Advances in Nanostructured Electrodes for Solid Oxide Cells by Infiltration or Exsolution
Abstract
1. Introduction
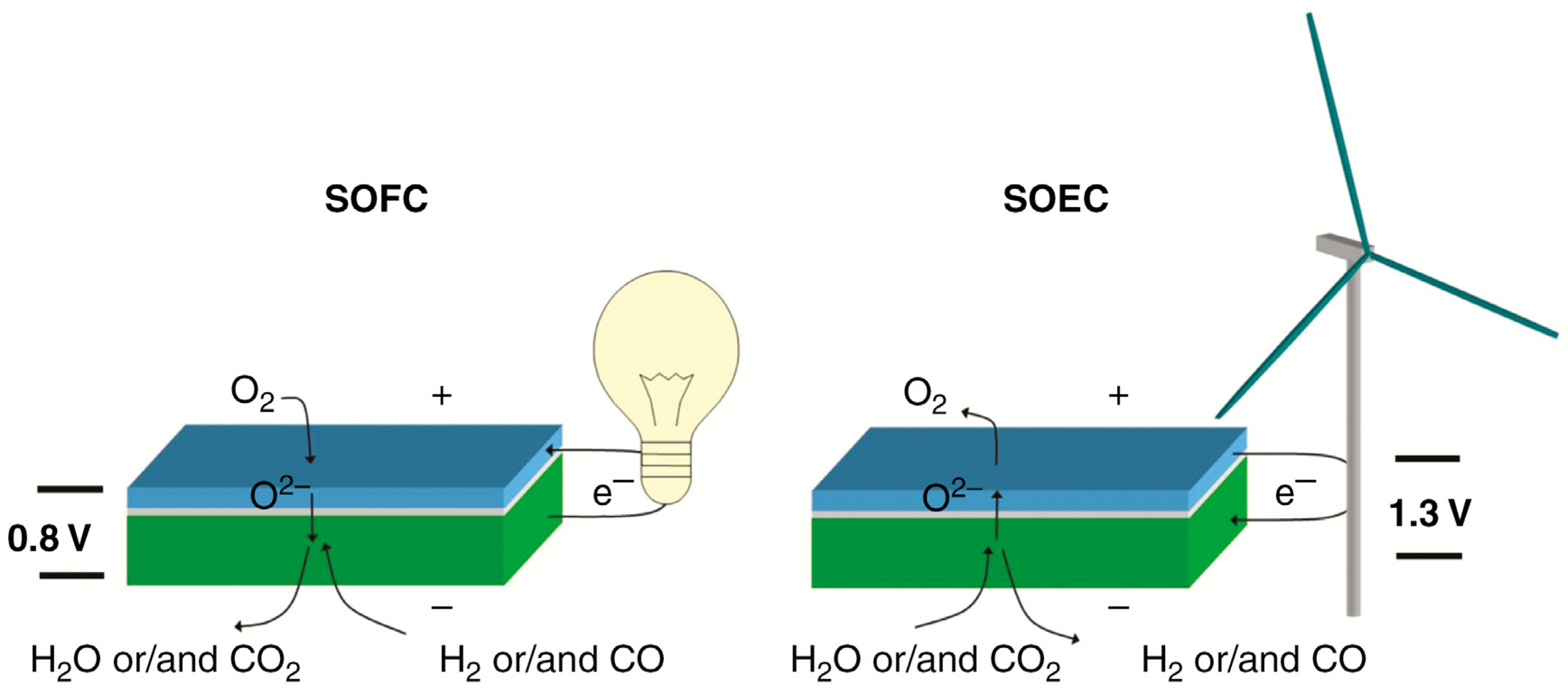
2. Infiltration
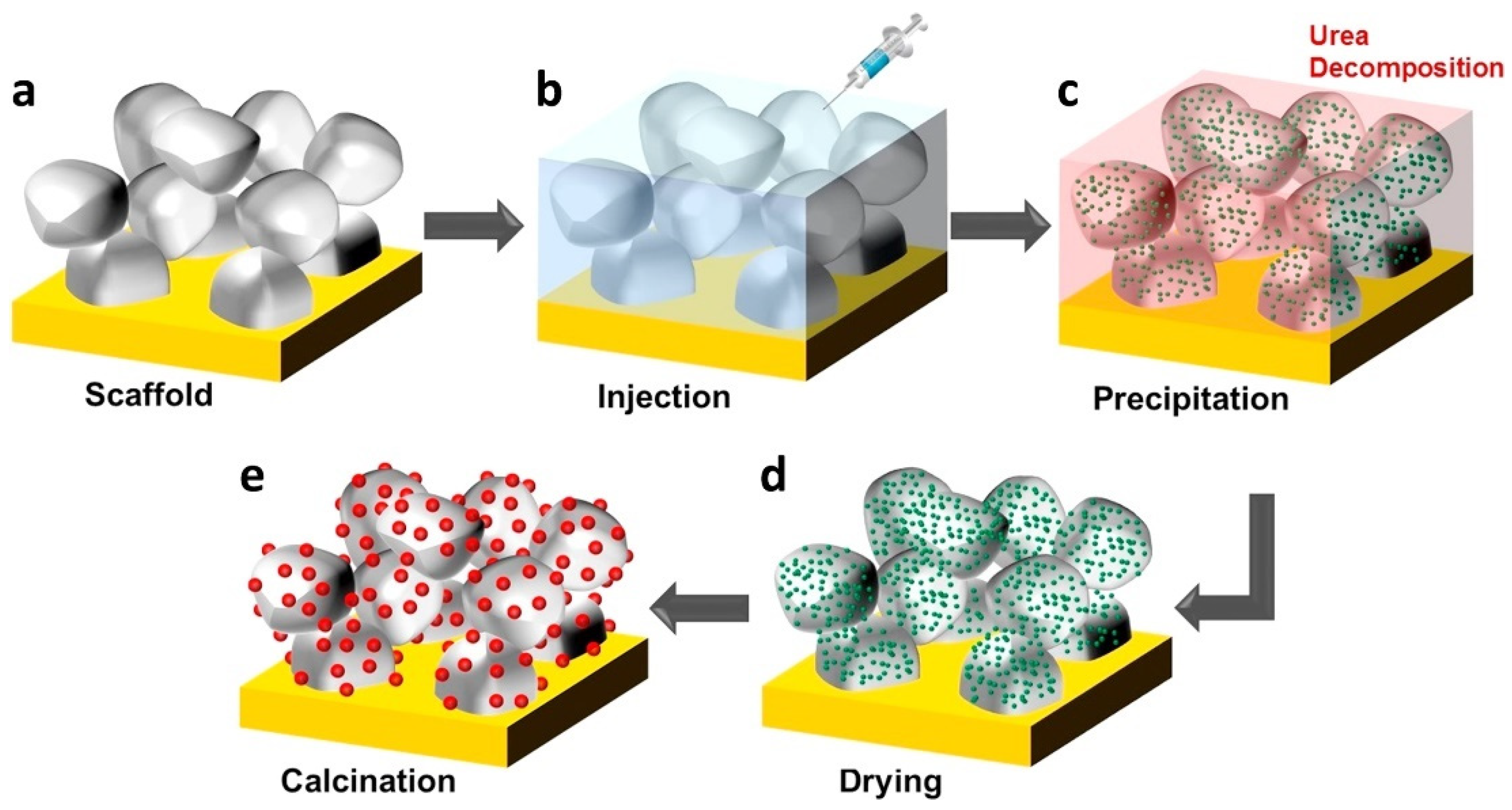
2.1. Metal Infiltration
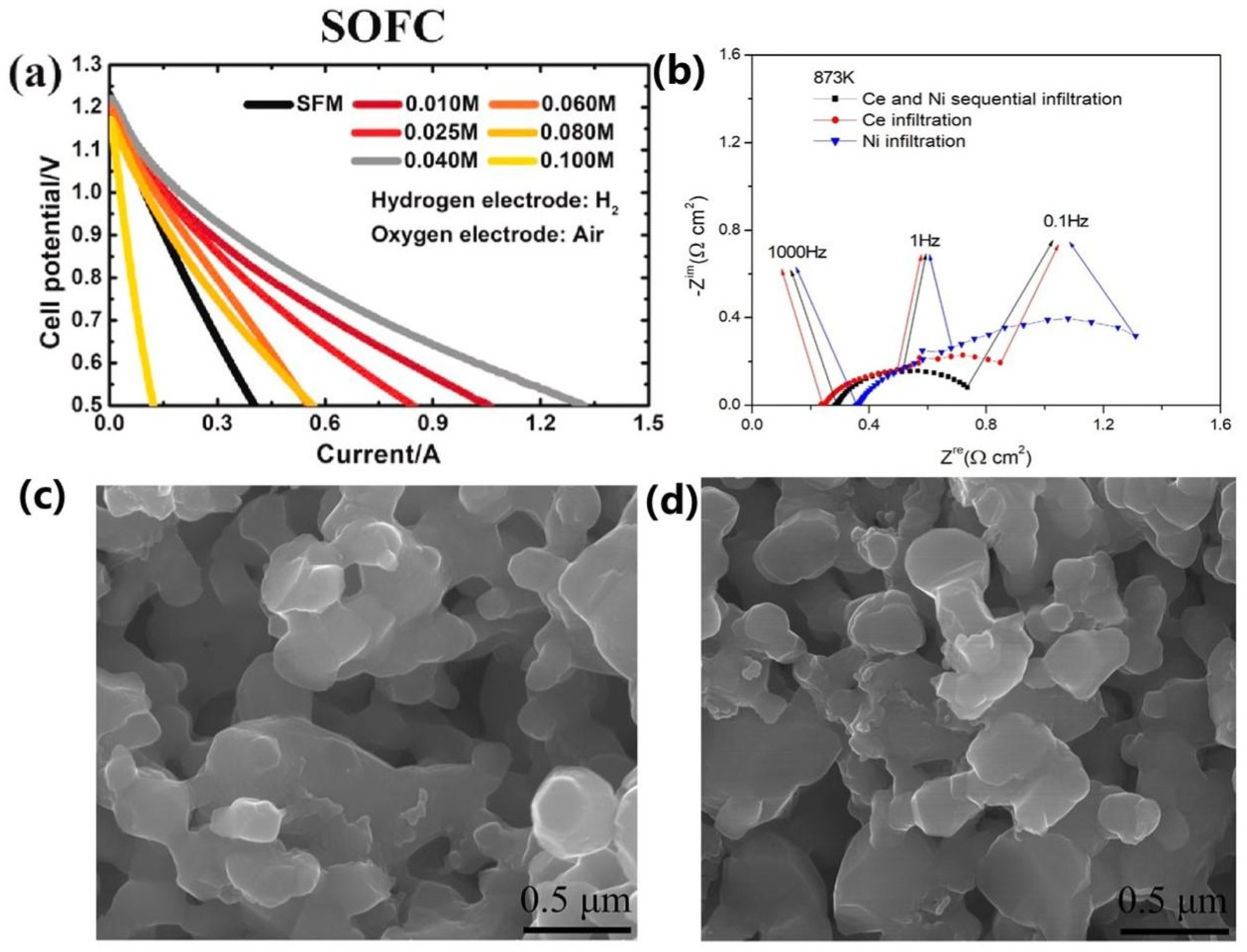
| Infiltrated Material | Cell Configuration Anode|Electrolyte| Cathode | T [°C] | Gas Composition | Potential [V] | Current Density [A cm−2] | Ref. |
|---|---|---|---|---|---|---|
| (Ce/Pr/Mn)Ox | LSM/YSZ|YSZ LSM/YSZ | 800 | H2/H2O (50:50) | 0.7 | 1.3 | [43] |
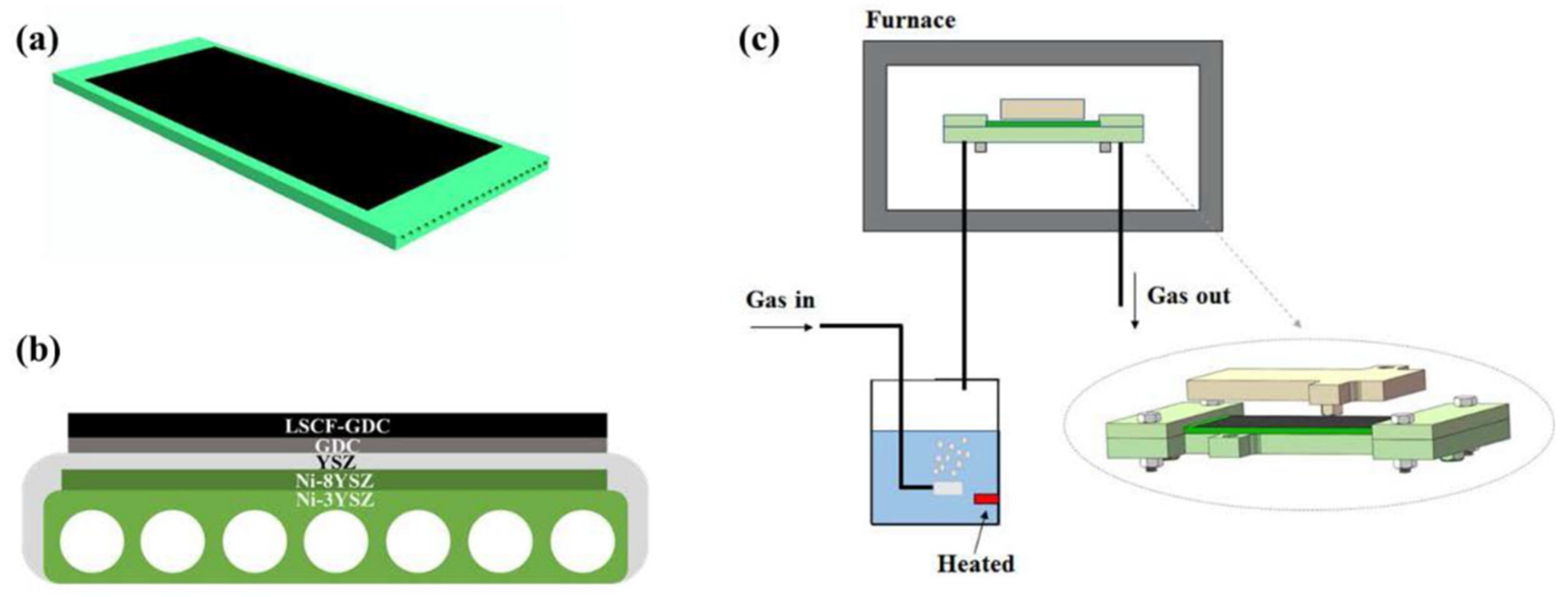
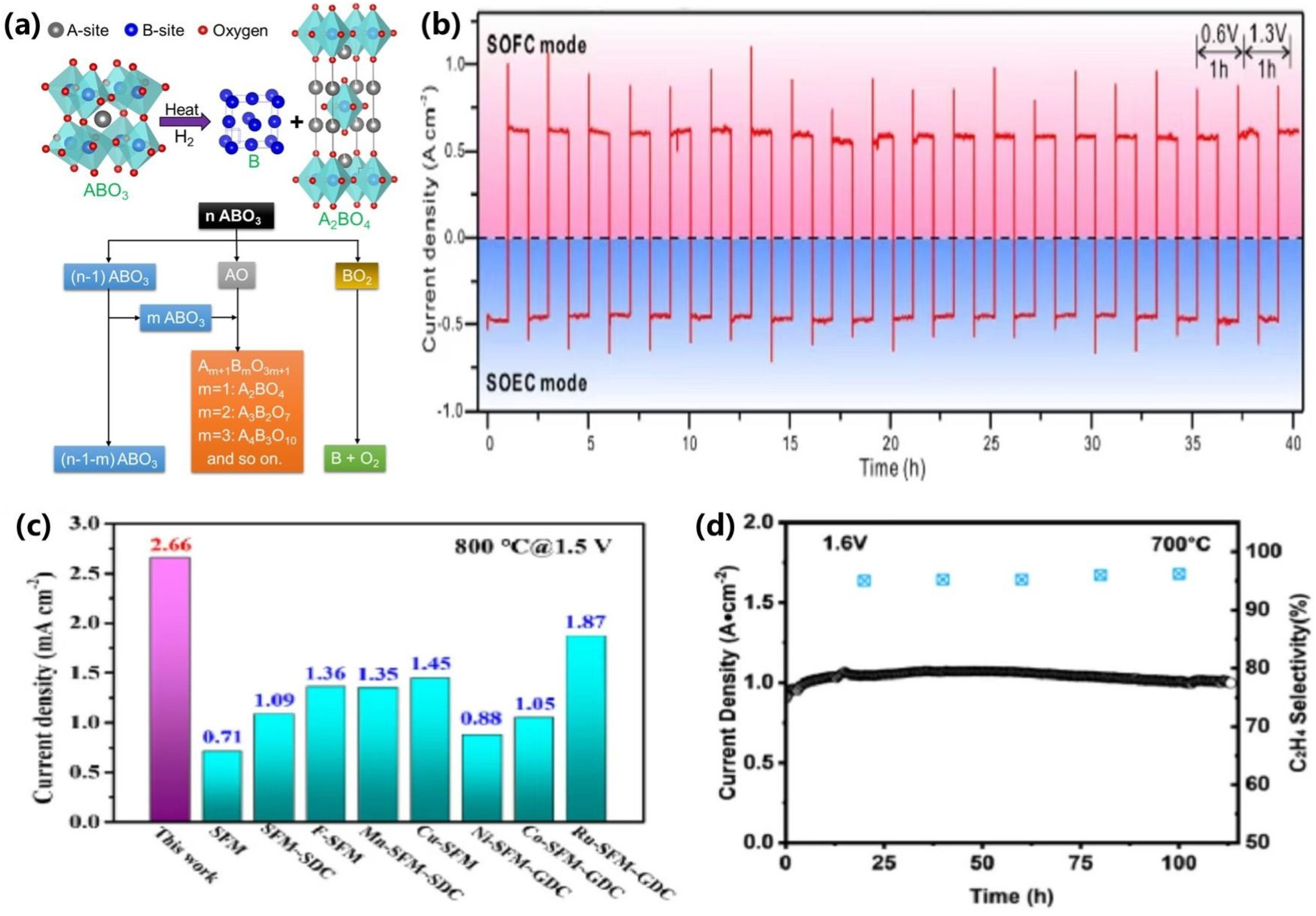
| Pt | Ni-YSZ|YSZ|SFM-SDC | 800 | Oxygen electrode: air Hydrogen electrode: 75% H2O-25% H2 | 1.5 | 3.28 | [47] |
| Ce/Ni | SSC|LSGM|NiO-YSZ | 600 | Oxygen electrode: air Hydrogen electrode: 17% H2O-25% H2-58% Ar | ~0.9 | 1.2 | [48] |
| Fe | Ni-YSZ|YSZ|LSCF-GDC | 800 | H2O/CO2/H2 | ~1.05 | 0.4 | [49] |
| PdO | LSM-YSZ|YSZ|Ni-YSZ | 750 | H2O (90 vol%) | 2.0 | 2.322 | [50] |
| Pr6O11 | LSCF|CGO|LSCF | 650 | / | / | / | [53] |
| Ni | SFM|LSGM|LSCF-GDC | 800 | H2 (3 vol% H2O) | ~0.6 | 0.7 | [54] |
| Ni | SFM-SDC|LSGM| SFM-SDC | 850 | Cathode: H2-H2O Anode: CH4-H2O | 0.5 | 1.022 | [56] |
| CeO2 | PBSCF-BZCYYb|BZCYYb|BZCYYb | 550 | Fuel electrode: CO2-Ar Oxygen electrode: Ar with 30% H2O | 1.5 | 1.12 | [57] |
| Ru | SFM-SDC|LSGM|SFM-SDC | 850 | Cathode: 74% H2O-26% H2 Anode: 5.8% CH4-91.2% N2-3% H2O | ~0.8 | 1.1 | [58] |
2.2. Perovskite Infiltration
| Infiltrated Material | Cell Configuration Anode|Electrolyte| Cathode | T [°C] | Gas Composition | Potential [V] | Current Density [A cm−2] | Ref. |
|---|---|---|---|---|---|---|
| SrTi0.3Fe0.6Co0.1O3-δ(STFC) | LSM-YSZ|YSZ|LSM-YSZ | 700 | Cathode: air Anode: 97% H2 + 3% H2O | ~0.8 | 0.5 | [55] |
| La0.6Sr0.4CoO3-δ (LSC) | LSCF|YSZ|LSCF | 800 | 50%: 50% (H2: H2O) | 1.4 | 1.75 | [60] |
| Sr2Fe1.5Mo0.5O6−δ (SFM) | LSM-YSZ|YSZ|LSCF-YSZ | 800 | Fuel electrode: pure CO2 Air electrode: air | 1.5 | 1.02 | [61] |
| La0.6Sr0.4CoO3-δ (LSC) | LSC-CGO|YSZ|NiO/YSZ | 750 | Fuel electrode: 90% H2O-10% H2 Air electrode: pure O2 | 1.3 | 1.07 | [62] |
| Sm0.5Sr0.5CoO3-δ (SSC) | LSCF-GDC|YSZ|NiO-YSZ | 750 | 76% H2O-24% H2 | 1.29 | 0.761 | [63] |
| Sm0.5Sr0.5CoO3-δ(SSC) | LSCF-GDC|YSZ|NiO-YSZ | 750 | Fuel electrode: 97% H2-3% H2O Air electrode: air | 1.29 | 2.1 | [40] |
| Sm0.5Sr0.5CoO3-δ (SSC) | LSCF-GDC|GDC|LSCF-GDC | 650 | Air | / | / | [64] |
2.3. Other Infiltration
| Infiltrated Material | Cell Configuration Anode|Electrolyte| Cathode | T [°C] | Gas Composition | Potential [V] | Current Density [A cm−2] | Ref. |
|---|---|---|---|---|---|---|
| SDC | LSCF-SDC|YSZ|LSCF-SDC | 800 | Cathode and anode: pure O2 | 0.03 | 0.5 | [69] |
| SrFe2O4-δ | LSM/YSZ|YSZ|Ni-YSZ | 800 | SOEC: Cathode: 60% steam Anode: air SOFC: 100% H2 | ~1.2 | 2.0 | [65] |
| Pd-GDC | LSCF|YSZ|LSCM | 850 | Cathode: 50% CO2/CO Anode: air | 1.5 | 0.364 | [66] |
| Gd0.2Ce0.8O1.9 (GDC) | LSM-YSZ|YSZ|SFM | 800 | 95% CO2/5% N2 | 1.6 | 0.446 | [70] |
| La2NiO4 | LSCF|YSZ|LSCF | 650 | O2 | / | / | [67] |
| La2NiO4+δ (LNO) | LSCN|GDC|LSCN | 750 | air | 0.104 | 0.5 | [73] |
| γ-Al₂O₃ | LSCF-SDC|LSGM|LSCF-SDC | 600 | Cathode: 95% CO2 + 5% N2 Anode: 36% C2H6 + 5% N2 + 59% Ar | / | / | [68] |
3. Exsolution
3.1. Exsolution of Single-Metal Nanoparticles
3.1.1. Co Nanoparticles
| Exsolved Metal | Electrode Composition | T [°C] | Gas Composition | Potential [V] | Current Density [A cm−2] | Ref. |
|---|---|---|---|---|---|---|
| Co | La0.5Sr0.5Fe0.8Co0.2O3-δ-SDC | 800 | 5% N2/95% CO2 | 1.6 | 1.80 | [78] |
| CeO2/Co | La0.7Ca0.3CrO3 | 800 | Pure CO2 | 1.3 | 1.05 | [79] |
| Co | Sr1.95Fe1.4Co0.1Mo0.4Ti0.1O6-δ (SFCMT) | 800 | Pure CO2 | 1.8 | 2.57 | [80] |
| Co | Sr2Fe1.3Co0.2Mo0.5O6-δ | 850 | 50% CO2–50% CO | 1.4 | 2.12 | [81] |
| Co | La0.5Ba0.5Mn0.9Co0.1O3-δ | 800 | Cathode: 10% H2O-90% H2 or 4% CO-96% CO2 Anode: air | 1.3 | 1.01 | [82] |
3.1.2. Cu Nanoparticles
| Exsolved Metal | Electrode Composition | T [°C] | Gas Composition | Potential [V] | Current Density [A cm−2] | Ref. |
|---|---|---|---|---|---|---|
| Cu | (La0.2Sr0.8)0.9Ti0.5Mn0.4Cu0.1O3−δ | 800 | Pure CO2 | 1.8 | 2.82 | [83] |
| Cu | Sr1.9Fe1.3Cu0.2Mo0.4Ti0.1O6-δ | 800 | CO2 | 1.8 | 3.21 | [85] |
| Cu | La0.43Sr0.37Cu0.12Ti0.88O3-δ | 900 | 3% H2O/H2 | ~0.7 | 1.5 | [86] |
| Cu | SrxTi0.7Cu0.2Mo0.1O3-δ | 800 | SOEC: pure CO2 SOFC: pure H2 | 1.8 | 2.16 | [87] |
| Cu | (LaSr)0.9Fe0.9Cu0.1O4 | 800 | Fuel cell: H2 and CH4 Electrolysis cell: 53.2% H2O/46.8% N2 | ~1.2 | 1.02 | [84] |
3.1.3. Ni Nanoparticles
| Exsolved Metal | Electrode Composition | T [°C] | Gas Composition | Potential [V] | Current Density [A cm−2] | Ref. |
|---|---|---|---|---|---|---|
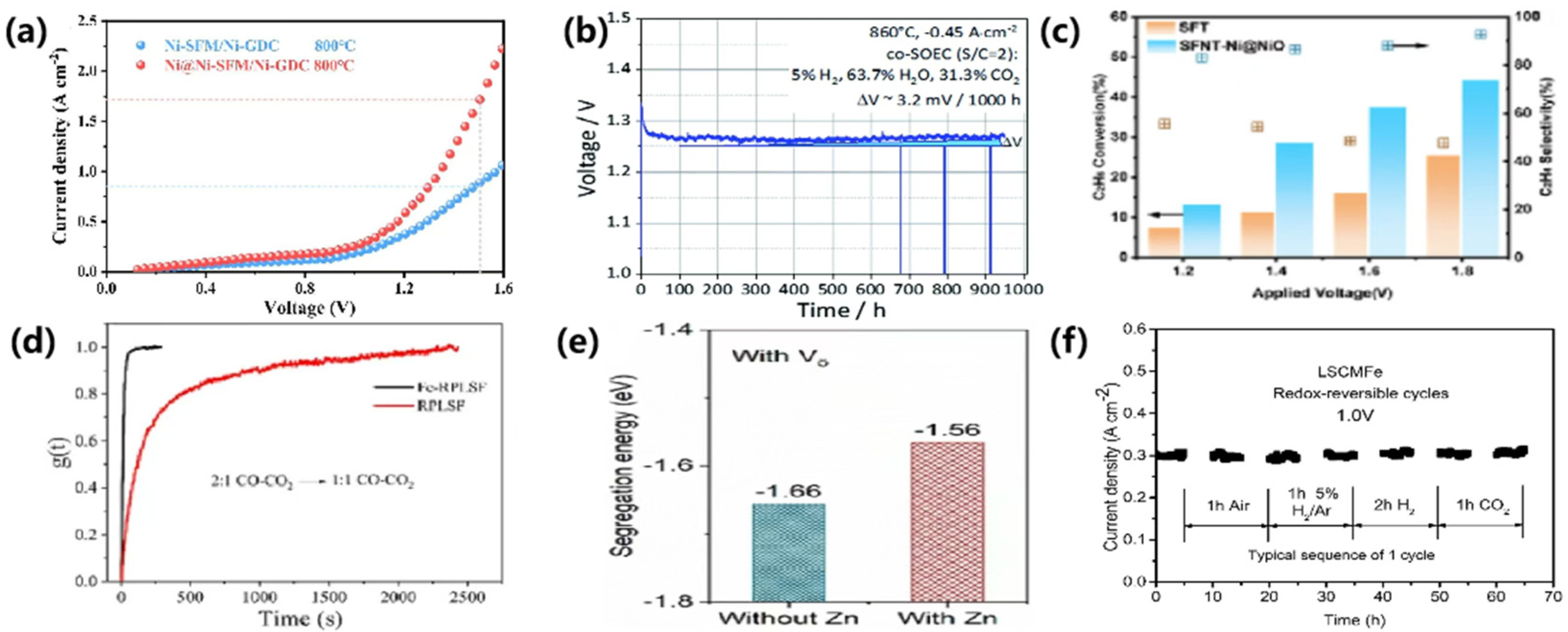
| Ni | Ni@Ni-SFM/NiGDC | 800 | Pure CO2 | 1.5 | 1.72 | [90] |
| Ni | La0.3Ca0.6Ni0.05MnxTi0.95-xO3-γ | 900 | 77.6% CO2-19.4% H2-3% H2O | 1.8 | 2.89 | [91] |
| Ni | (La4Srn4)0.9Ti0.9nNi0.1nO3n+2 (n = 5, 8, and 12) | 800 | 85% CO2-CO | 2.0 | 1.5 | [92] |
| Ni | La0.70Sr0.3Cr0.85Ni0.15O3−δ (L70SCrN) | 860 | H2-H2O-CO2 | 1.4 | ~1.0 | [93] |
| Ni | Sr3Fe1.4Ni0.1Ti0.5O7−δ | 800 | Anode: 50% C2H6 + 50% Ar Cathode: CO2 | 1.8 | ~1.3 | [94] |
3.1.4. Fe Nanoparticles
| Exsolved Metal | Electrode Composition | T [°C] | Gas Composition | Potential [V] | Current Density [A cm−2] | Ref. |
|---|---|---|---|---|---|---|
| Fe | Fe-RPLSF | 850 | Pure CO2 | 1.5 | 1.92 | [95] |
| Fe | (Pr0.5Ba0.5)1.8Fe1.8Mn0.2O5-δ | 850 | Pure CO2 | 1.8 | 1.6 | [100] |
| Fe | Sr2Fe1.4Zn0.1Mo0.5O6-δ | 850 | High purity CO2 | 1.6 | 2.74 | [96] |
| Fe | Sr2Fe1.5+xMo0.5O6-δ | 850 | CO2 | 1.6 | ~0.70 | [101] |
| Fe | La0.6Sr0.4Mn0.2Fe0.8O3-δ | 850 | 30% CO/CO2 | 1.5 | 2.04 | [102] |
| Fe | Sm0.2Ce0.8O2-δ | 800 | 50% CO/CO2 | 1.5 | 1.35 | [103] |
| Fe | (La0.75Sr0.25)0.9(Cr0.5Mn0.5)0.9Fe0.1O3-δ | 850 | Anode: CH4 Cathode: CO2 | 1.2 | 0.40 | [97] |
| Fe | La0.6Sr0.4FexO3-δ | 850 | Steam | 1.6 | 0.74 | [104] |
3.2. Exsolution of Alloy Nanoparticles
3.2.1. Co-Fe Alloy Nanoparticles

| Exsolved Metal | Electrode Composition | T [°C] | Gas Composition | Potential [V] | Current Density [A cm−2] | Ref. |
|---|---|---|---|---|---|---|
| Co-Fe | Pr4/3Ba2/3Co2/3Fe2/3Mn2/3O5+δ | 850 | Pure CO2 | 1.5 | 3.76 | [109] |
| Co-Fe | Pr0.4Sr0.6Co0.125Fe0.75Mo0.125O3-δ | 800 | Anode: air Cathode: H2/CO2 | 1.6 | 1.42 | [110] |
| Co-Fe | Sr2FeMo1-xCoxO6−δ (x = 0, 0.15, 0.25, 0.45) | 800 | Pure CO2 | 1.5 | 2.00 | [111] |
| Co-Fe | Sr2Ti0.8Co0.2FeO6-δ | 800 | Pure CO2 | 1.6 | 1.26 | [112] |
| Co-Fe | Sr1−xCexFe1−yCoyO3 | 800 | Pure CO2 | 1.3 | 1.30 | [115] |
| Co-Fe | Sr1.95Fe1.4Co0.1Mo0.5O6-δ | 800 | Anode: C2H6 Cathode: CO2 | 1.6 | 1.89 | [118] |
| Co-Fe | La0.7Sr0.2Co0.2Fe0.8O3 (LSCF) | 850 | Pure CH4 | 0.25 | 0.15 | [116] |
| Co-Fe | La0.6Sr0.4Ti0.3Fe0.5Co0.2O3-δ | 800 | Anode: 40% CH4 + 55% Ar + 5%N2 Cathode: 95% CO2-5% Ar | 1.5 | ~0.275 | [117] |
3.2.2. Ni-Fe Alloy Nanoparticles
| Exsolved Metal | Electrode Composition | T [°C] | Gas Composition | Potential [V] | Current Density [A cm−2] | Ref. |
|---|---|---|---|---|---|---|
| Ni-Fe | (La,Sr)FeO3 | 800 | 40% CO2/He | 1.1 | 0.29 | [121] |
| Ni-Fe | Sr2Fe1.5Mo0.5O6−δ | 800 | CO−CO2 (7:3) | 1.5 | 1.15 | [122] |
| Ni-Fe | Sr2Fe1.5Mo0.5O6-δ | 800 | Pure CO2 | 1.5 | 2.66 | [123] |
| Ni-Fe | La0.6Sr0.4Ni0.2Fe0.75Mo0.05O3-δ | 800 | Pure CO2 | 1.5 | 0.59 | [127] |
| Ni-Fe | La0.3Ca0.7Fe0.7Cr0.3O3-δ | 800 | Pure CO2 | 1.6 | 0.65 | [128] |
| Ni-Fe | La0.52Ca0.28Ni0.04Fe0.04Ti0.92O3/La0.52Ca0.28Ni0.03Co0.03Ti0.94O3 | 900 | Pure CO2 | 1.6 | 0.75 | [129] |
| Ni-Fe | Pr0.4Sr0.6Fe0.9Mo0.1O3 | 800 | Pure CO2 | 1.5 | 1.05 | [130] |
| Ni-Fe | Pr0.5Ba0.5Fe0.8Ni0.2O3-δ | 800 | Pure CO2 | 1.4 | 0.28 | [131] |
| Ni-Fe | Ce0.6Mn0.3Fe0.1O2−δ-NiFe-MnOx | 700 | Fuel electrode: CO2 + H2 Hydrogen electrode: 50% C2H6/Ar | 1.8 | 1.62 | [124] |
3.2.3. Others Alloy Nanoparticles
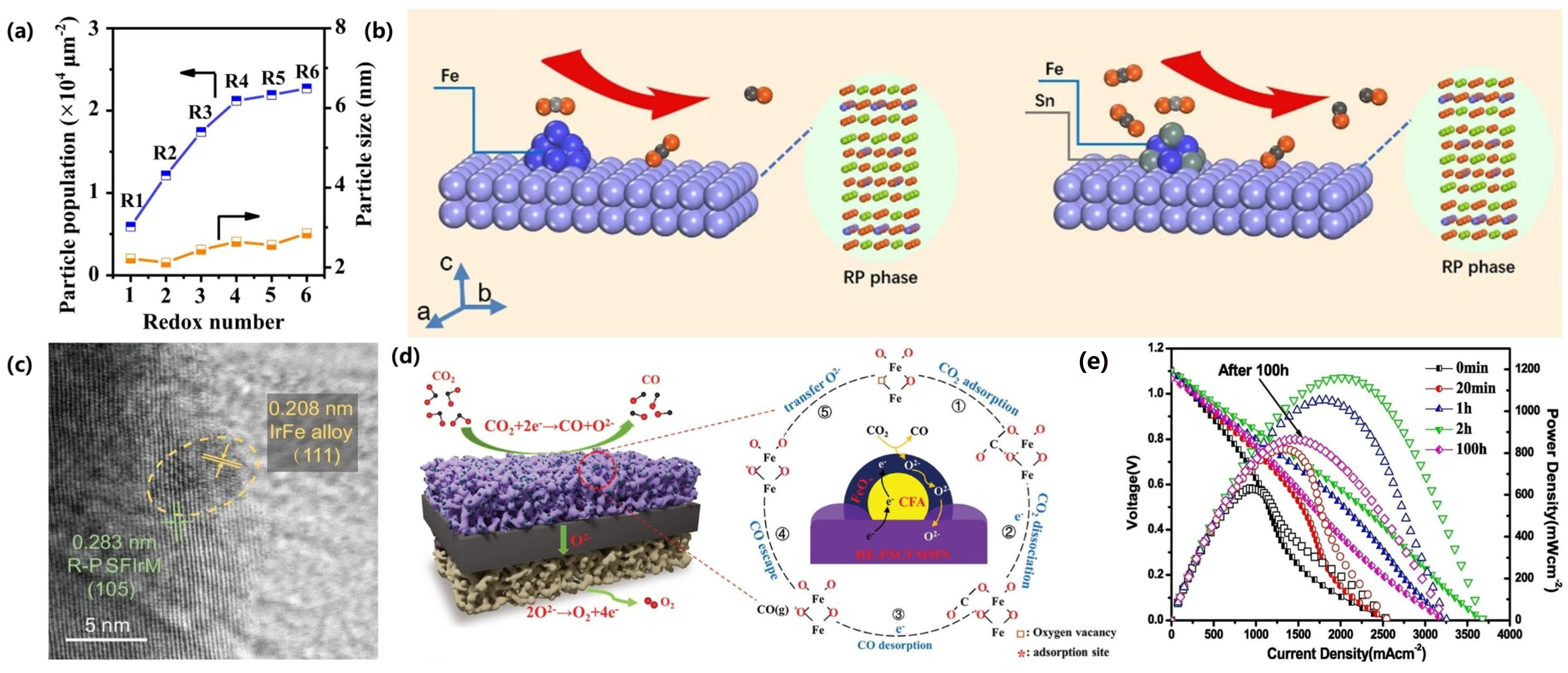
| Exsolved Metal | Electrode Composition | T [°C] | Gas Composition | Potential [V] | Current Density [A cm−2] | Ref. |
|---|---|---|---|---|---|---|
| Ru-Fe | Pr0.4Sr0.6Fe0.9Mo0.1O3-δ | 800 | Pure CO2 | 2.0 | 1.48 | [132] |
| Ru-Fe | Sr2Fe1.4Ru0.1Mo0.5O6−δ | 800 | 95% CO2 + 5% N2 | 1.6 | ~1.35 | [133] |
| Ni-Co | (La4Sr4)0.9Ti7.2Ni0.4Co0.4O2−δ | 850 | Pure CO2 | 2.0 | 1.53 | [134] |
| Ni-Cu | La0.7Sr0.3Cr0.5Mn0.5(NiCu)xO3−δ | 850 | Pure CO2 | 1.6 | 0.68 | [135] |
| Fe-Sn | Sr1.95Fe1.4Sn0.1Mo0.5O6-δ | 800 | Pure CO2 | 1.8 | 3.269 | [136] |
| Ir-Fe | Sr2Fe1.45Ir0.05Mo0.5O6-δ | 800 | 95% CO2/5% N2 | 1.6 | 1.46 | [137] |
| Fe-Cu | Sr2Fe1.25Cu0.25Mo0.5O6-δ | 800 | Pure CO2 | 1.4 | 1.70 | [138] |
| Fe-Cu | Pr0.8Sr1.2(CuFe)0.4Mo0.2Mn0.2Nb0.2O4-δ | 800 | Pure CO2 | 1.5 | 1.95 | [139] |
| Cu-Fe | Sr1.9Fe1.5Mo0.4Cu0.1O6-δ | 800 | Cathode: wet H2 (3% H2O) Anode: air | 0.99 | 1.2W cm−2 | [140] |
| Fe−Co−Ni | SrxFeCo0.2Ni0.2Mn0.1Mo0.5O6−δ | 800 | Cathode: 15%CO2/Ar Anode: air (60 mLmin−1) | 1.2 | 0.19 | [141] |
| Ni-Cu | Ce0.9(NixCu1-x)0.1O2-δ (x = 0–1) | 700 | C2H4 | 1.0 | 0.51 | [143] |
4. Topotactic Ion Exchange
| Exsolved Metal | Electrode Composition | T [°C] | Gas Composition | Potential [V] | Current Density [A cm−2] | Ref. |
|---|---|---|---|---|---|---|
| CO-Fe | PrBaMn1.7Co0.3O5+δ + 12 wt% Fe -GDC | 800 | 97% H2/3% H2O | ~0.90 | 1.20 | [146] |
| Fe-Ni | PrBaMn1.7Ni0.3O5+δ + 12 wt% Fe -GDC | 800 | 97% H2/3% H2O | 0.80 | ~1.30 | [147] |
| BaFe2O4 | BaCe0.25Fe0.75O3-δ-xV2O3 | 600 | 50% N2/50% O2 | 0.95 | 0.20 | [148] |
| Fe-Ni | La0.6Sr0.4Co0.2Fe0.8O3-δ-GDC | 800 | CO2 | 1.49 | 1.60 | [149] |
| Fe-Ni | La0.6Sr0.2Ti0.85Ni0.15O3-δ + Fe2O3 | 700 | 10% CO2-10% CH4-80% He | / | / | [150] |
5. Summary and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J. Energy access challenge and the role of fossil fuels in meeting electricity demand: Promoting renewable energy capacity for sustainable development. Geosci. Front. 2024, 15, 101873. [Google Scholar] [CrossRef]
- Bilgili, F.; Önderol, S.; Kuşkaya, S.; Alnour, M.; Hoque, M.E.; Balsalobre-Lorente, D. Renewable energy and technology adoption: Mitigating CO2 emissions through implementation strategies. In Natural Resources Forum; Blackwell Publishing Ltd.: Oxford, UK, 2024. [Google Scholar] [CrossRef]
- He, D.; Wu, S.; Cao, X.; Chen, D.; Zhang, L.; Zhang, Y.; Luo, Y. Dynamic trap of Ni at elevated temperature for yielding high-efficiency methane dry reforming catalyst. Appl. Catal. B Environ. Energy 2024, 346, 123728. [Google Scholar] [CrossRef]
- Qi, Y.; Stern, N.; He, J.-K.; Lu, J.-Q.; Liu, T.-L.; King, D.; Wu, T. The policy-driven peak and reduction of China’s carbon emissions. Adv. Clim. Change Res. 2020, 11, 65–71. [Google Scholar] [CrossRef]
- Razmjoo, A.; Gakenia Kaigutha, L.; Vaziri Rad, M.A.; Marzband, M.; Davarpanah, A.; Denai, M. A Technical analysis investigating energy sustainability utilizing reliable renewable energy sources to reduce CO2 emissions in a high potential area. Renew. Energy 2021, 164, 46–57. [Google Scholar] [CrossRef]
- Sun, X.; Chen, H.; Yin, Y.; Curnan, M.T.; Han, J.W.; Chen, Y.; Ma, Z. Progress of Exsolved Metal Nanoparticles on Oxides as High Performance (Electro)Catalysts for the Conversion of Small Molecules. Small 2021, 17, 2005383. [Google Scholar] [CrossRef]
- Ye, L.; Xie, K. High-temperature electrocatalysis and key materials in solid oxide electrolysis cells. J. Energy Chem. 2021, 54, 736–745. [Google Scholar] [CrossRef]
- Tian, Y.; Abhishek, N.; Yang, C.; Yang, R.; Choi, S.; Chi, B.; Pu, J.; Ling, Y.; Irvine, J.T.S.; Kim, G. Progress and potential for symmetrical solid oxide electrolysis cells. Matter 2022, 5, 482–514. [Google Scholar] [CrossRef]
- Chen, D.; Barreau, M.; Turczyniak-Surdacka, S.; Sobczak, K.; Strawski, M.; Salle, A.L.G.L.; Efimenko, A.; Teschner, D.; Petit, C.; Zafeiratos, S. Ceria nanoparticles as promoters of CO2 electroreduction on Ni/YSZ: An efficient preparation strategy and insights into the catalytic promotion mechanism. Nano Energy 2022, 101, 107564. [Google Scholar] [CrossRef]
- Rabuni, M.F.; Vatcharasuwan, N.; Li, T.; Li, K. High performance micro-monolithic reversible solid oxide electrochemical reactor. J. Power Sources 2020, 458, 228026. [Google Scholar] [CrossRef]
- Li, X.; Zhou, L.; Li, Q.; Kalu, A.; Liu, C.; Guan, B.; Salem Molouk, A.F.; Liu, X.; Li, W. Nanocomposite electrodes as a new opportunity to transform the performance of solid oxide cells. J. Mater. Chem. A 2023, 11, 25803–25824. [Google Scholar] [CrossRef]
- Seong, A.; Kim, J.; Kwon, O.; Jeong, H.Y.; Gorte, R.J.; Vohs, J.M.; Kim, G. Self-reconstructed interlayer derived by in-situ Mn diffusion from La0.5Sr0.5MnO3 via atomic layer deposition for an efficient bi-functional electrocatalyst. Nano Energy 2020, 71, 104564. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, L.; Paredes Navia, S.A.; Hinerman, A.; Gerdes, K.; Song, X. Synergetic Interaction of Additive Dual Nanocatalysts to Accelerate Oxygen Reduction Reaction in Fuel Cell Cathodes. ACS Catal. 2019, 9, 6664–6671. [Google Scholar] [CrossRef]
- Zhao, J.; Shen, Y.; Gu, J.; Wang, Y.; Zhao, Y.; Bu, Y. Optimizing water electrolysis activity in Mo-doped Sr2FeCoO6-δ perovskites by balancing oxygen vacancies and structural stability. J. Power Sources 2024, 592, 233928. [Google Scholar] [CrossRef]
- Chen, X.; Sun, X.; Zhou, J.; Zhou, D.; Xiaofei, Z.; Meng, J. Effects of CoO and Bi2O3 single/dual sintering aids doping on structure and properties of Ce0.8Nd0.2O1.9. Ceram. Int. 2020, 46, 22727–22732. [Google Scholar] [CrossRef]
- Aliotta, C.; Costa, M.; Liotta, L.F.; La Parola, V.; Magnacca, G.; Deganello, F. Peculiar Properties of the La0.25Ba0.25Sr0.5Co0.8Fe0.2O3−δ Perovskite as Oxygen Reduction Electrocatalyst. Molecules 2023, 28, 1621. [Google Scholar] [CrossRef]
- Wolf, S.E.; Winterhalder, F.E.; Vibhu, V.; de Haart, L.G.J.; Guillon, O.; Eichel, R.-A.; Menzler, N.H. Solid oxide electrolysis cells—Current material development and industrial application. J. Mater. Chem. A 2023, 11, 17977–18028. [Google Scholar] [CrossRef]
- Acosta, M.; Baiutti, F.; Tarancón, A.; MacManus-Driscoll, J.L. Nanostructured Materials and Interfaces for Advanced Ionic Electronic Conducting Oxides. Adv. Mater. Interfaces 2019, 6, 1900462. [Google Scholar] [CrossRef]
- Helal, H.; Ahrouch, M.; Rabehi, A.; Zappa, D.; Comini, E. Nanostructured Materials for Enhanced Performance of Solid Oxide Fuel Cells: A Comprehensive Review. Crystals 2024, 14, 306. [Google Scholar] [CrossRef]
- Mogensen, M.B.; Chen, M.; Frandsen, H.L.; Graves, C.; Hansen, J.B.; Hansen, K.V.; Hauch, A.; Jacobsen, T.; Jensen, S.H.; Skafte, T.L.; et al. Reversible solid-oxide cells for clean and sustainable energy. Clean Energy 2019, 3, 175–201. [Google Scholar] [CrossRef]
- Kim, S.; Jun, A.; Kwon, O.; Kim, J.; Yoo, S.; Jeong, H.Y.; Shin, J.; Kim, G. Nanostructured Double Perovskite Cathode with Low Sintering Temperature for Intermediate Temperature Solid Oxide Fuel Cells. ChemSusChem 2015, 8, 3153–3158. [Google Scholar] [CrossRef]
- Chrzan, A.; Karczewski, J.; Gazda, M.; Szymczewska, D.; Jasinski, P. La 0.6 Sr 0.4 Co 0.2 Fe 0.8 O 3-δ oxygen electrodes for solid oxide cells prepared by polymer precursor and nitrates solution infiltration into gadolinium doped ceria backbone. J. Eur. Ceram. Soc. 2017, 37, 3559–3564. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, Y.; Zhang, X.; Zhang, H.; Jiang, G.; Zhao, W.; Guo, J.; Chen, F.; Yan, M.; Zhang, Y.; et al. Robust redox-reversible perovskite type steam electrolyser electrode decorated with in situ exsolved metallic nanoparticles. J. Mater. Chem. A 2020, 8, 582–591. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, Y.; Huang, K.; Nicholas, J.D. Atomic Layer Deposited Zirconia Overcoats as On-Board Strontium Getters for Improved Solid Oxide Fuel Cell Nanocomposite Cathode Durability. ACS Appl. Energy Mater. 2020, 3, 4057–4067. [Google Scholar] [CrossRef]
- Chen, D.; Guan, Z.; Zhang, D.; Trotochaud, L.; Crumlin, E.; Nemsak, S.; Bluhm, H.; Tuller, H.L.; Chueh, W.C. Constructing a pathway for mixed ion and electron transfer reactions for O2 incorporation in Pr0.1Ce0.9O2−x. Nat. Catal. 2020, 3, 116–124. [Google Scholar] [CrossRef]
- Lee, Y.H.; Ren, H.; Wu, E.A.; Fullerton, E.E.; Meng, Y.S.; Minh, N.Q. All-Sputtered, Superior Power Density Thin-Film Solid Oxide Fuel Cells with a Novel Nanofibrous Ceramic Cathode. Nano Lett. 2020, 20, 2943–2949. [Google Scholar] [CrossRef] [PubMed]
- Duta, L.; Popescu, A.C. Current Research in Pulsed Laser Deposition. Coatings 2021, 11, 274. [Google Scholar] [CrossRef]
- Chasta, G.; Himanshu; Dhaka, M.S. A review on materials, advantages, and challenges in thin film based solid oxide fuel cells. Int. J. Energy Res. 2022, 46, 14627–14658. [Google Scholar] [CrossRef]
- Akkurt, S.; SindiraÇ, C.; ÖZmen Egesoy, T.; ErĞEn, E. A review on new cobalt-free cathode materials for reversible solid oxide fuel cells. J. Met. Mater. Miner. 2023, 33, 1654. [Google Scholar] [CrossRef]
- Connor, P.A.; Yue, X.; Savaniu, C.D.; Price, R.; Triantafyllou, G.; Cassidy, M.; Kerherve, G.; Payne, D.J.; Maher, R.C.; Cohen, L.F.; et al. Tailoring SOFC Electrode Microstructures for Improved Performance. Adv. Energy Mater. 2018, 8, 1800120. [Google Scholar] [CrossRef]
- Vohs, J.M.; Gorte, R.J. High-Performance SOFC Cathodes Prepared by Infiltration. Adv. Mater. 2009, 21, 943–956. [Google Scholar] [CrossRef]
- Irvine, J.T.S.; Neagu, D.; Verbraeken, M.C.; Chatzichristodoulou, C.; Graves, C.; Mogensen, M.B. Evolution of the electrochemical interface in high-temperature fuel cells and electrolysers. Nat. Energy 2016, 1, 15014. [Google Scholar] [CrossRef]
- Zhao, C.; Li, Y.; Zhang, W.; Zheng, Y.; Lou, X.; Yu, B.; Chen, J.; Chen, Y.; Liu, M.; Wang, J. Heterointerface engineering for enhancing the electrochemical performance of solid oxide cells. Energy Environ. Sci. 2020, 13, 53–85. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, X.; Xie, K.; Wang, G.; Bao, X. High-Temperature CO2 Electrolysis in Solid Oxide Electrolysis Cells: Developments, Challenges, and Prospects. Adv. Mater. 2019, 31, 1902033. [Google Scholar] [CrossRef]
- Hauch, A.; Küngas, R.; Blennow, P.; Hansen, A.B.; Hansen, J.B.; Mathiesen, B.V.; Mogensen, M.B. Recent advances in solid oxide cell technology for electrolysis. Science 2020, 370, eaba6118. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zou, Y.; Chen, K.; Li, N.; Li, D.; Jiang, S.P. A critical review of the nano-structured electrodes of solid oxide cells. Chem. Commun. 2022, 58, 10619–10626. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Y.; Hou, Y.; Li, X.; Peng, Z.; Du, Q.; Ni, M.; Jiao, K. Reconstruction and optimization of LSCF cathode microstructure based on Kinetic Monte Carlo method and Lattice Boltzmann method. Chem. Eng. J. 2022, 436, 132144. [Google Scholar] [CrossRef]
- Li, S.; Xiong, R.; Han, Z.; He, R.; Li, S.; Zhou, H.; Yu, C.; Cheng, S.; Xie, J. Unveiling low-tortuous effect on electrochemical performance toward ultrathick LiFePO4 electrode with 100 mg cm−2 area loading. J. Power Sources 2021, 515, 230588. [Google Scholar] [CrossRef]
- Wang, R.-T.; Chang, H.-Y.; Wang, J.-C. An Overview on the Novel Core-Shell Electrodes for Solid Oxide Fuel Cell (SOFC) Using Polymeric Methodology. Polymers 2021, 13, 2774. [Google Scholar] [CrossRef]
- Joong Yoon, K.; Biswas, M.; Kim, H.-J.; Park, M.; Hong, J.; Kim, H.; Son, J.-W.; Lee, J.-H.; Kim, B.-K.; Lee, H.-W. Nano-tailoring of infiltrated catalysts for high-temperature solid oxide regenerative fuel cells. Nano Energy 2017, 36, 9–20. [Google Scholar] [CrossRef]
- Tzelepis, S.; Kavadias, K.A.; Marnellos, G.E.; Xydis, G. A review study on proton exchange membrane fuel cell electrochemical performance focusing on anode and cathode catalyst layer modelling at macroscopic level. Renew. Sustain. Energy Rev. 2021, 151, 111543. [Google Scholar] [CrossRef]
- Futamura, S.; Muramoto, A.; Tachikawa, Y.; Matsuda, J.; Lyth, S.M.; Shiratori, Y.; Taniguchi, S.; Sasaki, K. SOFC anodes impregnated with noble metal catalyst nanoparticles for high fuel utilization. Int. J. Hydrogen Energy 2019, 44, 8502–8518. [Google Scholar] [CrossRef]
- Orera, A.; Betato, A.; Silva-Treviño, J.; Larrea, Á.; Laguna-Bercero, M.Á. Advanced metal oxide infiltrated electrodes for boosting the performance of solid oxide cells. J. Mater. Chem. A 2022, 10, 2541–2549. [Google Scholar] [CrossRef]
- Xia, J.; Wang, C.; Wang, X.; Bi, L.; Zhang, Y. A perspective on DRT applications for the analysis of solid oxide cell electrodes. Electrochim. Acta 2020, 349, 136328. [Google Scholar] [CrossRef]
- Bertei, A.; Ruiz-Trejo, E.; Tariq, F.; Yufit, V.; Atkinson, A.; Brandon, N.P. Validation of a physically-based solid oxide fuel cell anode model combining 3D tomography and impedance spectroscopy. Int. J. Hydrogen Energy 2016, 41, 22381–22393. [Google Scholar] [CrossRef]
- Lyagaeva, J.G.; Vdovin, G.K.; Medvedev, D.A. Distinguishing Bulk and Grain Boundary Transport of a Proton-Conducting Electrolyte by Combining Equivalent Circuit Scheme and Distribution of Relaxation Times Analyses. J. Phys. Chem. C 2019, 123, 21993–21997. [Google Scholar] [CrossRef]
- Lu, J.; Hu, Y.; Zhang, M.; Hu, Q.; Wu, J. Optimization of Pt infiltrated Sr2Fe2-xMoxO6-δ-Ce0.8Sm0.2O1.9 oxygen electrode for reversible solid oxide cell and CH4-assisted electrolysis process. Int. J. Hydrogen Energy 2024, 55, 786–795. [Google Scholar] [CrossRef]
- Ishihara, T.; Tan, Z.; Song, J.T.; Takagaki, A. Sequential-infiltration of Ce and Ni in NiO-YSZ fuel electrode for tubular type solid oxide reversible cells (SORC) using LaGaO3 electrolyte film. Solid State Ion. 2022, 379, 115914. [Google Scholar] [CrossRef]
- Jeong, H.-Y.; Kim, S.-W.; Bae, Y.; Yoon, K.J.; Lee, J.-H.; Hong, J. Effect of Fe infiltration to Ni/YSZ solid-oxide-cell fuel electrode on steam/CO2 co-electrolysis. Int. J. Energy Res. 2019, 43, 4949–4958. [Google Scholar] [CrossRef]
- Tan, Y.; Gao, S.; Xiong, C.; Chi, B. Nano-structured LSM-YSZ refined with PdO/ZrO2 oxygen electrode for intermediate temperature reversible solid oxide cells. Int. J. Hydrogen Energy 2020, 45, 19823–19830. [Google Scholar] [CrossRef]
- Ivanov, A.I.; Kolotygin, V.A.; Patrakeev, M.V.; Markov, A.A.; Bredikhin, S.I.; Kharton, V.V. Oxygen Nonstoichiometry and Transport Properties of Mixed-Conducting Ce0.6–xLa0.4PrxO2–δ. Russ. J. Electrochem. 2018, 54, 486–492. [Google Scholar] [CrossRef]
- Sharma, R.K.; Khamidy, N.I.; Rapenne, L.; Charlot, F.; Moussaoui, H.; Laurencin, J.; Djurado, E. Highly efficient architectured Pr6O11 oxygen electrode for solid oxide fuel cell. J. Power Sources 2019, 419, 171–180. [Google Scholar] [CrossRef]
- Khoshkalam, M.; Faghihi-Sani, M.A.; Tong, X.; Chen, M.; Hendriksen, P.V. Enhanced Activity of Pr6O11and CuO Infiltrated Ce0.9Gd0.1O2Based Composite Oxygen Electrodes. J. Electrochem. Soc. 2020, 167, 24505. [Google Scholar] [CrossRef]
- Xu, J.; Wan, S.; Wang, Y.; Huang, S.; Yuan, Z.; Chen, F.; Zhang, Y.; Liu, T. Enhancing performance of molybdenum doped strontium ferrite electrode by surface modification through Ni infiltration. Int. J. Hydrogen Energy 2021, 46, 10876–10891. [Google Scholar] [CrossRef]
- Zhang, S.-L.; Wang, H.; Lu, M.Y.; Li, C.-X.; Li, C.-J.; Barnett, S.A. Electrochemical performance and stability of SrTi0.3Fe0.6Co0.1O3-δ infiltrated La0.8Sr0.2MnO3Zr0.92Y0.16O2-δ oxygen electrodes for intermediate-temperature solid oxide electrochemical cells. J. Power Sources 2019, 426, 233–241. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Meng, X.; Liu, T.; Chen, F. Ni infiltrated Sr2Fe1.5Mo0.5O6-δ-Ce0.8Sm0.2O1.9 electrode for methane assisted steam electrolysis process. Electrochem. Commun. 2017, 79, 63–67. [Google Scholar] [CrossRef]
- Ye, Y.; Lee, W.; Pan, J.; Sun, X.; Zhou, M.; Li, J.; Zhang, N.; Han, J.W.; Chen, Y. Tuning the product selectivity of CO2/H2O co-electrolysis using CeO2-modified proton-conducting electrolysis cells. Energy Environ. Sci. 2023, 16, 3137–3145. [Google Scholar] [CrossRef]
- Liu, T.; Liu, H.; Zhang, X.; Lei, L.; Zhang, Y.; Yuan, Z.; Chen, F.; Wang, Y. A robust solid oxide electrolyzer for highly efficient electrochemical reforming of methane and steam. J. Mater. Chem. A 2019, 7, 13550–13558. [Google Scholar] [CrossRef]
- Vasudevan, S.; Manickam, M.; Sivasubramanian, R.J.D.T. A sol–gel derived LaCoO3 perovskite as an electrocatalyst for Al–air batteries. Dalton Trans. 2024, 53, 3713–3721. [Google Scholar] [CrossRef]
- Vibhu, V.; Yildiz, S.; Vinke, I.C.; Eichel, R.A.; Bassat, J.M.; de Haart, L.G.J. High Performance LSC Infiltrated LSCF Oxygen Electrode for High Temperature Steam Electrolysis Application. J. Electrochem. Soc. 2019, 166, F102–F108. [Google Scholar] [CrossRef]
- Sun, L.; Zheng, Q.; Li, N.; Chen, C.; Zhan, Z. Direct electrolysis of CO2 in solid oxide cells supported on ceramic fuel electrodes with straight open pores and coated catalysts. Solid. State Ion. 2020, 344, 115154. [Google Scholar] [CrossRef]
- Tong, X.; Ovtar, S.; Brodersen, K.; Hendriksen, P.V.; Chen, M. Large-area solid oxide cells with La0.6Sr0.4CoO3-δ infiltrated oxygen electrodes for electricity generation and hydrogen production. J. Power Sources 2020, 451, 227742. [Google Scholar] [CrossRef]
- Song, Z.; Pan, H.; Wan, G.; Wu, A.; Chen, Q.; Guan, W.; Singhal, S.C. Enhancing durability of solid oxide cells for hydrogen production from seawater by designing nano-structured Sm0.5Sr0.5Co3-δ infiltrated air electrodes. Int. J. Hydrogen Energy 2023, 48, 27095–27104. [Google Scholar] [CrossRef]
- Lu, M.Y.; Yang, T.; Scipioni, R.; Chart, Y.A.; Furlong, A.; Barnett, S.A. Sm0.5Sr0.5CoO3−δ. Surface Modification of La0.6Sr0.4Co0.2Fe0.8O3−δ. -Ce0.9Gd0.12−δ. Composite Oxygen Electrodes for Solid Oxide Electrochemical Cells. J. Electrochem. Soc. 2020, 167, 164504. [Google Scholar] [CrossRef]
- Brito, M.E.; Morishita, H.; Yamada, J.; Nishino, H.; Uchida, H. Further improvement in performances of La0.6Sr0.4Co0.2Fe0.8O3−δ—doped ceria composite oxygen electrodes with infiltrated doped ceria nanoparticles for reversible solid oxide cells. J. Power Sources 2019, 427, 293–298. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, Y.; Abernathy, H.; Pineault, R.; Addis, R.; Song, X.; Hackett, G.; Kalapos, T. Enabling durable hydrogen production and preventing the catastrophic delamination in the solid oxide electrolysis cells by infiltrating SrFe2O4-δ solutions into LSM/YSZ -based air electrode. J. Power Sources 2023, 580, 233389. [Google Scholar] [CrossRef]
- Lee, S.; Woo, S.H.; Shin, T.H.; Irvine, J.T.S. Pd and GDC Co-infiltrated LSCM cathode for high-temperature CO2 electrolysis using solid oxide electrolysis cells. Chem. Eng. J. 2021, 420, 127706. [Google Scholar] [CrossRef]
- Ghamarinia, M.; Babaei, A.; Zamani, C. Electrochemical characterization of La2NiO4-infiltrated La0.6Sr0.4Co0.2Fe0.8O3-δ by analysis of distribution of relaxation times. Electrochim. Acta 2020, 353, 136520. [Google Scholar] [CrossRef]
- Song, Y.; Lin, L.; Feng, W.; Zhang, X.; Dong, Q.; Li, X.; Lv, H.; Liu, Q.; Yang, F.; Liu, Z.; et al. Interfacial Enhancement by γ-Al2O3 of Electrochemical Oxidative Dehydrogenation of Ethane to Ethylene in Solid Oxide Electrolysis Cells. Angew. Chem. Int. Ed. 2019, 58, 16043–16046. [Google Scholar] [CrossRef]
- Lv, H.; Zhou, Y.; Zhang, X.; Song, Y.; Liu, Q.; Wang, G.; Bao, X. Infiltration of Ce0.8Gd0.2O1.9 nanoparticles on Sr2Fe1.5Mo0.5O6-δ cathode for CO2 electroreduction in solid oxide electrolysis cell. J. Energy Chem. 2019, 35, 71–78. [Google Scholar] [CrossRef]
- Maas, K.; Villepreux, E.; Cooper, D.; Salas-Colera, E.; Rubio-Zuazo, J.; Castro, G.R.; Renault, O.; Jimenez, C.; Roussel, H.; Mescot, X.; et al. Tuning Memristivity by Varying the Oxygen Content in a Mixed Ionic–Electronic Conductor. Adv. Funct. Mater. 2020, 30, 1909942. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kolchugin, A.A.; Sadykov, V.A.; Sadovskaya, E.M.; Filonova, E.A.; Eremeev, N.F.; Bogdanovich, N.M. Structure, transport properties and electrochemical behavior of the layered lanthanide nickelates doped with calcium. Int. J. Hydrogen Energy 2018, 43, 17373–17386. [Google Scholar] [CrossRef]
- Liu, Y.; Shuang, J.; Tong, X.; Yang, S.; Yang, Y.; Wei, M. Enhanced performance and stability of La2NiO4+δ impregnated La0.8Sr0.2Co0.8Ni0.2O3-δ oxygen electrodes for solid oxide electrolysis cells. Electrochim. Acta 2019, 298, 852–857. [Google Scholar] [CrossRef]
- Kim, K.J.; Han, H.; Defferriere, T.; Yoon, D.; Na, S.; Kim, S.J.; Dayaghi, A.M.; Son, J.; Oh, T.-S.; Jang, H.M.; et al. Facet-Dependent in Situ Growth of Nanoparticles in Epitaxial Thin Films: The Role of Interfacial Energy. J. Am. Chem. Soc. 2019, 141, 7509–7517. [Google Scholar] [CrossRef]
- Tomboc, G.M.; Tesfaye Gadisa, B.; Jun, M.; Chaudhari, N.K.; Kim, H.; Lee, K. Carbon Transition-metal Oxide Electrodes: Understanding the Role of Surface Engineering for High Energy Density Supercapacitors. Chem.—Asian J. 2020, 15, 1628–1647. [Google Scholar] [CrossRef] [PubMed]
- Neagu, D.; Papaioannou, E.I.; Ramli, W.K.W.; Miller, D.N.; Murdoch, B.J.; Ménard, H.; Umar, A.; Barlow, A.J.; Cumpson, P.J.; Irvine, J.T.S.; et al. Demonstration of chemistry at a point through restructuring and catalytic activation at anchored nanoparticles. Nat. Commun. 2017, 8, 1855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, M.-R.; Luo, J.-L. In Situ Exsolved Metal Nanoparticles: A Smart Approach for Optimization of Catalysts. Chem. Mater. 2020, 32, 5424–5441. [Google Scholar] [CrossRef]
- Li, J.; Liu, Q.; Song, Y.; Lv, H.; Feng, W.; Shen, Y.; Guan, C.; Zhang, X.; Wang, G. In-situ exsolution of cobalt nanoparticles from La0.5Sr0.5Fe0.8Co0.2O3-δ cathode for enhanced CO2 electrolysis performance. Green. Chem. Eng. 2022, 3, 250–258. [Google Scholar] [CrossRef]
- Lu, L.; He, D.; Fang, R.; Ni, C.; Irvine, J.T.S. Co nanoparticles decorated with in-situ exsolved oxygen-storage CeO2 for an efficient and stable electrolysis of pure CO2. J. Power Sources 2023, 580, 233424. [Google Scholar] [CrossRef]
- Lu, C.; Xu, C.; Sun, W.; Ren, R.; Qiao, J.; Wang, Z.; Sun, K.; Pan, G.; Cao, Y. Enhancing catalytic activity of CO2 electrolysis by building efficient and durable heterostructure for solid oxide electrolysis cell cathode. J. Power Sources 2023, 574, 233134. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Yang, Z.; Chen, Y.; Peng, S. A highly active and durable electrode with in situ exsolved Co nanoparticles for solid oxide electrolysis cells. J. Power Sources 2020, 478, 229082. [Google Scholar] [CrossRef]
- Gan, J.; Hou, N.; Yao, T.; Fan, L.; Gan, T.; Huang, Z.; Zhao, Y.; Li, Y. A high performing perovskite cathode with in situ exsolved Co nanoparticles for H2O and CO2 solid oxide electrolysis cell. Catal. Today 2021, 364, 89–96. [Google Scholar] [CrossRef]
- Yang, X.; Sun, W.; Ma, M.; Xu, C.; Ren, R.; Qiao, J.; Wang, Z.; Li, Z.; Zhen, S.; Sun, K. Achieving Highly Efficient Carbon Dioxide Electrolysis by In Situ Construction of the Heterostructure. ACS Appl. Mater. Interfaces 2021, 13, 20060–20069. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Yang, X.; Ma, M.; Sun, J.; Ren, R.; Xu, C.; Qiao, J.; Sun, W.; Sun, K.; Wang, Z. Boosting catalytic and CO2 adsorption ability by in situ Cu nanoparticle exsolution for solid oxide electrolysis cell cathode. Ceram. Int. 2023, 49, 27214–27221. [Google Scholar] [CrossRef]
- Jo, S.; Jeong, H.G.; Kim, Y.H.; Neagu, D.; Myung, J.-h. Stability and activity controls of Cu nanoparticles for high-performance solid oxide fuel cells. Appl. Catal. B Environ. 2021, 285, 119828. [Google Scholar] [CrossRef]
- Fu, L.; Zhou, J.; Yang, J.; Lian, Z.; Wang, J.; Cheng, Y.; Wu, K. Exsolution of Cu nanoparticles in (LaSr)0.9Fe0.9Cu0.1O4 Ruddlesden-Popper oxide as symmetrical electrode for solid oxide cells. Appl. Surf. Sci. 2020, 511, 145525. [Google Scholar] [CrossRef]
- Yang, X.; Sun, K.; Sun, W.; Ma, M.; Ren, R.; Qiao, J.; Wang, Z.; Zhen, S.; Xu, C. Surface reconstruction of defective SrTi0.7Cu0.2Mo0.1O3-δ perovskite oxide induced by in-situ copper nanoparticle exsolution for high-performance direct CO2 electrolysis. J. Eur. Ceram. Soc. 2023, 43, 3414–3420. [Google Scholar] [CrossRef]
- Khan, M.S.; Lee, S.-B.; Song, R.-H.; Lee, J.-W.; Lim, T.-H.; Park, S.-J. Fundamental mechanisms involved in the degradation of nickel–yttria stabilized zirconia (Ni–YSZ) anode during solid oxide fuel cells operation: A review. Ceram. Int. 2016, 42, 35–48. [Google Scholar] [CrossRef]
- Ayyanusamy, P.; Alphonse, R.; Minakshi, M.; Sivasubramanian, R. Synthesis of Amorphous Nickel-Cobalt Hydroxides for Ni−Zn Batteries. Chem.—A Eur. J. 2024, 30, e202402325. [Google Scholar] [CrossRef]
- Hu, F.; Ling, Y.; Fang, S.; Sui, L.; Xiao, H.; Huang, Y.; Wang, S.; He, B.; Zhao, L. Engineering dual-exsolution on self-assembled cathode to achieve efficient electrocatalytic CO2 reduction. Appl. Catal. B Environ. 2023, 337, 122968. [Google Scholar] [CrossRef]
- Zhang, N.; Naden, A.; Zhang, L.; Yang, X.; Connor, P.; Irvine, J. Enhanced CO2 Electrolysis Through Mn Substitution Coupled with Ni Exsolution in Lanthanum Calcium Titanate Electrodes. Adv. Mater. 2024, 36, e2308481. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, J.; Sun, Y.; Yue, X.; Yang, J.; Fu, L.; Deng, Q.; Zhao, H.; Yin, C.; Wu, K. Tuning exsolution of nanoparticles in defect engineered layered perovskite oxides for efficient CO2 electrolysis. J. Energy Chem. 2023, 84, 219–227. [Google Scholar] [CrossRef]
- Amaya-Dueñas, D.-M.; Chen, G.; Weidenkaff, A.; Sata, N.; Han, F.; Biswas, I.; Costa, R.; Friedrich, K.A. A-site deficient chromite with in situ Ni exsolution as a fuel electrode for solid oxide cells (SOCs). J. Mater. Chem. A 2021, 9, 5685–5701. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, W.; Xu, C.; Zhang, L.; Ren, R.; Qiao, J.; Wang, Z.; Rooney, D.; Sun, K. Tuning the Product Selectivity of C2H6 Electrolysis Using a Core–Shell-Structured Ni@NiO-Modified Anode in a Ceramic Electrochemical Reactor. ACS Sustain. Chem. Eng. 2024, 12, 2289–2299. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Wang, R.; Zhang, C.; Li, G.; Davey, K.; Zhang, S.; Guo, Z. Progress and perspectives on iron-based electrode materials for alkali metal-ion batteries: A critical review. Chem. Soc. Rev. 2024, 53, 4154–4229. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Zhou, J.; Zhang, Z.; Li, S.; Ni, J.; Wu, K.; Irvine, J.T.S. Iron-based electrode materials for solid oxide fuel cells and electrolysers. Energy Environ. Sci. 2021, 14, 6287–6319. [Google Scholar] [CrossRef]
- Liu, C.; Li, S.; Gao, J.; Bian, L.; Hou, Y.; Wang, L.; Peng, J.; Bao, J.; Song, X.; An, S. Enhancing CO2 Catalytic Adsorption on an Fe Nanoparticle-Decorated LaSrFeO4 + δ Cathode for CO2 Electrolysis. ACS Appl. Mater. Interfaces 2021, 13, 8229–8238. [Google Scholar] [CrossRef]
- Liu, S.; Yang, M.; Xu, R.; Xiang, X.; Yang, G.; Xu, H.; Xiao, G.; Ran, R.; Zhou, W.; Shao, Z. In situ passivation of Fe nanoparticles exsolved from perovskite cathodes through zinc doping for CO2 electrolysis. Green Chem. 2023, 25, 9826–9836. [Google Scholar] [CrossRef]
- Dong, C.; Jiang, F.; Yang, L.; Wang, C.; Xie, K. Enhancing electrocatalytic reforming of CO2/CH4 with in situ exsolved metal-oxide interfaces in a solid oxide electrolysis cell. Sep. Purif. Technol. 2022, 299, 121714. [Google Scholar] [CrossRef]
- Tan, Y.; Tang, J.; Yang, C.; Tian, Y.; Wang, Z.; Pu, J.; Chi, B. Double perovskite decorated with in situ exsolved Fe nanoparticles as active catalyst for CO2 electrolysis. Ceram. Int. 2023, 49, 40797–40803. [Google Scholar] [CrossRef]
- Gao, X.; Ye, L.; Xie, K. Voltage-driven reduction method to optimize in-situ exsolution of Fe nanoparticles at Sr2Fe1.5+xMo0.5O6-δ interface. J. Power Sources 2023, 561, 232740. [Google Scholar] [CrossRef]
- Choi, J.; Park, S.; Han, H.; Kim, M.; Park, M.; Han, J.; Kim, W.B. Highly efficient CO2 electrolysis to CO on Ruddlesden–Popper perovskite oxide with in situ exsolved Fe nanoparticles. J. Mater. Chem. A 2021, 9, 8740–8748. [Google Scholar] [CrossRef]
- Jiang, Y.; Ye, L.; Zhang, S.; Xia, C. Doped ceria with exsolved Fe0 nanoparticles as a Sr-free cathode for CO2 electrolysis in SOECs at reduced temperatures. J. Mater. Chem. A 2022, 10, 9380–9383. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, L.; Zhang, Y.; Gan, L. Enhanced steam electrolysis with exsolved iron nanoparticles in perovskite cathode. Int. J. Hydrogen Energy 2022, 47, 24287–24296. [Google Scholar] [CrossRef]
- Zhuang, G.; Chen, Y.; Zhuang, Z.; Yu, Y.; Yu, J. Oxygen vacancies in metal oxides: Recent progress towards advanced catalyst design. Sci. China Mater. 2020, 63, 2089–2118. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Guan, K.; Meng, J.; Wei, Z.; Liu, X.; Meng, J. Enhanced Anode Performance and Coking Resistance by In Situ Exsolved Multiple-Twinned Co–Fe Nanoparticles for Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2019, 12, 461–473. [Google Scholar] [CrossRef]
- Kujawska, K.; Koliński, W.; Bochentyn, B. Forming Ni-Fe and Co-Fe Bimetallic Structures on SrTiO3-Based SOFC Anode Candidates. Fuels 2024, 5, 564–573. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Q.; Luo, J.-L. CO2-to-CO conversion on layered perovskite within situ exsolved Co–Fe alloy nanoparticles: An active and stable cathode for solid oxide electrolysis cells. J. Mater. Chem. A 2016, 4, 17521–17528. [Google Scholar] [CrossRef]
- Bae, K.T.; Jeong, I.; Akromjon, A.; Im, H.-N.; Lee, K.T. Robust and efficient Fe/Mn bimetal doped Pr4/3Ba2/3Co2/3Fe2/3Mn2/3O5+δ double perovskite catalysts for direct CO2 electrolysis. Chem. Eng. J. 2023, 472, 145015. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, D.; Wang, Y.; Li, Y.; Ren, C.; Ding, M.; Liu, T. Promoting catalysis activity with optimizable self-generated Co-Fe alloy nanoparticles for efficient CO2 electrolysis performance upgrade. Nano Res. 2023, 16, 10992–10999. [Google Scholar] [CrossRef]
- Xi, X.; Wang, X.-W.; Fan, Y.; Wang, Q.; Lu, Y.; Li, J.; Shao, L.; Luo, J.-L.; Fu, X.-Z. Efficient bifunctional electrocatalysts for solid oxide cells based on the structural evolution of perovskites with abundant defects and exsolved CoFe nanoparticles. J. Power Sources 2021, 482, 228981. [Google Scholar] [CrossRef]
- Sun, X.; Ye, Y.; Zhou, M.; Chen, H.; Li, Y.; Chen, P.; Dong, D.; Ling, Y.; Khan, M.; Chen, Y. Layered-perovskite oxides with in situ exsolved Co–Fe alloy nanoparticles as highly efficient electrodes for high-temperature carbon dioxide electrolysis. J. Mater. Chem. A 2022, 10, 2327–2335. [Google Scholar] [CrossRef]
- Li, B.; He, S.; Li, J.; Yue, X.; Irvine, J.T.S.; Xie, D.; Ni, J.; Ni, C. A Ce/Ru Codoped SrFeO3−δ Perovskite for a Coke-Resistant Anode of a Symmetrical Solid Oxide Fuel Cell. ACS Catal. 2020, 10, 14398–14409. [Google Scholar] [CrossRef]
- He, S.; Li, M.; Hui, J.; Yue, X. In-situ construction of ceria-metal/titanate heterostructure with controllable architectures for efficient fuel electrochemical conversion. Appl. Catal. B Environ. 2021, 298, 120588. [Google Scholar] [CrossRef]
- Wu, M.; Ni, J.; Ni, C. Achieving High-Efficiency CO2 Electrolysis for SrFeO3-δ-Based Symmetric Electrodes. ACS Sustain. Chem. Eng. 2023, 11, 10717–10726. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.J.; Ferree, M.; Gunduz, S.; Co, A.C.; Kim, M.; Ozkan, U.S. In-situ exsolution of bimetallic CoFe nanoparticles on (La,Sr)FeO3 perovskite: Its effect on electrocatalytic oxidative coupling of methane. Appl. Catal. B Environ. 2023, 321, 122026. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, S.; Li, R.; Yu, J.; Zhang, X.; Li, M.; Zheng, X.; Zhu, J.; Song, Y.; Wang, G.; et al. In situ exsolved CoFe alloy nanoparticles for stable anodic methane reforming in solid oxide electrolysis cells. Joule 2024, 8, 2016–2032. [Google Scholar] [CrossRef]
- Qin, M.; Zhang, S.; Sun, W.; Xu, C.; Qiao, J.; Wang, Z.; Zhen, S.; Sun, K. Novel Sr1.95Fe1.4Co0.1Mo0.5O6-δ anode heterostructure for efficient electrochemical oxidative dehydrogenation of ethane to ethylene by solid oxide electrolysis cells. Ceram. Int. 2023, 49, 30178–30186. [Google Scholar] [CrossRef]
- Kang, Q.; Lai, D.; Tang, W.; Lu, Q.; Gao, F. Intrinsic activity modulation and structural design of NiFe alloy catalysts for an efficient oxygen evolution reaction. Chem. Sci. 2021, 12, 3818–3835. [Google Scholar] [CrossRef]
- An, W.; Gatewood, D.; Dunlap, B.; Turner, C.H. Catalytic activity of bimetallic nickel alloys for solid-oxide fuel cell anode reactions from density-functional theory. J. Power Sources 2011, 196, 4724–4728. [Google Scholar] [CrossRef]
- Deka, D.J.; Kim, J.; Gunduz, S.; Aouine, M.; Millet, J.-M.M.; Co, A.C.; Ozkan, U.S. Investigation of hetero-phases grown via in-situ exsolution on a Ni-doped (La,Sr)FeO3 cathode and the resultant activity enhancement in CO2 reduction. Appl. Catal. B Environ. 2021, 286, 119917. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zhang, S.; Ren, C.; Jing, Y.; Cheng, F.; Wu, Q.; Lund, P.; Fan, L. Mutual Conversion of CO–CO2 on a Perovskite Fuel Electrode with Endogenous Alloy Nanoparticles for Reversible Solid Oxide Cells. ACS Appl. Mater. Interfaces 2022, 14, 9138–9150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jiang, Y.; Han, H.; Li, Y.; Xia, C. Perovskite Oxyfluoride Ceramic with In Situ Exsolved Ni–Fe Nanoparticles for Direct CO2 Electrolysis in Solid Oxide Electrolysis Cells. ACS Appl. Mater. Interfaces 2022, 14, 28854–28864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, C.; Ren, R.; Qiao, J.; Wang, Z.; Sun, W.; Sun, K. Self-Assembly Dual Active Site Nanocomposite Anode Ce0.6Mn0.3Fe0.1O2−δ/NiFe/MnOx for Electrooxidative Dehydrogenation of Ethane to Ethylene. ACS Appl. Mater. Interfaces 2024, 16, 3451–3459. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, W.; Xu, C.; Ren, R.; Yang, X.; Qiao, J.; Wang, Z.; Sun, K. Attenuating a metal–oxygen bond of a double perovskite oxide via anion doping to enhance its catalytic activity for the oxygen reduction reaction. J. Mater. Chem. A 2020, 8, 14091–14098. [Google Scholar] [CrossRef]
- Lv, H.; Lin, L.; Zhang, X.; Song, Y.; Matsumoto, H.; Zeng, C.; Ta, N.; Liu, W.; Gao, D.; Wang, G.; et al. In Situ Investigation of Reversible Exsolution/Dissolution of CoFe Alloy Nanoparticles in a Co-Doped Sr2Fe1.5Mo0.5O6−δ Cathode for CO2 Electrolysis. Adv. Mater. 2020, 32, 1906193. [Google Scholar] [CrossRef]
- Li, P.; Xuan, Y.; Jiang, B.; Zhang, S.; Xia, C. Hollow La0.6Sr0.4Ni0.2Fe0.75Mo0.05O3-δ electrodes with exsolved FeNi3 in quasi-symmetrical solid oxide electrolysis cells for direct CO2 electrolysis. Electrochem. Commun. 2022, 134, 107188. [Google Scholar] [CrossRef]
- Ansari, H.M.; Bass, A.S.; Ahmad, N.; Birss, V.I. Unraveling the evolution of exsolved Fe–Ni alloy nanoparticles in Ni-doped La0.3Ca0.7Fe0.7Cr0.3O3−δ and their role in enhancing CO2–CO electrocatalysis. J. Mater. Chem. A 2022, 10, 2280–2294. [Google Scholar] [CrossRef]
- Xu, M.; Liu, C.; Naden, A.B.; Früchtl, H.; Bühl, M.; Irvine, J.T.S. Electrochemical Activation Applied to Perovskite Titanate Fibers to Yield Supported Alloy Nanoparticles for Electrocatalytic Application. Small 2022, 19, 2204682. [Google Scholar] [CrossRef]
- Li, P.; Yang, P.; Liu, F.; Xiao, W.; Yan, F.; Gan, T.; Zhao, K.; Fu, D. Enhancing catalytic activity of CO2 electrolysis via B-site cation doped perovskite cathode in solid oxide electrolysis cell. Ceram. Int. 2023, 49, 12980–12989. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, C.; Wang, Y.; Xu, M.; Ling, Y.; Pu, J.; Ciucci, F.; Irvine, J.T.S.; Chi, B. Phase transition with in situ exsolution nanoparticles in the reduced Pr0.5Ba0.5Fe0.8Ni0.2O3−δ electrode for symmetric solid oxide cells. J. Mater. Chem. A 2022, 10, 16490–16496. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, W.; Wang, Z.; Ren, C.; Wang, Y.; Ding, M.; Liu, T. Efficient electrochemical CO2 reduction reaction on a robust perovskite type cathode with in-situ exsolved Fe-Ru alloy nanocatalysts. Sep. Purif. Technol. 2023, 304, 122287. [Google Scholar] [CrossRef]
- Lv, H.; Lin, L.; Zhang, X.; Li, R.; Song, Y.; Matsumoto, H.; Ta, N.; Zeng, C.; Fu, Q.; Wang, G.; et al. Promoting exsolution of RuFe alloy nanoparticles on Sr2Fe1.4Ru0.1Mo0.5O6−δ via repeated redox manipulations for CO2 electrolysis. Nat. Commun. 2021, 12, 5665. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, J.; Zhou, Z.; Zhang, Q.; Wang, J.; Sun, Y.; Yin, C.; Xue, Z.; Wang, K.; Wu, K. Exsoluble Ni–Co alloy nanoparticles anchored on a layered perovskite for direct CO2 electrolysis. Mater. Lett. 2024, 362, 135926. [Google Scholar] [CrossRef]
- Ma, G.; Xu, Y.; Xie, K. Enhanced Electrolysis of CO2 with Metal–Oxide Interfaces in Perovskite Cathode in Solid Oxide Electrolysis Cell. Catalysts 2022, 12, 1607. [Google Scholar] [CrossRef]
- Lv, J.; Sun, W.; Xu, C.; Yang, X.; Ma, M.; Zhang, L.; Zhang, S.; Qiao, J.; Zhen, S.; Sun, K. Enhancing the catalytic activity and CO2 chemisorption ability of the perovskite cathode for soild oxide electrolysis cell through in situ Fe-Sn alloy nanoparticles. Sep. Purif. Technol. 2022, 294, 121127. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, T.; Li, R.; Lv, H.; Ta, N.; Zhang, X.; Song, Y.; Liu, Q.; Feng, W.; Wang, G.; et al. In situ electrochemical reconstruction of Sr2Fe1.45Ir0.05Mo0.5O6-δ perovskite cathode for CO2 electrolysis in solid oxide electrolysis cells. Natl. Sci. Rev. 2023, 10, nwad078. [Google Scholar] [CrossRef]
- Xi, X.; Fan, Y.; Zhang, J.; Luo, J.-L.; Fu, X.-Z. In situ construction of hetero-structured perovskite composites with exsolved Fe and Cu metallic nanoparticles as efficient CO2 reduction electrocatalysts for high performance solid oxide electrolysis cells. J. Mater. Chem. A 2022, 10, 2509–2518. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, T.; Du, K.; Zhang, Q.; Liu, M.; Yang, C. A High-Entropy Layered Perovskite Coated with In Situ Exsolved Core-Shell CuFe@FeOx Nanoparticles for Efficient CO2 Electrolysis. Adv. Mater. 2023, 36, 2312119. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Zhang, P.; Han, X.; Chen, H.; Wei, B.; Lü, Z. Surface regulating of copper-iron nanoparticles bimetallic exsolution for enhanced performance and durability of double perovskite anode in solid oxide fuel cells. Fuel 2023, 353, 129140. [Google Scholar] [CrossRef]
- López-García, A.; Almar, L.; Escolástico, S.; Hungría, A.B.; Carrillo, A.J.; Serra, J.M. Tuning Ternary Alloyed Nanoparticle Composition and Morphology by Exsolution in Double Perovskite Electrodes for CO2 Electrolysis. ACS Appl. Energy Mater. 2022, 5, 13269–13283. [Google Scholar] [CrossRef]
- Wu, W.; Wang, L.-C.; Hu, H.; Bian, W.; Gomez, J.Y.; Orme, C.J.; Ding, H.; Dong, Y.; He, T.; Li, J.; et al. Electrochemically Engineered, Highly Energy-Efficient Conversion of Ethane to Ethylene and Hydrogen below 550 °C in a Protonic Ceramic Electrochemical Cell. ACS Catal. 2021, 11, 12194–12202. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, L.; Xie, K. Electrochemical dehydrogenation of ethane to ethylene in solid oxide electrolyzer. Int. J. Hydrogen Energy 2023, 48, 30238–30246. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.K.; Liu, J.; Curcio, A.; Jang, J.-S.; Kim, I.-D.; Ciucci, F.; Jung, W. Nanoparticle Ex-solution for Supported Catalysts: Materials Design, Mechanism and Future Perspectives. ACS Nano 2021, 15, 81–110. [Google Scholar] [CrossRef]
- Neagu, D.; Oh, T.-S.; Miller, D.N.; Ménard, H.; Bukhari, S.M.; Gamble, S.R.; Gorte, R.J.; Vohs, J.M.; Irvine, J.T.S. Nano-socketed nickel particles with enhanced coking resistance grown in situ by redox exsolution. Nat. Commun. 2015, 6, 8120. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.; Kwon, O.; Kim, K.; Kim, S.; Kim, H.; Shin, J.; Jeong, H.Y.; Sengodan, S.; Han, J.W.; Kim, G. Cation-swapped homogeneous nanoparticles in perovskite oxides for high power density. Nat. Commun. 2019, 10, 697. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.; Kwon, O.; Kim, S.; Jeong, H.Y.; Kim, G. Ni-Fe Bimetallic Nanocatalysts Produced by Topotactic Exsolution in Fe deposited PrBaMn1.7Ni0.3O5+δ for Dry Reforming of Methane. J. Electrochem. Soc. 2020, 167, 064518. [Google Scholar] [CrossRef]
- Tang, S.; Fu, M.; Qin, Z.; Gao, Y.; Tao, Z. Topological Ion Optimized Composite Cathode for Proton-Conducting Solid Oxide Fuel Cells. Adv. Funct. Mater. 2005, 35, 2501995. [Google Scholar] [CrossRef]
- Lv, H.; Wang, S.; Shen, Y.; Zhang, X.; Song, Y.; Li, R.; Ta, N.; Liu, T.; Wang, G.; Bao, X. Iron-triggered exsolution of FeNi alloy nanoparticles via topotactic cation exchange on Pr0.7Sr0.3Cr0.9Ni0.1O3−δ perovskite for CO2 electrolysis. Next Energy 2023, 1, 100024. [Google Scholar] [CrossRef]
- Joo, S.; Seong, A.; Kwon, O.; Kim, K.; Lee, J.H.; Gorte, R.J.; Vohs, J.M.; Han, J.W.; Kim, G. Highly active dry methane reforming catalysts with boosted in situ grown Ni-Fe nanoparticles on perovskite via atomic layer deposition. Sci. Adv. 2020, 6, eabb1573. [Google Scholar] [CrossRef]
- Johnson, E.; Koh, A. Recent Advances in Smart Emulsion Materials: From Synthesis to Applications. Adv. Eng. Mater. 2024, 26, 2400995. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, M.; Li, F.; Fang, S.; He, D.; Lu, J.; Zhang, Y.; Cao, X.; Liu, J.; Chen, D.; Luo, Y. Advances in Nanostructured Electrodes for Solid Oxide Cells by Infiltration or Exsolution. Materials 2025, 18, 1802. https://doi.org/10.3390/ma18081802
Dai M, Li F, Fang S, He D, Lu J, Zhang Y, Cao X, Liu J, Chen D, Luo Y. Advances in Nanostructured Electrodes for Solid Oxide Cells by Infiltration or Exsolution. Materials. 2025; 18(8):1802. https://doi.org/10.3390/ma18081802
Chicago/Turabian StyleDai, Mingyue, Futao Li, Shujuan Fang, Dedong He, Jichang Lu, Yu Zhang, Xiaohua Cao, Jiangping Liu, Dingkai Chen, and Yongming Luo. 2025. "Advances in Nanostructured Electrodes for Solid Oxide Cells by Infiltration or Exsolution" Materials 18, no. 8: 1802. https://doi.org/10.3390/ma18081802
APA StyleDai, M., Li, F., Fang, S., He, D., Lu, J., Zhang, Y., Cao, X., Liu, J., Chen, D., & Luo, Y. (2025). Advances in Nanostructured Electrodes for Solid Oxide Cells by Infiltration or Exsolution. Materials, 18(8), 1802. https://doi.org/10.3390/ma18081802






