Smartphone-Assisted Plasmonic Nanosensor for Visual and Specific Sensing of Toxic Cyanide Ions by β−Cyclodextrin Templated Gold-Rich/Silver Bimetallic Alloy Nanoparticles
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials and Instruments
2.2. Preparation of β-CD Functionalized Ag/Au-Rich Alloy NPs
2.3. Sensitive and Specific Detection Protocol of CN−
2.4. Real Sample Analysis
3. Results and Discussion
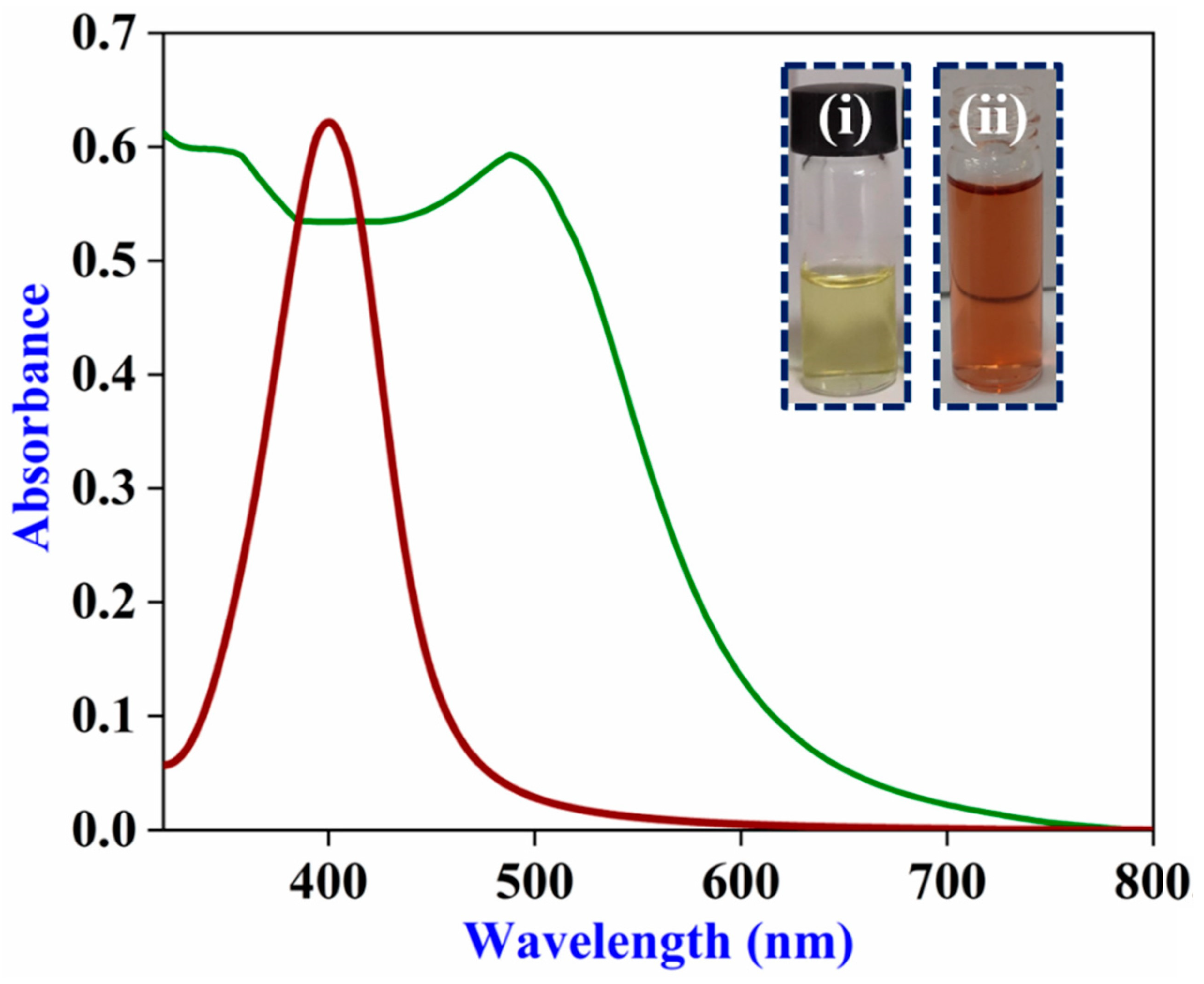
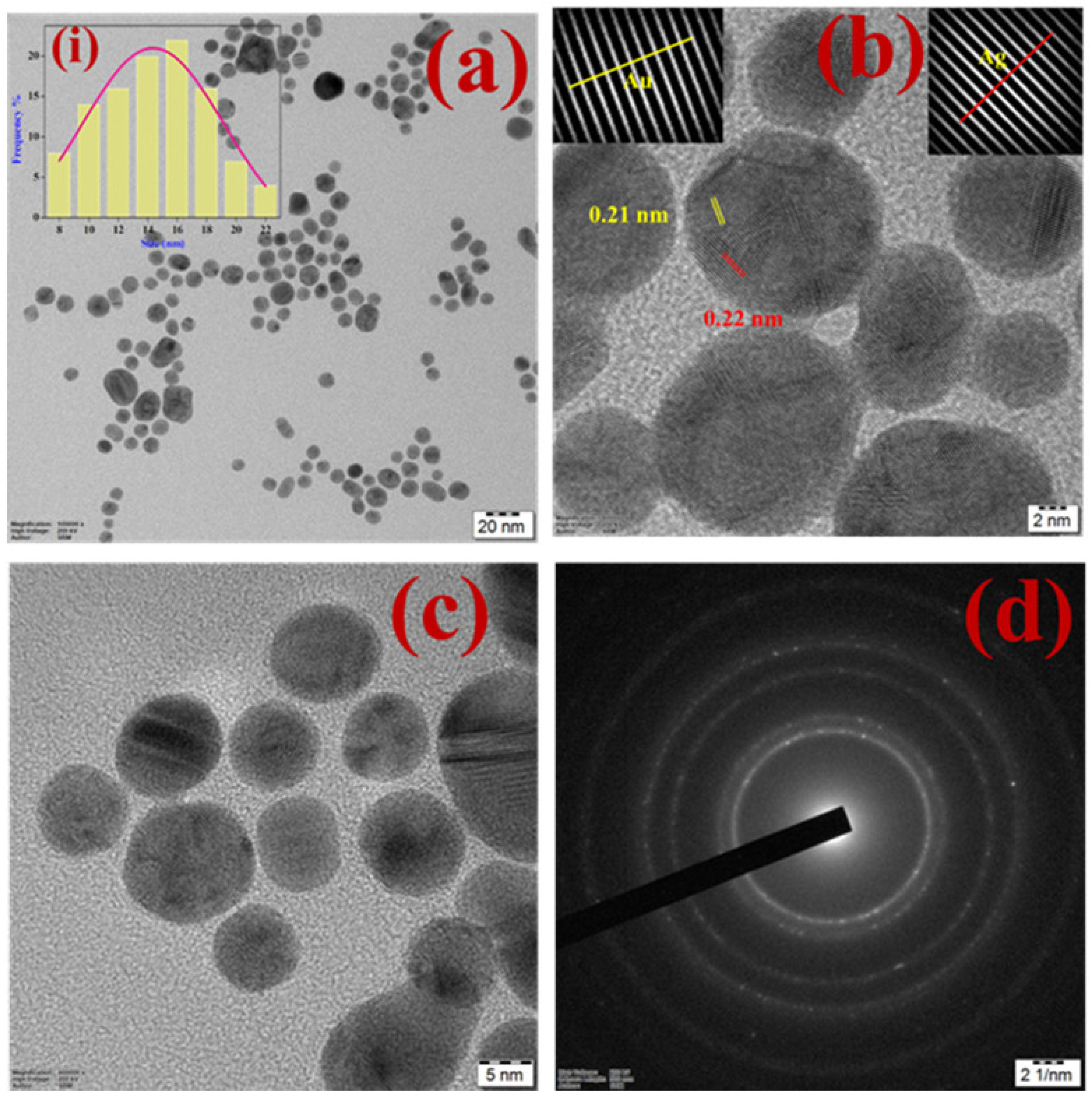
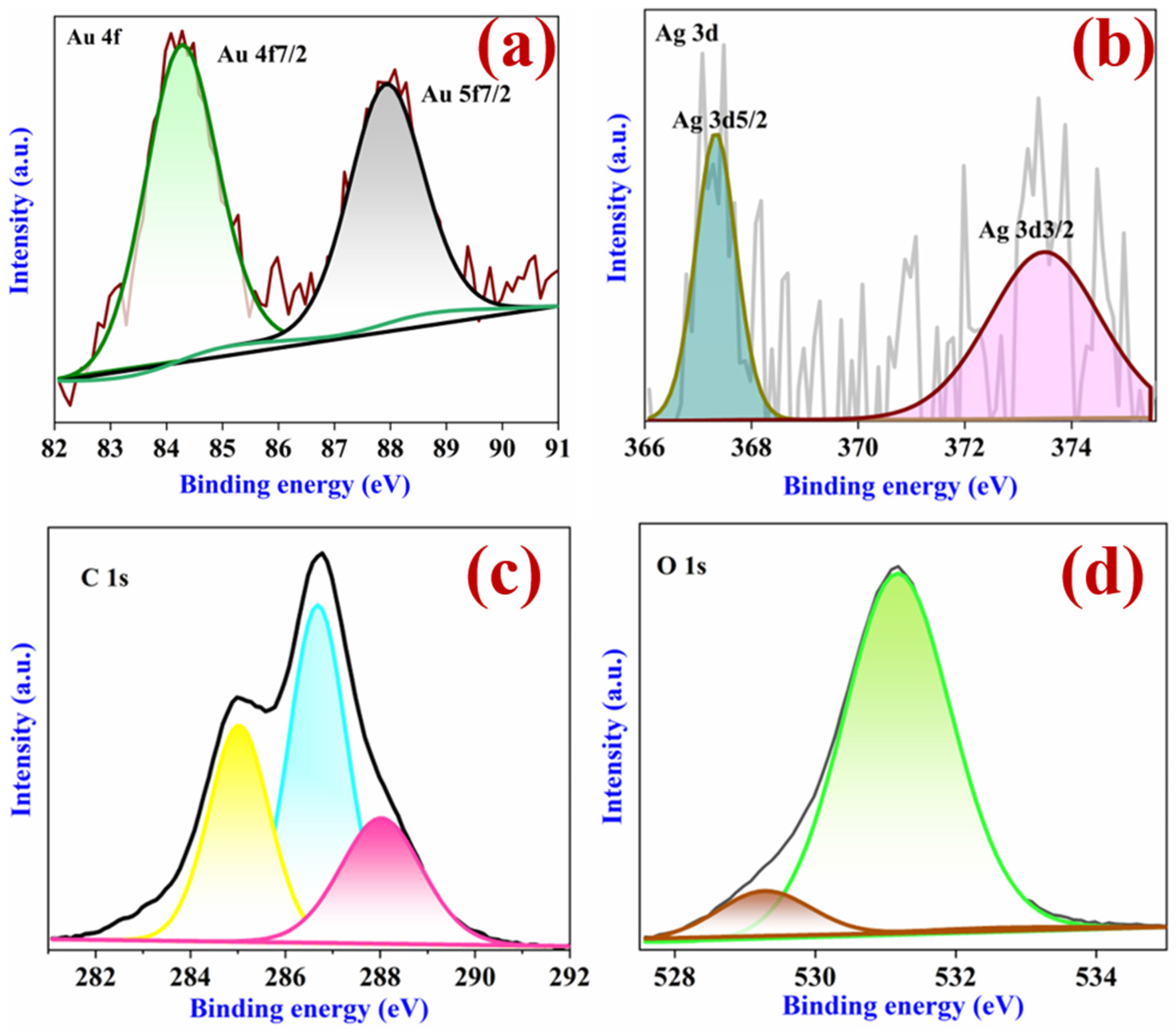
3.1. Structural Characterization of β-CD-Ag/Au-Rich Alloy NPs
3.2. Optimization of the Present Work
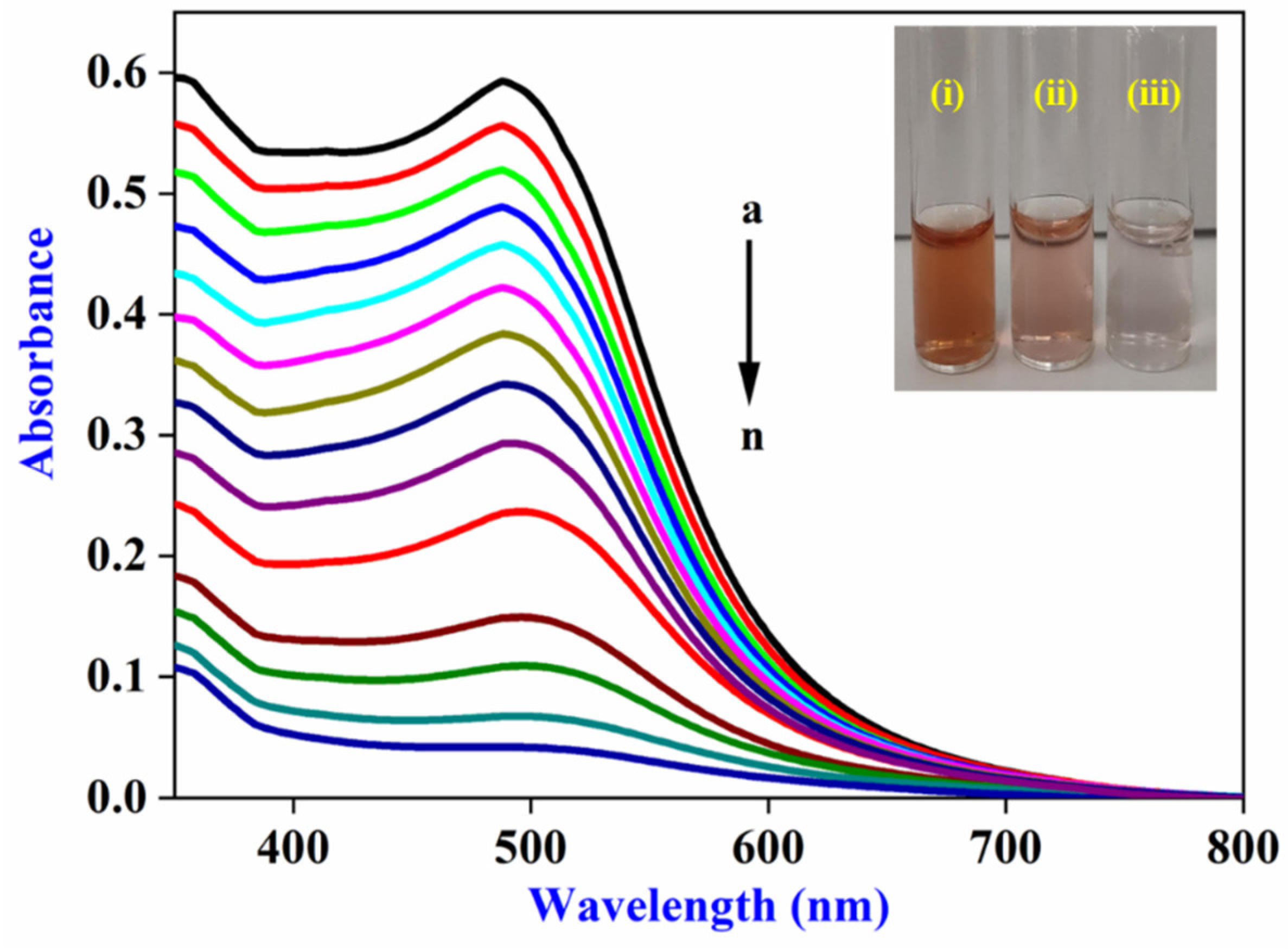
3.3. Spectrophotometric and Colorimetric Detection of CN−
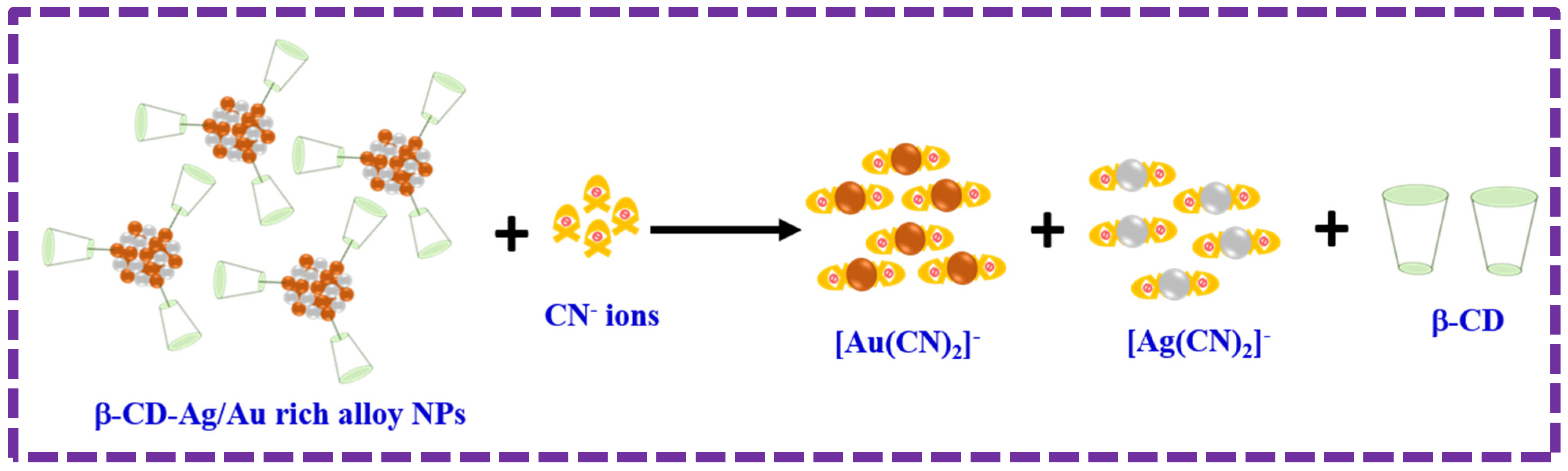
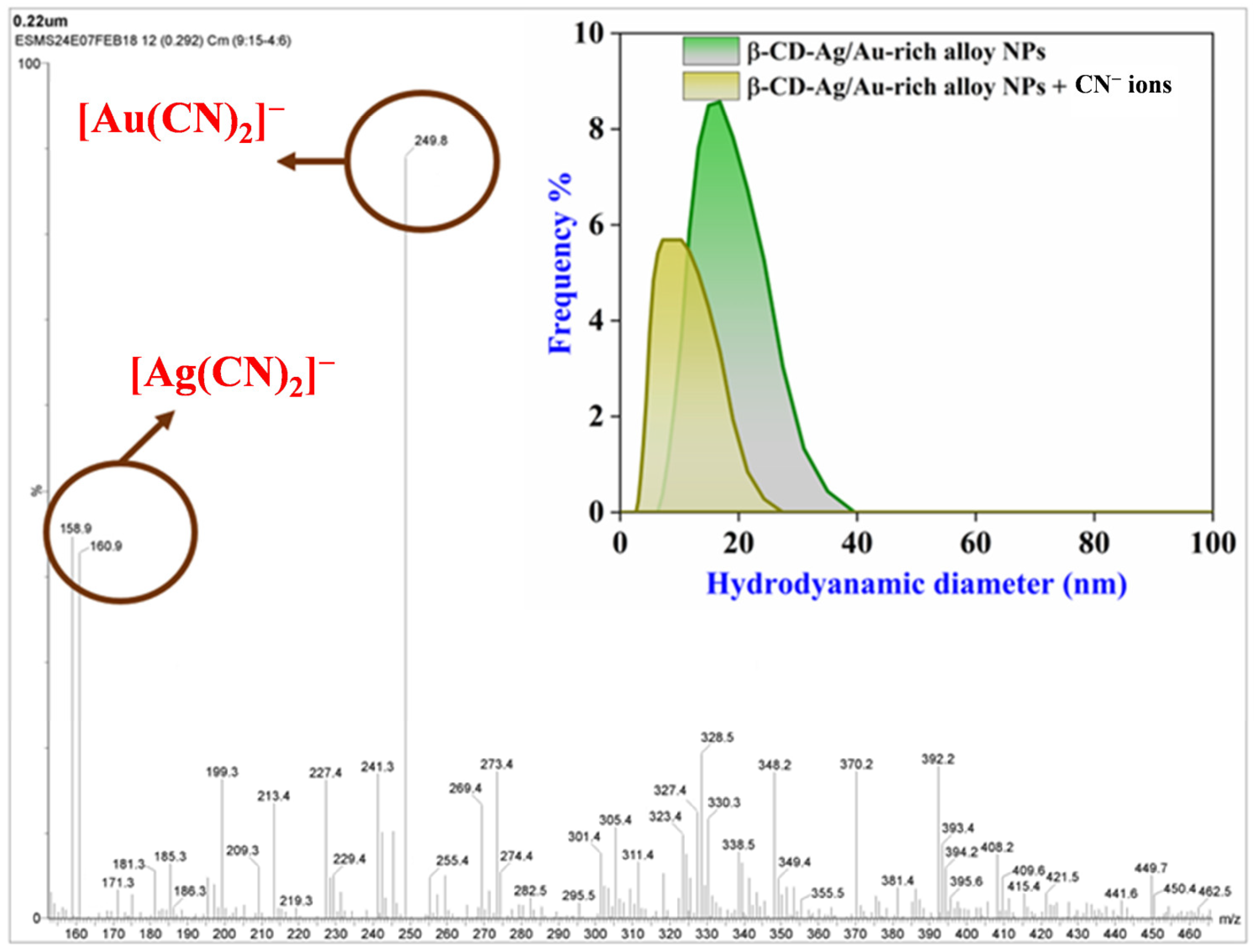
3.4. Sensing Mechanism
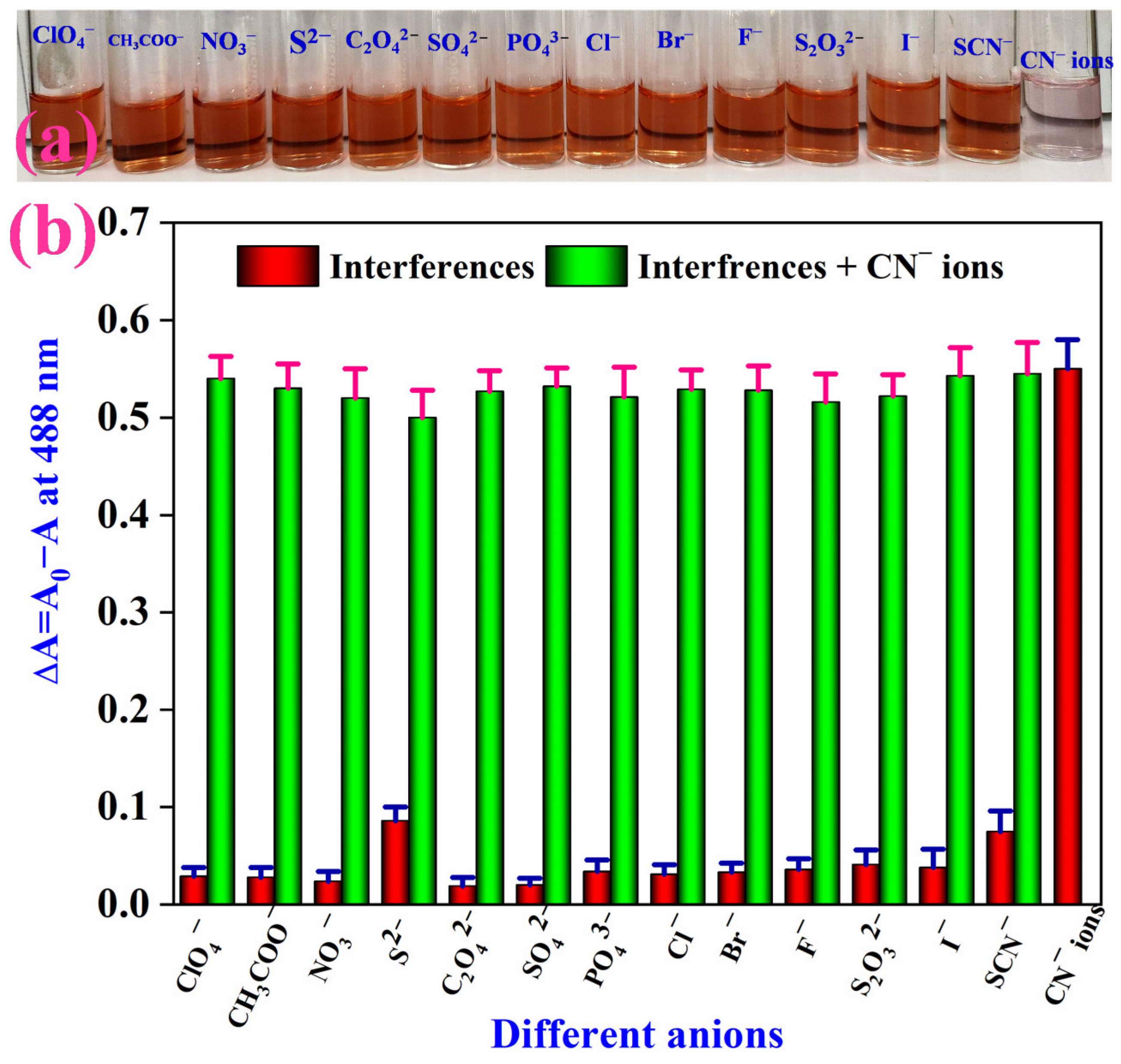
3.5. Selectivity
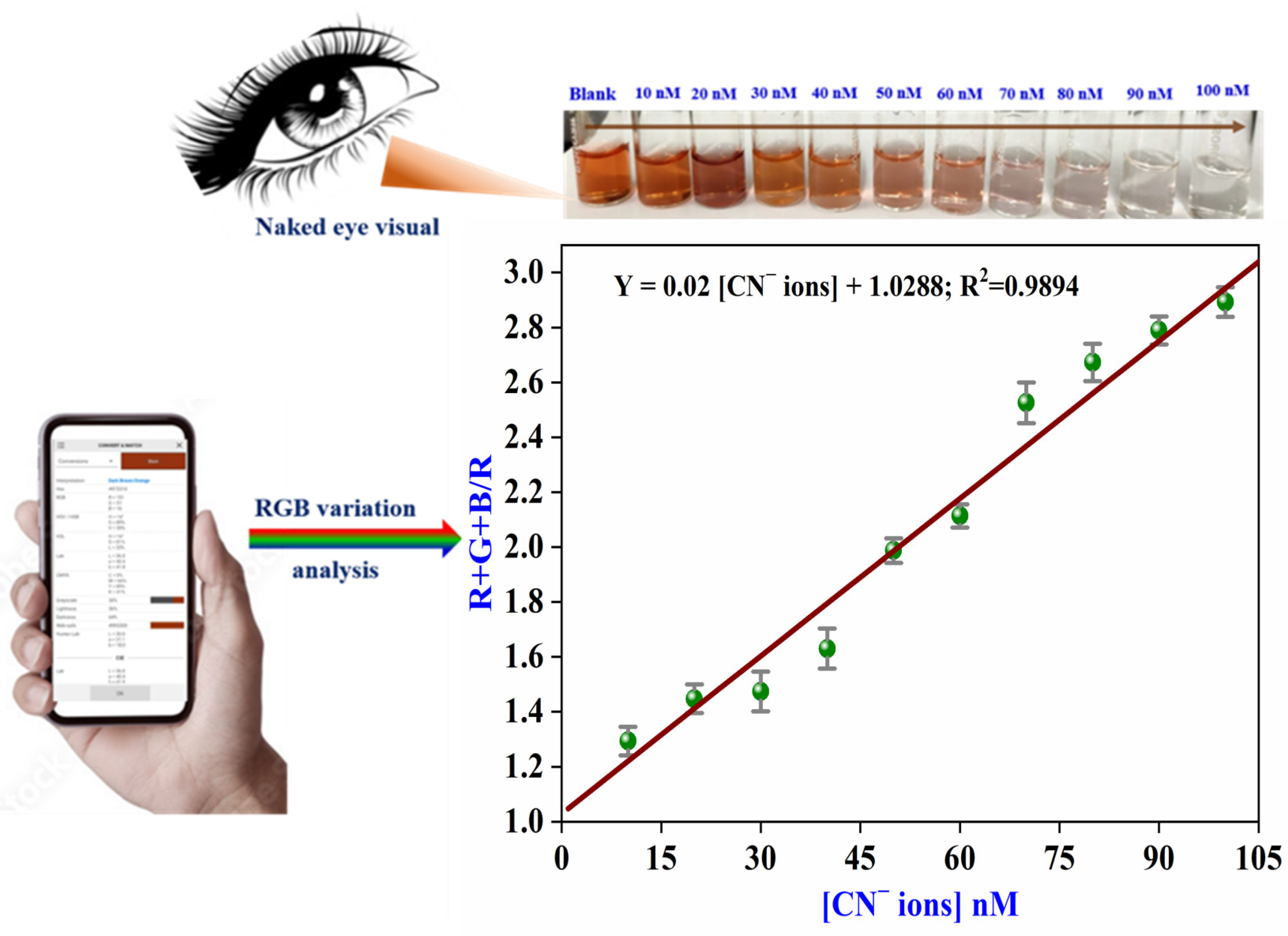
3.6. Smartphone-Based Colorimetry
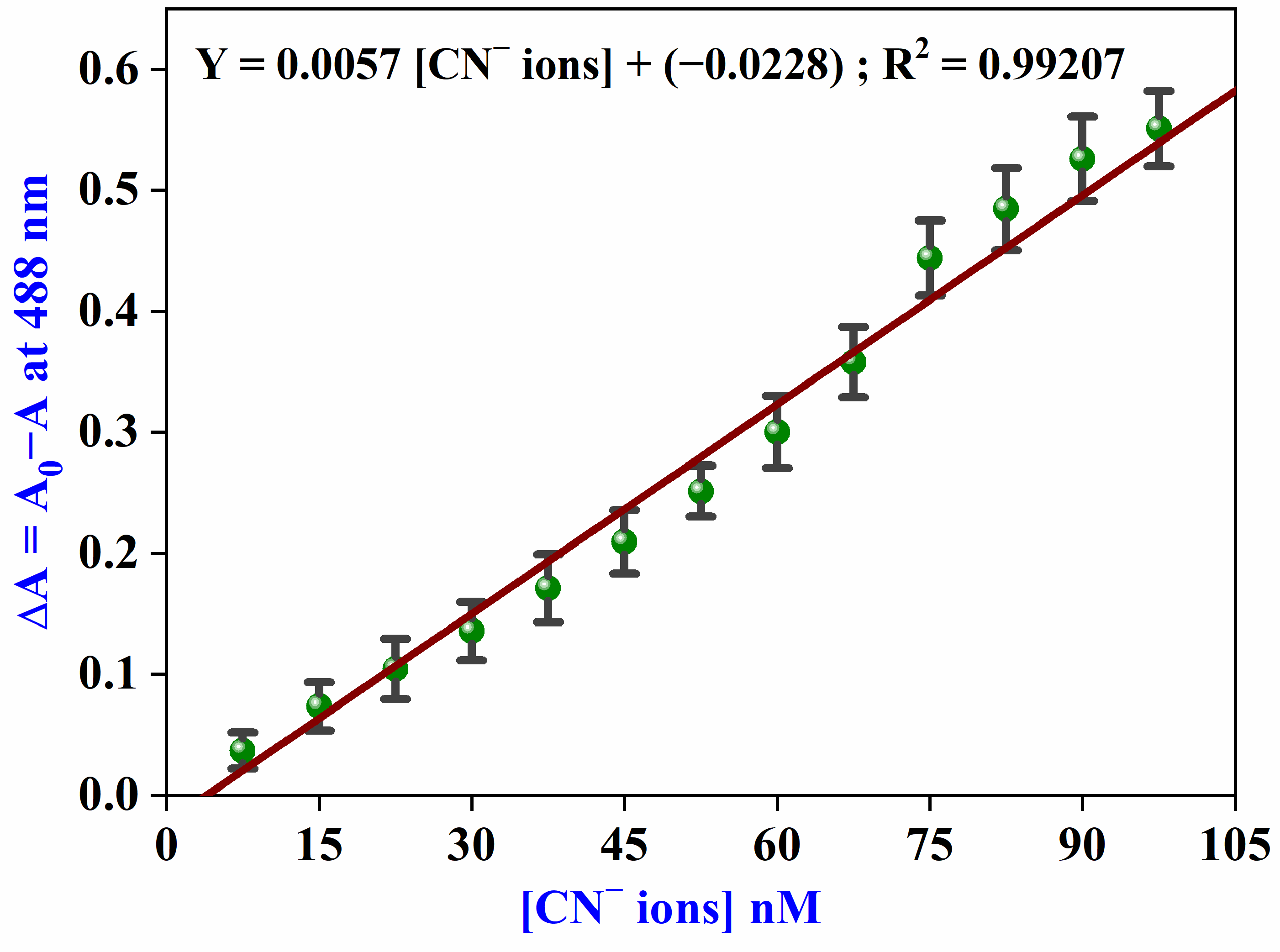
3.7. Analytical Parameters
3.8. Practical Utility and Reproducibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajamanikandan, R.; Sasikumar, K.; Kosame, S.; Ju, H. Optical sensing of toxic cyanide anions using noble metal nanomaterials. Nanomaterials 2023, 13, 290. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, X.; Kim, H.N.; Yoon, J. Sensors for the optical detection of cyanide ion. Chem. Soc. Rev. 2010, 39, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, L.; Chen, X.; Yoon, J. Recent progress in the development of fluorometric and colorimetric chemosensors for detection of cyanide ions. Chem. Soc. Rev. 2014, 43, 4312–4324. [Google Scholar] [PubMed]
- Kuyucak, N.; Akcil, A. Cyanide and removal options from effluents in gold mining and metallurgical processes. Miner. Eng. 2013, 50, 13–29. [Google Scholar] [CrossRef]
- Phuong, N.T.T.; Phung, V.D.; Le, T.B.N.; Chi, T.; Hien, B.T.T.; Tho, L.H.; Mai, N.X.D.; Phan, T.B.; Tran, N.H.T.; Ju, H. Ultrasensitive monitoring of cyanide concentrations in water using a Aucore–Agshell hybrid-coating-based fiber optical sensor. Langmuir 2023, 39, 15799–15807. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Cheng, X.; Huo, L.; Lu, L. Gold-nanocluster-based fluorescent sensors for highly sensitive and selective detection of cyanide in water. Adv. Funct. Mater. 2010, 20, 951–956. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Gaumerd, V.; Capello, Y.; Bonnin, Q.; Renard, P.Y.; Romieu, A. Fluorogenic detection of cyanide ions in pure aqueous media through an intramolecular crossed-benzoin reaction: Limitations unveiled and possible solutions. Analyst 2025, 150, 168–176. [Google Scholar]
- Bhandari, R.K.; Manandhar, E.; Oda, R.P.; Rockwood, G.A.; Logue, B.A. Simultaneous high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS-MS) analysis of cyanide and thiocyanate from swine plasma. Anal. Bioanal. Chem. 2014, 406, 727–734. [Google Scholar] [CrossRef]
- Amirjani, A.; Rahbarimehr, E. Recent advances in functionalization of plasmonic nanostructures for optical sensing. Microchim. Acta 2021, 188, 57. [Google Scholar] [CrossRef]
- Stewart, M.E.; Anderton, C.R.; Thompson, L.B.; Maria, J.; Gray, S.K.; Rogers, J.A.; Nuzzo, R.G. Nanostructured plasmonic sensors. Chem. Rev. 2008, 108, 494–521. [Google Scholar]
- Yang, T.; Huang, H.; Zhu, F.; Lin, Q.; Zhang, L.; Liu, J. Recent progresses in nanobiosensing for food safety analysis. Sensors 2016, 16, 1118. [Google Scholar] [CrossRef] [PubMed]
- Putri, L.A.; Prabowo, Y.D.; Dewi DM, M.; Mumtazah, Z.; Adila, F.P.; Fadillah, G.; Wasisto, H.S. Review of noble metal nanoparticle-based colorimetric sensors for food safety monitoring. ACS Appl. Nano Mater. 2024, 7, 19821–19853. [Google Scholar]
- Shang, L.; Jin, L.; Dong, S. Sensitive turn-on fluorescent detection of cyanide based on the dissolution of fluorophore functionalized gold nanoparticles. Chem. Commun. 2009, 21, 3077–3079. [Google Scholar]
- Wei, S.C.; Hsu, P.H.; Lee, Y.F.; Lin, Y.W.; Huang, C.C. Selective detection of iodide and cyanide anions using gold-nanoparticle-based fluorescent probes. ACS Appl. Mater. Interfaces 2012, 4, 2652–2658. [Google Scholar]
- Seok, J.S.; Ju, H. Plasmonic optical biosensors for detecting C-reactive protein: A review. Micromachines 2020, 11, 895. [Google Scholar] [CrossRef]
- Tran NH, T.; Phan, T.B.; Nguyen, T.T.; Ju, H. Coupling of silver nanoparticle-conjugated fluorescent dyes into optical fiber modes for enhanced signal-to-noise ratio. Biosens. Bioelectron. 2021, 176, 112900. [Google Scholar]
- Amirjani, A.; Haghshenas, D.F. Ag nanostructures as the surface plasmon resonance (SPR)˗ based sensors: A mechanistic study with an emphasis on heavy metallic ions detection. Sens. Actuators B Chem. 2018, 273, 1768–1779. [Google Scholar]
- Tran, V.T.; Ju, H. Fluorescence based on surface plasmon coupled emission for ultrahigh sensitivity immunoassay of cardiac troponin I. Biomedicines 2021, 9, 448. [Google Scholar] [CrossRef]
- Xu, C.; Nie, W.; Peng, H.; Li, H.; Wan, J. Enhancing dust concentration monitoring in high particulate matter environments: A dual-light source particulate matter sensor approach based on Mie scattering. Sens. Actuators A Phys. 2025, 387, 116348. [Google Scholar]
- Du, Y.; Zhao, Y.; Qu, Y.; Chen, C.H.; Chen, C.M.; Chuang, C.H.; Zhu, Y. Enhanced light–matter interaction of graphene–gold nanoparticle hybrid films for high-performance SERS detection. J. Mater. Chem. C 2014, 2, 4683–4691. [Google Scholar]
- Zeng, J.B.; Cao, Y.Y.; Chen, J.J.; Wang, X.D.; Yu, J.F.; Yu, B.B.; Chen, X. Au@ Ag core/shell nanoparticles as colorimetric probes for cyanide sensing. Nanoscale 2014, 6, 9939–9943. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.Y.; Chen, Y.; Liu, R.S.; Huang, J.; Wang, D.; Cao, Z.; Zeng, J. Matrix colorimetry for high-resolution visual detection of free cyanide with Au@ Au–Ag yolk–Shell nanoparticles. J. Mater. Chem. C 2021, 9, 4661–4669. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Zhang, J.; Huo, K.; Gu, J.; Zhou, Y.; Liu, X. Bimetallic-based colorimetric sensor for highly selective, stable and sensitive detection of iodide ions. Microchem. J. 2024, 199, 110098. [Google Scholar] [CrossRef]
- Raveendran, P.; Fu, J.; Wallen, S.L. A simple and “green” method for the synthesis of Au, Ag, and Au–Ag alloy nanoparticles. Green Chem. 2006, 8, 34–38. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, C.; Gao, W.; Han, Z.; Liu, T.; Li, C.; Zheng, H. Ag-Au alloy nanoparticles: Synthesis and in situ monitoring SERS of plasmonic catalysis. Sens. Actuators B Chem. 2016, 231, 609–614. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Zhou, X.; Wen, C.Y.; Yu, J.; Han, X.; Zeng, J. A convenient colorimetric method for sensitive and specific detection of cyanide using Ag@ Au core–shell nanoparticles. Sens. Actuators B Chem. 2016, 228, 366–372. [Google Scholar] [CrossRef]
- Fu, W.; Fu, X.; Li, Z.; Liu, Z.; Li, X. Advances in smartphone assisted sensors for on-site detection of food safety based on fluorescence on-off-on mode: A review. Chem. Eng. J. 2024, 489, 151225. [Google Scholar] [CrossRef]
- Rajamanikandan, R.; Shanmugaraj, K.; Ilanchelian, M.; Ju, H. Cysteamine-decorated gold nanoparticles for plasmon-based colorimetric on-site sensors for detecting cyanide ions using the smart-phone color ratio and for catalytic reduction of 4-nitrophenol. Chemosphere 2023, 316, 137836. [Google Scholar] [CrossRef]
- Azizi, N.; Hallaj, T.; Samadi, N. A dual-mode fluorometric and smartphone-based colorimetric sensor for cyanide detection using tungsten disulfide quantum dots and silver nanoparticles. J. Food Compos. Anal. 2024, 129, 106081. [Google Scholar] [CrossRef]
- Pande, S.; Ghosh, S.K.; Praharaj, S.; Panigrahi, S.; Basu, S.; Jana, S.; Pal, T. Synthesis of normal and inverted gold− silver core− shell architectures in β-cyclodextrin and their applications in SERS. J. Phys. Chem. C 2007, 111, 10806–10813. [Google Scholar] [CrossRef]
- Rajamanikandan, R.; Sasikumar, K.; Ju, H. β-Cyclodextrin Functionalized Au@ Ag Core-Shell Nanoparticles: Plasmonic Sensors for Cysteamine and Efficient Nanocatalysts for Nitrobenzene-to-Aniline Conversion. Biosensors 2024, 14, 544. [Google Scholar] [CrossRef] [PubMed]
- Rajamanikandan, R.; Ilanchelian, M. β-Cyclodextrin protected gold nanoparticle-based cotton swabs as an effective candidate for specific sensing of trace levels of cyanide. Anal. Methods 2019, 11, 97–104. [Google Scholar]
- Link, S.; Wang, Z.L.; El-Sayed, M.A. Alloy formation of gold− silver nanoparticles and the dependence of the plasmon absorption on their composition. J. Phys. Chem. B 1999, 103, 3529–3533. [Google Scholar]
- Pham TT, H.; Dien, N.D.; Vu, X.H. Facile synthesis of silver/gold alloy nanoparticles for ultra-sensitive rhodamine B detection. RSC Adv. 2021, 11, 21475–21488. [Google Scholar]
- Zheng, D.; Hu, C.; Gan, T.; Dang, X.; Hu, S. Preparation and application of a novel vanillin sensor based on biosynthesis of Au–Ag alloy nanoparticles. Sens. Actuators B Chem. 2010, 148, 247–252. [Google Scholar]
- Pham, T.T.H.; Vu, X.H.; Dien, N.D.; Trang, T.T.; Van Truong, N.; Thanh, T.D.; Tan, P.M.; Ca, N.X. The structural transition of bimetallic Ag–Au from core/shell to alloy and SERS application. RSC Adv. 2020, 10, 24577–24594. [Google Scholar]
- Selva Sharma, A.; Ilanchelian, M. Comprehensive multi spectroscopic analysis on the interaction and corona formation of human serum albumin with gold/silver alloy nanoparticles. J. Phys. Chem. B 2015, 119, 9461–9476. [Google Scholar]
- Dougherty, G.M.; Rose, K.A.; Tok JB, H.; Pannu, S.S.; Chuang, F.Y.; Sha, M.Y.; Penn, S.G. The zeta potential of surface-functionalized metallic nanorod particles in aqueous solution. Electrophoresis 2008, 29, 1131–1139. [Google Scholar]
- Dinparvar, S.; Bagirova, M.; Allahverdiyev, A.M.; Abamor, E.S.; Safarov, T.; Aydogdu, M.; Aktas, D. A nanotechnology-based new approach in the treatment of breast cancer: Biosynthesized silver nanoparticles using Cuminum cyminum L. seed extract. J. Photochem. Photobiol. B Biol. 2020, 208, 111902. [Google Scholar] [CrossRef]
- Akhondi, M.; Jafari, A.H.; Jamalizadeh, E. Selective colorimetric detection of HgII using silver nanoparticles modified with Apple and Nigella Sativa seed extracts and β-Cyclodextrin. J. Environ. Chem. Eng. 2020, 8, 103566. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, M.; Zhou, Z.; Luo, D.; Xu, G.; Xiong, Z. β-CD@ AgNPs with peroxisase-like activity for colorimetric determination of chiral tryptophan. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 323, 124871. [Google Scholar]
- Li, J.J.; Qiao, D.; Yang, S.Z.; Weng, G.J.; Zhu, J.; Zhao, J.W. Colorimetric determination of cysteine based on inhibition of GSH-Au/Pt NCs as peroxidase mimic. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 248, 119257. [Google Scholar]
- Li, S.; Wei, T.; Tang, M.; Chai, F.; Qu, F.; Wang, C. Facile synthesis of bimetallic Ag-Cu nanoparticles for colorimetric detection of mercury ion and catalysis. Sens. Actuators B Chem. 2018, 255, 1471–1481. [Google Scholar]
- Bi, N.; Zhang, Y.; Xi, Y.; Hu, M.; Song, W.; Xu, J.; Jia, L. Colorimetric response of lysine-caped gold/silver alloy nanocomposites for mercury (II) ion detection. Colloids Surf. B Biointerfaces 2021, 205, 111846. [Google Scholar]
- Geng, Z.; Miao, Y.; Zhang, G.; Liang, X. Colorimetric biosensor based on smartphone: State-of-art. Sens. Actuators A Phys. 2023, 349, 114056. [Google Scholar]
- Qian, S.; Cui, Y.; Cai, Z.; Li, L. Applications of smartphone-based colorimetric biosensors. Biosens. Bioelectron. X 2022, 11, 100173. [Google Scholar]
- Fernandes, G.M.; Silva, W.R.; Barreto, D.N.; Lamarca, R.S.; Gomes PC, F.L.; da SPetruci, J.F.; Batista, A.D. Novel approaches for colorimetric measurements in analytical chemistry—A review. Anal. Chim. Acta 2020, 1135, 187–203. [Google Scholar]
- Kap, Ö.; Kılıç, V.; Hardy, J.G.; Horzum, N. Smartphone-based colorimetric detection systems for glucose monitoring in the diagnosis and management of diabetes. Analyst 2021, 146, 2784–2806. [Google Scholar]
- Dong, Z.Z.; Yang, C.; Vellaisamy, K.; Li, G.; Leung, C.H.; Ma, D.L. Construction of a nano biosensor for cyanide anion detection and its application in environmental and biological systems. ACS Sens. 2017, 2, 1517–1522. [Google Scholar]
- Zhang, R.L.; Jia, S.L.; Wang, K.M. Au@ Ag Core/Shell Nanorods Based Colorimetric Assay for the Rapid and Selective Detection of Cyanide Anions. Sci. Adv. Mater. 2019, 11, 1739–1744. [Google Scholar]
- Medrano-Lopez, J.A.; Villalpando, I.; Salazar, M.I.; Torres-Torres, C. Hierarchical Nanobiosensors at the End of the SARS-CoV-2 Pandemic. Biosensors 2024, 14, 108. [Google Scholar] [CrossRef]
- Tan SC, L.; He, Z.; Wang, G.; Yu, Y.; Yang, L. Protein-templated metal nanoclusters: Molecular-like hybrids for biosensing, diagnostics and pharmaceutics. Molecules 2023, 28, 5531. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phuong Truong, N.N.; Rajamanikandan, R.; Sasikumar, K.; Ju, H. Smartphone-Assisted Plasmonic Nanosensor for Visual and Specific Sensing of Toxic Cyanide Ions by β−Cyclodextrin Templated Gold-Rich/Silver Bimetallic Alloy Nanoparticles. Materials 2025, 18, 1604. https://doi.org/10.3390/ma18071604
Phuong Truong NN, Rajamanikandan R, Sasikumar K, Ju H. Smartphone-Assisted Plasmonic Nanosensor for Visual and Specific Sensing of Toxic Cyanide Ions by β−Cyclodextrin Templated Gold-Rich/Silver Bimetallic Alloy Nanoparticles. Materials. 2025; 18(7):1604. https://doi.org/10.3390/ma18071604
Chicago/Turabian StylePhuong Truong, Nguyen Nam, Ramar Rajamanikandan, Kandasamy Sasikumar, and Heongkyu Ju. 2025. "Smartphone-Assisted Plasmonic Nanosensor for Visual and Specific Sensing of Toxic Cyanide Ions by β−Cyclodextrin Templated Gold-Rich/Silver Bimetallic Alloy Nanoparticles" Materials 18, no. 7: 1604. https://doi.org/10.3390/ma18071604
APA StylePhuong Truong, N. N., Rajamanikandan, R., Sasikumar, K., & Ju, H. (2025). Smartphone-Assisted Plasmonic Nanosensor for Visual and Specific Sensing of Toxic Cyanide Ions by β−Cyclodextrin Templated Gold-Rich/Silver Bimetallic Alloy Nanoparticles. Materials, 18(7), 1604. https://doi.org/10.3390/ma18071604






