. 2011, 159, 616–622. [Google Scholar]
- Zhao, X.; Miao, R.; Guo, M.; Shang, X.; Zhou, Y.; Zhu, J. Biochar enhanced polycyclic aromatic hydrocarbons degradation in soil planted with ryegrass: Bacterial community and degradation gene expression mechanisms. Sci. Total Environ. 2022, 838, 156076. [Google Scholar]
- Chen, B.; Yuan, M.; Qian, L. Enhanced bioremediation of PAH-contaminated soil by immobilized bacteria with plant residue and biochar as carriers. J. Soils Sediments 2012, 12, 1350–1359. [Google Scholar] [CrossRef]
- Hua, L.; Chen, Y.-X.; Wu, W.-X.; Ma, H.-R. Effect of bio-charcoal on the trans of polycyclic aromatic hydrocarbons in soil-plant system with composted sludge application. Huan Jing Ke Xue = Huanjing Kexue 2009, 30, 2419–2424. [Google Scholar]
- Pathak, H.K.; Seth, C.S.; Chauhan, P.K.; Dubey, G.; Singh, G.; Jain, D.; Upadhyay, S.K.; Dwivedi, P.; Khoo, K.S. Recent advancement of nano-biochar for the remediation of heavy metals and emerging contaminants: Mechanism, adsorption kinetic model, plant growth and development. Environ. Res. 2024, 255, 119136. [Google Scholar] [PubMed]
- Denyes, M.J.; Langlois, V.S.; Rutter, A.; Zeeb, B.A. The use of biochar to reduce soil PCB bioavailability to Cucurbita pepo and Eisenia fetida. Sci. Total Environ. 2012, 437, 76–82. [Google Scholar] [PubMed]
- Rizwan, M.; Murtaza, G.; Zulfiqar, F.; Moosa, A.; Iqbal, R.; Ahmed, Z.; Irshad, S.; Khan, I.; Li, T.; Chen, J.; et al. Sustainable manufacture and application of biochar to improve soil properties and remediate soil contaminated with organic impurities: A systematic review. Front. Environ. Sci. 2023, 11, 1277240. [Google Scholar]
- Hoang, S.A.; Lamb, D.; Seshadri, B.; Sarkar, B.; Choppala, G.; Kirkham, M.B.; Bolan, N.S. Rhizoremediation as a green technology for the remediation of petroleum hydrocarbon-contaminated soils. J. Hazard. Mater. 2021, 401, 123282. [Google Scholar]
- Ogbonnaya, U.; Semple, K. Impact of Biochar on Organic Contaminants in Soil: A Tool for Mitigating Risk? Agronomy 2013, 3, 349–375. [Google Scholar] [CrossRef]
- Zhen, M.; Chen, H.; Liu, Q.; Song, B.; Wang, Y.; Tang, J. Combination of rhamnolipid and biochar in assisting phytoremediation of petroleum hydrocarbon contaminated soil using Spartina anglica. J. Environ. Sci. 2019, 85, 107–118. [Google Scholar]
- Valizadeh, S.; Lee, S.S.; Baek, K.; Choi, Y.J.; Jeon, B.-H.; Rhee, G.H.; Andrew Lin, K.-Y.; Park, Y.-K. Bioremediation strategies with biochar for polychlorinated biphenyls (PCBs)-contaminated soils: A review. Environ. Res. 2021, 200, 111757. [Google Scholar]
- Hayat, A.; Hussain, I.; Soja, G.; Iqbal, M.; Shahid, N.; Syed, J.H.; Yousaf, S. Organic and chemical amendments positively modulate the bacterial proliferation for effective rhizoremediation of PCBs-contaminated soil. Ecol. Eng. 2019, 138, 412–419. [Google Scholar]
- Pino, N.J.; Muñera, L.M.; Peñuela, G.A. Bioaugmentation with Immobilized Microorganisms to Enhance Phytoremediation of PCB-Contaminated Soil. Soil Sediment Contam. Int. J. 2016, 25, 419–430. [Google Scholar]
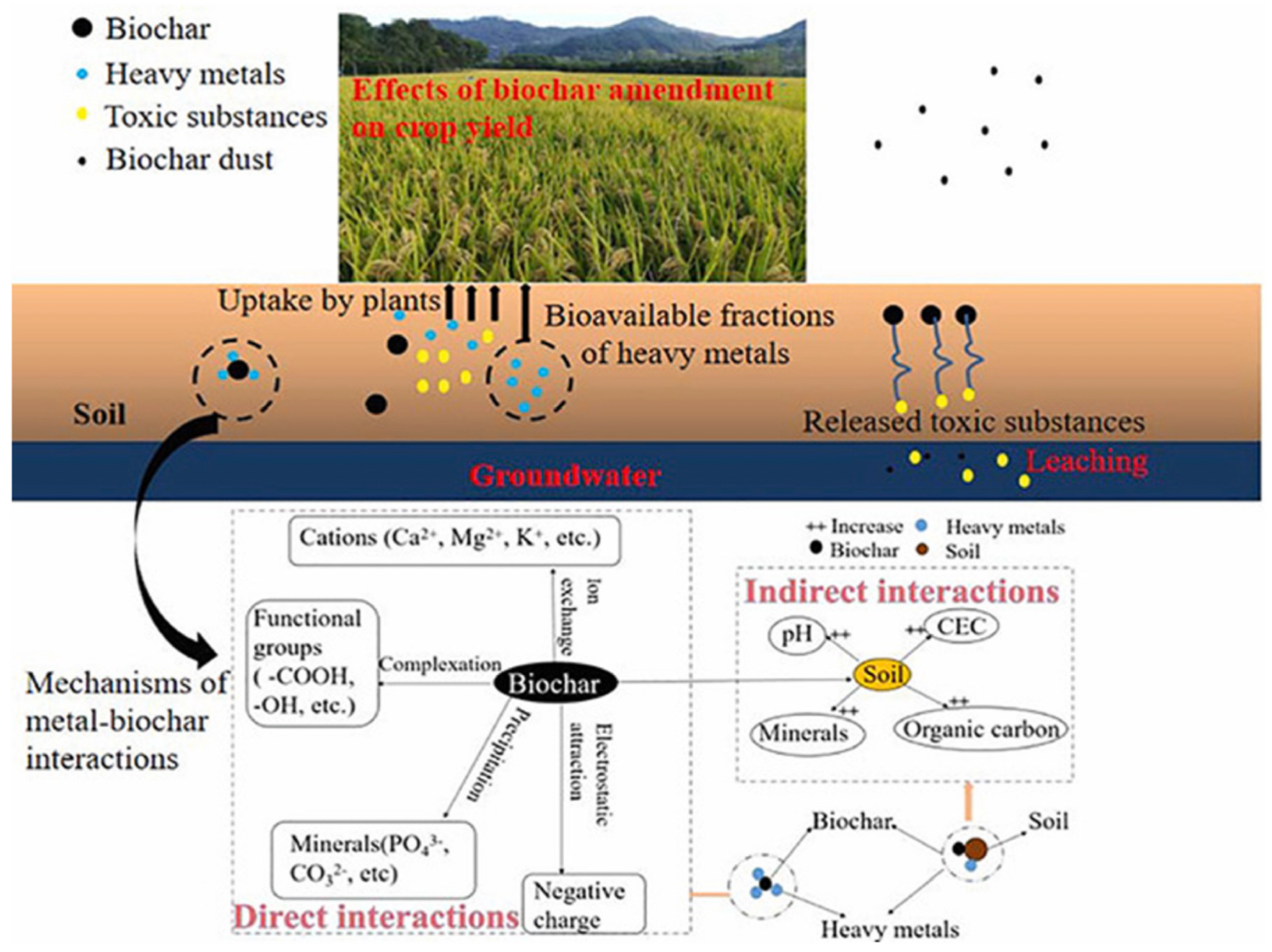
- Liang, M.; Lu, L.; He, H.; Li, J.; Zhu, Z.; Zhu, Y. Applications of Biochar and Modified Biochar in Heavy Metal Contaminated Soil: A Descriptive Review. Sustainability 2021, 13, 14041. [Google Scholar] [CrossRef]
- Gong, H.; Zhao, L.; Rui, X.; Hu, J.; Zhu, N. A review of pristine and modified biochar immobilizing typical heavy metals in soil: Applications and challenges. J. Hazard. Mater. 2022, 432, 128668. [Google Scholar] [PubMed]
- Wu, J.; Li, Z.; Huang, D.; Liu, X.; Tang, C.; Parikh, S.J.; Xu, J. A novel calcium-based magnetic biochar is effective in stabilization of arsenic and cadmium co-contamination in aerobic soils. J. Hazard. Mater. 2020, 387, 122010. [Google Scholar] [PubMed]
- Qiao, J.-t.; Li, X.-m.; Li, F.-b. Roles of different active metal-reducing bacteria in arsenic release from arsenic-contaminated paddy soil amended with biochar. J. Hazard. Mater. 2018, 344, 958–967. [Google Scholar] [PubMed]
- Wen, E.; Yang, X.; Chen, H.; Shaheen, S.M.; Sarkar, B.; Xu, S.; Song, H.; Liang, Y.; Rinklebe, J.; Hou, D.; et al. Iron-modified biochar and water management regime-induced changes in plant growth, enzyme activities, and phytoavailability of arsenic, cadmium and lead in a paddy soil. J. Hazard. Mater. 2021, 407, 124344. [Google Scholar]
- Islam, M.S.; Magid, A.S.I.A.; Chen, Y.; Weng, L.; Ma, J.; Arafat, M.Y.; Khan, Z.H.; Li, Y. Effect of calcium and iron-enriched biochar on arsenic and cadmium accumulation from soil to rice paddy tissues. Sci. Total Environ. 2021, 785, 147163. [Google Scholar]
- Liu, M.; Almatrafi, E.; Zhang, Y.; Xu, P.; Song, B.; Zhou, C.; Zeng, G.; Zhu, Y. A critical review of biochar-based materials for the remediation of heavy metal contaminated environment: Applications and practical evaluations. Sci. Total Environ. 2022, 806, 150531. [Google Scholar]
- Alaboudi, K.A.; Ahmed, B.; Brodie, G. Effect of biochar on Pb, Cd and Cr availability and maize growth in artificial contaminated soil. Ann. Agric. Sci. 2019, 64, 95–102. [Google Scholar]
- Rafique, M.I.; Usman, A.R.A.; Ahmad, M.; Al-Wabel, M.I. Immobilization and mitigation of chromium toxicity in aqueous solutions and tannery waste-contaminated soil using biochar and polymer-modified biochar. Chemosphere 2021, 266, 129198. [Google Scholar]
- Murad, H.A.; Ahmad, M.; Bundschuh, J.; Hashimoto, Y.; Zhang, M.; Sarkar, B.; Ok, Y.S. A remediation approach to chromium-contaminated water and soil using engineered biochar derived from peanut shell. Environ. Res. 2022, 204, 112125. [Google Scholar]
- Medha, I.; Chandra, S.; Vanapalli, K.R.; Samal, B.; Bhattacharya, J.; Das, B.K. (3-Aminopropyl)triethoxysilane and iron rice straw biochar composites for the sorption of Cr (VI) and Zn (II) using the extract of heavy metals contaminated soil. Sci. Total Environ. 2021, 771, 144764. [Google Scholar]
- Chen, X.; Dai, Y.; Fan, J.; Xu, X.; Cao, X. Application of iron-biochar composite in topsoil for simultaneous remediation of chromium-contaminated soil and groundwater: Immobilization mechanism and long-term stability. J. Hazard. Mater. 2021, 405, 124226. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Fang, Z.; Tsang, P.E.; Zheng, L.; Cheng, W.; Fang, J.; Zhao, D. Remediation of hexavalent chromium contaminated soil by biochar-supported zero-valent iron nanoparticles. J. Hazard. Mater. 2016, 318, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.K.; Noman, A.; Alhaithloul, H.A.S.; Adeel, M.; Rui, Y.; Shah, T.; Zhu, S.; Shang, J. Goethite-modified biochar ameliorates the growth of rice (Oryza sativa L.) plants by suppressing Cd and As-induced oxidative stress in Cd and As co-contaminated paddy soil. Sci. Total Environ. 2020, 717, 137086. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, X.; Gao, M.; Song, Z. Effect of Fe–Mn–Ce modified biochar composite on microbial diversity and properties of arsenic-contaminated paddy soils. Chemosphere 2020, 250, 126249. [Google Scholar] [CrossRef]
- Wan, X.; Li, C.; Parikh, S.J. Simultaneous removal of arsenic, cadmium, and lead from soil by iron-modified magnetic biochar. Environ. Pollut. 2020, 261, 114157. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Chen, X.; Xu, Z.; Xu, X.; Zhao, L.; Qiu, H.; Cao, X. One-pot synthesis of nZVI-embedded biochar for remediation of two mining arsenic-contaminated soils: Arsenic immobilization associated with iron transformation. J. Hazard. Mater. 2020, 398, 122901. [Google Scholar] [CrossRef]
- Fan, J.; Cai, C.; Chi, H.; Reid, B.J.; Coulon, F.; Zhang, Y.; Hou, Y. Remediation of cadmium and lead polluted soil using thiol-modified biochar. J. Hazard. Mater. 2020, 388, 122037. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Wang, F.; Wang, L.; Liu, J.; Hashimoto, Y.; Hosomi, M. Arsenic immobilization and removal in contaminated soil using zero-valent iron or magnetic biochar amendment followed by dry magnetic separation. Sci. Total Environ. 2021, 768, 144521. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, P.; Jeyakumar, P.; Bolan, N.; Wang, H.; Gao, B.; Wang, S.; Wang, B. Biochar as a potential strategy for remediation of contaminated mining soils: Mechanisms, applications, and future perspectives. J. Environ. Manag. 2022, 313, 114973. [Google Scholar] [CrossRef]
- Mujtaba Munir, M.A.; Liu, G.; Yousaf, B.; Ali, M.U.; Cheema, A.I.; Rashid, M.S.; Rehman, A. Bamboo-biochar and hydrothermally treated-coal mediated geochemical speciation, transformation and uptake of Cd, Cr, and Pb in a polymetal(iod)s-contaminated mine soil. Environ. Pollut. 2020, 265, 114816. [Google Scholar] [CrossRef]
- Sun, T.; Xu, Y.; Sun, Y.; Wang, L.; Liang, X.; Jia, H. Crayfish shell biochar for the mitigation of Pb contaminated water and soil: Characteristics, mechanisms, and applications. Environ. Pollut. 2021, 271, 116308. [Google Scholar] [CrossRef] [PubMed]
- Naeem, I.; Masood, N.; Turan, V.; Iqbal, M. Prospective usage of magnesium potassium phosphate cement combined with Bougainvillea alba derived biochar to reduce Pb bioavailability in soil and its uptake by Spinacia oleracea L. Ecotoxicol. Environ. Saf. 2021, 208, 111723. [Google Scholar] [CrossRef] [PubMed]
- Turan, V. Potential of pistachio shell biochar and dicalcium phosphate combination to reduce Pb speciation in spinach, improved soil enzymatic activities, plant nutritional quality, and antioxidant defense system. Chemosphere 2020, 245, 125611. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Y.; Tang, L.; Hussain, B.; Usman, M.; Gurajala, H.K.; Rashid, M.S.; He, Z.; Yang, X. Efficiency of lime, biochar, Fe containing biochar and composite amendments for Cd and Pb immobilization in a co-contaminated alluvial soil. Environ. Pollut. 2020, 257, 113609. [Google Scholar] [CrossRef]
- Mandal, S.; Pu, S.; Shangguan, L.; Liu, S.; Ma, H.; Adhikari, S.; Hou, D. Synergistic construction of green tea biochar supported nZVI for immobilization of lead in soil: A mechanistic investigation. Environ. Int. 2020, 135, 105374. [Google Scholar] [CrossRef]
- Qian, W.; Liang, J.-Y.; Zhang, W.-X.; Huang, S.-T.; Diao, Z.-H. A porous biochar supported nanoscale zero-valent iron material highly efficient for the simultaneous remediation of cadmium and lead contaminated soil. J. Environ. Sci. 2022, 113, 231–241. [Google Scholar] [CrossRef]
- Ye, J.; Liao, W.; Zhang, P.; Li, J.; Nabi, M.; Wang, S.; Cai, Y.; Li, F. Fe1-xS/biochar combined with thiobacillus enhancing lead phytoavailability in contaminated soil: Preparation of biochar, enrichment of thiobacillus and their function on soil lead. Environ. Pollut. 2020, 267, 115447. [Google Scholar] [CrossRef]
- Gu, P.; Zhang, Y.; Xie, H.; Wei, J.; Zhang, X.; Huang, X.; Wang, J.; Lou, X. Effect of cornstalk biochar on phytoremediation of Cd-contaminated soil by Beta vulgaris var cicla L. Ecotoxicol. Environ. Saf. 2020, 205, 111144. [Google Scholar] [CrossRef]
- Wei, L.; Huang, Y.; Huang, L.; Huang, Q.; Li, Y.; Li, X.; Yang, S.; Liu, C.; Liu, Z. Combined biochar and soda residues increases maize yields and decreases grain Cd/Pb in a highly Cd/Pb-polluted acid Udults soil. Agric. Ecosyst. Environ. 2021, 306, 107198. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, K.; Zhan, W.; Huang, L.; Liu, Y.; Li, T.; Yang, Z.; Liao, Q.; Chen, R.; Zhang, C.; et al. Highly effective stabilization of Cd and Cu in two different soils and improvement of soil properties by multiple-modified biochar. Ecotoxicol. Environ. Saf. 2021, 207, 111294. [Google Scholar] [CrossRef]
- Gong, H.; Huang, J.; Ding, Z.; Chi, J. A potential method using magnetically modified wheat straw biochars for soil Cd extraction. Ecol. Eng. 2021, 166, 106240. [Google Scholar]
- Khum-in, V.; Suk-in, J.; In-ai, P.; Piaowan, K.; Phaimisap, Y.; Supanpaiboon, W.; Phenrat, T. Combining biochar and zerovalent iron (BZVI) as a paddy field soil amendment for heavy cadmium (Cd) contamination decreases Cd but increases zinc and iron concentrations in rice grains: A field-scale evaluation. Process Saf. Environ. Prot. 2020, 141, 222–233. [Google Scholar]
- Yang, S.; Xiao, Q.; Li, B.; Zhou, T.; Cen, Q.; Liu, Z.; Zhou, Y. Insights into remediation of cadmium and lead contaminated-soil by Fe-Mn modified biochar. J. Environ. Chem. Eng. 2024, 12, 112771. [Google Scholar]
- Xu, C.; Zhao, J.; Yang, W.; He, L.; Wei, W.; Tan, X.; Wang, J.; Lin, A. Evaluation of biochar pyrolyzed from kitchen waste, corn straw, and peanut hulls on immobilization of Pb and Cd in contaminated soil. Environ. Pollut. 2020, 261, 114133. [Google Scholar]
- Pan, H.; Yang, X.; Chen, H.; Sarkar, B.; Bolan, N.; Shaheen, S.M.; Wu, F.; Che, L.; Ma, Y.; Rinklebe, J.; et al. Pristine and iron-engineered animal- and plant-derived biochars enhanced bacterial abundance and immobilized arsenic and lead in a contaminated soil. Sci. Total Environ. 2021, 763, 144218. [Google Scholar]
- Chen, X.; Lewis, S.; Heal, K.V.; Lin, Q.; Sohi, S.P. Biochar engineering and ageing influence the spatiotemporal dynamics of soil pH in the charosphere. Geoderma 2021, 386, 114919. [Google Scholar]
- Rehman, M.Z.U.; Batool, Z.; Ayub, M.A.; Hussaini, K.M.; Murtaza, G.; Usman, M.; Naeem, A.; Khalid, H.; Rizwan, M.; Ali, S. Effect of acidified biochar on bioaccumulation of cadmium (Cd) and rice growth in contaminated soil. Environ. Technol. Innov. 2020, 19, 101015. [Google Scholar]
- Zhu, J.; Gao, W.; Ge, L.; Zhao, W.; Zhang, G.; Niu, Y. Immobilization properties and adsorption mechanism of nickel(II) in soil by biochar combined with humic acid-wood vinegar. Ecotoxicol. Environ. Saf. 2021, 215, 112159. [Google Scholar]
- Mailakeba, C.D.; B.K, R.R. Biochar application alters soil Ni fractions and phytotoxicity of Ni to pakchoi (Brassica rapa L. ssp. chinensis L.) plants. Environ. Technol. Innov. 2021, 23, 101751. [Google Scholar]
- Ali, U.; Shaaban, M.; Bashir, S.; Fu, Q.; Zhu, J.; Shoffikul Islam, M.; Hu, H. Effect of rice straw, biochar and calcite on maize plant and Ni bio-availability in acidic Ni contaminated soil. J. Environ. Manag. 2020, 259, 109674. [Google Scholar]
- Ali, A.; Shaheen, S.M.; Guo, D.; Li, Y.; Xiao, R.; Wahid, F.; Azeem, M.; Sohail, K.; Zhang, T.; Rinklebe, J.; et al. Apricot shell- and apple tree-derived biochar affect the fractionation and bioavailability of Zn and Cd as well as the microbial activity in smelter contaminated soil. Environ. Pollut. 2020, 264, 114773. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Ali, A.; Arockiam Jeyasundar, P.G.S.; Bashir, S.; Hussain, Q.; Wahid, F.; Ali, E.F.; Abdelrahman, H.; Li, R.; Antoniadis, V.; et al. Effects of sheep bone biochar on soil quality, maize growth, and fractionation and phytoavailability of Cd and Zn in a mining-contaminated soil. Chemosphere 2021, 282, 131016. [Google Scholar] [PubMed]
- Azeem, M.; Ali, A.; Arockiam Jeyasundar, P.G.S.; Li, Y.; Abdelrahman, H.; Latif, A.; Li, R.; Basta, N.; Li, G.; Shaheen, S.M.; et al. Bone-derived biochar improved soil quality and reduced Cd and Zn phytoavailability in a multi-metal contaminated mining soil. Environ. Pollut. 2021, 277, 116800. [Google Scholar] [PubMed]
- Tu, C.; Wei, J.; Guan, F.; Liu, Y.; Sun, Y.; Luo, Y. Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil. Environ. Int. 2020, 137, 105576. [Google Scholar] [CrossRef]
- Rehman, M.; Liu, L.; Bashir, S.; Saleem, M.H.; Chen, C.; Peng, D.; Siddique, K.H.M. Influence of rice straw biochar on growth, antioxidant capacity and copper uptake in ramie (Boehmeria nivea L.) grown as forage in aged copper-contaminated soil. Plant Physiol. Biochem. 2019, 138, 121–129. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, R.; Ji, S.; Xie, L.; Zhang, H. Effects of biochar derived from sewage sludge and sewage sludge/cotton stalks on the immobilization and phytoavailability of Pb, Cu, and Zn in sandy loam soil. J. Hazard. Mater. 2021, 419, 126468. [Google Scholar] [CrossRef]
- Van Poucke, R.; Meers, E.; Tack, F.M.G. Leaching behavior of Cd, Zn and nutrients (K, P, S) from a contaminated soil as affected by amendment with biochar. Chemosphere 2020, 245, 125561. [Google Scholar]
- Gao, R.; Hu, H.; Fu, Q.; Li, Z.; Xing, Z.; Ali, U.; Zhu, J.; Liu, Y. Remediation of Pb, Cd, and Cu contaminated soil by co-pyrolysis biochar derived from rape straw and orthophosphate: Speciation transformation, risk evaluation and mechanism inquiry. Sci. Total Environ. 2020, 730, 139119. [Google Scholar]
- Méndez, A.; Paz-Ferreiro, J.; Araujo, F.; Gascó, G. Biochar from pyrolysis of deinking paper sludge and its use in the treatment of a nickel polluted soil. J. Anal. Appl. Pyrolysis 2014, 107, 46–52. [Google Scholar]
- Ali, U.; Shaaban, M.; Bashir, S.; Gao, R.; Fu, Q.; Zhu, J.; Hu, H. Rice straw, biochar and calcite incorporation enhance nickel (Ni) immobilization in contaminated soil and Ni removal capacity. Chemosphere 2020, 244, 125418. [Google Scholar]
- Lian, W.; Shi, W.; Tian, S.; Gong, X.; Yu, Q.; Lu, H.; Liu, Z.; Zheng, J.; Wang, Y.; Bian, R.; et al. Preparation and application of biochar from co-pyrolysis of different feedstocks for immobilization of heavy metals in contaminated soil. Waste Manag. 2023, 163, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-H.; Xie, Y.; Zhou, G.; Li, Z.; Ye, A.; Huang, X.; Xie, Y.; Shi, L.; Cao, X.; Zhang, J.; et al. The effects of short-term, long-term, and reapplication of biochar on the remediation of heavy metal-contaminated soil. Ecotoxicol. Environ. Saf. 2022, 248, 114316. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, J.; Pei, H.; Li, Q.; Cheng, D.; Zhou, J.; Pei, G.; Wang, Y.; Liu, F. Effective remediation and phytotoxicity assessment of oxytetracycline and Cd co-contaminated soil using biochar. Environ. Technol. Innov. 2024, 35, 103649. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; van Hullebusch, E.D.; Amrane, A.; Rtimi, S. Mechanisms and adsorption capacities of biochar for the removal of organic and inorganic pollutants from industrial wastewater. Int. J. Environ. Sci. Technol. 2020, 18, 3273–3294. [Google Scholar]
- Li, G.; Chen, F.; Jia, S.; Wang, Z.; Zuo, Q.; He, H. Effect of biochar on Cd and pyrene removal and bacteria communities variations in soils with culturing ryegrass (Lolium perenne L.). Environ. Pollut. 2020, 265, 114887. [Google Scholar] [CrossRef]
- Shang, X.; Wu, S.; Liu, Y.; Zhang, K.; Guo, M.; Zhou, Y.; Zhu, J.; Li, X.; Miao, R. Rice husk and its derived biochar assist phytoremediation of heavy metals and PAHs co-contaminated soils but differently affect bacterial community. J. Hazard. Mater. 2024, 466, 133684. [Google Scholar] [CrossRef]
- Murtaza, G.; Ahmed, Z.; Eldin, S.M.; Ali, I.; Usman, M.; Iqbal, R.; Rizwan, M.; Abdel-Hameed, U.K.; Haider, A.A.; Tariq, A. Biochar as a Green Sorbent for Remediation of Polluted Soils and Associated Toxicity Risks: A Critical Review. Separations 2023, 10, 197. [Google Scholar] [CrossRef]
- Anae, J.; Ahmad, N.; Kumar, V.; Thakur, V.K.; Gutierrez, T.; Yang, X.J.; Cai, C.; Yang, Z.; Coulon, F. Recent advances in biochar engineering for soil contaminated with complex chemical mixtures: Remediation strategies and future perspectives. Sci. Total Environ. 2021, 767, 144351. [Google Scholar] [CrossRef]
- Lu, Q. Insights into the remediation of cadmium-pyrene co-contaminated soil by electrokinetic and the influence factors. Chemosphere 2020, 254, 126861. [Google Scholar] [CrossRef]
- Lan, J.; Wen, F.; Ren, Y.; Liu, G.; Jiang, Y.; Wang, Z.; Zhu, X. An overview of bioelectrokinetic and bioelectrochemical remediation of petroleum-contaminated soils. Environ. Sci. Ecotechnol. 2023, 16, 100278. [Google Scholar] [CrossRef]
- Huang, D.; Xu, Q.; Cheng, J.; Lu, X.; Zhang, H. Electrokinetic Remediation and Its Combined Technologies for Removal of Organic Pollutants from Contaminated Soils. Int. J. Electrochem. Sci. 2012, 7, 4528–4544. [Google Scholar]
- Ma, L.; Li, Z.; Qiao, M.; Liu, J.; Jia, B.; Yang, B.; Liu, Y. Enhancing electrokinetic remediation of TPH-Cr(VI) co-contaminated soils with biochar-immobilized bacteria as biological permeable reactive barriers. Chem. Eng. J. 2023, 478, 147301. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J. Effect of ageing on biochar properties and pollutant management. Chemosphere 2022, 292, 133427. [Google Scholar] [PubMed]
- Nguyen, T.-B.; Sherpa, K.; Bui, X.-T.; Nguyen, V.-T.; Vo, T.-D.-H.; Ho, H.-T.-T.; Chen, C.-W.; Dong, C.-D. Biochar for soil remediation: A comprehensive review of current research on pollutant removal. Environ. Pollut. 2023, 337, 122571. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Zdarta, J. Fabrication of engineered biochar for remediation of toxic contaminants in soil matrices and soil valorization. Chemosphere 2024, 358, 142101. [Google Scholar]
- Roy, K.; Kar, S.; Das, R.N. Chapter 1—Background of QSAR and Historical Developments. In Understanding the Basics of QSAR for Applications in Pharmaceutical Sciences and Risk Assessment; Roy, K., Kar, S., Das, R.N., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 1–46. [Google Scholar]
- Keswani, M.; Han, Z. Chapter 4—Post-CMP Cleaning. In Developments in Surface Contamination and Cleaning; Kohli, R., Mittal, K.L., Eds.; William Andrew Publishing: Oxford, UK, 2015; pp. 145–183. [Google Scholar]
- Barati, M.; Bakhtiari, F.; Mowla, D.; Safarzadeh, S. Comparison of the effects of poultry manure and its biochar on barley growth in petroleum-contaminated soils. Int. J. Phytoremediat. 2018, 20, 98–103. [Google Scholar]
- Naja, G.; Volesky, B. The Mechanism of Metal Cation and Anion Biosorption. In Microbial Biosorption of Metals; Kotrba, P., Mackova, M., Macek, T., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 19–58. [Google Scholar]
- Hosmane, N.S. Chapter 6—Review of Bonding Theories for d-Block Metal Complexes. In Advanced Inorganic Chemistry; Hosmane, N.S., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 89–114. [Google Scholar]
- Harland, C.E. Ion Exchange: Theory and Practice; The Royal Society of Chemistry: London, UK, 1994. [Google Scholar]
- Chen, B.; Zhou, D.; Zhu, L. Transitional Adsorption and Partition of Nonpolar and Polar Aromatic Contaminants by Biochars of Pine Needles with Different Pyrolytic Temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar]
- Chen, B.; Chen, Z. Sorption of naphthalene and 1-naphthol by biochars of orange peels with different pyrolytic temperatures. Chemosphere 2009, 76, 127–133. [Google Scholar]
- Xiang, W.; Zhang, X.; Chen, K.; Fang, J.; He, F.; Hu, X.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Enhanced adsorption performance and governing mechanisms of ball-milled biochar for the removal of volatile organic compounds (VOCs). Chem. Eng. J. 2020, 385, 123842. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar]
- Binh, Q.A.; Nguyen, H.-H. Investigation the isotherm and kinetics of adsorption mechanism of herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) on corn cob biochar. Bioresour. Technol. Rep. 2020, 11, 100520. [Google Scholar]
- Zhu, X.M.; Chen, B.L.; Zhu, L.Z.; Xing, B.S. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [PubMed]
- Yao, R.J.; Li, H.Q.; Yang, J.S.; Wang, X.P.; Xie, W.P.; Zhang, X. Biochar Addition Inhibits Nitrification by Shifting Community Structure of Ammonia-Oxidizing Microorganisms in Salt-Affected Irrigation-Silting Soil. Microorganisms 2022, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Liu, S.; Ye, S.; Yang, H.; Song, B.; Qin, F.; Shen, M.; Tan, C.; Zeng, G.; Tan, X. Potential hazards of biochar: The negative environmental impacts of biochar applications. J. Hazard. Mater. 2021, 420, 126611. [Google Scholar]
- Liao, S.; Pan, B.; Li, H.; Zhang, D.; Xing, B. Detecting Free Radicals in Biochars and Determining Their Ability to Inhibit the Germination and Growth of Corn, Wheat and Rice Seedlings. Environ. Sci. Technol. 2014, 48, 8581–8587. [Google Scholar]
- Liu, Y.; Dai, Q.; Jin, X.; Dong, X.; Peng, J.; Wu, M.; Liang, N.; Pan, B.; Xing, B. Negative Impacts of Biochars on Urease Activity: High pH, Heavy Metals, Polycyclic Aromatic Hydrocarbons, or Free Radicals? Environ. Sci. Technol. 2018, 52, 12740–12747. [Google Scholar]
- Wang, Y.-Y.; Jing, X.-R.; Li, L.-L.; Liu, W.-J.; Tong, Z.-H.; Jiang, H. Biotoxicity Evaluations of Three Typical Biochars Using a Simulated System of Fast Pyrolytic Biochar Extracts on Organisms of Three Kingdoms. ACS Sustain. Chem. Eng. 2016, 5, 481–488. [Google Scholar]
- Dutta, T.; Kwon, E.; Bhattacharya, S.S.; Jeon, B.H.; Deep, A.; Uchimiya, M.; Kim, K.H. Polycyclic aromatic hydrocarbons and volatile organic compounds in biochar and biochar-amended soil: A review. GCB Bioenergy 2016, 9, 990–1004. [Google Scholar]
- Feng, D.; Zhao, Y.; Zhang, Y.; Zhang, Z.; Zhang, L.; Sun, S. In-situ steam reforming of biomass tar over sawdust biochar in mild catalytic temperature. Biomass Bioenergy 2017, 107, 261–270. [Google Scholar]
- Huang, C.; Zhang, C.; Huang, D.; Wang, D.; Tian, S.; Wang, R.; Yang, Y.; Wang, W.; Qin, F. Influence of surface functionalities of pyrogenic carbonaceous materials on the generation of reactive species towards organic contaminants: A review. Chem. Eng. J. 2021, 404, 127066. [Google Scholar]
- Oh, T.K.; Shinogi, Y.; Chikushi, J.; Lee, Y.H.; Choi, B. Effect of Aqueous Extract of Biochar on Germination and Seedling Growth of Lettuce (Lactuca sativa L.). J. Fac. Agric. Kyushu Univ. 2012, 57, 55–60. [Google Scholar]
- Lyu, H.; Zhang, H.; Dong, J.; Shen, B.; Cheng, Z.; Yu, J.; Li, R.; Shao, N.; Tang, J. Pyrolysis temperature matters: Biochar-derived dissolved organic matter modulates aging behavior and biotoxicity of microplastics. Water Res. 2024, 250, 121064. [Google Scholar]
| Materials | Elemental Content (%) | BET-SA (m2/g) | Pore Volume (cm3/g) | pHPZC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O | S | Vmicro | Vmeso | Vtotal | |||
| CS | 46.24 | 5.98 | 1.97 | 45.42 | 0.07 | 2.6 | 0.0011 | 0.0035 | 0.0086 | - |
| CS-300 | 55.54 | 4.06 | 1.72 | 37.75 | 0.05 | 3.3 | 0.0006 | 0.0040 | 0.0103 | 3.21 |
| CS-500 | 57.25 | 2.26 | 1.60 | 37.04 | 0.04 | 4.2 | 0.0006 | 0.0041 | 0.0117 | 3.54 |
| CS-800 | 59.36 | 1.12 | 1.48 | 36.11 | 0.04 | 19.6 | 0.0003 | 0.0059 | 0.0156 | 4.05 |
| MBM | 18.24 | 3.53 | 4.63 | 70.10 | 1.62 | 42.1 | 0.0034 | 0.1020 | 0.1250 | - |
| MBM-500 | 15.08 | 0.96 | 2.07 | 61.34 | 1.13 | 67.3 | 0.0022 | 0.1540 | 0.1800 | 4.32 |
| BS | 66.57 | 6.20 | 0.44 | 36.17 | 0.01 | 4.4 | 0.0005 | 0.0002 | 0.0007 | - |
| BS-500 | 85.74 | 3.12 | 0.41 | 10.33 | 0.01 | 7.2 | 0.0001 | 0.0005 | 0.0014 | 4.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, F.; Wang, Y.; Wei, Y. Advancements in Biochar for Soil Remediation of Heavy Metals and/or Organic Pollutants. Materials 2025, 18, 1524. https://doi.org/10.3390/ma18071524
Meng F, Wang Y, Wei Y. Advancements in Biochar for Soil Remediation of Heavy Metals and/or Organic Pollutants. Materials. 2025; 18(7):1524. https://doi.org/10.3390/ma18071524
Chicago/Turabian StyleMeng, Fanyue, Yanming Wang, and Yuexing Wei. 2025. "Advancements in Biochar for Soil Remediation of Heavy Metals and/or Organic Pollutants" Materials 18, no. 7: 1524. https://doi.org/10.3390/ma18071524
APA StyleMeng, F., Wang, Y., & Wei, Y. (2025). Advancements in Biochar for Soil Remediation of Heavy Metals and/or Organic Pollutants. Materials, 18(7), 1524. https://doi.org/10.3390/ma18071524





