Anti-Swelling Aramid-Nanofiber-Reinforced Zwitterionic Polymer Hydrogel for Strain Sensors
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Aramid Nanofiber (ANF) Dispersion
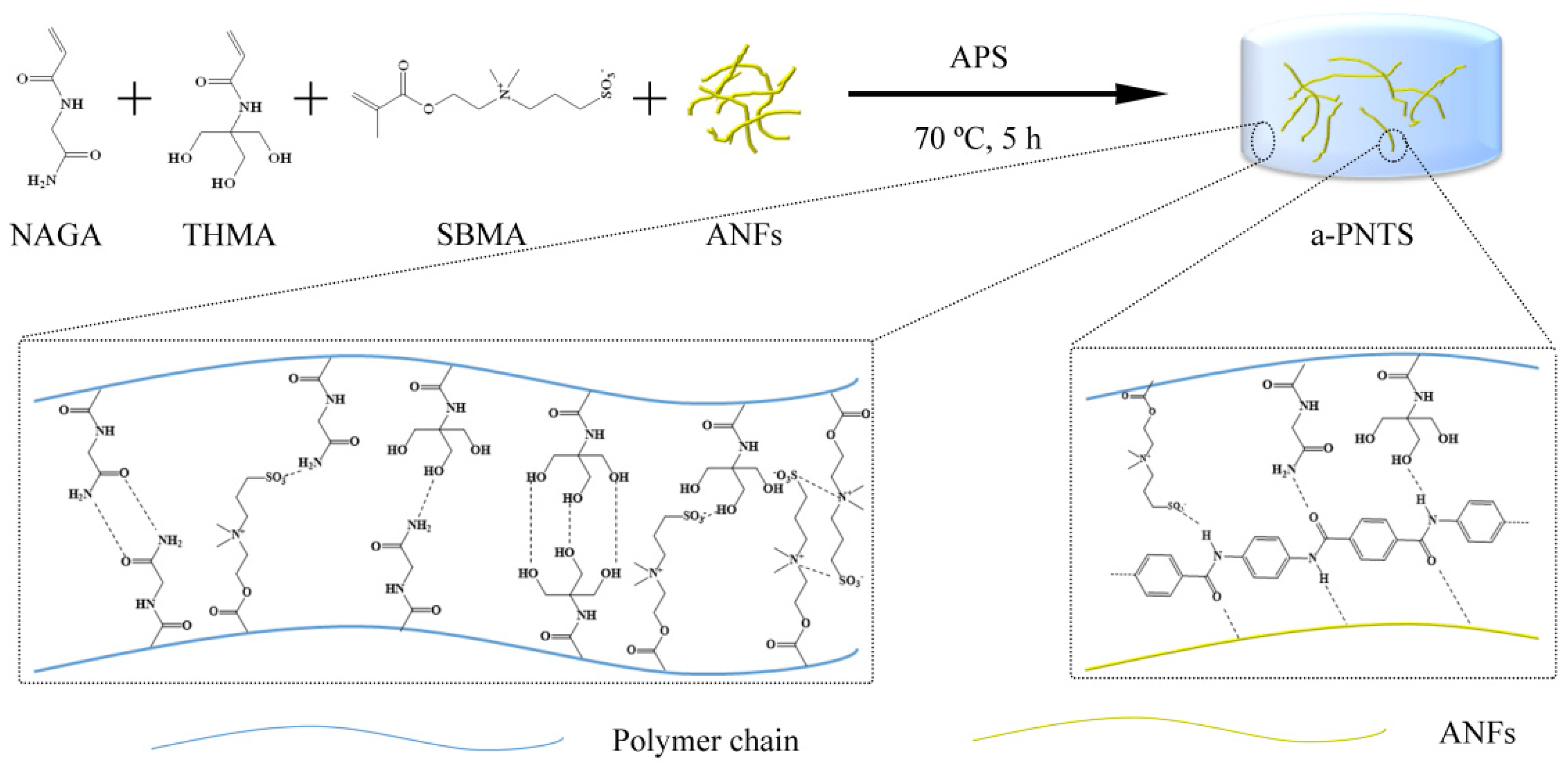
2.3. Preparation of a Series of a-PNTS, PNTS, PNT and PNS Hydrogels
2.4. Material Characterization
2.5. Measurement of Mechanical Properties
2.6. Measurement of Swelling Ratio
2.7. Measurement of Ionic Conductivity
3. Results and Discussion
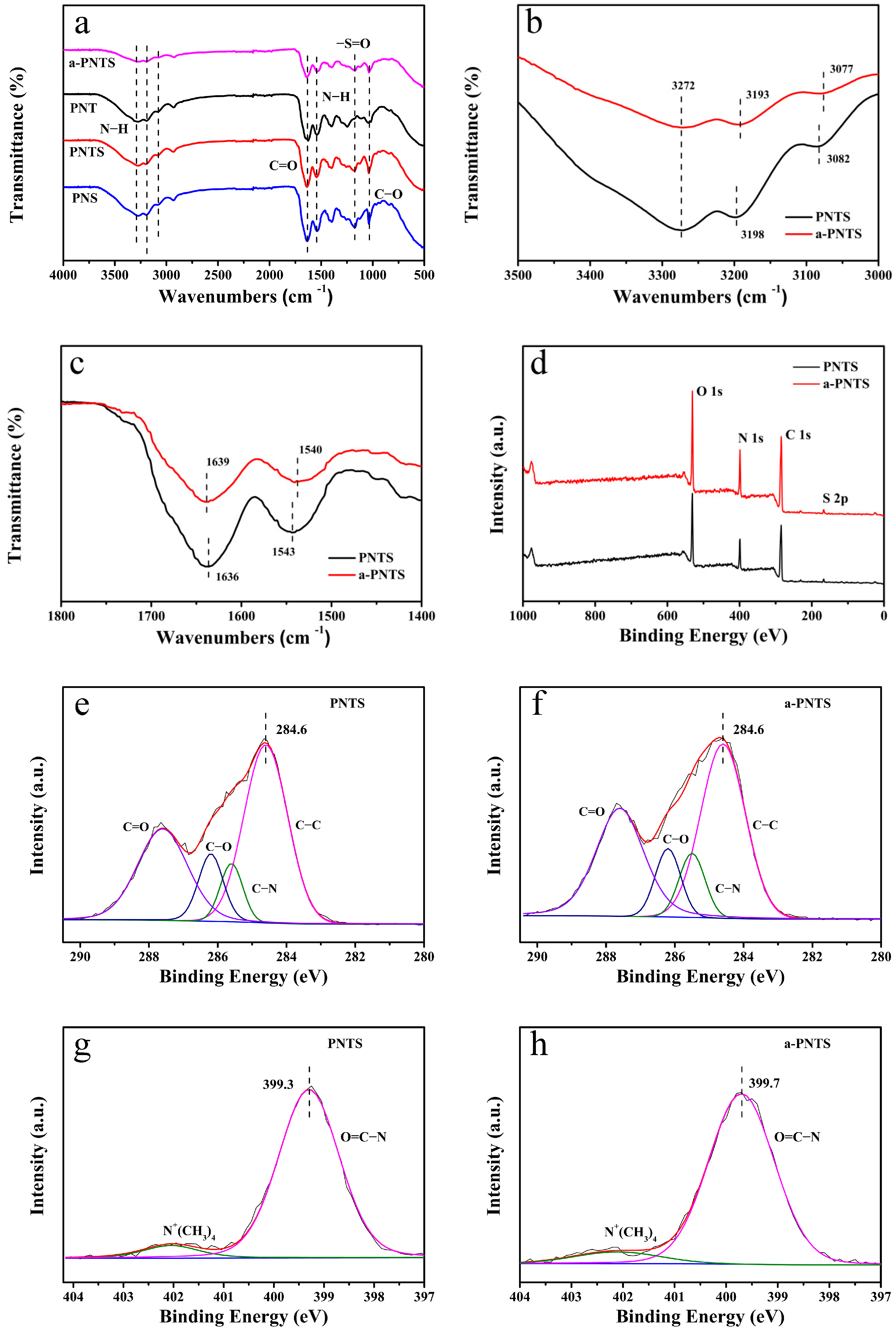
3.1. Chemical Composition of Hydrogels
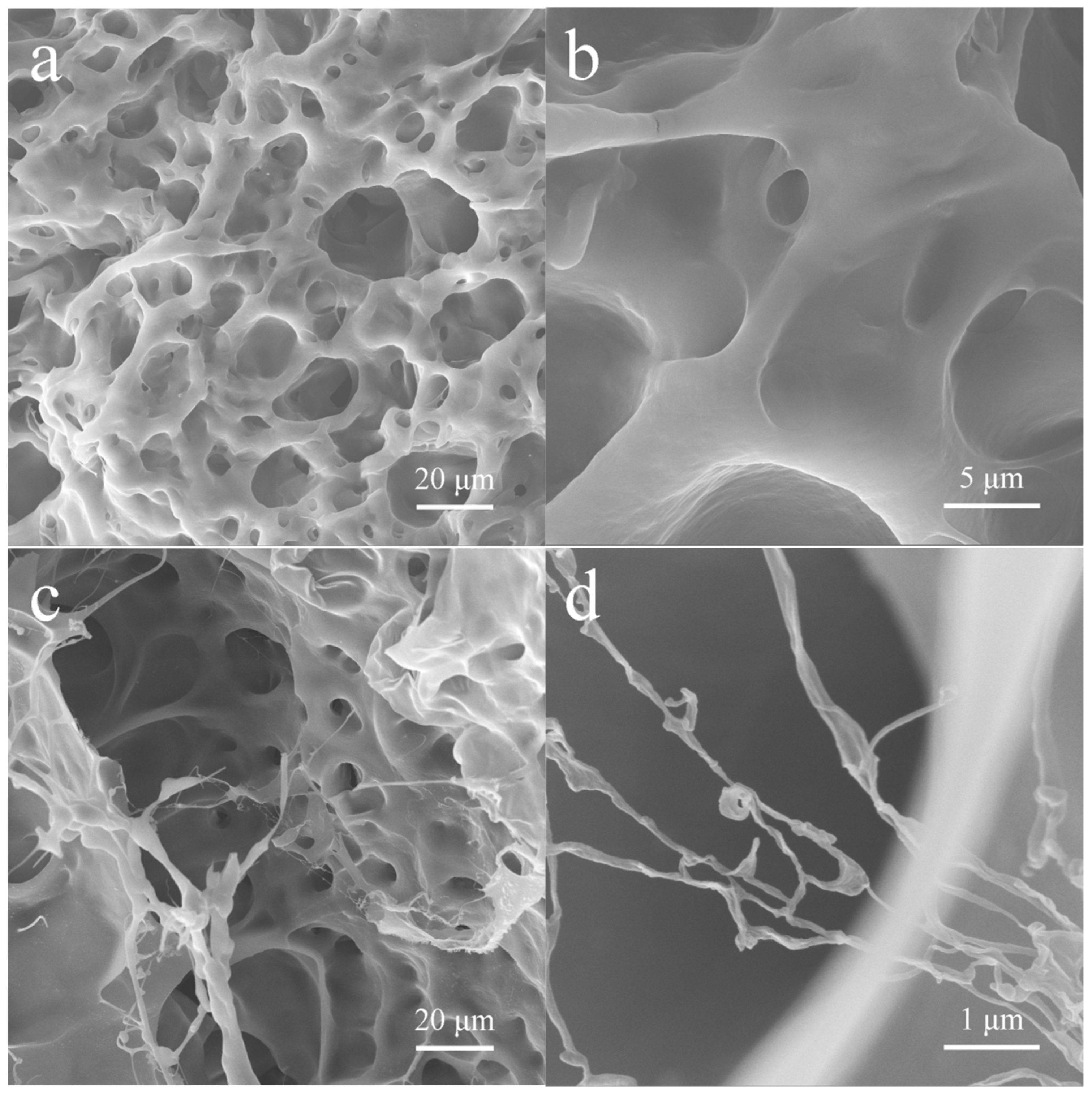
3.2. The Internal Microstructure
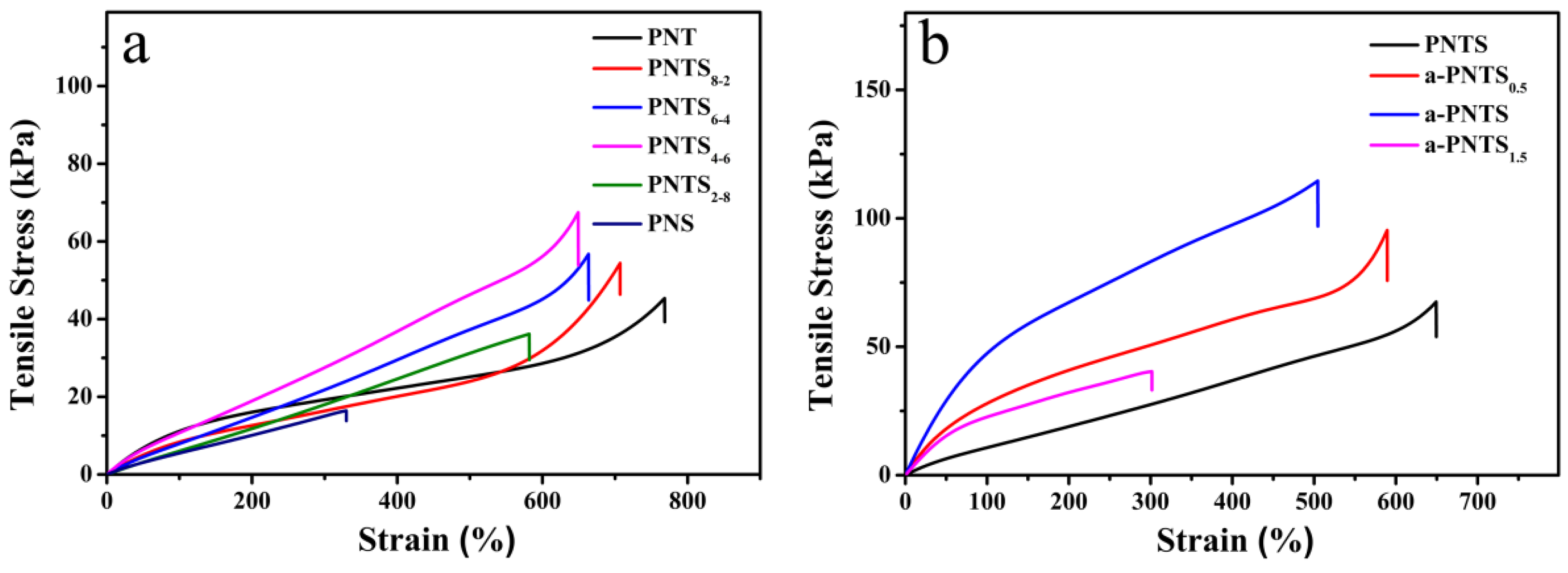
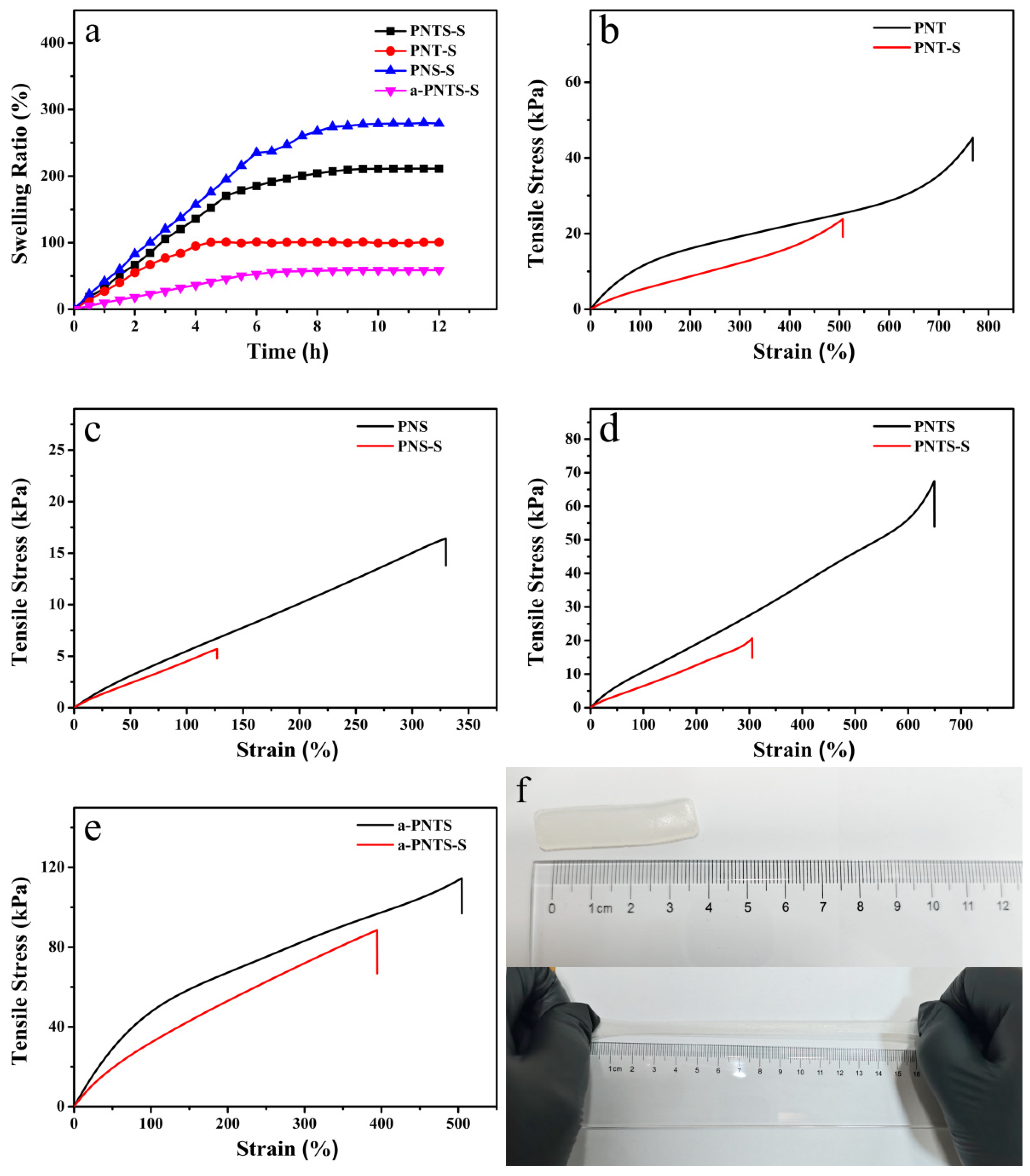
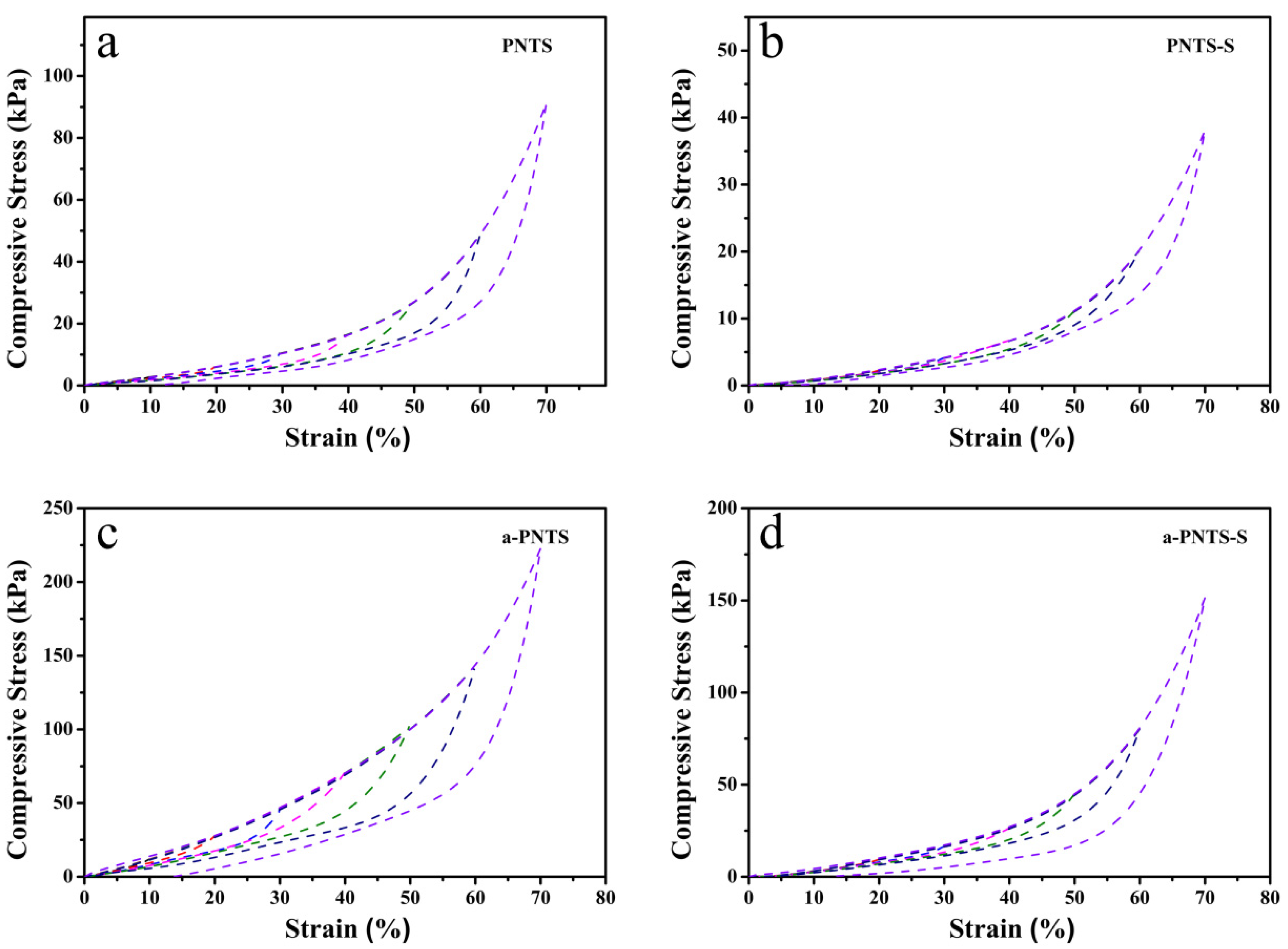
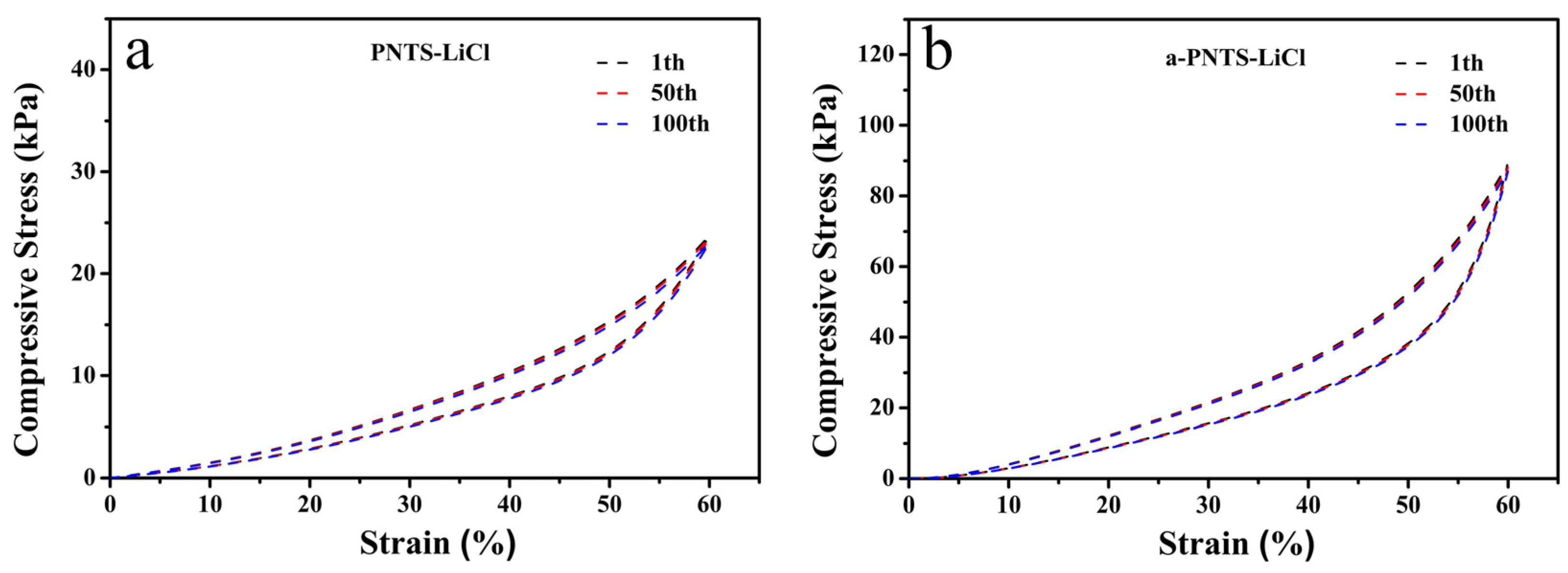
3.3. Mechanical Properties of Hydrogels
3.4. Swelling Behavior of Hydrogels
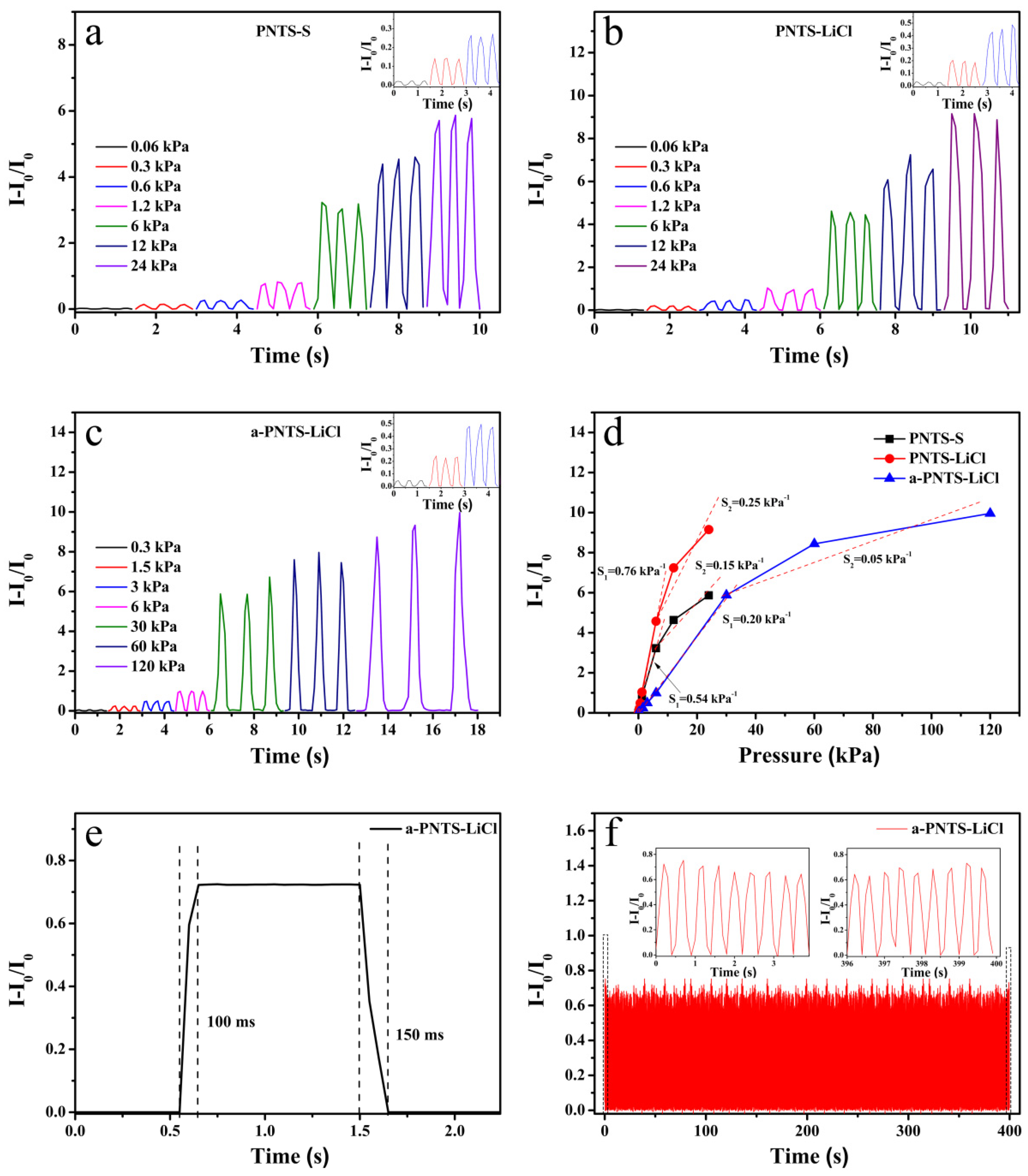
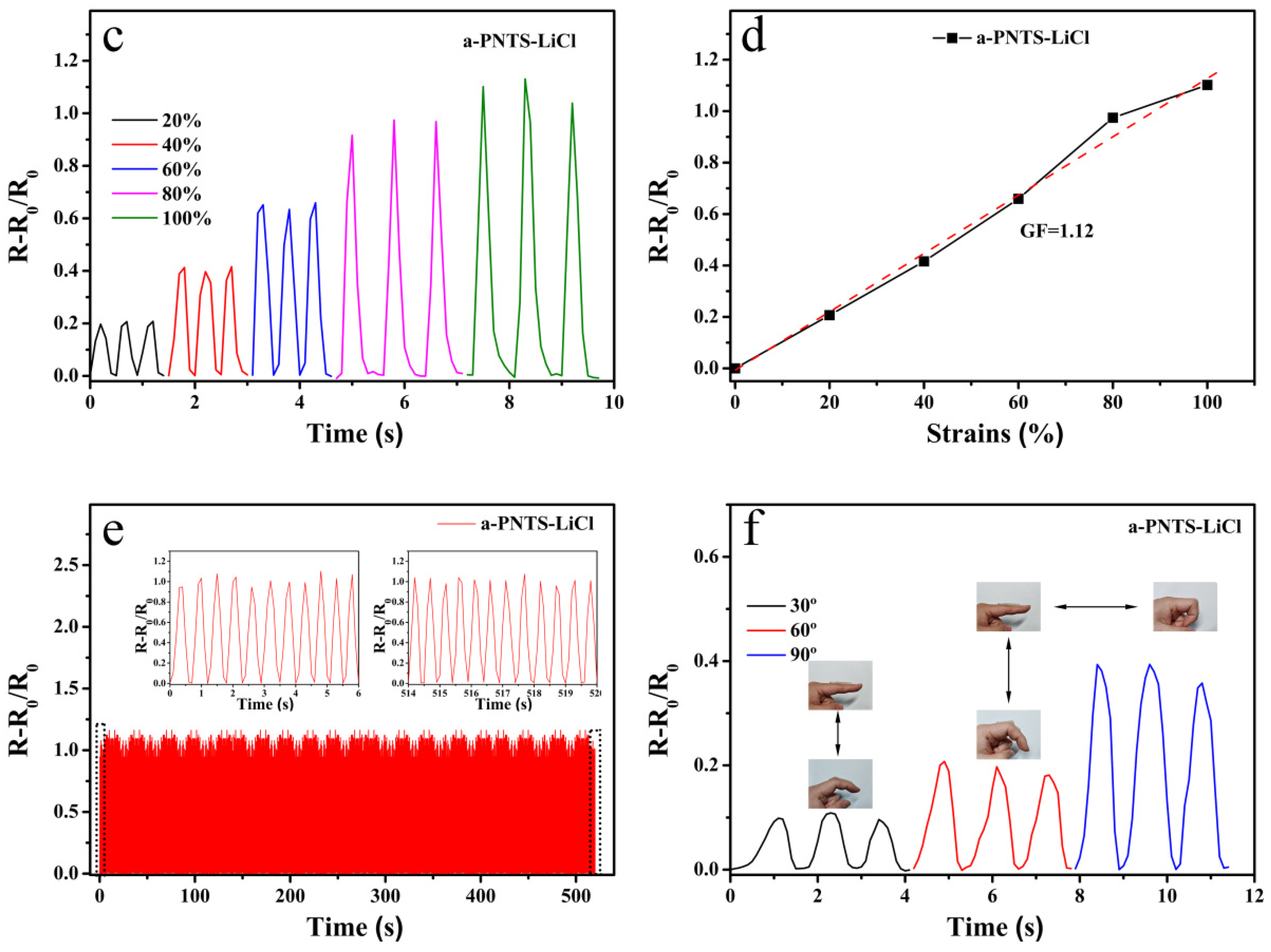
3.5. The Strain-Sensing Performance
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, J.; Lee, Y.; Cho, S.; Choe, A.; Yeo, J.; Ro, Y.G.; Kim, J.; Kang, D.; Lee, S.; Ko, H. Soft sensors and actuators for wearable human-machine interfaces. Chem. Rev. 2024, 124, 1464–1534. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Jiang, T.; Wang, M.; Li, T.; Song, Y.; Liu, J.; Wang, Z.; Jiang, H. Bioinspired monopolar controlled ionic hydrogels for flexible non-contact human-machine interfaces. Adv. Funct. Mater. 2024, 34, 2408338. [Google Scholar] [CrossRef]
- Chen, G.; Matsuhisa, N.; Liu, Z.; Qi, D.; Cai, P.; Jiang, Y.; Wan, C.; Cui, Y.; Leow, W.R.; Liu, Z.; et al. Plasticizing silk protein for on-skin stretchable electrodes. Adv. Mater. 2018, 30, 1800129. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhuang, Q.; Ding, Y.; Zhang, C.; Song, X.; Chen, Z.; Zhang, Y.; Mei, Q.; Zhao, X.; Huang, Q.; et al. Wet-adaptive electronic skin. Adv. Mater. 2023, 35, 2305630. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, L.; Liu, Y.; Leng, J. Metamaterial-based electronic skin with conformality and multisensory integration. Adv. Funct. Mater. 2024, 34, 2406789. [Google Scholar] [CrossRef]
- Wu, R.; Ma, L.; Patil, A.; Meng, Z.; Liu, S.; Hou, C.; Zhang, Y.; Yu, W.; Guo, W.; Liu, X.Y. Graphene decorated carbonized cellulose fabric for physiological signal monitoring and energy harvesting. J. Mater. Chem. A 2020, 8, 12665–12673. [Google Scholar] [CrossRef]
- Du, Y.; Kim, J.H.; Kong, H.; Li, A.A.; Jin, M.L.; Kim, D.H.; Wang, Y. Biocompatible electronic skins for cardiovascular health monitoring. Adv. Healthc. Mater. 2024, 13, 2302461. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, Y.; Li, Z. Emerging intelligent wearable devices for cardiovascular health monitoring. Nano Today 2024, 59, 102544. [Google Scholar] [CrossRef]
- Dang, G.; Chen, K.; Luo, Y.; Hu, R.; Huang, Y.; Tang, H.; Huang, B.; Sun, J.; Liu, X.; Wu, Y.; et al. Flexible fibrous visible light sensors based on spiropyran for wearable devices, electronic skins, and thermal management fabrics. Small Sci. 2024, 4, 2400018. [Google Scholar] [CrossRef]
- Hu, R.; Li, J.; Wu, F.; Lu, Z.; Deng, C. A dual function flexible sensor for independent temperature and pressure sensing. Chem. Eng. J. 2024, 391, 152135. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, H.; Zhao, Y.; Liu, H.; Nei, Z.; Xu, F.; Zhu, J.; Huang, W. Robust fiber-shaped flexible temperature sensors for safety monitoring with ultrahigh sensitivity. Adv. Mater. 2024, 36, 2310613. [Google Scholar] [CrossRef]
- Qiu, J.; Liu, S.; Guo, Y.; Yang, L.; Jiang, K. Anisotropic flexible pressure/strain sensors: Recent advances, fabrication techniques, and future prospects. Chem. Eng. J. 2025, 504, 158799. [Google Scholar] [CrossRef]
- Lyu, T.; Han, Y.; Chen, Z.; Fan, X.; Tian, Y. Hydrogels and hydrogel derivatives for atmospheric water harvesting. Mater. Today Sustain. 2024, 25, 100693. [Google Scholar] [CrossRef]
- Shu, Q.; Gu, Y.; Xia, W.; Lu, X.; Pang, Y.; Teng, J.; Liu, B.; Li, Y. Injectable hydrogels for bioelectronics: A viable alternative to traditional hydrogels. Chem. Eng. J. 2024, 495, 153391. [Google Scholar] [CrossRef]
- Guo, W.; Ma, M. Conductive nanocomposite hydrogels for flexible wearable sensors. J. Mater. Chem. A 2024, 12, 9339–9371. [Google Scholar]
- Lu, Y.; Zhong, W. A biocompatible, highly adhesive zwitterionic polymer hydrogel with high ionic conductivity, anti-freezing and moisturizing for wearable strain sensor. Chem. Eng. J. 2024, 490, 151691. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.; Zhao, G.; Zhang, Z.; Zhao, X.; Wan, X.; Zhang, Y.; Wang, Z.L.; Li, L. Stretchable unsymmetrical piezoelectric BaTiO3 composite hydrogel for triboelectric nanogenerators and multimodal sensors. ACS Nano 2022, 16, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, W.; Yang, W.; Chen, J.; Peng, Q.; Wang, T.; Yang, D.; Wang, J.; Zhang, H.; Zeng, H. Stretchable, compressible, and conductive hydrogel for sensitive wearable soft sensors. J. Colloid Interface Sci. 2022, 618, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dai, T.; Wu, H.; Ye, M.; Yuan, G.; Jia, H. Tannic acid-Fe3+ activated rapid polymerization of ionic conductive hydrogels with high mechanical properties, self-healing, and self-adhesion for flexible wearable sensors. Compos. Sci. Technol. 2022, 221, 109345. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, X.; Shu, H.; Zhong, W. Ultrahigh conductivity and antifreezing zwitterionic sulfobetaine hydrogel electrolyte for low-temperature resistance flexible supercapacitors. J. Mater. Chem. A 2023, 11, 9097–9111. [Google Scholar] [CrossRef]
- Fu, D.; Lu, Y.; Peng, Z.; Zhong, W. A zwitterionic hydrogel with a surprising function of increasing the ionic conductivity of alkali metal chloride or sulfuric acid water-soluble electrolyte. J. Mater. Chem. A 2023, 11, 13532–13551. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, F.; Mi, H.; Wu, Z.; Ji, C.; Yang, C.; Qiu, J. Kinetics-boosted effect enabled by zwitterionic hydrogel electrolyte for highly reversible zinc anode in zinc-ion hybrid micro-supercapacitors. Adv. Energy Mater. 2022, 12, 2202219. [Google Scholar] [CrossRef]
- Wang, H.; Meng, L.; Ye, Y.; Wu, J.; Zhu, S.; Liu, Y.; Li, K.; Yang, X.; Wei, M.; Wang, M.; et al. Antibacterial zwitterionic hydrogel for flexible and wearable ultrafast-response strain sensors with low hysteresis. Giant 2024, 17, 100234. [Google Scholar] [CrossRef]
- Liu, J.; Deng, J.; Zhao, F.; Wu, T.; Xing, J. A highly stretchable, adhesive, anti-freezing hydrogel with prominent antibacterial property rapidly prepared by photopolymerization for wound dressing. Colloids Surf. A Physicochem. Eng. Asp. 2024, 683, 133087. [Google Scholar] [CrossRef]
- Zhang, Z.; Raffa, P. Anti-freezing conductive zwitterionic composite hydrogels for stable multifunctional sensors. Eur. Polym. J. 2023, 199, 112484. [Google Scholar] [CrossRef]
- Niu, Y.; Liu, H.; He, R.; Li, Z.; Ren, H.; Gao, B.; Guo, H.; Genin, G.M.; Xu, F. The new generation of soft and wearable electronics for health monitoring in varying environment: From normal to extreme conditions. Mater. Today 2020, 41, 219–242. [Google Scholar] [CrossRef]
- Su, X.; Hao, D.; Xu, X.; Guo, X.; Li, Z.; Jiang, L. Hydrophilic/hydrophobic heterogeneity anti-biofouling hydrogels with well-regulated rehydration. ACS Appl. Mater. Interfaces 2020, 12, 25316–25323. [Google Scholar] [CrossRef]
- Qin, Z.; Yu, X.; Wu, H.; Li, J.; Lv, H.; Yang, X. Nonswellable and tough supramolecular hydrogel based on strong micelle cross-linkings. Biomacromolecules 2019, 20, 3399–3407. [Google Scholar] [CrossRef]
- Delplace, V.; Nickerson, P.E.B.; Ortin-Martinez, A.; Baker, A.E.G.; Wallace, V.A.; Shoichet, S. Nonswelling, ultralow content inverse electron-demand diels–alder hyaluronan hydrogels with tunable gelation time: Synthesis and in vitro evaluation. Adv. Funct. Mater. 2020, 30, 1903978. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, L. Encapsulation of hydrogel sensors. Chem. Eng. J. 2024, 484, 149631. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, L.; Song, B.; Bai, X.; Huang, Z.; Bu, X.; Chen, T.; Fu, H.; Guo, P. High-strain sensitive zwitterionic hydrogels with swelling-resistant and controllable rehydration for sustainable wearable sensor. J. Colloid Interface Sci. 2022, 620, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, W. Poly(N-acryloyl glycinamide): A fascinating polymer that exhibits a range of properties from UCST to high-strength hydrogels. Chem. Commun. 2018, 54, 10540. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, Y.; Li, S.; Deng, L.; Zhang, J.; Huang, P.; Feng, Z.; Kong, D.; Wang, W.; Dong, A. Skin-adaptable, long-lasting moisture, and temperature-tolerant hydrogel dressings for accelerating burn wound healing without secondary damage. ACS Appl. Mater. Interfaces 2021, 13, 59695–59707. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Xu, Z.; Liang, Q.; Liu, B.; Li, H.; Wu, Y.; Zhang, Y.; Lin, Z.; Wu, M.; Ruan, C.; et al. Direct 3D printing of high strength biohybrid gradient hydrogel scaffolds for efficient repair of osteochondral defect. Adv. Funct. Mater. 2018, 28, 1706644. [Google Scholar] [CrossRef]
- Yang, M.; Cao, K.; Sui, L.; Qi, Y.; Zhu, J.; Waas, A.; Arruda, E.M.; Kieffer, J.; Thouless, M.D.; Kotov, N.A. Dispersions of aramid nanofibers: A new nanoscale building block. ACS Nano 2011, 5, 6945–6954. [Google Scholar] [CrossRef]
- Lin, J.; Bang, S.H.; Malakooti, M.H.; Sodano, H.A. Isolation of aramid nanofibers for high strength and toughness polymer nanocomposites. ACS Appl. Mater. Interfaces 2017, 9, 11167–11175. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhang, X.N.; Song, Y.; Zhao, Y.; Chen, L.; Su, F.; Li, L.; Wu, Z.L.; Zheng, Q. Ultrastiff and tough supramolecular hydrogels with a dense and robust hydrogen bond network. Chem. Mater. 2019, 31, 1430–1440. [Google Scholar] [CrossRef]
- Zhang, X.N.; Wang, Y.J.; Sun, S.; Hou, L.; Wu, P.; Wu, Z.L.; Zheng, Q. A tough and stiff hydrogel with tunable water content and mechanical properties based on the synergistic effect of hydrogen bonding and hydrophobic interaction. Macromolecules 2018, 51, 8136–8146. [Google Scholar] [CrossRef]
- Zhang, H.J.; Sun, T.L.; Zhang, A.K.; Ikura, Y.; Nakajima, T.; Nonoyama, T.; Kurokawa, T.; Ito, O.; Ishitobi, H.; Gong, J.P. Tough physical double-network hydrogels based on amphiphilic triblock copolymers. Adv. Mater. 2016, 28, 4884–4890. [Google Scholar] [CrossRef]
- Li, W. A semi-interpenetrating poly(ionic liquid) network-driven low hysteresis and transparent hydrogel as a self-powered multifunctional sensor. Adv. Funct. Mater. 2024, 34, 2401607. [Google Scholar]
- Li, Y.; Yan, J.; Liu, Y.; Xie, X. Super tough and intelligent multibond network physical hydrogels facilitated by Ti3C2Tx Mxene nanosheets. ACS Nano 2022, 16, 1567–1577. [Google Scholar] [CrossRef]
- Guo, X.; Lu, Y.; Fu, D.; Yu, C.; Yang, X.; Zhong, W. Ultrahigh ionic conductivity and alkaline tolerance of poly(amidoxime)-based hydrogel for high performance piezoresistive sensor. Chem. Eng. J. 2023, 452, 139208. [Google Scholar] [CrossRef]
- Song, X.; Guo, J.; Zhang, Y.; Guan, F.; Tao, J.; Yao, Q.; Ji, X. Multi-network hydrogel-based stretchable, self-adhesive, and self-healing self-powered flexible sensors for multi-scale, dynamic and static strain/pressure sensing. Colloids Surf. A Physicochem. Eng. Asp. 2025, 708, 136035. [Google Scholar] [CrossRef]
- Shen, K.; Xu, K.; Zhang, M.; Yu, J.; Yang, Y.; Zhao, X.; Zhang, Q.; Wu, Y.; Zhang, Y.; Cheng, Y. Multiple hydrogen bonds reinforced conductive hydrogels with robust elasticity and ultra-durability as multifunctional ionic skins. Chem. Eng. J. 2023, 451, 138525. [Google Scholar] [CrossRef]
- Sun, C.; Chen, J.; Han, Z.; Zhang, Y.; Yang, F.; Xu, H.; Liu, C.; Shen, C. Unique framework effect induced by uniform silk fibroin dynamic nanospheres enables multiscale hydrogel with outstanding elastic resilience and strain sensing performanc. Int. J. Biol. Macromol. 2024, 281, 136422. [Google Scholar] [CrossRef]
- Wang, F.; Song, D.; Zhou, C.; Li, X.; Huang, Y.; Xu, W.; Liu, G.; Zhou, S. Mxene-based skin-like hydrogel sensor and machine learning assisted hand writing recognition. ACS Appl. Mater. Interfaces 2024, 16, 41583–41595. [Google Scholar] [CrossRef]
- Wang, H.; Ding, Q.; Luo, Y.; Wu, Z.; Yu, J.; Chen, H.; Zhou, Y.; Zhang, H.; Tao, K.; Chen, X.; et al. High-performance hydrogel sensors enabled multimodal and accurate human–machine interaction system for active rehabilitation. Adv. Mater. 2023, 36, 2309868. [Google Scholar] [CrossRef]
- Lu, X.; Si, Y.; Zhang, S.; Yu, J.; Ding, B. In situ synthesis of mechanically robust, transparent nanofiber-reinforced hydrogels for highly sensitive multiple sensing. Adv. Funct. Mater. 2021, 31, 2103117. [Google Scholar] [CrossRef]
- He, S.; Liu, Z.; Wu, X.; Liu, J.; Fang, H.; Shao, W. Novel flexible hydrogels based on carboxymethyl guar gum and polyacrylic acid for ultra-highly sensitive and reliable strain and pressure sensors. Carbohydr. Polym. 2024, 324, 121515. [Google Scholar] [CrossRef]


| Sample | NAGA (mg) | THMA (mg) | SBMA (mg) | ANFs (mg) | DI Water (mL) |
|---|---|---|---|---|---|
| PNS | 400 | 0 | 200 | 0 | 2 |
| PNT | 400 | 200 | 0 | 0 | 2 |
| PNTS | 400 | 80 | 120 | 0 | 2 |
| PNTS2-8 | 400 | 40 | 160 | 0 | 2 |
| PNTS6-4 | 400 | 120 | 80 | 0 | 2 |
| PNTS8-2 | 400 | 160 | 40 | 0 | 2 |
| a-PNTS-0.5 | 400 | 80 | 120 | 3 | 2 |
| a-PNTS | 400 | 80 | 120 | 6 | 2 |
| a-PNTS-1.5 | 400 | 80 | 120 | 9 | 2 |
| Sample | C (at.%) | N (at.%) | O (at.%) | S (at.%) |
|---|---|---|---|---|
| PNTS | 64.9 | 13.0 | 21.1 | 1.0 |
| a-PNTS | 64.0 | 14.1 | 20.8 | 1.1 |
| Sample | C−C (284. 6 eV) | C−N (285.5 eV) | C−O (286.2 eV) | C=O (287.6 eV) |
|---|---|---|---|---|
| PNTS | 49.5 | 8.5 | 10.5 | 31.5 |
| a-PNTS | 45.8 | 9.7 | 10.2 | 34.4 |
| Sample | C−N | −N+ (CH3)2 (402.1 eV) |
|---|---|---|
| PNTS | 92.7 | 7.3 |
| a-PNTS | 92.1 | 7.9 |
| Material | Sensitivity (Detection Rage) | Detection Limit | Response Time, Recovery Time | Ref |
|---|---|---|---|---|
| PAAm/AKP/Alg-Zn2+ | 0.35 kPa−1 (<5 kPa) 0.05 kPa−1 (5–50 kPa) | - | 200 ms, 200 ms | [43] |
| P(AA-APA)-Fe3+ | 0.015 kPa−1 (<20 kPa) 0.0072 kPa−1 (20–70 kPa) 0.0022 kPa−1 (70–150 kPa) | 1 kPa | 180 ms, 170 ms | [44] |
| APS/AgNW | 0.30 kPa−1 (<1 kPa) 0.033 kPa−1 (1–14 kPa) 2.27 MPa−1 (14–64 kPa) 0.32 MPa−1 (64–250 kPa) | - | 271 ms, 296 ms | [45] |
| PMAA@MXene | 5.15 MPa−1 (<0.09 MPa) 0.09 MPa−1 (0.09–1.82 MPa) | - | 0.75 s, - | [46] |
| NaCl-TA-PAM | 0.95 kPa−1 (<0.448 kPa) 0.17 kPa−1 (0.638–1.641 kPa) | 16 Pa | 20 ms, 40 ms | [47] |
| SFRHs | 0.038 kPa−1 (<16.5 kPa) 0.008 kPa−1 (16.5–28 kPa) | 1 kPa | - | [48] |
| CMGG/PAA/LS/Al3+ | 0.104 kPa−1 (<2.5 kPa) 0.015 kPa−1 (16.5–24 kPa) | - | 200 ms, 200 ms | [49] |
| PNTS-LiCl | 0.76 kPa−1 (<6 kPa) 0.25 kPa−1 (6–24 kPa) | 60 Pa | 100 ms, 150 ms | This work |
| a-PNTS-LiCl | 0.20 kPa−1 (<30 kPa) 0.05 kPa−1 (30–120 kPa) | 300 Pa | 100 ms, 150 ms | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Zhong, W. Anti-Swelling Aramid-Nanofiber-Reinforced Zwitterionic Polymer Hydrogel for Strain Sensors. Materials 2025, 18, 1800. https://doi.org/10.3390/ma18081800
Chen Z, Zhong W. Anti-Swelling Aramid-Nanofiber-Reinforced Zwitterionic Polymer Hydrogel for Strain Sensors. Materials. 2025; 18(8):1800. https://doi.org/10.3390/ma18081800
Chicago/Turabian StyleChen, Zeyu, and Wenbin Zhong. 2025. "Anti-Swelling Aramid-Nanofiber-Reinforced Zwitterionic Polymer Hydrogel for Strain Sensors" Materials 18, no. 8: 1800. https://doi.org/10.3390/ma18081800
APA StyleChen, Z., & Zhong, W. (2025). Anti-Swelling Aramid-Nanofiber-Reinforced Zwitterionic Polymer Hydrogel for Strain Sensors. Materials, 18(8), 1800. https://doi.org/10.3390/ma18081800






