Testing the Corrosion Rate of Prestressed Concrete Beams Under Variable Temperature and Humidity Conditions
Abstract
1. Introduction
Significance and Novelty of Researche
2. Materials and Methods
2.1. Accelerated Process of Chloride Migration in Concrete
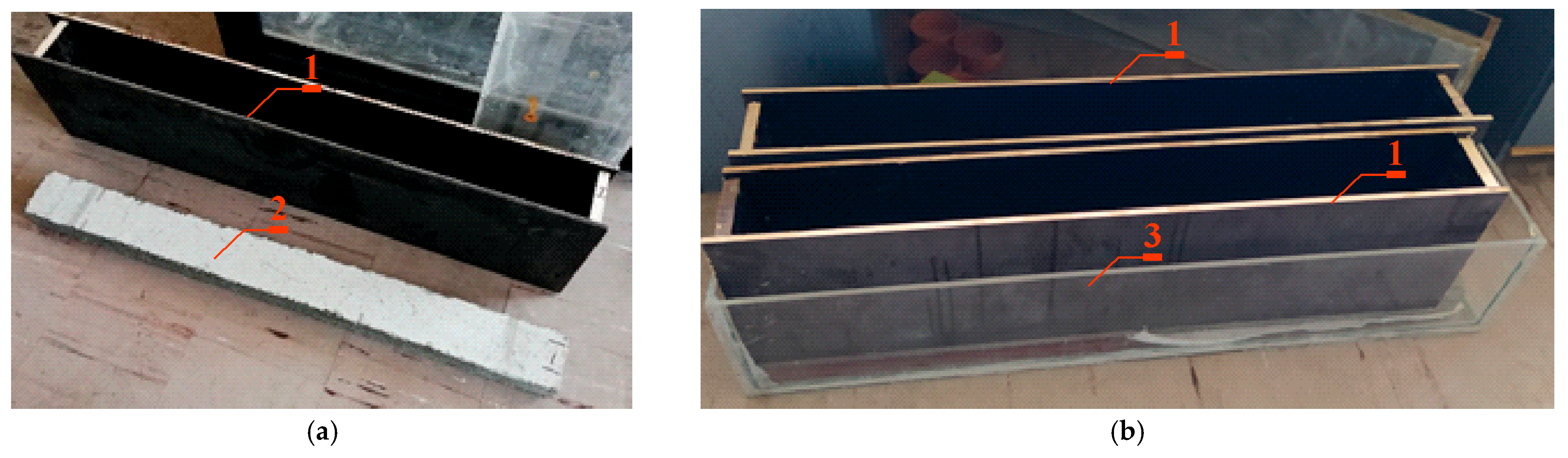
2.1.1. Preparation of Samples for Migration Tests
2.1.2. Migration Tests
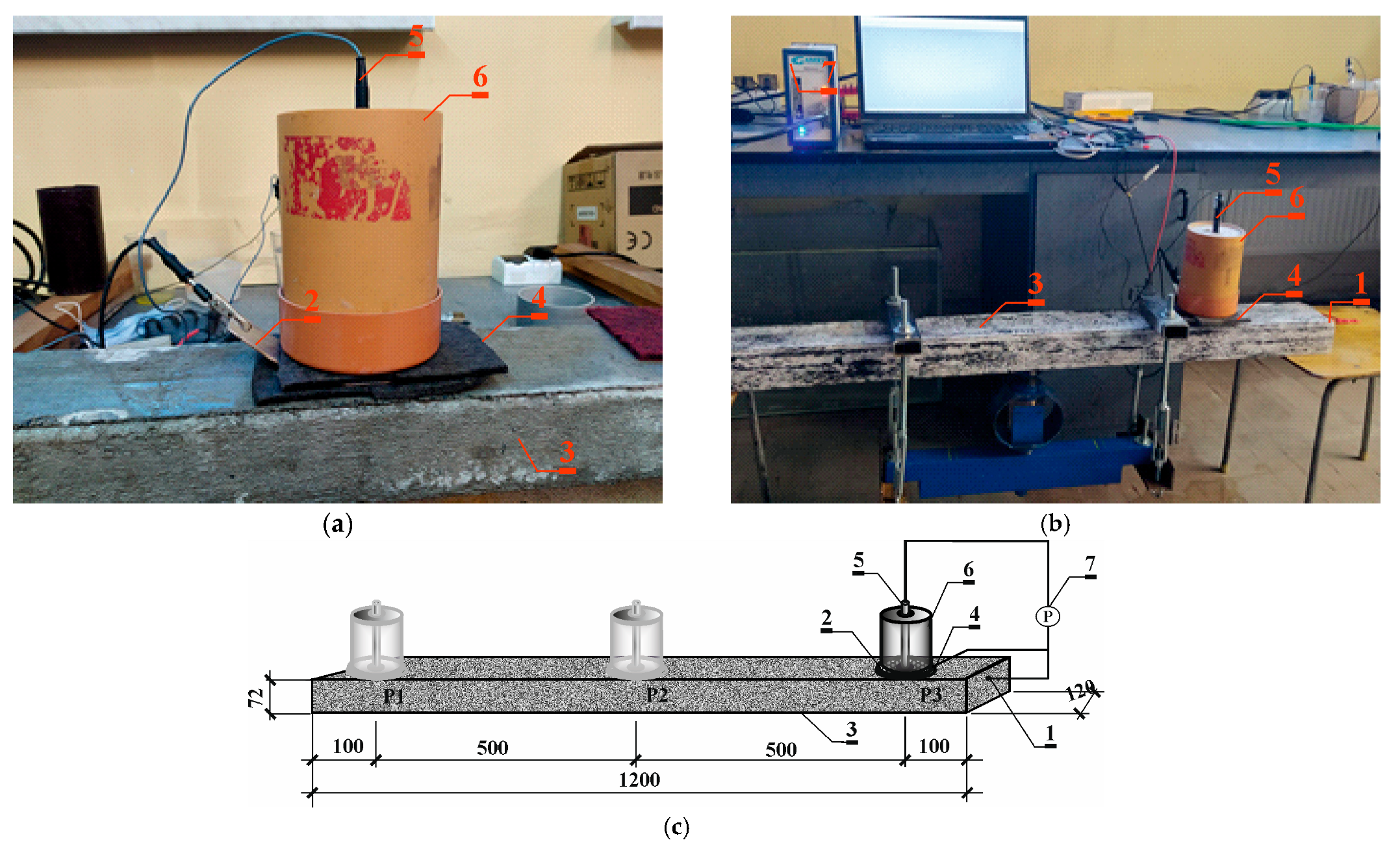
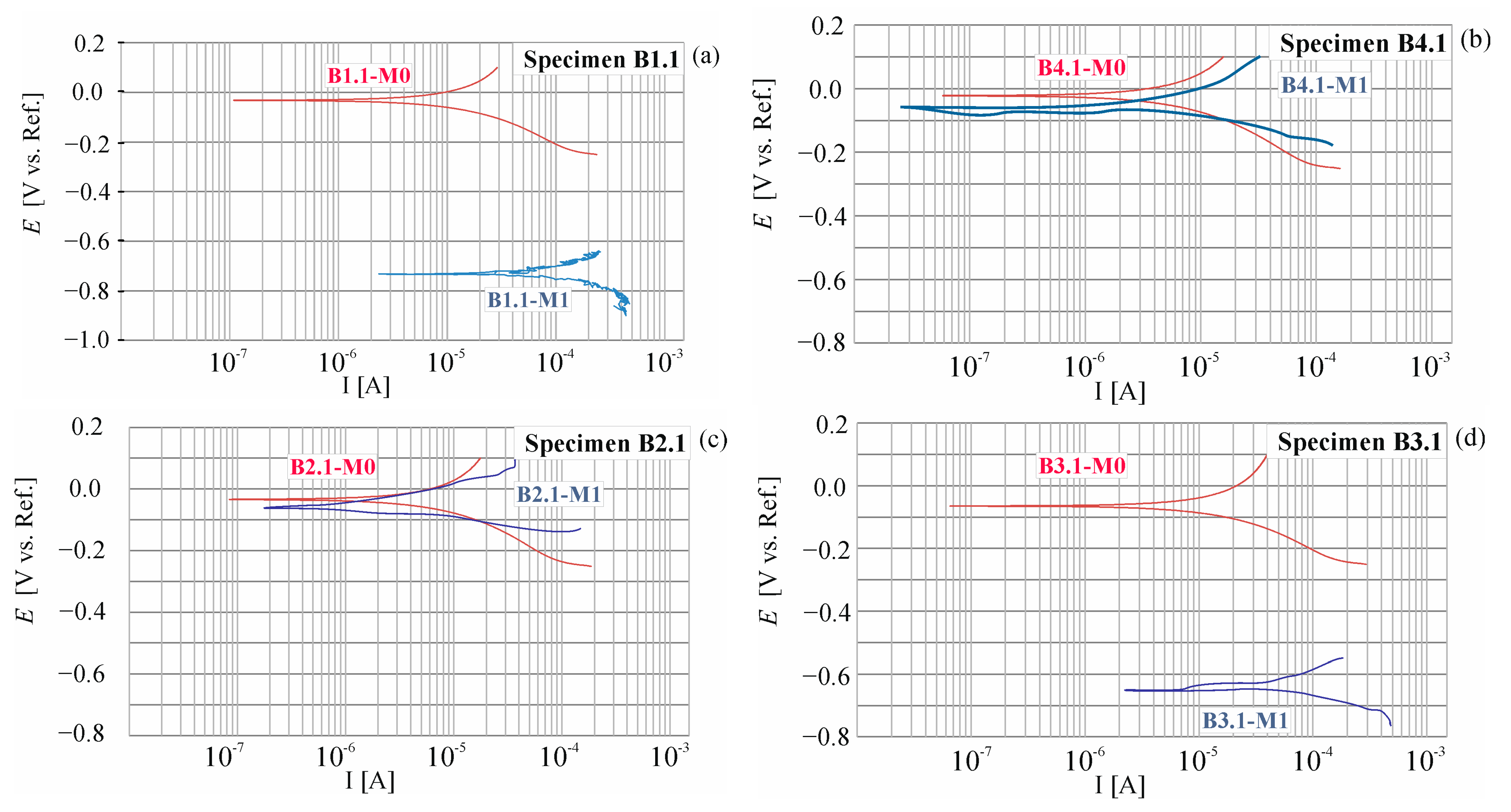
2.1.3. Control of the Corrosion Process in the Process of Accelerated Migration
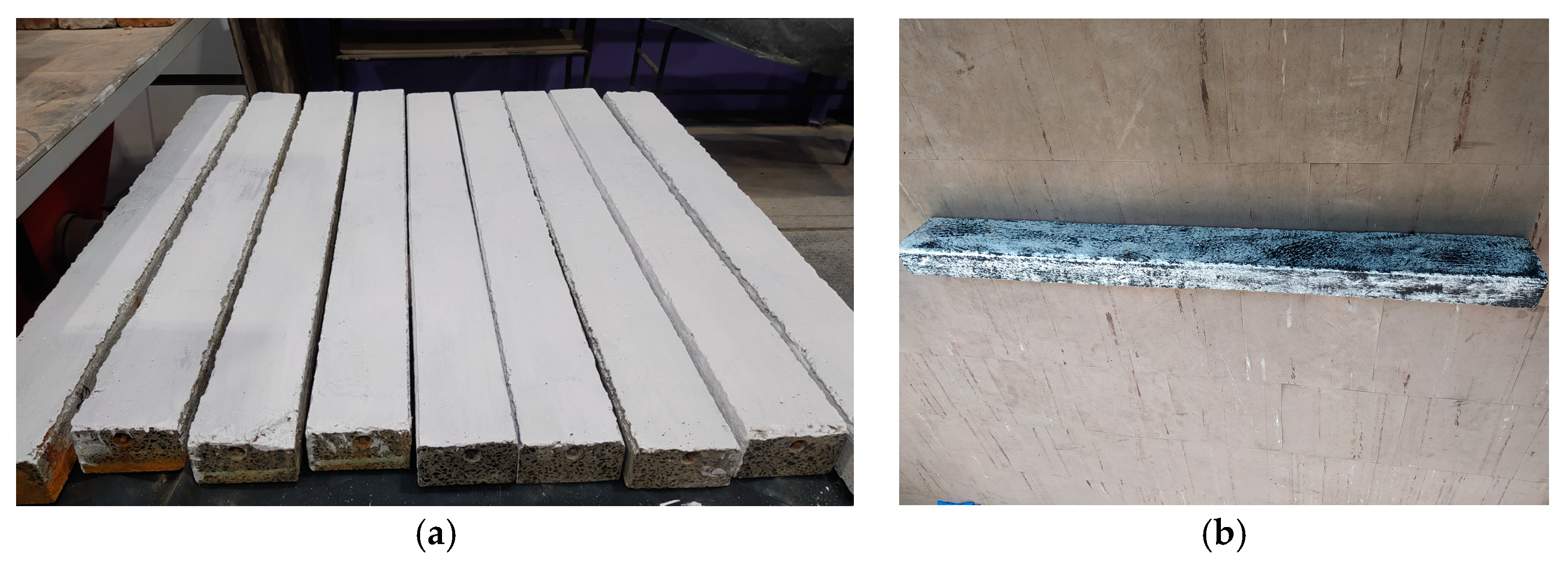
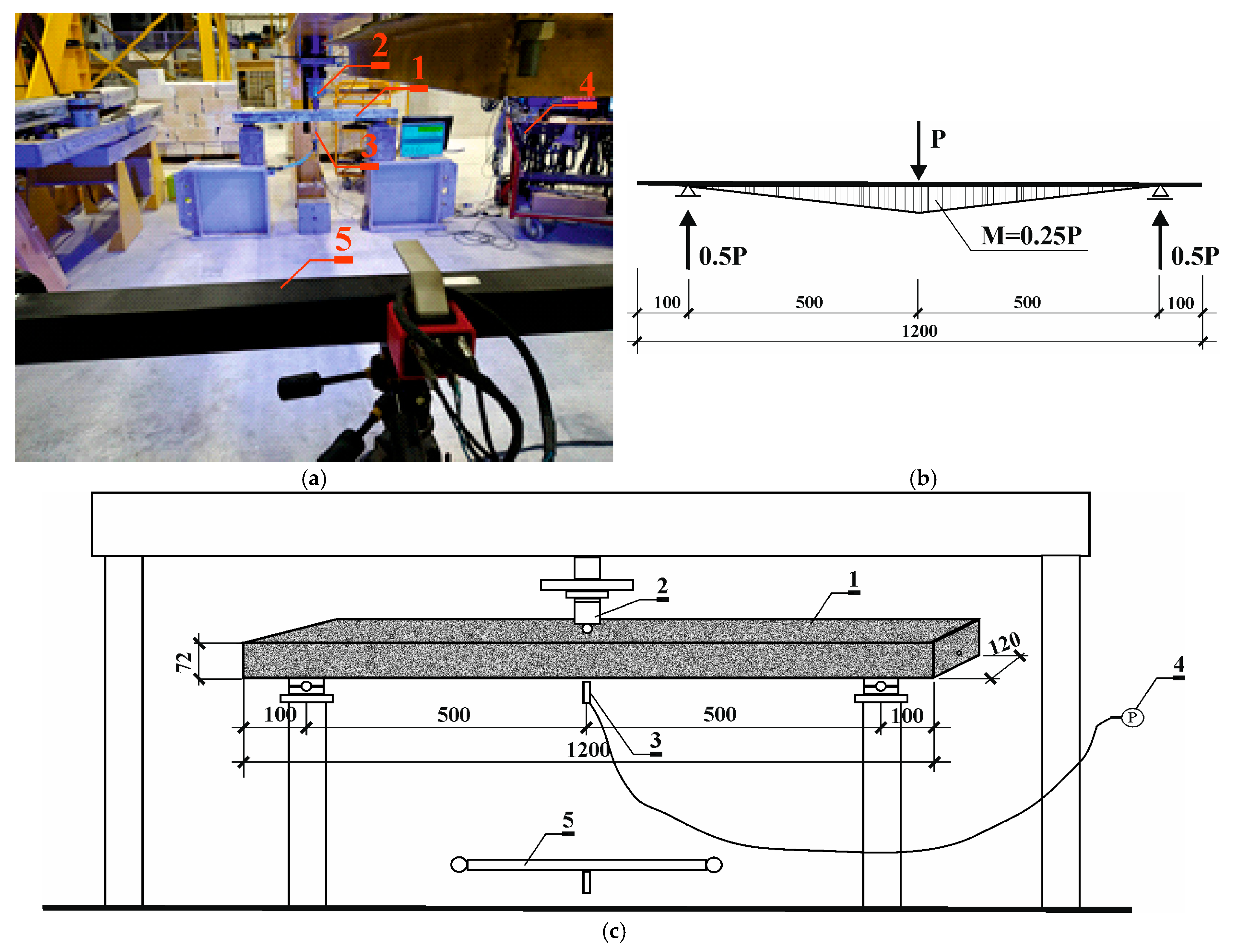
2.2. Determination of Scratching Condition in Prestressed Concrete Beams Using Aramis Optical System
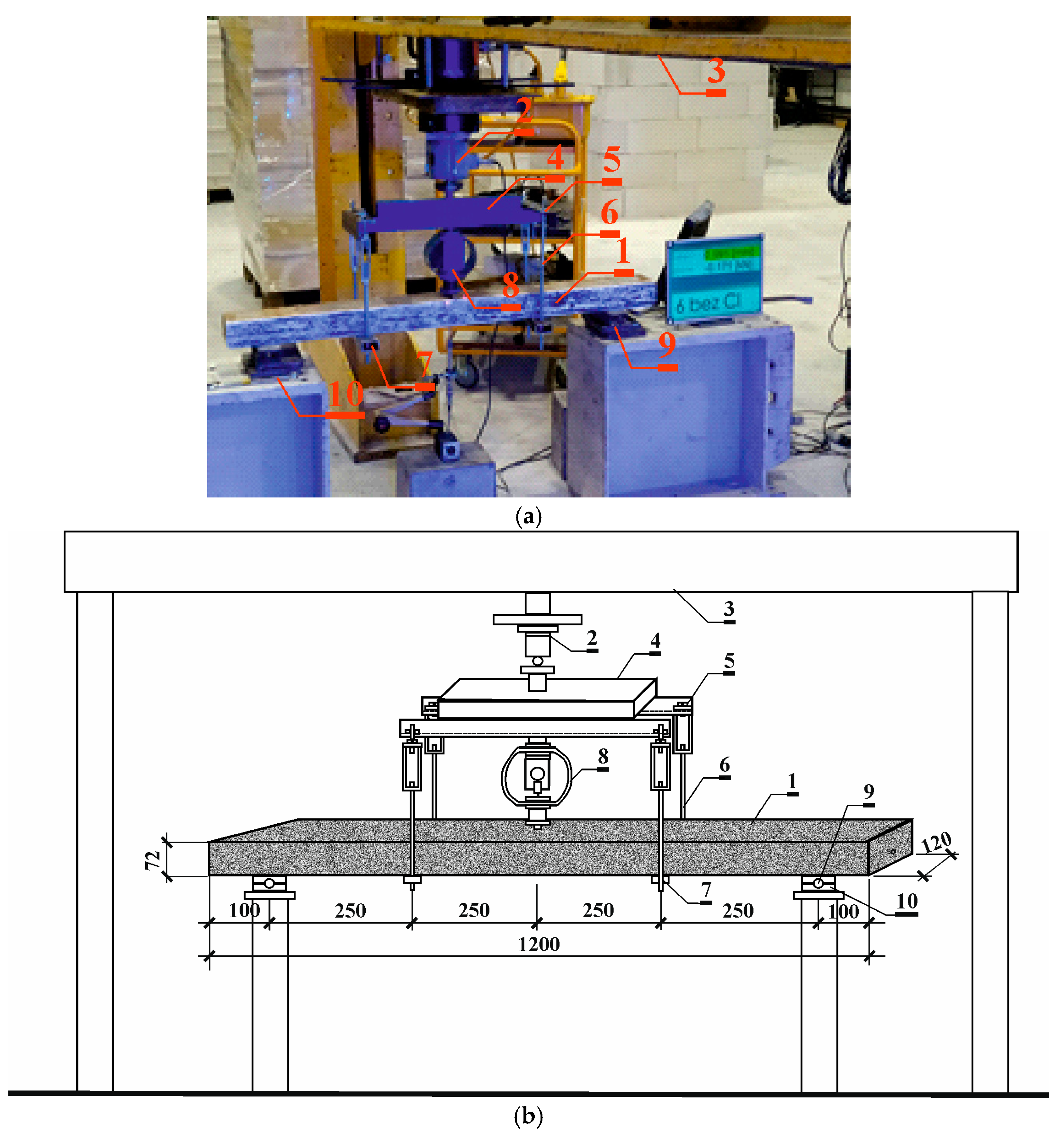
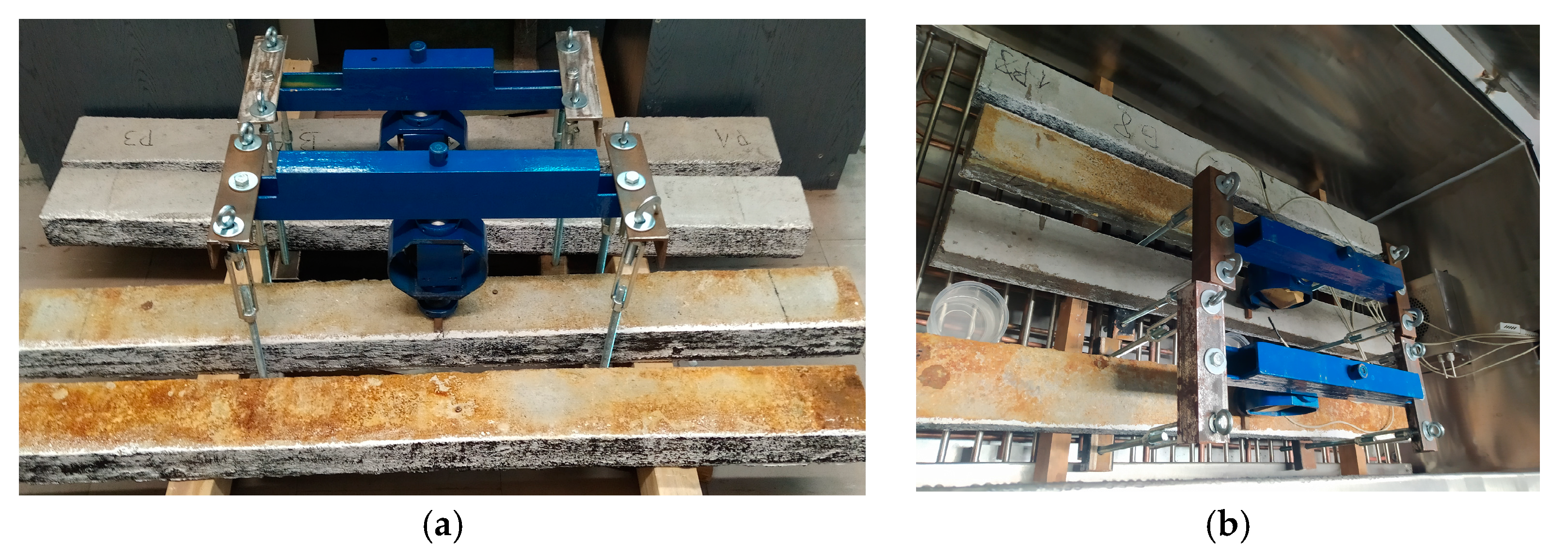
2.3. Destructive Testing of Prestressed Beams Using the Aramis Optical System
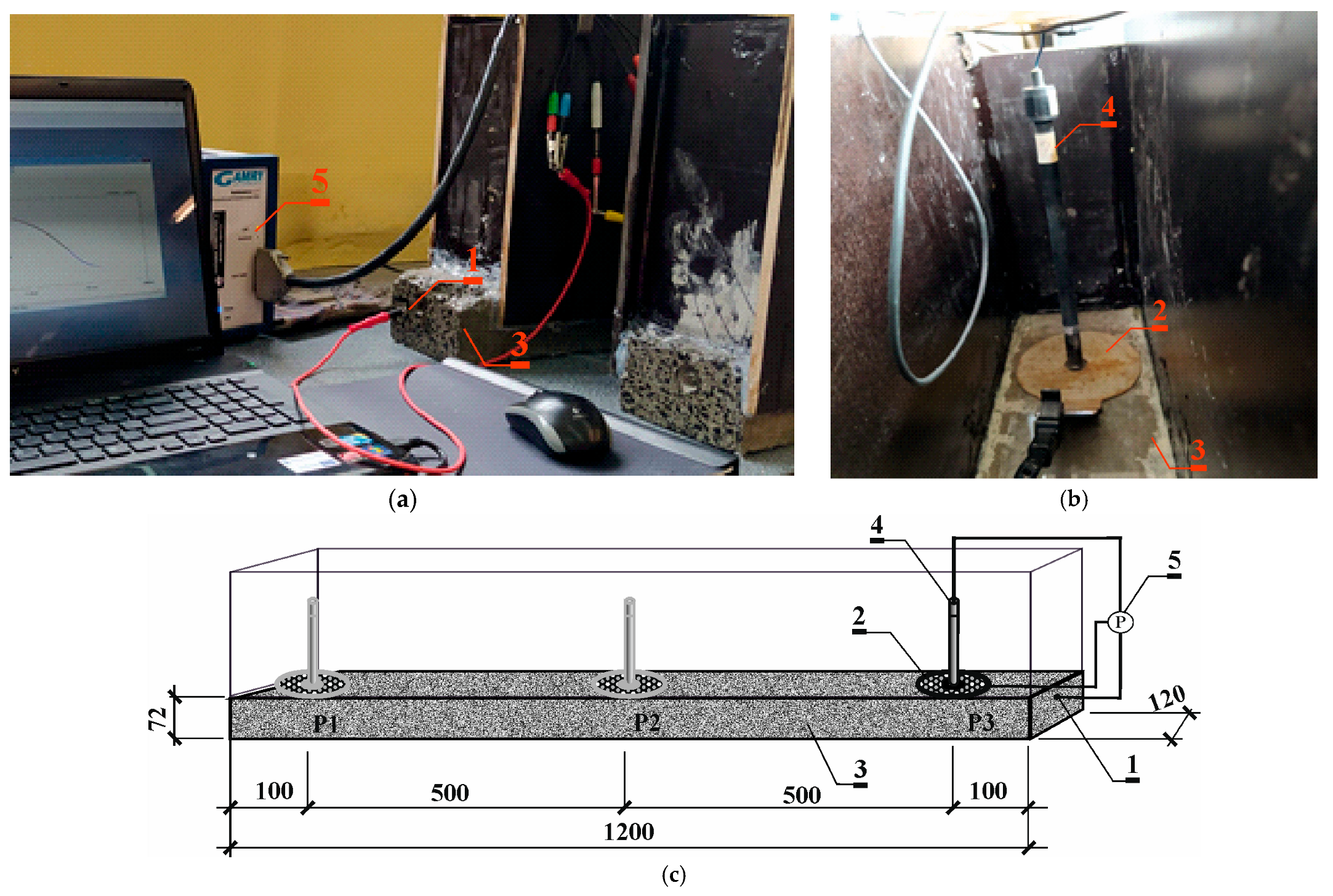
2.4. Testing the Effect of Temperature Increase on the Development of Corrosion Processes
Control of the Corrosion Process Under Different Environmental Conditions
3. Results and Discussion
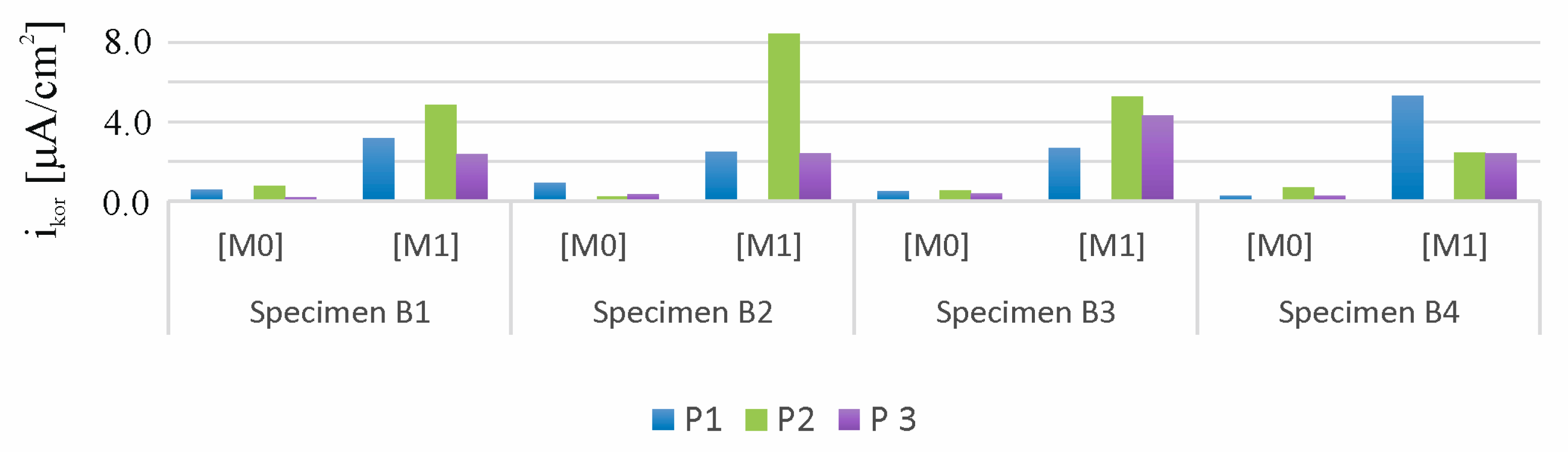
3.1. Results of the Accelerated Corrosion Process
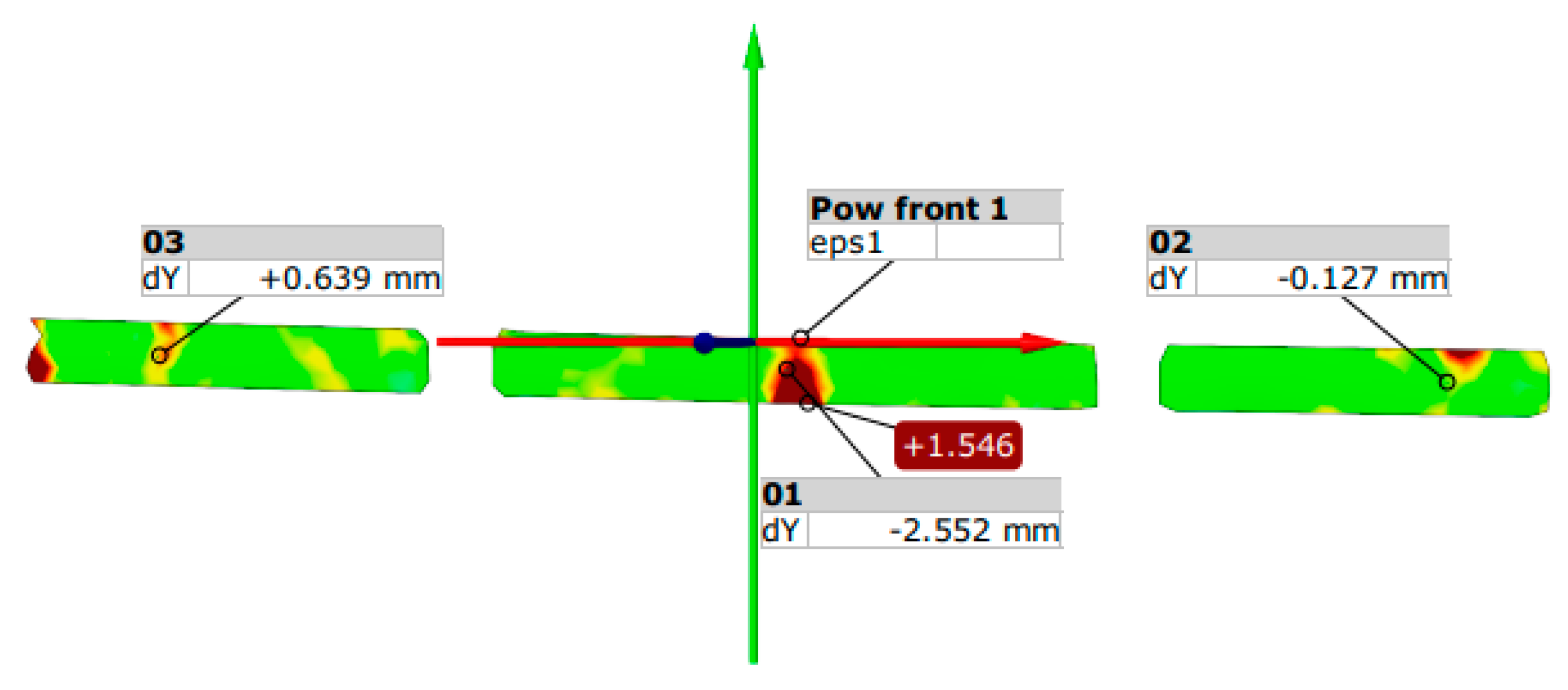
3.2. Results of Scratching Condition in Prestressed Concrete Beams Using Aramis Optical System
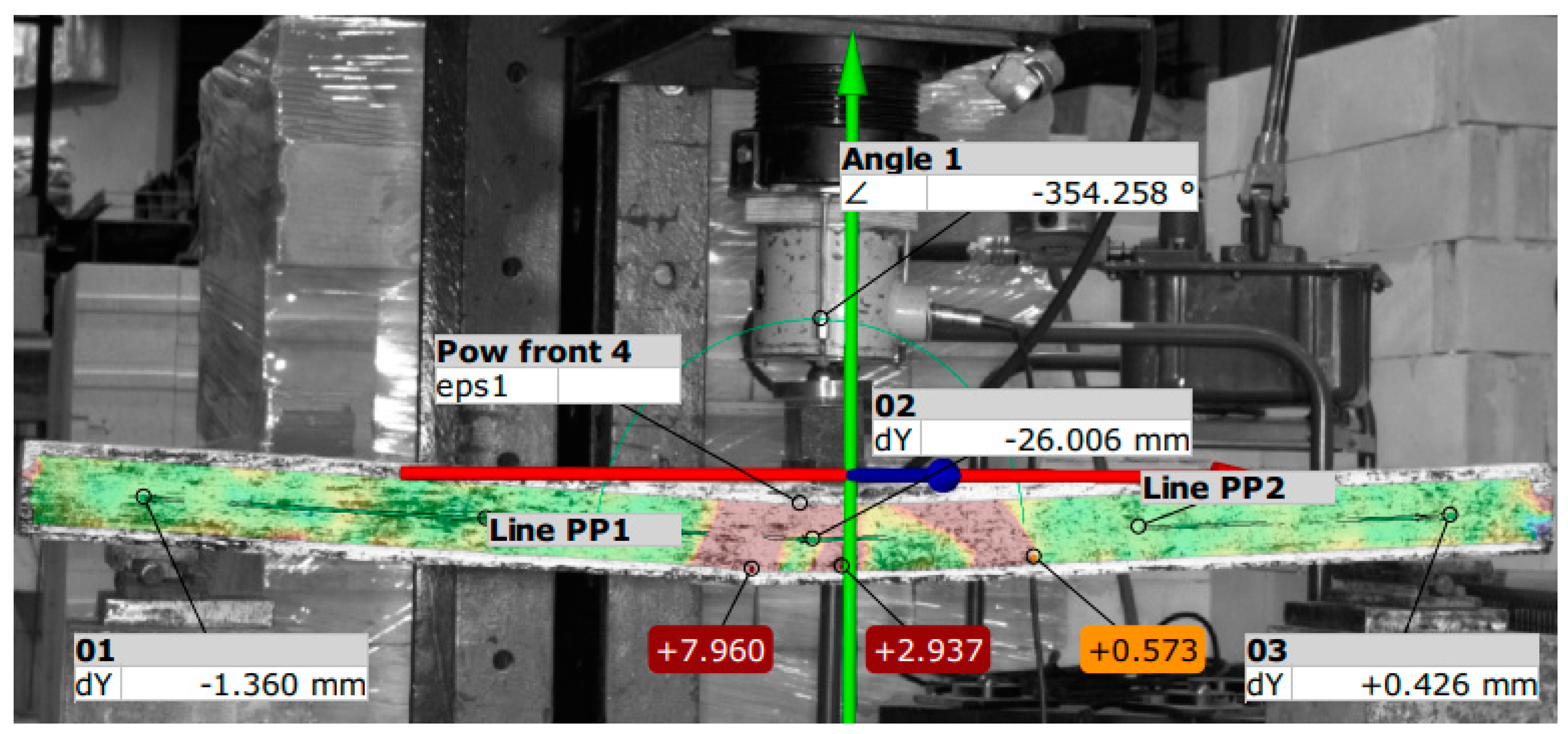
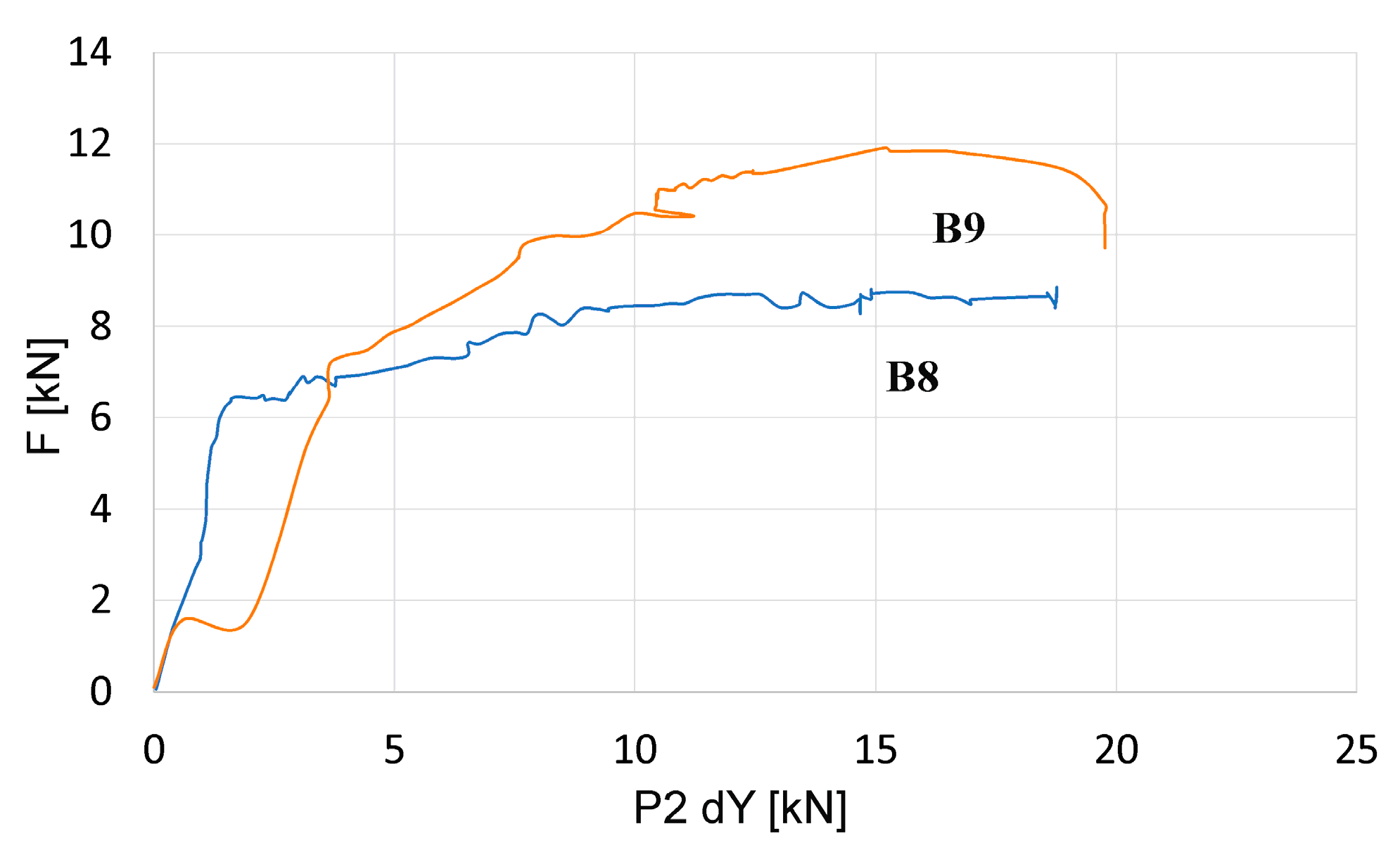
3.3. Results of Destructive Testing of Prestressed Beams Using the Aramis Optical System
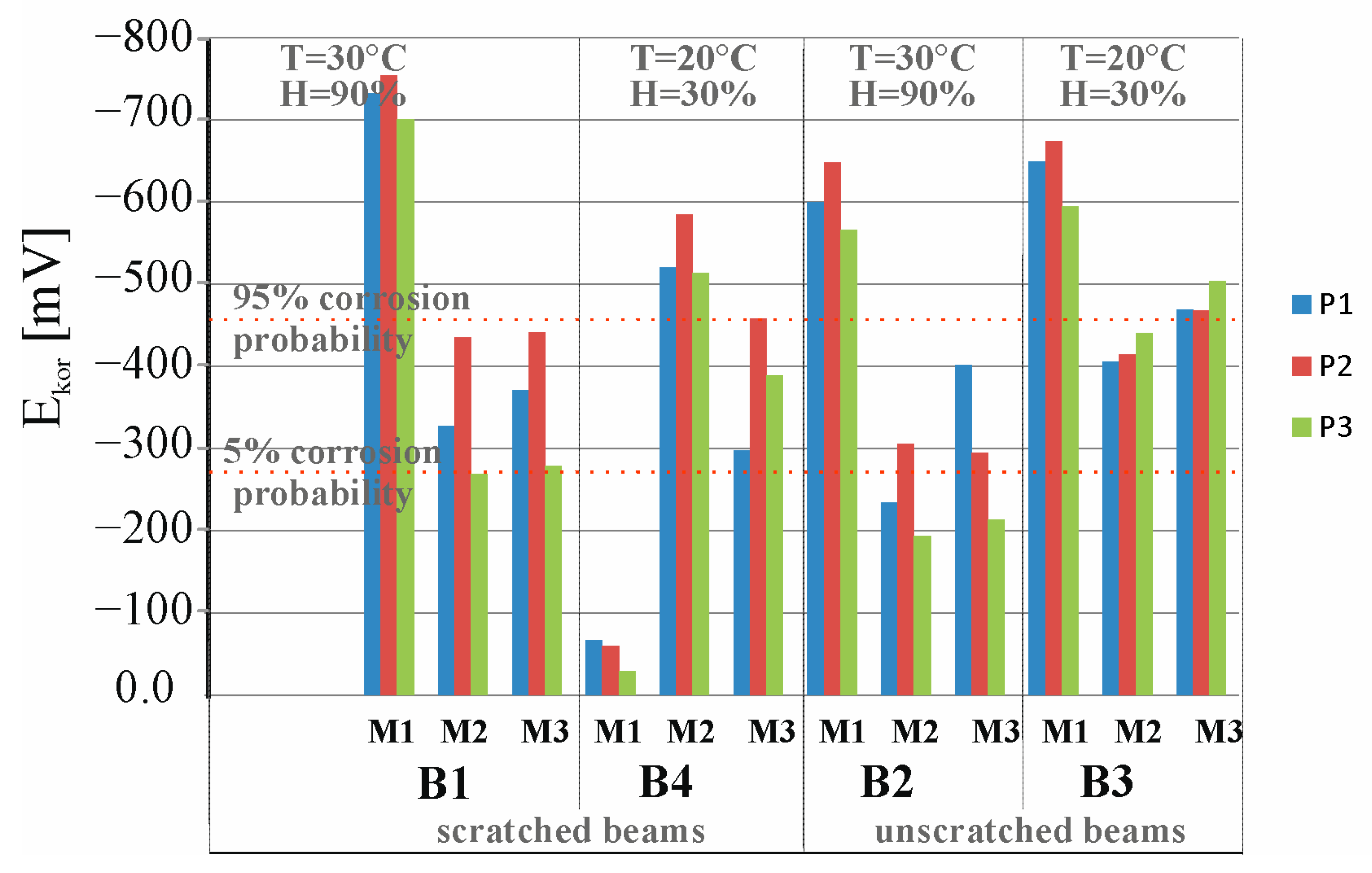
3.4. Results of Corrosion Measurement in the Climate Chamber
4. Conclusions
- The use of the chloride ion migration method proved to be an effective method for initiating and accelerating corrosion processes in all test items subjected to this process.
- Based on the strength tests, it can be concluded that in all elements the first scratches appeared in the middle of the span of the beams.
- A very important and so far overlooked issue is the effect of chloride ion content on the mechanical properties of concrete. The content of chloride ions in concrete can contribute to changes in the elastic and strength properties of concrete. A 30% decrease in deflection values and a 46% decrease in strain values in beams subjected to accelerated chloride migration before the test may indicate, on the one hand, the effect of a change in the adhesion of reinforcing strings due to corrosion and, on the other hand, a change in the mechanical properties of concrete.
- In order to accurately assess the load-bearing capacity of prestressed concrete beams, tests would have to be conducted on a larger number of elements.
- An increase in temperature is not the only condition implying an increase in the corrosion rate in both scratched and additionally loaded elements and unloaded elements. More important is the ease of access to oxygen, which, when the environment is very humid, is inhibited probably by filling the concrete pores with water.
- The conducted tests should be extended in order to observe the progress of corrosion processes over time, perhaps with a simultaneous change in the environmental conditions set in the climate chamber.
- It is also necessary to perform strength tests on the beams after the corrosion tests are completed in order to further observe the influence of corrosion processes on the values of deflection and deformation on prestressed concrete beams.
- It is also necessary to carry out more tests to confirm the preliminary test results obtained.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Measure No. | Time Days | Ecorr mV | ba mV | bc mV | Rp kΩ | RpA kΩcm2 | Icorr μA/cm2 | Vr mm/year |
|---|---|---|---|---|---|---|---|---|
| B1-M0-P1 | 0 | −32 | 216 | 203 | 3.10 | 85.87 | 0.53 | 0.006 |
| B1-M1-P1 | 30 | −732 | 118 | 172 | 0.35 | 9.70 | 3.13 | 0.036 |
| B2-M0-P1 | 0 | −34 | 2580 | 320 | 5.00 | 138.50 | 0.89 | 0.010 |
| B2-M1-P1 | 30 | −599 | 276 | 184 | 0.71 | 19.67 | 2.44 | 0.028 |
| B3-M0-P1 | 0 | −65.1 | 133 | 155 | 2.40 | 66.48 | 0.47 | 0.005 |
| B3-M1-P1 | 30 | −648 | 205 | 115 | 0.44 | 12.19 | 2.62 | 0.030 |
| B4-M0-P1 | 0 | −22 | 128 | 197 | 5.70 | 157.89 | 0.21 | 0.002 |
| B4-M1-P1 | 30 | −66 | 169 | 121 | 0.21 | 5.82 | 5.26 | 0.061 |
| B1-M0-P2 | 0 | −42 | 208 | 203 | 2.10 | 58.17 | 0.77 | 0.009 |
| B1-M1-P2 | 30 | −753 | 134 | 199 | 0.26 | 7.20 | 4.83 | 0.056 |
| B2-M0-P2 | 0 | −51 | 69 | 168 | 3.69 | 102.21 | 0.21 | 0.002 |
| B2-M1-P2 | 30 | −647 | 1000 | 170 | 0.27 | 7.48 | 8.44 | 0.098 |
| B3-M0-P2 | 0 | −75 | 95 | 141 | 1.72 | 47.64 | 0.52 | 0.006 |
| B3-M1-P2 | 30 | −673 | 169 | 121 | 0,21 | 5.82 | 5.26 | 0.061 |
| B4-M0-P2 | 0 | −44 | 228 | 215 | 2.60 | 72.02 | 0.67 | 0.008 |
| B4-M1-P2 | 30 | −59 | 124 | 270 | 0.55 | 15.24 | 2.42 | 0.028 |
| B1-M0-P3 | 0 | −19 | 713 | 47 | 4.80 | 132.96 | 0.14 | 0.002 |
| B1-M1-P3 | 30 | −700 | 52 | 127 | 0.25 | 6.93 | 2.31 | 0.027 |
| B2-M0-P3 | 0 | −20 | 216 | 216 | 5,30 | 146.81 | 0.32 | 0.025 |
| B2-M1-P3 | 30 | −565 | 107 | 132 | 0,39 | 10.80 | 2.38 | 0.028 |
| B3-M0-P3 | 0 | −66 | 95 | 130 | 2.54 | 70.36 | 0.34 | 0.004 |
| B3-M1-P3 | 30 | −594 | 112 | 159 | 0.24 | 6.65 | 4.29 | 0.050 |
| B4-M0-P3 | 0 | −20 | 209 | 230 | 7.00 | 193.90 | 0.25 | 0.003 |
| B4-M1-P3 | 30 | −29 | 152 | 211 | 0.58 | 16.07 | 2.39 | 0.028 |
| Element No. | F kN | 01 dY mm | 02 dY mm | 03 dY mm | eps1 % | Angle ° | Element No. | P kN | 01 dY mm | 02 dY mm | 03 dY mm | eps1 % | Angle ° |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 | 5.1 | −0.13 | −2.6 | −0.64 | +1.6 | - | B4 | 6.9 | −0.46 | −2.54 | −1.48 | +1.4 | 359.4 |
| B5 | 3.3 | −0.41 | −2.9 | −0.64 | +1.4 | - | B6 | 4.7 | −0.16 | −3.04 | −0.01 | +2.1 | 359.3 |
| B8 | 8.8 | −1.74 | −15.04 | +0.44 | +6.3 | 356.7 | B9 | 11.9 | −1.27 | −15.8 | −0.39 | +3.9 | 356.7 |
| Measure No. | Time Days | Ecorr mV | ba mV | bc mV | Rp kΩ | RpA kΩcm2 | Icorr μA/cm2 | Vr mm/year |
|---|---|---|---|---|---|---|---|---|
| Climatic chamber: temperature 30 °C; humidity 90% | ||||||||
| B1-M2-P1 | 30 | −327 | 76 | 61 | 0.42 | 11.63 | 1.26 | 0.015 |
| B1-M3-P1 | 60 | −371 | 389 | 339 | 1.26 | 34.90 | 2.25 | 0.026 |
| B1-M2-P2 | 30 | −435 | 82 | 100 | 0.76 | 21.05 | 0.93 | 0.011 |
| B1-M3-P2 | 60 | −441 | 61 | 60 | 2.50 | 69.25 | 0.19 | 0.002 |
| B1-M2-P3 | 30 | −269 | 191 | 429 | 0.82 | 22.71 | 2.53 | 0.029 |
| B1-M3-P3 | 60 | −279 | 1000 | 2690 | 4.65 | 128.81 | 2.46 | 0.029 |
| B2-M2-P1 | 30 | −233 | 132 | 114 | 4.97 | 137.67 | 0.19 | 0.043 |
| B2-M3-P1 | 60 | −401 | 463 | 362 | 1.79 | 49.58 | 1.78 | 0.007 |
| B2-M2-P2 | 30 | −305 | 216 | 248 | 1.07 | 29.64 | 1.69 | 0.003 |
| B2-M3-P2 | 60 | −294 | 68 | 131 | 2.60 | 72.02 | 0.27 | 0.000 |
| B2-M2-P3 | 30 | −193 | 97 | 213 | 5.92 | 163.98 | 0.18 | 0.002 |
| B2-M3-P3 | 60 | −213 | 149 | 60 | 6.53 | 180.88 | 0.10 | 0.001 |
| B5-M1-P1 | 0 | −206 | 197 | 106 | 6.96 | 192.79 | 0.16 | 0.002 |
| B5-M2-P1 | 30 | −218 | 66 | 42 | 1.70 | 47.09 | 0.24 | 0.003 |
| B5-M3-P1 | 60 | −129 | 77 | 54 | 4.10 | 113.57 | 0.12 | 0.001 |
| B5-M1-P2 | 0 | −205 | 195 | 105 | 6.25 | 173.13 | 0.17 | 0.002 |
| B5-M2-P2 | 30 | −206 | 26 | 70 | 3.10 | 85.87 | 0.10 | 0.001 |
| B5-M3-P2 | 60 | −135 | 88 | 46 | 3.20 | 88.64 | 0.15 | 0.002 |
| B5-M1-P3 | 0 | −230 | 183 | 103 | 11.57 | 320.49 | 0.09 | 0.001 |
| B5-M2-P3 | 30 | −185 | 73 | 34 | 3.20 | 88.64 | 0.11 | 0.001 |
| B5-M3-P3 | 60 | −178 | 113 | 74 | 3.80 | 105.26 | 0.18 | 0.002 |
| B7-M1-P1 | 0 | −197 | 195 | 103 | 6.77 | 187.53 | 0.16 | 0.002 |
| B7-M2-P1 | 30 | −144 | 49 | 54 | 3.70 | 102.49 | 0.11 | 0.001 |
| B7-M3-P1 | 60 | −139 | 36 | 73 | 8.20 | 227.14 | 0.05 | 0.001 |
| B7-M1-P2 | 0 | −198 | 180 | 116 | 6.65 | 184,21 | 0.17 | 0.002 |
| B7-M2-P2 | 30 | −182 | 90 | 45 | 3.46 | 95.84 | 0.14 | 0.002 |
| B7-M3-P2 | 60 | −112 | 53 | 50 | 3.00 | 83.10 | 0.13 | 0.002 |
| B7-M1-P3 | 0 | −192 | 189 | 110 | 7.45 | 206.37 | 0.15 | 0.002 |
| B7-M2-P3 | 30 | −170 | 90 | 54 | 2.40 | 66.48 | 0.22 | 0.003 |
| B7-M3-P3 | 60 | −173 | 80 | 55 | 1.90 | 52.63 | 0.27 | 0.003 |
| Measure No. | Time Days | Ecorr mV | ba mV | bc mV | Rp kΩ | RpA kΩcm2 | Icorr μA/cm2 | Vr mm/year |
|---|---|---|---|---|---|---|---|---|
| Laboratory: temperature 20 °C; humidity 30% | ||||||||
| B3-M2-P1 | 30 | −405 | 9950 | 450 | 3.10 | 85.87 | 2.18 | 0.025 |
| B3-M3-P1 | 60 | −469 | 1000 | 358 | 0.67 | 18.56 | 6.17 | 0.072 |
| B3-M2-P2 | 30 | −414 | 1508 | 485 | 5.80 | 160.66 | 0.99 | 0.012 |
| B3-M3-P2 | 60 | −468 | 4438 | 442 | 0.65 | 18.01 | 9.69 | 0.112 |
| B3-M2-P3 | 30 | −440 | 333 | 118 | 1.27 | 35.18 | 1.08 | 0.012 |
| B3-M3-P3 | 60 | −503 | 1000 | 504 | 1.10 | 30.47 | 4.78 | 0.055 |
| B4-M2-P1 | 30 | −520 | 1000 | 232 | 0.45 | 12.47 | 6.56 | 0.076 |
| B4-M3-P1 | 60 | −297 | 255 | 35 | 0.63 | 17.45 | 0.77 | 0.009 |
| B4-M2-P2 | 30 | −584 | 1000 | 307 | 0.75 | 20.78 | 4.91 | 0.057 |
| B4-M3-P2 | 60 | −458 | 3483 | 226 | 0.69 | 19.11 | 4.82 | 0.056 |
| B4-M2-P3 | 30 | −513 | 288 | 327 | 0.43 | 11.91 | 5.58 | 0.065 |
| B4-M3-P3 | 60 | −388 | 1000 | 375 | 1.20 | 33.24 | 3.56 | 0.041 |
| B6-M2-P1 | 0 | −206 | 45 | 44 | 4.30 | 119.11 | 0.08 | 0.001 |
| B6-M3-P1 | 30 | −224 | 189 | 40 | 8.50 | 235.45 | 0.06 | 0.001 |
| B6-M4-P1 | 60 | −235 | 95 | 77 | 2.50 | 69.25 | 0.27 | 0.003 |
| B6-M2-P2 | 0 | −177 | 62 | 50 | 4.51 | 124.93 | 0.10 | 0.001 |
| B6-M3-P2 | 30 | −107 | 266 | 32 | 6.23 | 172.57 | 0.07 | 0.001 |
| B6-M4-P2 | 60 | −197 | 149 | 79 | 3.40 | 94.18 | 0.24 | 0.003 |
| B6-M1-P3 | 0 | −141 | 11 | 49 | 17.92 | 496.38 | 0.01 | 0.000 |
| B6-M2-P3 | 30 | −11 | 161 | 42 | 9.50 | 263.15 | 0.05 | 0.001 |
| B6-M3-P3 | 60 | −228 | 157 | 96 | 2.40 | 66.48 | 0.39 | 0.005 |
| B10-M1-P1 | 0 | −262 | 319 | 136 | 3.60 | 99.72 | 0.42 | 0.005 |
| B10-M2-P1 | 30 | −285 | 35 | 36 | 6.10 | 168.97 | 0.05 | 0.001 |
| B10-M3-P1 | 60 | −195 | 93 | 104 | 4.92 | 136.28 | 0.16 | 0.002 |
| B10-M1-P2 | 0 | −217 | 189 | 76 | 5.30 | 146.81 | 0.16 | 0.002 |
| B10-M2-P2 | 30 | −157 | 40 | 40 | 4.20 | 116.34 | 0.07 | 0.001 |
| B10-M3-P2 | 60 | −179 | 154 | 103 | 2.87 | 79.50 | 0.34 | 0.004 |
| B10-M1-P3 | 0 | −219 | 101 | 82 | 4.50 | 124.65 | 0.16 | 0.002 |
| B10-M2-P3 | 30 | −168 | 40 | 40 | 4.93 | 136.56 | 0.06 | 0.001 |
| B10-M3-P3 | 60 | −212 | 33 | 50 | 3.50 | 96.95 | 0.09 | 0.001 |
References
- Ajdukiewicz Andrzej i Mames, J. Konstrukcje z Betonu Sprężonego; Stowarzyszenie Producentów Cementu: Kraków, Poland, 2003. [Google Scholar]
- Niu, D.; Miao, Y. Experimental study on fatigue performance of corroded highway bridges based on vehicle loading. Tumu Gongcheng Xuebao/China Civ. Eng. J. 2018, 51, 1–10. [Google Scholar]
- Lu, Z.H.; Wu, S.Y.; Tang, Z.; Zhao, Y.G.; Li, W. Effect of chloride-induced corrosion on the bond behaviors between steel strands and concrete. Mater. Struct. Constr. 2021, 54, 129. [Google Scholar] [CrossRef]
- Yu, Q.-Q.; Gu, X.-L.; Zeng, Y.-H.; Zhang, W.-P. Flexural behavior of Corrosion-Damaged prestressed concrete beams. Eng. Struct. 2022, 272, 114985. [Google Scholar] [CrossRef]
- Madaj, A.; Mossor, K. Korozja stali sprężającej w konstrukcjach kablobetonowych. Przyczyny, skutki, zapobieganie. Arch. Inst. Inżynierii Lądowej 2018, 26, 93–108. [Google Scholar] [CrossRef]
- Sigvaldsen, M.; Ersdal, G.; Markeset, G.; Samarakoon, S.; Langeteig, M. Capacity of concrete structures with corroded reinforcement and prestressing tendons. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1201, 012052. [Google Scholar] [CrossRef]
- Valiente, A. Stress corrosion failure of large diameter pressure pipelines of prestressed concrete. Eng. Fail. Anal. 2001, 8, 245–261. [Google Scholar] [CrossRef]
- Cherry, B.W.; Price, S.M. Pitting, crevice and stress corrosion cracking studies of cold drawn eutectoid steels. Corros. Sci. 1980, 20, 1163–1183. [Google Scholar] [CrossRef]
- Li, F.M.; Yuan, Y.S.; Jiang, J.H.; Wang, B. Stress corrosion cracking susceptibility of steel strands in concrete contaminated with salt. In Proceedings of the 1st International Conference of Microstructure Related Durability of Cementitious Composites, Nanjing, China, 13–15 October 2008; pp. 463–472. [Google Scholar]
- Xu, F.; Chen, Y.; Zheng, X.; Ma, R.; Tian, H. Experimental study on corrosion and mechanical behavior of Main Cable Wires Considering the effect of strain. Materials 2019, 12, 753. [Google Scholar] [CrossRef]
- Hansson, C.M.; Poursaee, A.; Laurent, A. Macrocell and microcell corrosion of steel in ordinary Portland cement and high performance concretes. Cem. Concr. Res. 2006, 36, 2098–2102. [Google Scholar] [CrossRef]
- Ahmad, S. Reinforcement corrosion in concrete structures, its monitoring and service life prediction—A review. Cem. Concr. Compos. 2003, 25, 459–471. [Google Scholar] [CrossRef]
- Li, F.; Yuan, Y.; Li, C.Q. Corrosion propagation of prestressing steel strands in concrete subject to chloride attack. Constr. Build. Mater. 2011, 25, 3878–3885. [Google Scholar] [CrossRef]
- Zhao, Y.; Dou, Y. Study on fatigue damage law of bonding between steel strand and concrete under corrosive environment of chloride. Chem. Eng. Trans. 2017, 62, 1033–1038. [Google Scholar] [CrossRef]
- Szweda, Z.; Jasinski, R. Static Analysis of Prestressed Floor Slabs HC500 with Changes in Tendon Adhesion to Concrete Induced by Penetration of Chloride Ions. IOP Conf. Ser. Mater. Sci. Eng. 2019, 471, 052035. [Google Scholar] [CrossRef]
- Kioumarsi, M.; Benenato, A.; Ferracuti, B.; Imperatore, S. Residual flexural capacity of corroded prestressed reinforced concrete beams. Metals 2021, 11, 442. [Google Scholar] [CrossRef]
- Belletti, B.; Ravasini, S.; Sirico, A.; Montero, J.S.; Torres, J.; Rebolledo, N.; Saura, P. Experimental investigation on shear behaviour of corroded and repaired prestressed concrete beams. In Bridge Maintenance, Safety, Management, Digitalization and Sustainability, Proceedings of the 12th International Conference on Bridge Maintenance, Safety and Management (IABMAS 2024), Copenhagen, Denmark, 24–28 June 2024; Taylor & Francis: Abingdon, UK, 2024; pp. 525–532. [Google Scholar] [CrossRef]
- Rinaldi, Z.; Imperatore, S.; Valente, C. Experimental evaluation of the flexural behavior of corroded P/C beams. Constr. Build. Mater. 2010, 24, 2267–2278. [Google Scholar] [CrossRef]
- El Menoufy, A.; Soudki, K. Flexural behavior of corroded pretensioned girders repaired with CFRP sheets. PCI J. 2014, 59, 129–143. [Google Scholar] [CrossRef]
- ElBatanouny, M.K.; Nanni, A.; Ziehl, P.H.; Matta, F. Condition assessment of prestressed concrete beams using cyclic and monotonic load tests. ACI Struct. J. 2015, 112, 81–90. [Google Scholar] [CrossRef]
- Enevoldsen, J.N.; Hansson, C.M.; Hope, B.B. The influence of internal relative humidity on the rate of corrosion of steel embedded in concrete and mortar. Cem. Concr. Res. 1994, 24, 1373–1382. [Google Scholar] [CrossRef]
- Pour-Ghaz, M.; Isgor, O.B.; Ghods, P. The effect of temperature on the corrosion of steel in concrete. Part 1: Simulated polarization resistance tests and model development. Corros. Sci. 2009, 51, 415–425. [Google Scholar] [CrossRef]
- Jaśniok, T.; Jaśniok, M. Influence of rapid changes of moisture content in concrete and temperature on corrosion rate of reinforcing steel. Procedia Eng. 2015, 108, 316–323. [Google Scholar] [CrossRef]
- López, W.; González, J.A.; Andrade, C. Influence of temperature on the service life of rebars. Cem. Concr. Res. 1993, 23, 1130–1140. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, C.; Sarría, J. Influence of relative humidity and temperature on-site corrosion rates. Mater. Constr. 1998, 1998, 5–17. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, C.; Sarría, J. Corrosion rate evolution in concrete structures exposed to the atmosphere. Cem. Concr. Compos. 2002, 24, 55–64. [Google Scholar] [CrossRef]
- Bouteiller, V.; Cherrier, J.F.; L’Hostis, V.; Rebolledo, N.; Andrade, C.; Marie-Victoire, E. Influence of humidity and temperature on the corrosion of reinforced concrete prisms. Eur. J. Environ. Civ. Eng. 2012, 16, 471–480. [Google Scholar] [CrossRef]
- Yang, R.; Yang, Y.; Zhang, X.; Wang, X. Experimental Study on Secondary Anchorage Bond Performance of Residual Stress after Corrosion Fracture at Ends of Prestressed Steel Strands. Materials 2023, 16, 7441. [Google Scholar] [CrossRef]
- Szweda, Z.; Gołaszewski, J.; Ghosh, P.; Lehner, P.; Konečný, P. Comparison of Standardized Methods for Determining the Diffusion Coefficient of Chloride in Concrete with Thermodynamic Model of Migration. Materials 2023, 16, 637. [Google Scholar] [CrossRef]
- Castellote, M.; Andrade, C.; Alonso, C. Chloride transference numbers in steady-state migration tests. Mag. Concr. Res. 2000, 52, 93–100. [Google Scholar] [CrossRef]
- Szweda, Z.; Mazurkiewicz, J.; Konečný, P.; Ponikiewski, T. Effect of Imperial Smelting Process Slag Addition in Self Compacting Concrete Concrete on the Efficiency of Electrochemical Chloride Extraction. Materials 2023, 16, 5159. [Google Scholar] [CrossRef]
- Moshtaghi, M.; Eškinja, M.; Mori, G.; Griesser, T.; Safyari, M.; Cole, I. The effect of HPAM polymer for enhanced oil recovery on corrosion behaviour of a carbon steel and interaction with the inhibitor under simulated brine conditions. Corros. Sci. 2023, 217, 111118. [Google Scholar] [CrossRef]
- Ajdukiewicz, C.; Gajewski, M.; Mossakowski, P. Zastosowanie systemu optycznej korelacji obrazu Aramis do identyfikacji rys w elementach betonowych. In Proceedings of the TRANSCOMP—XIV International Conference Computer Systems Aided Science, Industry and Transport, Zakopane, Polska, 6–9 December 2011; pp. 1–8. [Google Scholar]
- Szweda, Z.; Kuziak, J.; Sozańska-Jędrasik, L.; Czachura, D. Analysis of the Effect of Protective Properties of Concretes with Similar Composition on the Corrosion Rate of Reinforcing Steel Induced by Chloride Ions. Materials 2023, 16, 3889. [Google Scholar] [CrossRef]
- Scully, J.R. Polarization Resistance Method for Determination of Instantaneous Corrosion Rates. Corrosion 2000, 56, 199–218. [Google Scholar] [CrossRef]
- Raczkiewicz, W. Use of polypropylene fi bres to increase the resistance of reinforcement to chloride corrosion in concretes. Sci. Eng. Compos. Mater. 2021, 22, 555–567. [Google Scholar] [CrossRef]
- Raczkiewicz, W.; Wójcicki, A. Temperature impact on the assessment of reinforcement corrosion risk in concrete by galvanostatic pulse method. Appl. Sci. 2020, 10, 1089. [Google Scholar] [CrossRef]
- Szweda, Z.; Skórkowski, A.; Konečný, P. The Influence of Corrosion Processes on the Degradation of Concrete Cover. Materials 2024, 17, 1398. [Google Scholar] [CrossRef] [PubMed]
- Szweda, Z. The influence of chloride ions content on the mechanical properties of concrete. Struct. Environ. 2024, 16, 158–165. [Google Scholar] [CrossRef]
- Szweda, Z.; Krak, M.; Czerniak, S.; Skórkowski, A.; Małek, J. Testing the Corrosion Rate of Prestressed Beams Under Variable Temperature and Humidity Conditions. Available online: https://zenodo.org/records/14840580 (accessed on 9 February 2025).
- Cheng, L.; Maruyama, I. A Prediction Method for the Corrosion Rate of Steel Rebar in Carbonated Mortar under Variable Environmental Conditions. J. Adv. Concr. Technol. 2023, 21, 611–630. [Google Scholar] [CrossRef]
- Alhozaimy, A.; Hussain, R.R.; Al-Zaid, R.; Al-Negheimish, A. Coupled effect of ambient high relative humidity and varying temperature marine environment on corrosion of reinforced concrete. Constr. Build. Mater. 2012, 28, 670–679. [Google Scholar] [CrossRef]








| Compressive Strength [MPa] | Density [kg/m3] | Porosity [%] |
|---|---|---|
| 58.3 | 2359 | 12 |
| Constituent % mass | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | Eq. Na2O | SO3 | Cl |
| 19.38 | 4.57 | 3.59 | 63.78 | 1.38 | 0.58 | 0.21 | 0.59 | 3.26 | 0.069 |
| Sand (0–2) * mm [kg/m3] | Gravel (2–8) * mm [kg/m3] | Type of Cement | Cement [kg/m3] | w/c |
|---|---|---|---|---|
| 800 | 800 | CEM I 42.5 R ** | 260 | 0.3 |
| Constituent % mass | C | Si | Mn | Cr | P | S |
| 0.7–0.9 | 0.15–0.30 | 0.60–0.90 | 0.30 | 0.035 | 0.035 |
| Beam Number | B1 | B2 | B3 | B4 | B5 | B6 | B7 | B8 | B9 | B10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Chloride migration | + | + | + | + | − | − | − | − | − | − |
| Scratch condition | + | − | − | + | + | + | − | − | − | − |
| Placement in the climate chamber | + | + | − | − | + | − | + | − | − | − |
| Destruction | − | − | − | − | − | − | − | + | + | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szweda, Z.; Krak, M.; Czarniak, S.; Skórkowski, A.; Małek, J.; Sikorski, J.; Trojan, J.; Konečný, P.; Vacek, M.; Marek, J. Testing the Corrosion Rate of Prestressed Concrete Beams Under Variable Temperature and Humidity Conditions. Materials 2025, 18, 1553. https://doi.org/10.3390/ma18071553
Szweda Z, Krak M, Czarniak S, Skórkowski A, Małek J, Sikorski J, Trojan J, Konečný P, Vacek M, Marek J. Testing the Corrosion Rate of Prestressed Concrete Beams Under Variable Temperature and Humidity Conditions. Materials. 2025; 18(7):1553. https://doi.org/10.3390/ma18071553
Chicago/Turabian StyleSzweda, Zofia, Michał Krak, Szymon Czarniak, Artur Skórkowski, Jacek Małek, Jakub Sikorski, Jakub Trojan, Petr Konečný, Miroslav Vacek, and Jakub Marek. 2025. "Testing the Corrosion Rate of Prestressed Concrete Beams Under Variable Temperature and Humidity Conditions" Materials 18, no. 7: 1553. https://doi.org/10.3390/ma18071553
APA StyleSzweda, Z., Krak, M., Czarniak, S., Skórkowski, A., Małek, J., Sikorski, J., Trojan, J., Konečný, P., Vacek, M., & Marek, J. (2025). Testing the Corrosion Rate of Prestressed Concrete Beams Under Variable Temperature and Humidity Conditions. Materials, 18(7), 1553. https://doi.org/10.3390/ma18071553







