Monitoring Antioxidant Consumption and Build-Up in Polypropylene During Open-Loop and Closed-Loop Mechanical Recycling
Abstract
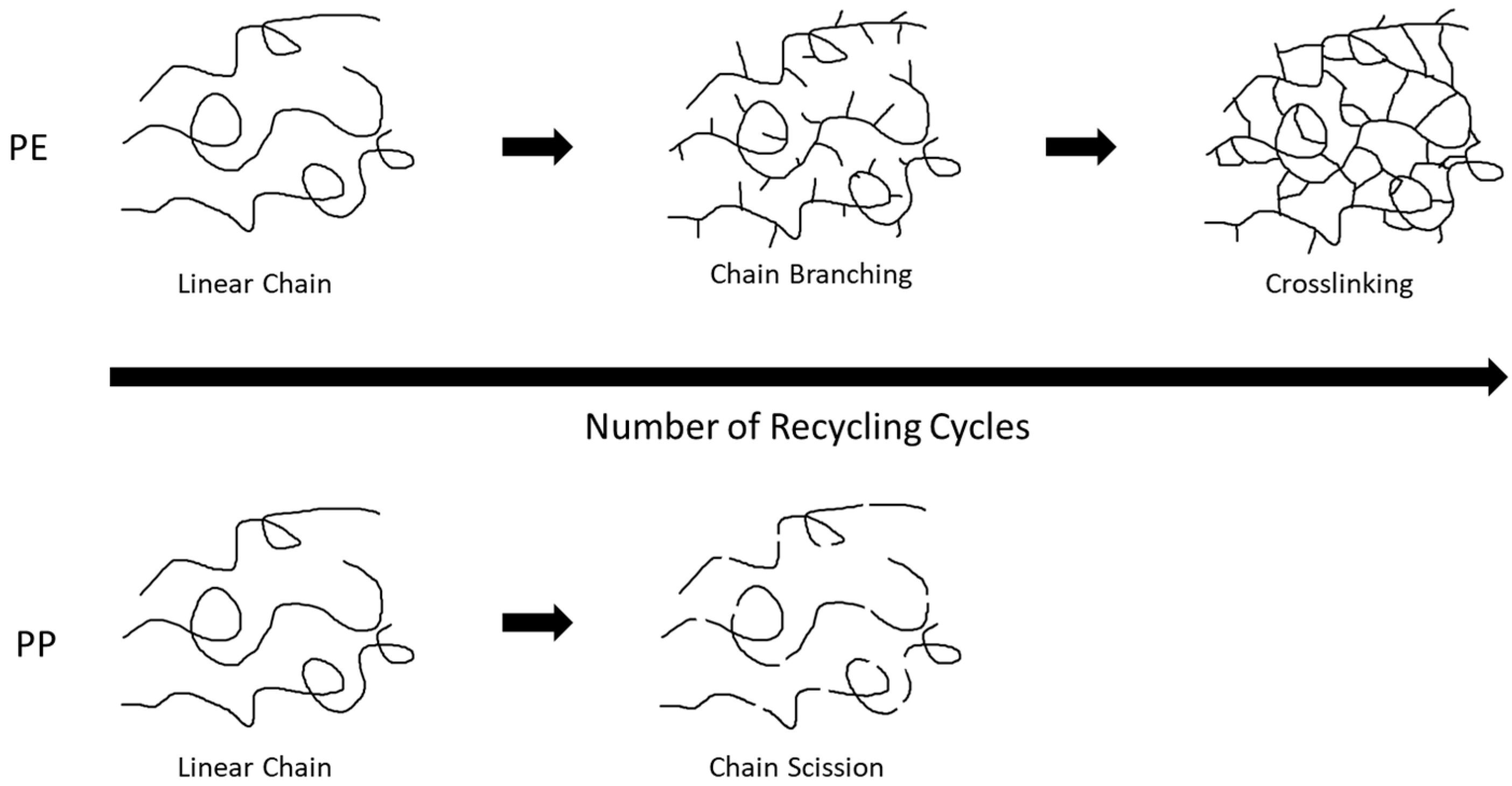
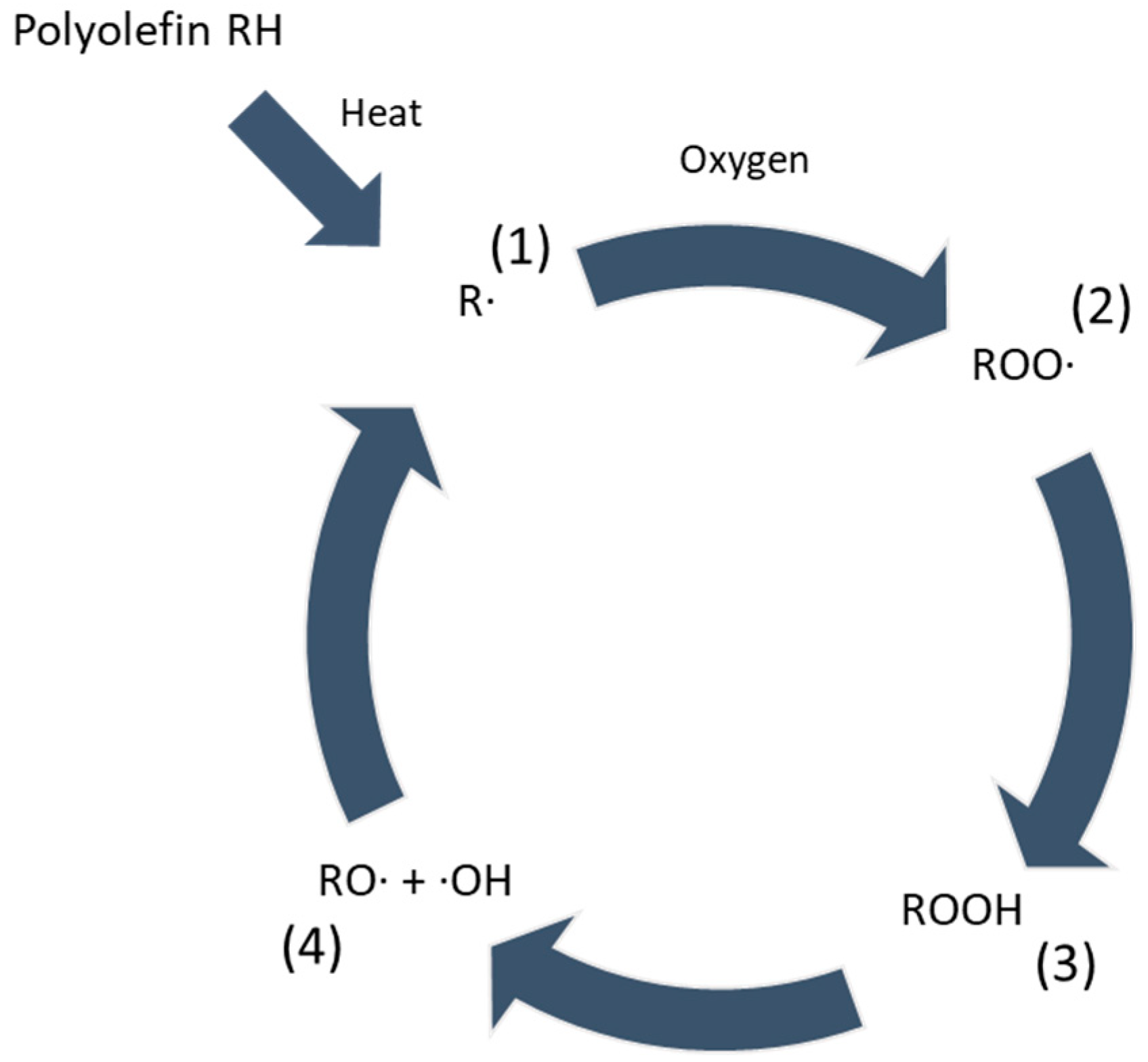
:1. Introduction
2. Experimental
2.1. Materials
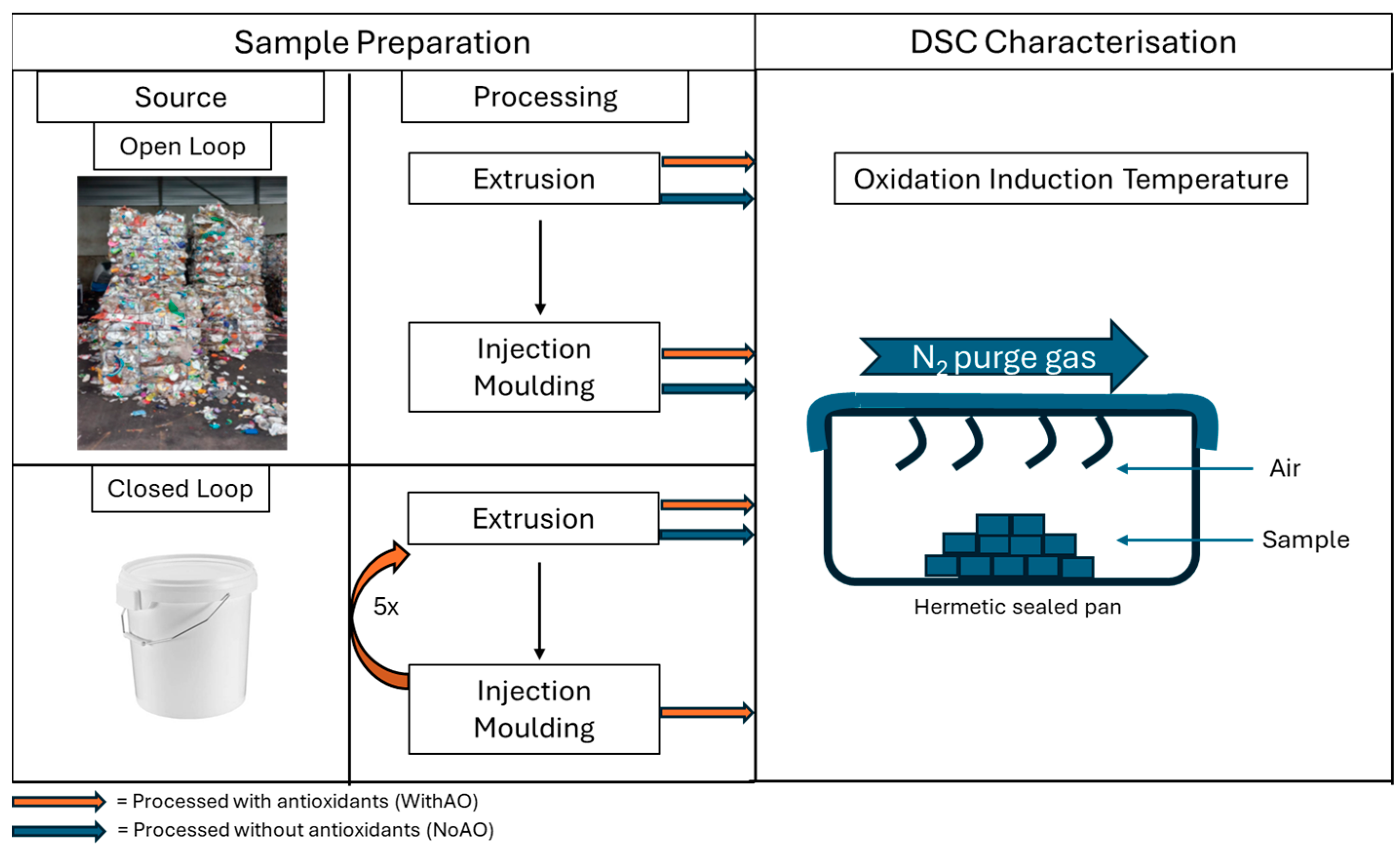
2.2. Sample Preparation
2.3. Characterisation
2.3.1. Thermal Analysis
2.3.2. Chemical Analysis
3. Results
3.1. Open-Loop Recycling
3.1.1. Composition
3.1.2. Oxidative Degradation
3.2. Closed-Loop Recycling
3.2.1. Composition
3.2.2. Oxidative Degradation
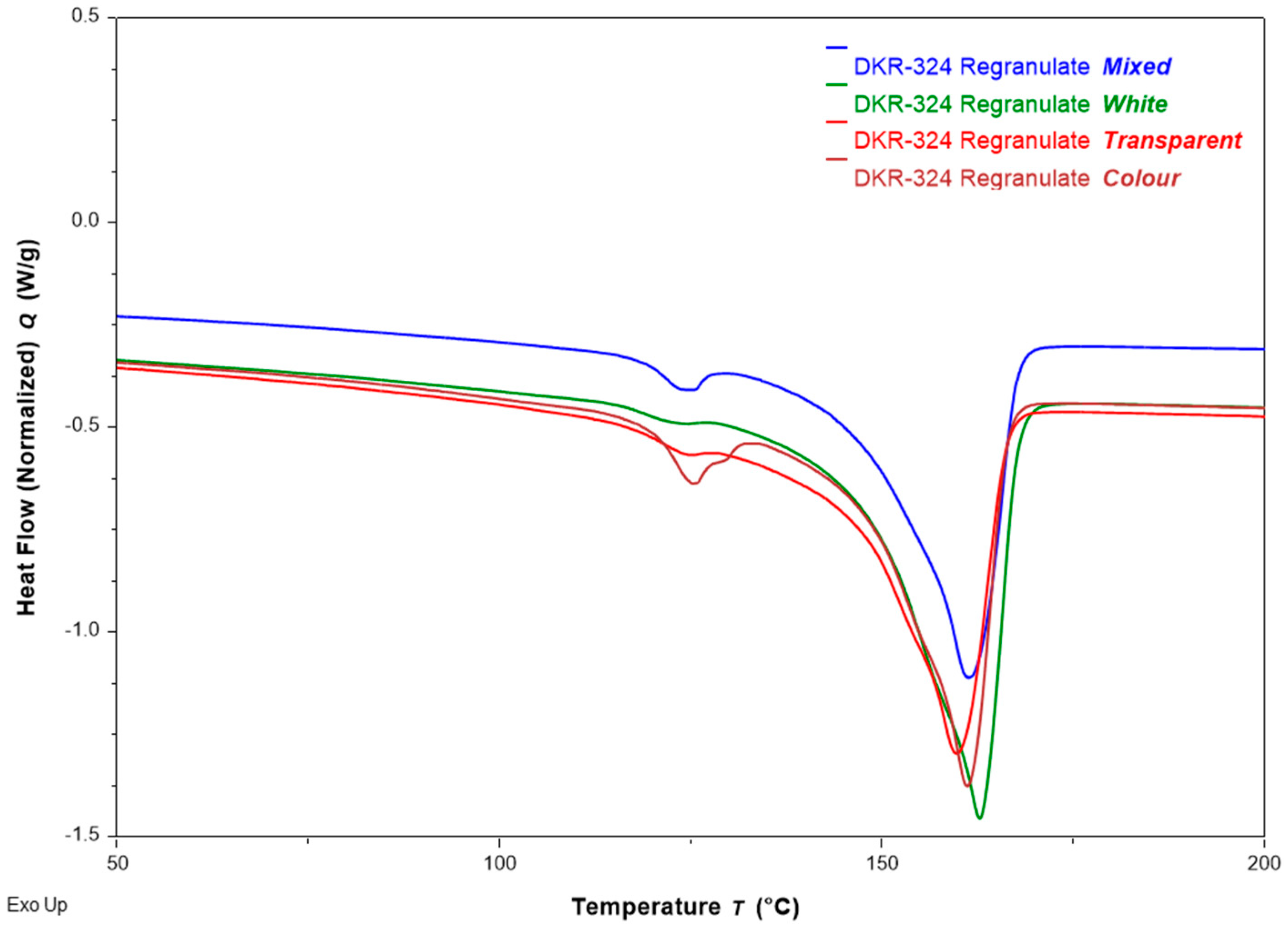
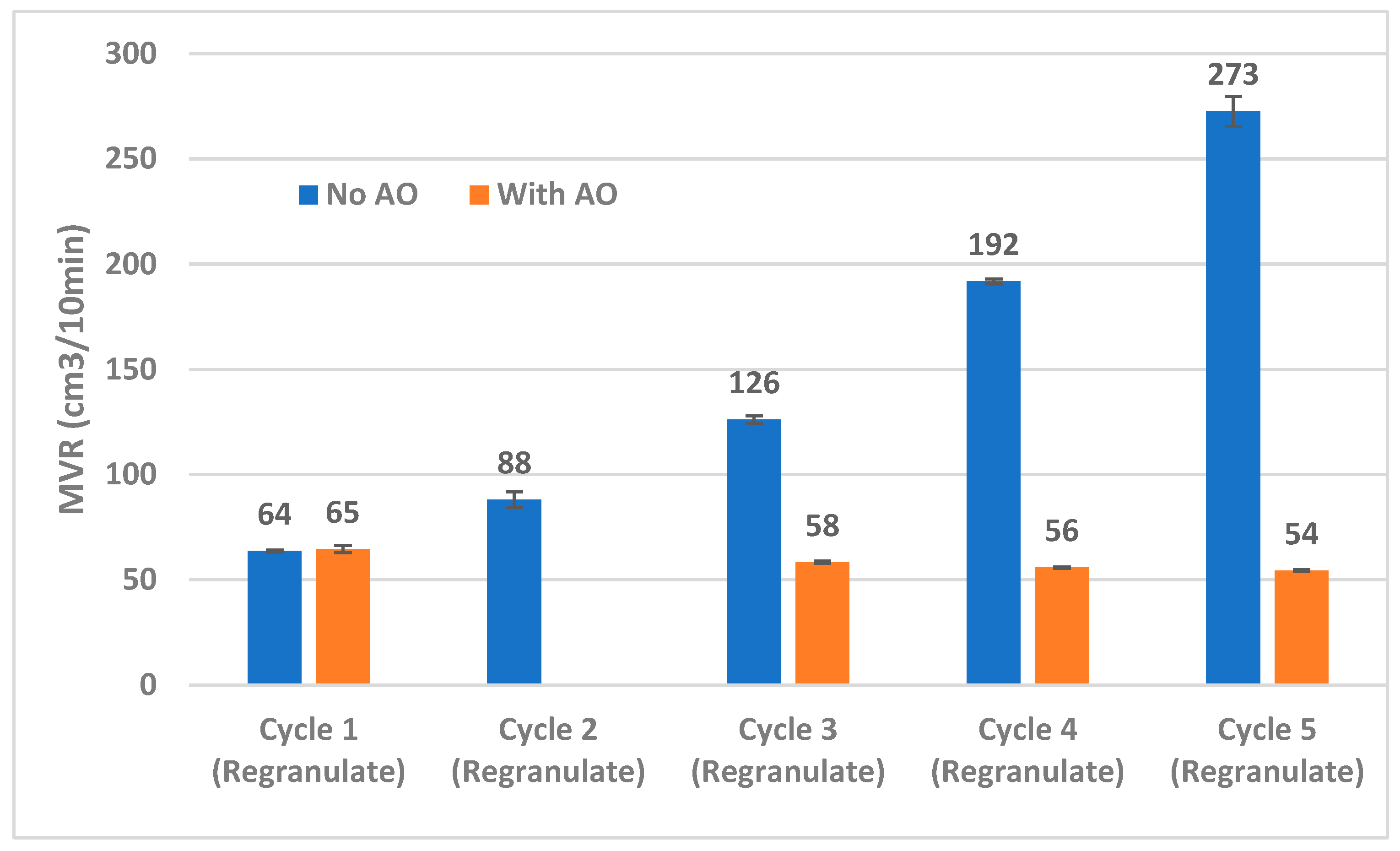
3.2.3. Melt Behavior
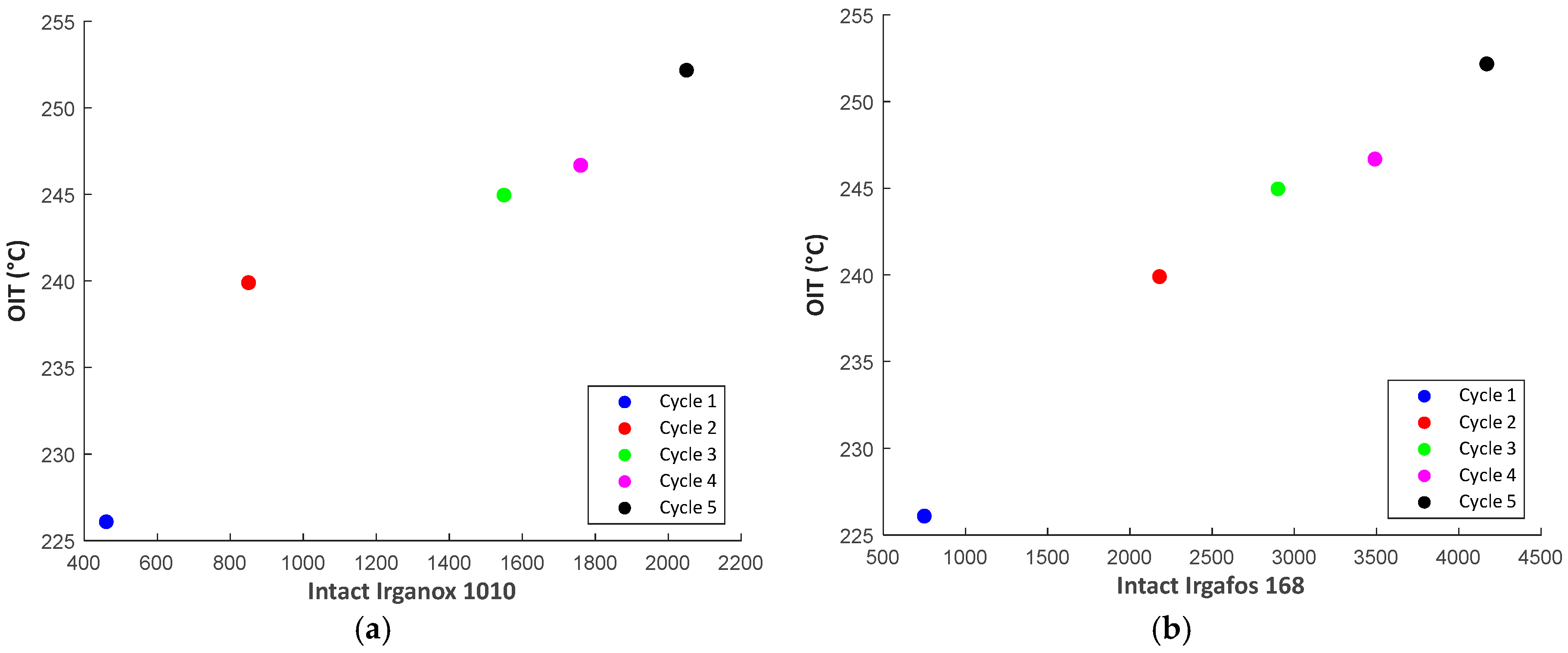
3.2.4. Correlation Plots
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di, J.; Reck, B.K.; Miatto, A.; Graedel, T.E. United States plastics: Large flows, short lifetimes, and negligible recycling. Resour. Conserv. Recycl. 2021, 167, 105440. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar]
- Walker, T.R.; Fequet, L. Current trends of unsustainable plastic production and micro(nano)plastic pollution. TrAC Trends Anal. Chem. 2023, 160, 116984. [Google Scholar]
- Narancic, T.; O’Connor, K.E. Plastic waste as a global challenge: Are biodegradable plastics the answer to the plastic waste problem? Microbiology 2019, 165, 129–137. [Google Scholar]
- Siddiqui, J.; Pandey, G. A Review of Plastic Waste Management Strategies. Int. Res. J. Environ. Sci. 2013, 2, 84–88. [Google Scholar]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, e2000415. [Google Scholar]
- Phelan, A.; Meissner, K.; Humphrey, J.; Ross, H. Plastic pollution and packaging: Corporate commitments and actions from the food and beverage sector. J. Clean. Prod. 2022, 331, 129827. [Google Scholar]
- Plastics Europe. Plastics—The Fast Facts 2023; Plastics Europe: Brussels, Belgium, 2023. [Google Scholar]
- Eurostat. 41% of Plastic Packaging Waste Recycled in 2022. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/w/ddn-20241024-3 (accessed on 19 December 2024).
- Kasper, J.B.; Parker, L.A.; Postema, S.; Höppener, E.M.; Leighton, A.H.; Finnegan, A.M.; Rutten, S.B.; Nijman, J.; Lasarati, A.; Soares, A.C.C.; et al. Losses and emissions in polypropylene recycling from household packaging waste. Waste Manag. 2025, 191, 230–241. [Google Scholar]
- Novák, I.; Popelka, A.; Špitalský, Z.; Krupa, I.; Tavman, S. Polyolefin in Packaging and Food Industry. In Polyolefin Compounds and Materials; Springer: Berlin/Heidelberg, Germany, 2015; pp. 181–199. [Google Scholar]
- Sam, S.T.; Nuradibah, M.A.; Ismail, H.; Noriman, N.Z.; Ragunathan, S. Recent Advances in Polyolefins/Natural Polymer Blends Used for Packaging Application. Polym.-Plast. Technol. Eng. 2014, 53, 631–644. [Google Scholar]
- Yin, S.; Tuladhar, R.; Shi, F.; Shanks, R.A.; Combe, M.; Collister, T. Mechanical reprocessing of polyolefin waste: A review. Polym. Eng. Sci. 2015, 55, 2899–2909. [Google Scholar] [CrossRef]
- Ambrogi, V.; Carfagna, C.; Cerruti, P.; Marturano, V. 4—Additives in Polymers. In Modification of Polymer Properties; William Andrew: Norwich, NY, USA, 2017; pp. 87–108. [Google Scholar]
- Shorten, D. Polyolefins for food packaging. Food Chem. 1982, 8, 109–119. [Google Scholar]
- Gijsman, P.; Fiorio, R. Long term thermo-oxidative degradation and stabilization of polypropylene (PP) and the implications for its recyclability. Polym. Degrad. Stab. 2023, 208, 110260. [Google Scholar]
- McKeen, L.W. Introduction to the Effect of Heat Aging on Plastics. In The Effect of Long Term Thermal Exposure on Plastics and Elastomers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 17–42. [Google Scholar]
- Qian, S.; Igarashi, T.; Nitta, K.-H. Thermal degradation behavior of polypropylene in the melt state: Molecular weight distribution changes and chain scission mechanism. Polym. Bull. 2011, 67, 1661–1670. [Google Scholar]
- Pfaendner, R. Restabilization—30 years of research for quality improvement of recycled plastics review. Polym. Degrad. Stab. 2022, 203, 110082. [Google Scholar] [CrossRef]
- Oswald, H.J.; Turi, E. The deterioration of polypropylene by oxidative degradation. Polym. Eng. Sci. 1965, 5, 152–158. [Google Scholar]
- Coleman, E.A. 23—Plastics Additives. In Applied Plastics Engineering Handbook; William Andrew: Norwich, NY, USA, 2011; pp. 419–428. [Google Scholar]
- Santos, A.; Agnelli, J.; Trevisan, D.; Manrich, S. Degradation and stabilization of polyolefins from municipal plastic waste during multiple extrusions under different reprocessing conditions. Polym. Degrad. Stab. 2002, 77, 441–447. [Google Scholar] [CrossRef]
- Karlsson, S. Recycled Polyolefins. Material Properties and Means for Quality Determination. In Long Term Properties of Polyolefins; Springer: Berlin, Germany, 2004; pp. 201–230. [Google Scholar]
- Ritter, A.; Michel, E.; Schmid, M.; Affolter, S. Interlaboratory test on polymers: Determination of antioxidants in polyolefins. Polym. Test. 2005, 24, 498–506. [Google Scholar] [CrossRef]
- Dopico-García, M.S.; López-Vilariñó, J.M.; González-Rodríguez, M.V. Antioxidant Content of and Migration from Commercial Polyethylene, Polypropylene, and Polyvinyl Chloride Packages. J. Agric. Food Chem. 2007, 55, 3225–3231. [Google Scholar]
- Green, S.; Bai, S.; Cheatham, M.; Cong, R.; Yau, W. Determination of antioxidants in polyolefins using total dissolution methodology followed by RPLC. J. Sep. Sci. 2010, 33, 3455–3462. [Google Scholar]
- ISO 11357-6:2018; Plastics—Differential Scanning Calorimetry (DSC)—Part 6: Determination of Oxidation Induction Time (Isothermal OIT) and Oxidation Induction Temperature (Dynamic OIT). ISO—International Organization for Standardization: Geneva, Switzerland, 2018.
- Peltzer, M.; Jimenez, A. Determination of oxidation parameters by DSC for polypropylene stabilized with hydroxytyrosol (3,4-dihydroxy-phenylethanol). J. Therm. Anal. Calorim. 2009, 96, 243–248. [Google Scholar]
- Huysman, S.; Debaveye, S.; Schaubroeck, T.; Meester, S.D.; Ardente, F.; Mathieux, F.; Dewulf, J. The recyclability benefit rate of closed-loop and open-loop systems: A case study on plastic recycling in Flanders. Resour. Conserv. Recycl. 2015, 101, 53–60. [Google Scholar]
- European Union. Commission Regulation (EU) 2022/1616. Off. J. Eur. Union 2022, 243, 3–46. [Google Scholar]
- Uekert, T.; Singh, A.; DesVeaux, J.S.; Ghosh, T.; Bhatt, A.; Yadav, G.; Afzal, S.; Walzberg, J.; Knauer, K.M.; Nicholson, S.R.; et al. Technical, Economic, and Environmental Comparison of Closed-Loop Recycling Technologies for Common Plastics. ACS Sustain. Chem. Eng. 2023, 11, 965–978. [Google Scholar]
- Traxler, I.; Laske, S.; Fischer, J. Closed-loop recycling of polypropylene: A case study on mechanical recycling of separately collected yogurt cups in Austria. Resour. Conserv. Recycl. 2024, 205, 107537. [Google Scholar]
- Der Grüne Punkt. Produktspezifikation 05/2012 Fraktions-Nr. 324. 2012. Available online: https://www.nedvang.nl/wp-content/uploads/2019/03/PP-DKR-324.pdf (accessed on 1 November 2024).
- ISO 527-1A:2019; Plastics—Determination of Tensile Properties—Part 1: General Principles. ISO—International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 1133:2022; Plastics—Determination of the Melt Mass-Flow Rate (MFR) and Melt Volume-Flow Rate (MVR) of Thermoplastics. Part 1: Standard Method. ISO—International Organization for Standardization: Geneva, Switzerland, 2022.
- Bashirgondabi; Ureel, Y.; Delva, L.; Fiorio, R.; Geem, K.M.V.; Ragaert, K. Accurate determination of polyethylene (PE) and polypropylene (PP) content in polyolefin blends using machine learning-assisted differential scanning calorimetry (DSC) analysis. Polym. Test. 2024, 131, 108353. [Google Scholar]
- Jansen, M.; Velzen, U.T.V.; Pretz, T. Handbook for Sorting of Plastic Packaging Waste Concentrates; Wageningen UR Food & Biobased Research: Wageningen, The Netherlands, 2015. [Google Scholar]
- Cecon, V.S.; Pham, T.; Curtzwiler, G.; Vorst, K. Assessment of Different Application Grades of Post-Consumer Recycled (PCR) Polyolefins from Material Recovery Facilities (MRFs) in the United States. ACS Appl. Polym. Mater. 2024, 6, 13065–13076. [Google Scholar]
- Blázquez-Blázquez, E.; Díez-Rodríguez, T.M.; Pérez, E.; Cerrada, M.L. Recycling of metallocene isotactic polypropylene: Importance of antioxidants. J. Therm. Anal. Calorim. 2022, 147, 13363–13374. [Google Scholar]
- Archodoulaki, V.M.; Seidler, S.L. Oxidation induction time studies on the thermal degradation behaviour of polyoxymethylene. Polym. Test. 2006, 25, 83–90. [Google Scholar]
- Peterson, J.D.; Vyazovkin, S.; Wight, C.A. Kinetics of the Thermal and Thermo-Oxidative Degradation of Polystyrene, Polyethylene and Poly(propylene). Macromol. Cheistry Phys. 2001, 202, 775–784. [Google Scholar]
- François-Heude; Richaud, E.; Leprovost, J.; Heninger, M.; Mestdagh, H.; Desnoux, E.; Colin, X. Real-time quantitative analysis of volatile products generated during solid-state polypropylene thermal oxidation. Polym. Test. 2013, 32, 907–917. [Google Scholar]



| Regranulate (Extrusion) | Mn (kDa) | Mw (kDa) | Mz (kDa) | Mw/Mn or Đ |
|---|---|---|---|---|
| Mixed | 34.3 | 205 | 640 | 5.98 |
| Color | 30.5 | 165 | 520 | 5.41 |
| Transparent | 33.3 | 185 | 560 | 5.55 |
| White | 32 | 162 | 510 | 5.06 |
| DKR-324 Type | Oxidation Induction Time (min) | |||
|---|---|---|---|---|
| Regranulate (Extrusion) | Dog-Bone (Extrusion + Injection Molding) | |||
| No AO | With AO | No AO | With AO | |
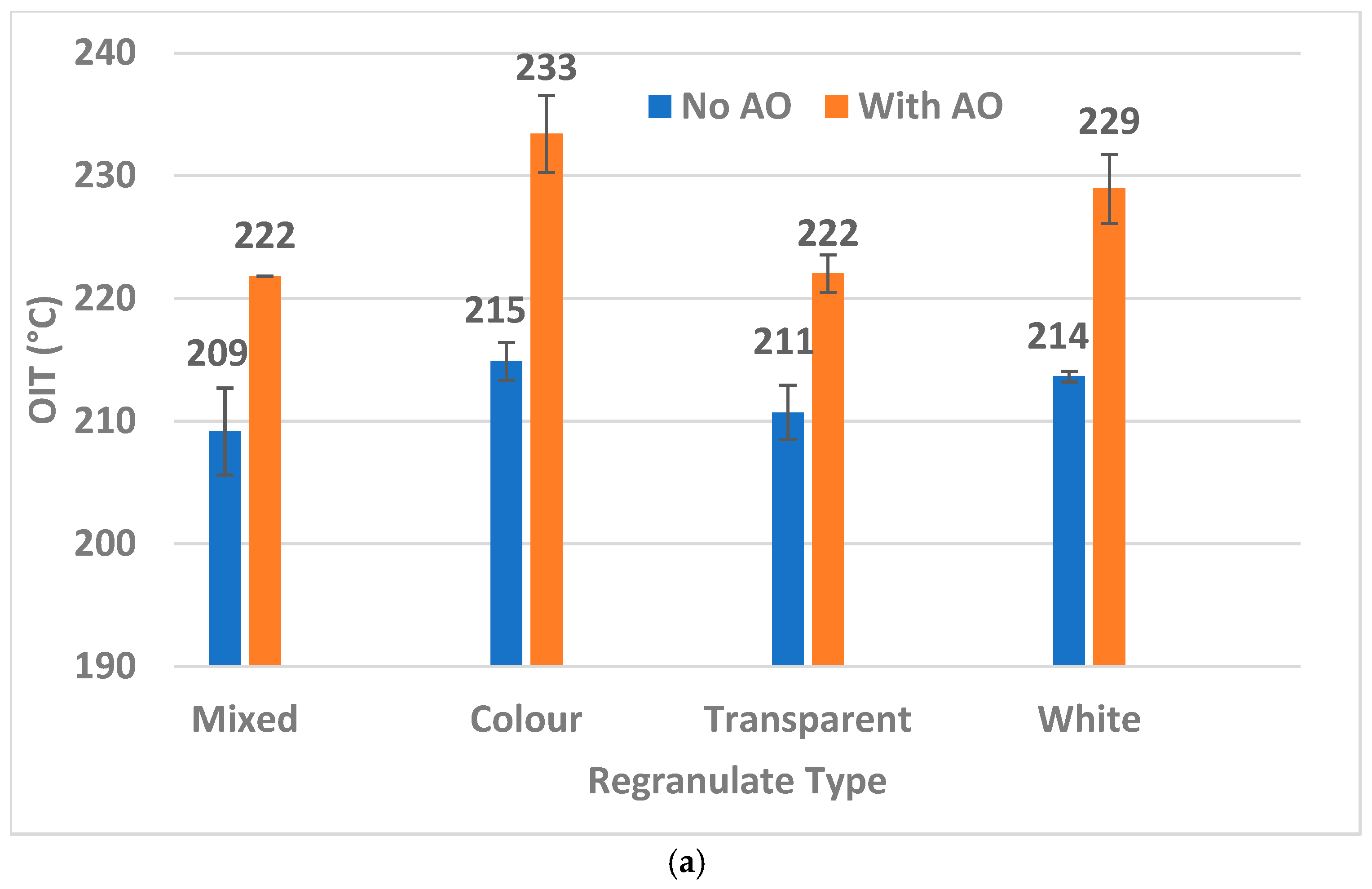
| Mixed | 3.3 | 12.5 | 2.5 | 4.7 |
| Color | 4.3 | 52.5 | 5.9 | 13.0 |
| Transparent | 5.0 | 11.4 | 4.0 | 10.0 |
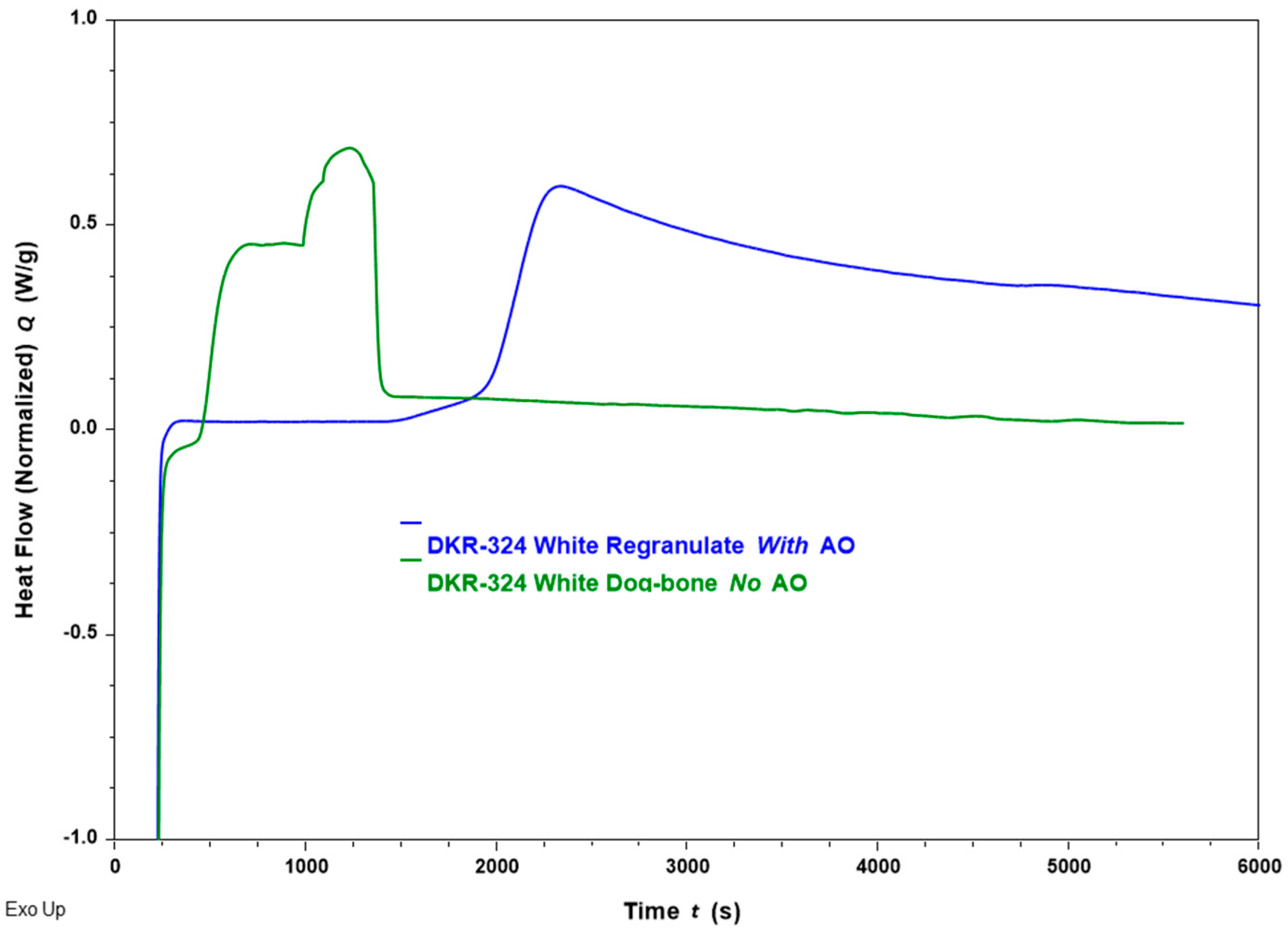
| White | 5.5 | 29.0 | 4.0 | 9.3 |
| Regranulate (Extrusion) | Irganox 1010 Total [ppm] | Irganox 1010 Intact [ppm] | Irganox 1010 Degraded [ppm] | Irgafos 168 Total [ppm] | Irgafos 168 Intact [ppm] | Irgafos 168 Degraded [ppm] |
|---|---|---|---|---|---|---|
| Mixed No AO | 190 | 170 | <50 | 580 | 150 | 430 |
| Mixed with AO | 470 | 430 | <50 | 1100 | 590 | 510 |
| White No AO | 310 | 220 | 70 | 580 | 190 | 390 |
| White with AO | 510 | 440 | 70 | 1000 | 500 | 500 |
| Dog-bone (Extrusion + Injection Molding) | Mn (kDa) | Mw (kDa) | Mz (kDa) | Mw/Mn or Đ |
|---|---|---|---|---|
| Cycle 1 w. AO | 36.6 | 180 | 650 | 4.92 |
| Cycle 2 w. AO | 31.1 | 178 | 620 | 5.72 |
| Cycle 3 w. AO | 30.3 | 179 | 600 | 5.91 |
| Cycle 4 w. AO | 29.7 | 177 | 570 | 5.96 |
| Cycle 5 w. AO | 37.7 | 181 | 570 | 4.8 |
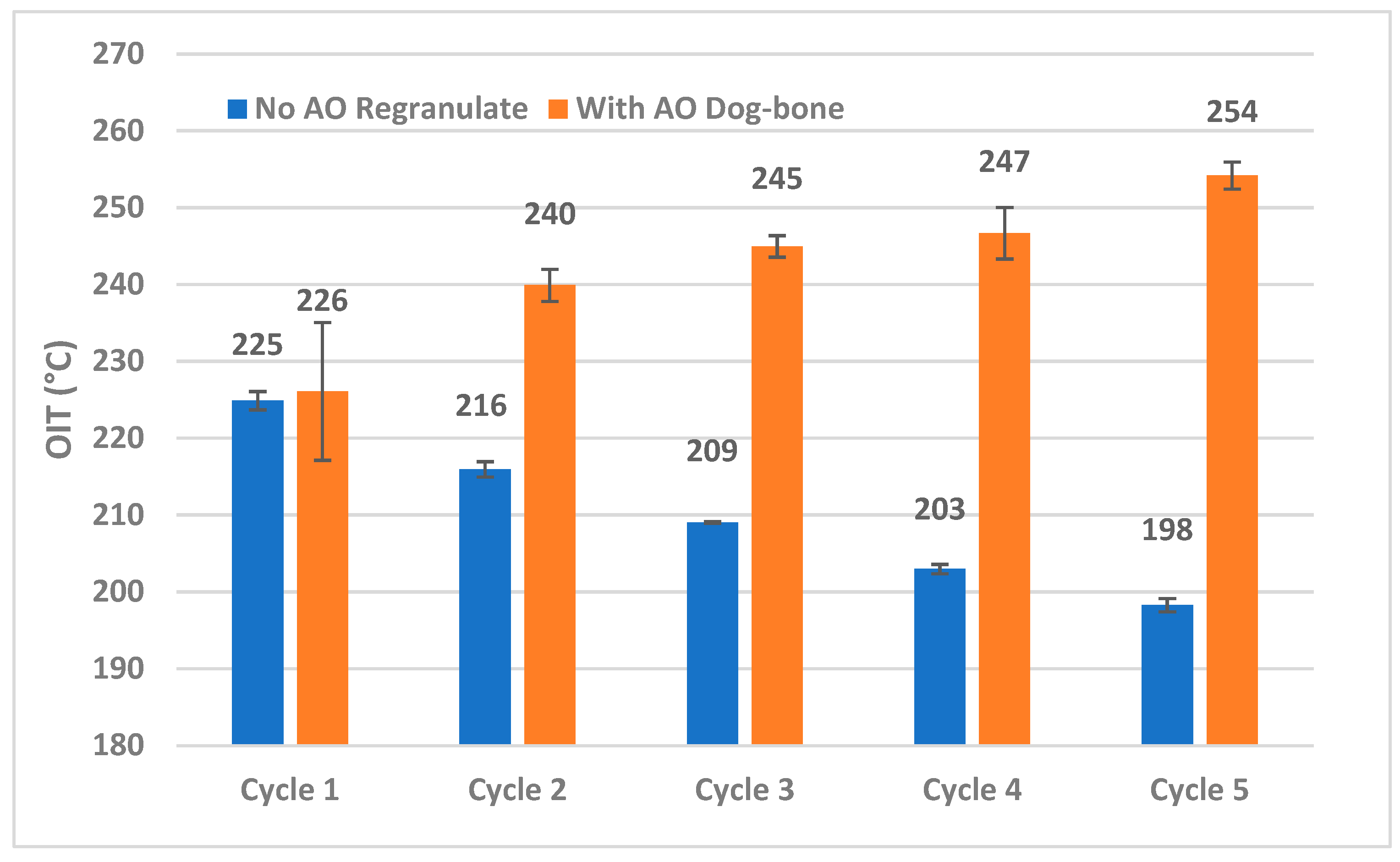
| Sample | OIt (min) | |
|---|---|---|
| No AO (Regranulates) | With AO (Dog-Bone) | |
| Cycle 1 | 2.3 | 2.9 |
| Cycle 2 | 1.3 | 7.1 |
| Cycle 3 | 1.0 | 9.2 |
| Cycle 4 | 0.7 | 21.4 |
| Cycle 5 | 0.1 | 36.6 |
| Dog-bone with AO | Irganox 1010 Total [ppm] | Irganox 1010 Intact [ppm] | Irganox 1010 Degraded [ppm] | Irgafos 168 Total [ppm] | Irgafos 168 Intact [ppm] | Irgafos 168 Degraded [ppm] |
|---|---|---|---|---|---|---|
| Cycle 1 | 500 | 460 | <50 | 1090 | 750 | 340 |
| Cycle 2 | 890 | 850 | <50 | 2420 | 2180 | 240 |
| Cycle 3 | 1620 | 1550 | 70 | 3210 | 2900 | 310 |
| Cycle 4 | 1850 | 1760 | 80 | 3870 | 3490 | 380 |
| Cycle 5 | 2210 | 2050 | 150 | 4650 | 4170 | 480 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knoben, N.; Vanhouttem, M.; Wypkema, A.; Subramanian, N. Monitoring Antioxidant Consumption and Build-Up in Polypropylene During Open-Loop and Closed-Loop Mechanical Recycling. Materials 2025, 18, 1640. https://doi.org/10.3390/ma18071640
Knoben N, Vanhouttem M, Wypkema A, Subramanian N. Monitoring Antioxidant Consumption and Build-Up in Polypropylene During Open-Loop and Closed-Loop Mechanical Recycling. Materials. 2025; 18(7):1640. https://doi.org/10.3390/ma18071640
Chicago/Turabian StyleKnoben, Niek, Max Vanhouttem, Aike Wypkema, and Nithya Subramanian. 2025. "Monitoring Antioxidant Consumption and Build-Up in Polypropylene During Open-Loop and Closed-Loop Mechanical Recycling" Materials 18, no. 7: 1640. https://doi.org/10.3390/ma18071640
APA StyleKnoben, N., Vanhouttem, M., Wypkema, A., & Subramanian, N. (2025). Monitoring Antioxidant Consumption and Build-Up in Polypropylene During Open-Loop and Closed-Loop Mechanical Recycling. Materials, 18(7), 1640. https://doi.org/10.3390/ma18071640






