Identification and Visualization Textile Fibers by Raman Imaging
Abstract
1. Introduction
2. Materials and Methods
3. Results
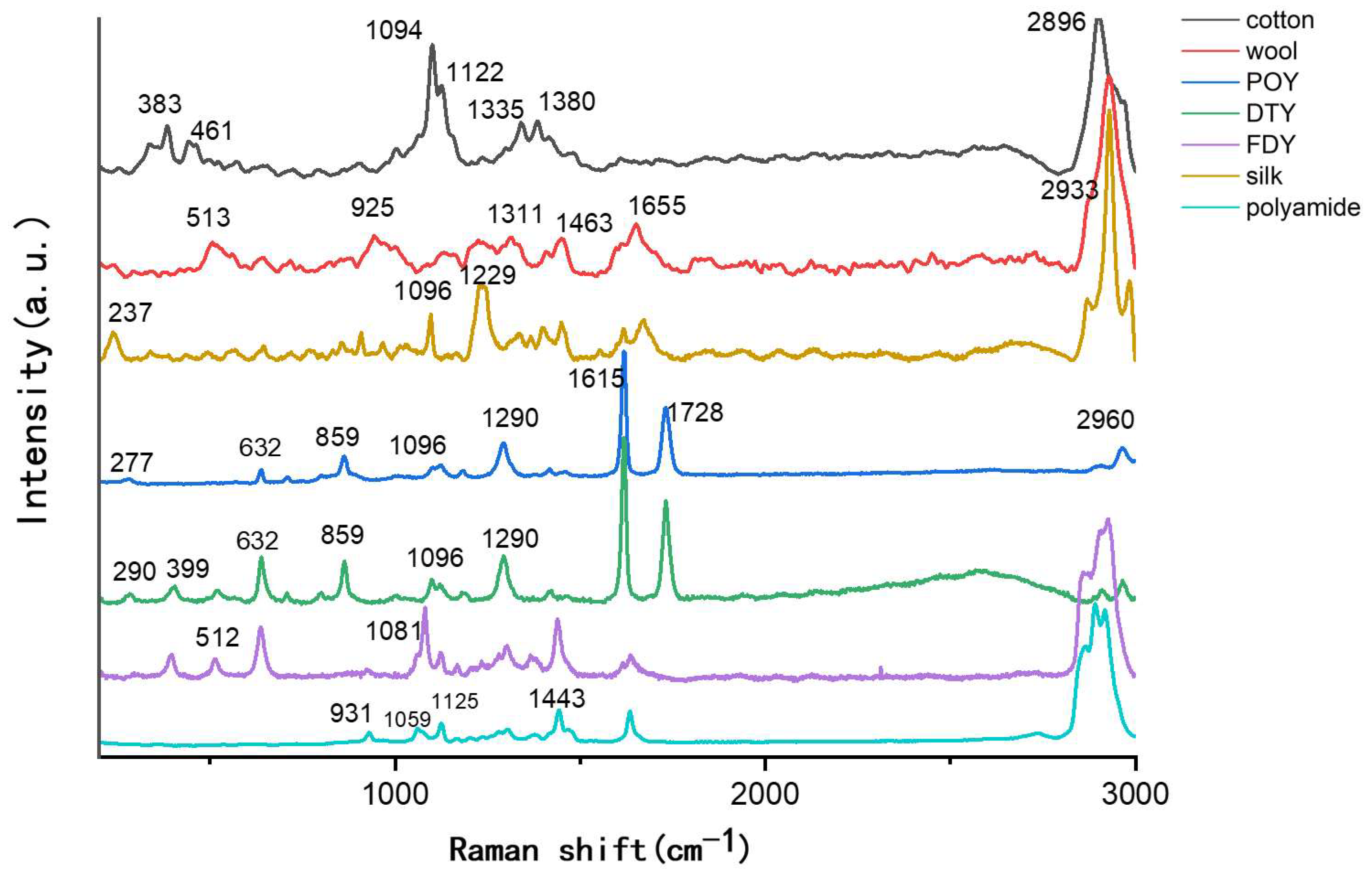
3.1. Raman Spectrum of Common Textile Fibers
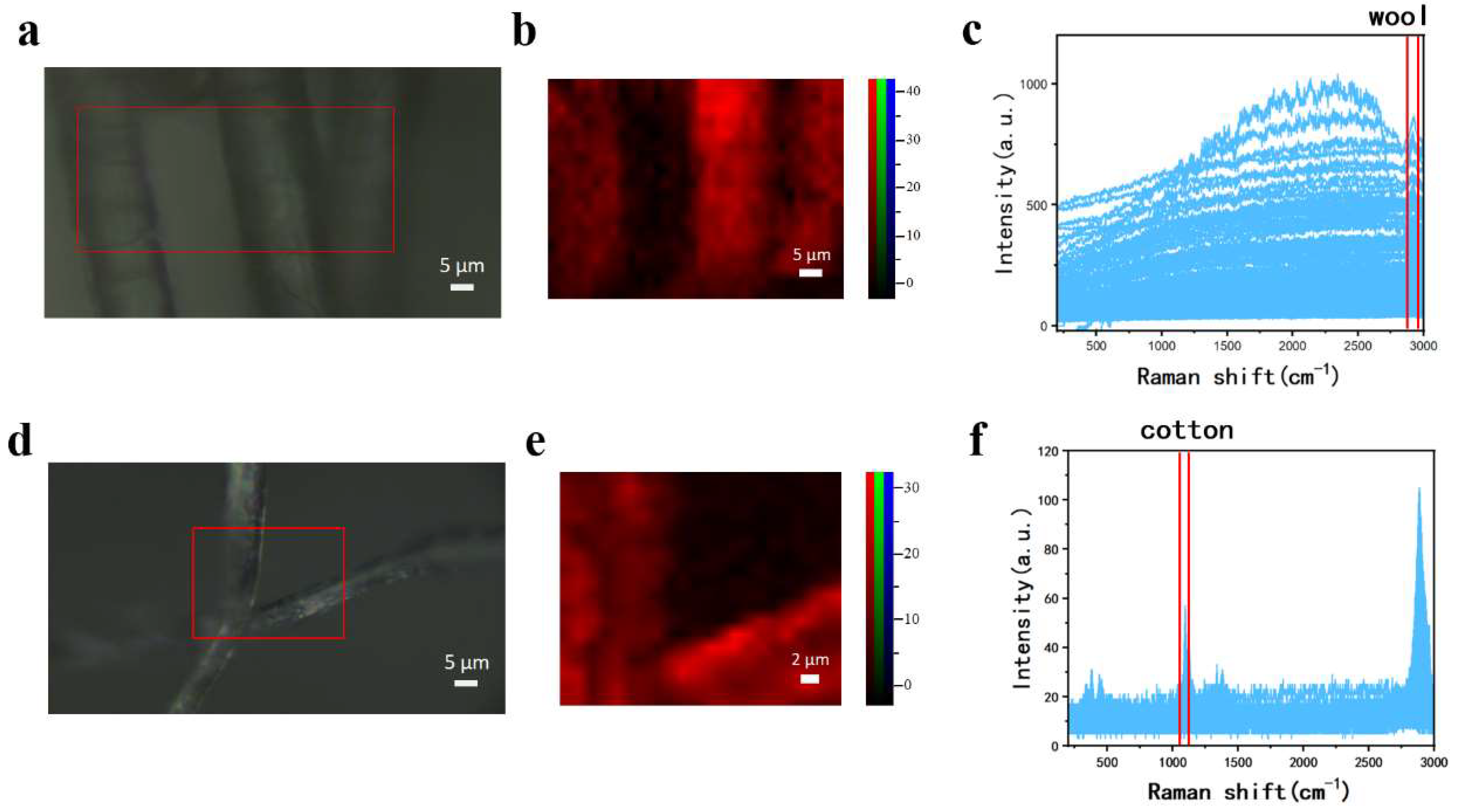
3.2. Raman Imaging of Single-Component Textile Fibers
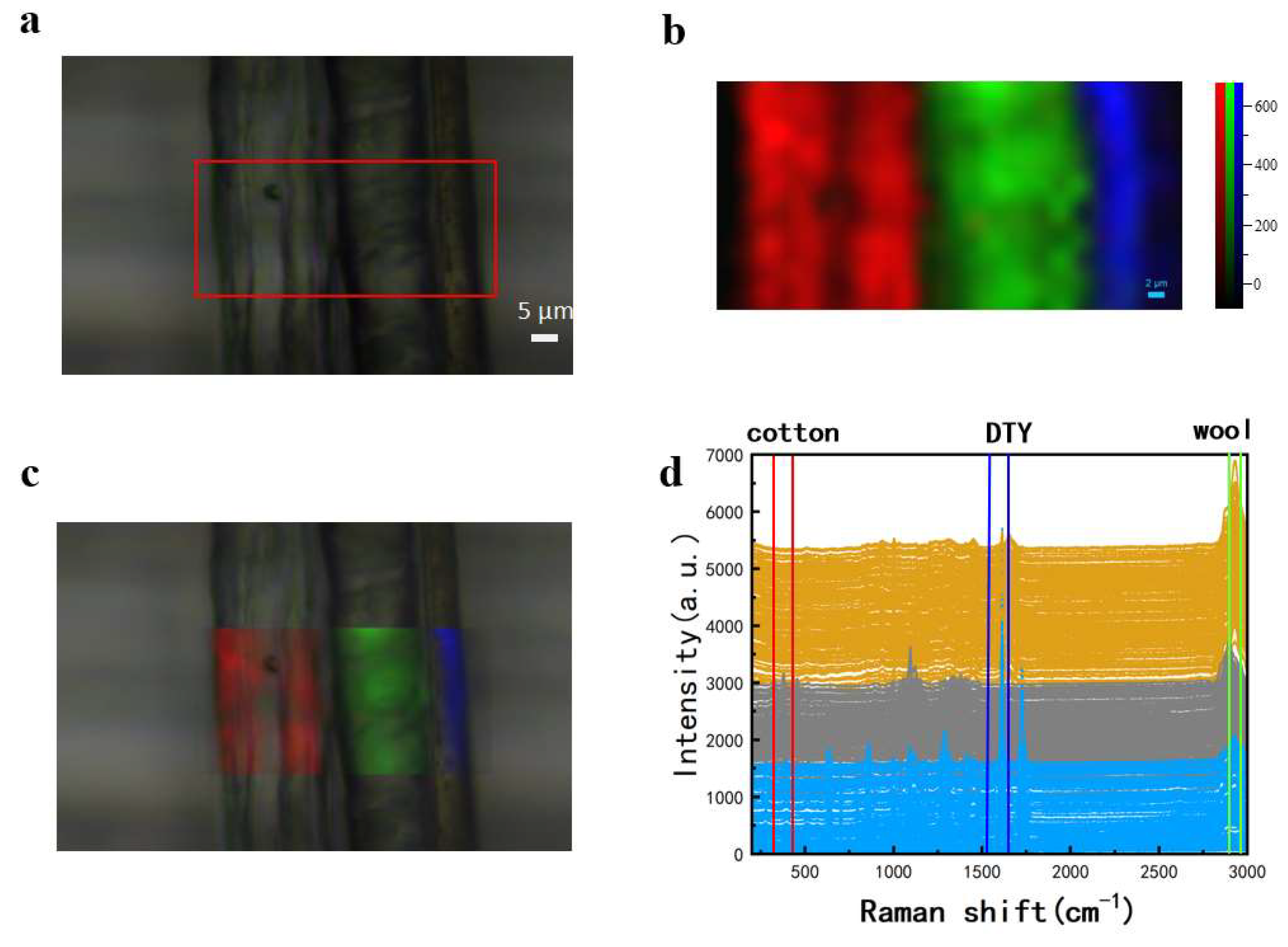
3.3. Raman Imaging of Multi-Component Textile Fibers
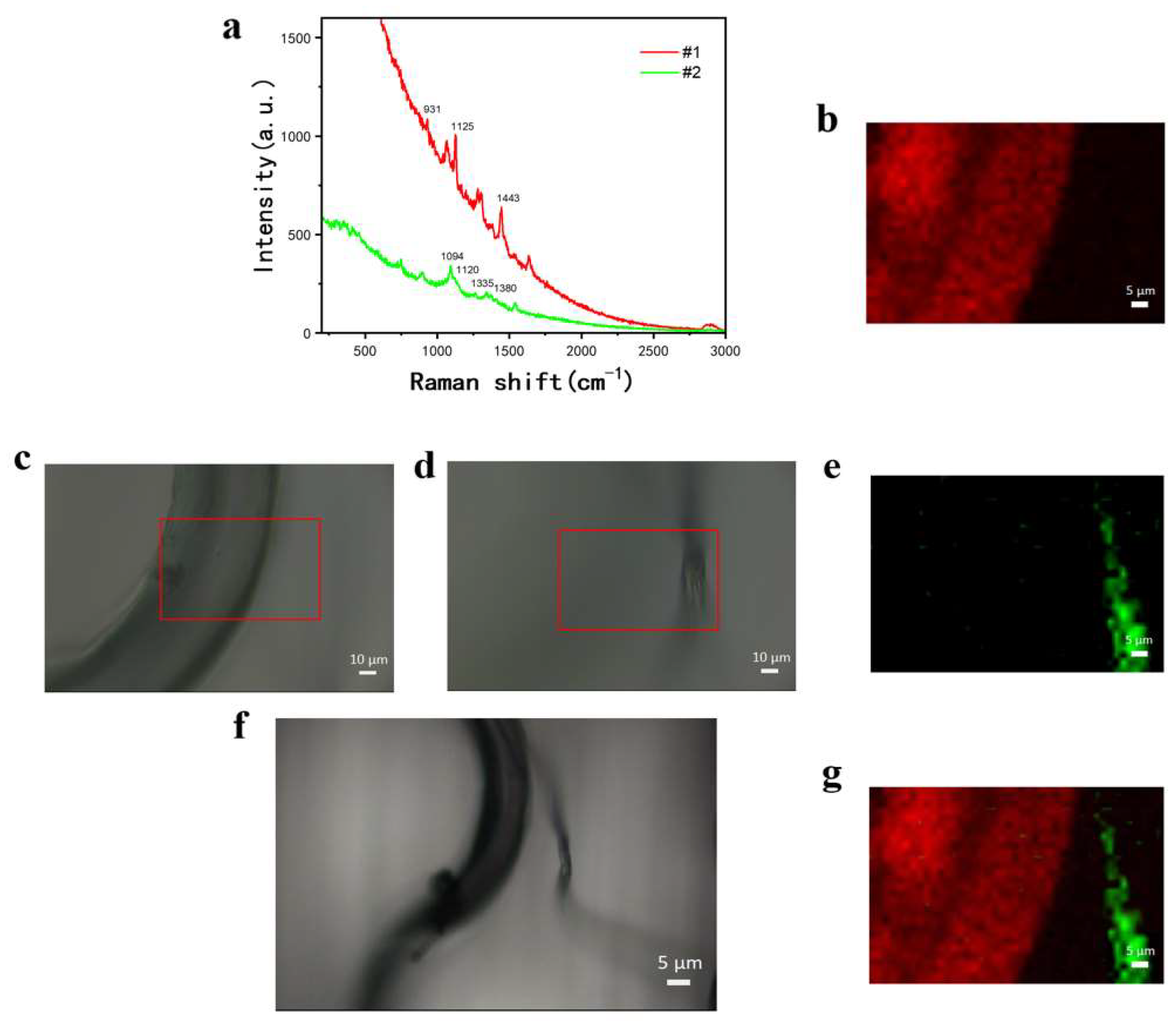
3.4. Raman Imaging of Dyed Blended Textile Fibers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, J.; Yu, L.; Ding, Q.; Wang, R. Textile fiber identification using Near-Infrared spectroscopy and pattern recognition. Autex Res. J. 2019, 19, 201–209. [Google Scholar]
- Huang, J.; He, H.; Lv, R.; Zhang, G.; Zhou, Z.; Wang, X. Non-destructive detection and classification of textile fibres based on hyperspectral imaging and 1D-CNN. Anal. Chim. Acta 2022, 1224, 340238. [Google Scholar] [PubMed]
- Graf, W.F.; Pearson, J.R.; Appadoo, D.R.T.; Robertson, E.G.; Ennis, C. The prediction of far-infrared spectra for molecular crystals of forensic interest—Phenylethylamine, ephedrine & pseudoephedrine. Forensic Chem. 2020, 17, 100204. [Google Scholar]
- Quintero Balbas, D.; Lanterna, G.; Cirrincione, C.; Fontana, R.; Striova, J. Non-invasive identification of textile fibres using near-infrared fibre optics reflectance spectroscopy and multivariate classification techniques. Eur. Phys. J. Plus 2022, 137, 85. [Google Scholar]
- Ryder, M.L.; Gabra-Sanders, T. A microscopic study of remains of textiles made from plant fibres. Oxf. J. Archaeol. 1987, 6, 91–108. [Google Scholar]
- Wu, J.; Sun, G.; Dai, L.; Xie, W. Qualitative analysis of textile fiber by Raman spectroscopy. J. Text. Res. 2011, 32, 28–33. [Google Scholar]
- Suomela, J.; Vajanto, K.; Räisänen, R. Seeking nettle textiles—Utilizing a combination of microscopic methods for fibre identification. Stud. Conserv. 2018, 63, 412–422. [Google Scholar]
- Gleba, M.; Harris, S. The first plant bast fibre technology: Identifying splicing in archaeological textiles. Archaeol. Anthropol. Sci. 2019, 11, 2329–2346. [Google Scholar] [CrossRef]
- Abbott, L.C.; Batchelor, S.N.; Smith, J.R.L.; Moore, J.N. Resonance Raman and UV–visible spectroscopy of black dyes on textiles. Forensic Sci. Int. 2010, 202, 54–63. [Google Scholar] [CrossRef]
- Buzzini, P.; Massonnet, G. The discrimination of colored acrylic, cotton, and wool textile fibers using micro-Raman spectroscopy. Part 1, in situ detection and characterization of dyes. J. Forensic Sci. 2013, 58, 1593–1600. [Google Scholar]
- Buzzini, P.; Massonnet, G. The Analysis of colored acrylic, cotton, and wool textile fibers using Micro-Raman spectroscopy. Part 2, Comparison with the traditional methods of fiber Examination. J. Forensic Sci. 2015, 60, 712–720. [Google Scholar] [PubMed]
- Mcgregor, B.A.; Liu, X.; Wang, X.G. Comparisons of the Fourier Transform Infrared Spectra of cashmere, guard hair, wool and other animal fibres. J. Text. Inst. 2018, 109, 813–822. [Google Scholar]
- Cassista, A.; Peters, A.D. Survey of red, green and blue cotton fibres. Can. Soc. Forensic Sci. J. 1997, 30, 225–231. [Google Scholar]
- Zięba-Palus, J.; Wąs-Gubała, J. An investigation into the use of micro-Raman spectroscopy for the analysis of car paints and single textile fibres. J. Mol. Struct. 2011, 993, 127–133. [Google Scholar]
- Buzzini, P.; Suzuki, E. Forensic applications of Raman spectroscopy for the in situ analyses of pigments and dyes in ink and paint evidence. J. Raman Spectrosc. 2016, 47, 16–27. [Google Scholar]
- Ren, J.; Mao, S.; Lin, J.; Xu, Y.; Zhu, Q.; Xu, N. Research progress of Raman Spectroscopy and Raman Imaging in pharmaceutical analysis. Curr. Pharm. Des. 2022, 28, e180522204991. [Google Scholar]
- López-López, M.; García-Ruiz, C. Infrared and Raman spectroscopy techniques applied to identification of explosives. TrAC Trends Anal. Chem. 2014, 54, 36–44. [Google Scholar]
- Chauhan, S.; Sharma, S. Applications of Raman spectroscopy in the analysis of biological evidence. Forensic Sci. Med. Pathol. 2024, 20, 1066–1090. [Google Scholar]
- Hermsen, A.; Schoettl, J.; Hertel, F.; Cerullo, M.; Schlueter, A.; Lehmann, C.W.; Mayer, C.; Jaeger, M. Green textile materials for Surface Enhanced Raman Spectroscopy identification of pesticides using a Raman handheld spectrometer for In-Field detection. Appl. Spectrosc. 2022, 76, 1222–1233. [Google Scholar]
- Zapata, F.; Ortega-Ojeda, F.E.; García-Ruiz, C. Forensic examination of textile fibres using Raman imaging and multivariate analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 268, 120695. [Google Scholar]
- He, Z.; Nam, S.; Fang, D. Raman spectroscopic assessment of fibers and seeds of six cotton genotypes. Agric. Environ. Lett. 2023, 8, e20102. [Google Scholar] [CrossRef]
- Puchowicz, D.; Giesz, P.; Kozanecki, M.; Cieślak, M. Surface-enhanced Raman spectroscopy (SERS) in cotton fabrics analysis. Talanta 2019, 195, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Dorota, P.; Malgorzata, C. Raman Spectroscopy in the analysis of textile structures. In Recent Developments in Atomic Force Microscopy and Raman Spectroscopy for Materials Characterization; Chandra, S.P., Samir, K.R., Eds.; IntechOpen: Rijeka, Croatia, 2021; pp. 1–21. [Google Scholar]
- Mather, R.R.; Wardman, R.H. Protein Fibres. In The Chemistry of Textile Fibres; Royal Society of Chemistry: Cambridge, UK, 2015; pp. 67–110. [Google Scholar]
- Mattiello, S.; Guzzini, A.; Del Giudice, A.; Santulli, C.; Antonini, M.; Lupidi, G.; Gunnella, R. Physico-Chemical Characterization of Keratin from Wool and Chicken Feathers Extracted Using Refined Chemical Methods. Polymers 2023, 15, 181. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.; Roux, C.; Wiggins, K.G. Forensic Examination of Fibres; CRC Press: Boca Raton, FL, USA, 2017; pp. 288–308. [Google Scholar]
- Prego, P.; García-Ruiz, C. Spectroscopic techniques for the forensic analysis of textile fibers. Appl. Spectrosc. Rev. 2016, 51, 258–281. [Google Scholar]
- Sobhani, Z.; Luo, Y.; Gibson, C.T.; Tang, Y.; Naidu, R.; Fang, C. Collecting microplastics in gardens: Case study (ii) from ropes. Environ. Technol. Innov. 2022, 26, 102322. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Chen, H. Identification and Visualization Textile Fibers by Raman Imaging. Materials 2025, 18, 1682. https://doi.org/10.3390/ma18071682
Liu K, Chen H. Identification and Visualization Textile Fibers by Raman Imaging. Materials. 2025; 18(7):1682. https://doi.org/10.3390/ma18071682
Chicago/Turabian StyleLiu, Kaili, and Huacai Chen. 2025. "Identification and Visualization Textile Fibers by Raman Imaging" Materials 18, no. 7: 1682. https://doi.org/10.3390/ma18071682
APA StyleLiu, K., & Chen, H. (2025). Identification and Visualization Textile Fibers by Raman Imaging. Materials, 18(7), 1682. https://doi.org/10.3390/ma18071682




