Study on the Microscale Mechanisms of Multi-Source Solid Waste Synergy in Enhancing Physicochemical Properties of Red Mud-Based Backfill Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
2.3. Experimental Methods
2.3.1. Uniaxial Compressive Strength Test
2.3.2. Microstructure Characterization
2.3.3. Environmental Performance Test
3. Results and Discussion
3.1. Physicochemical Performance Analysis
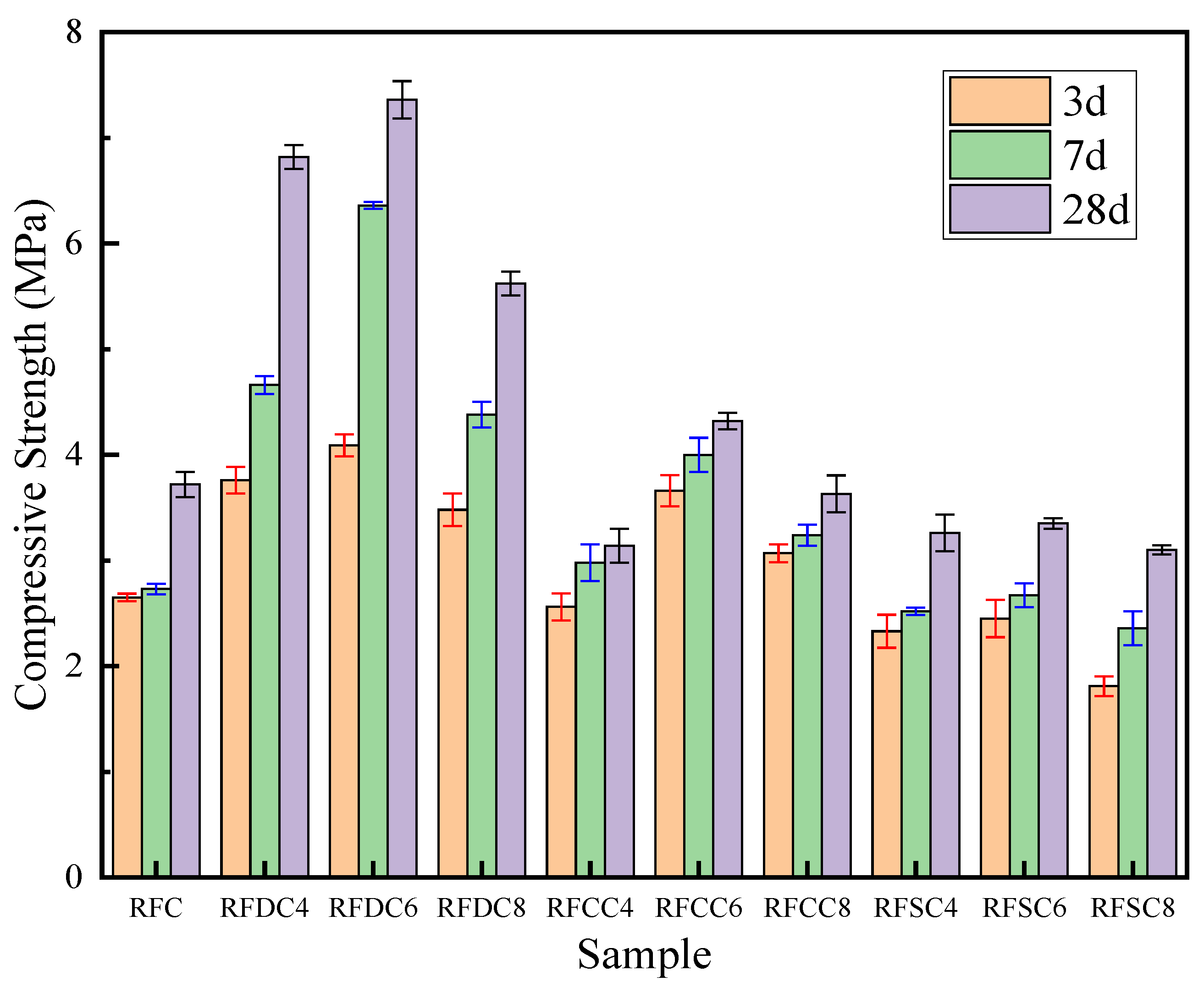
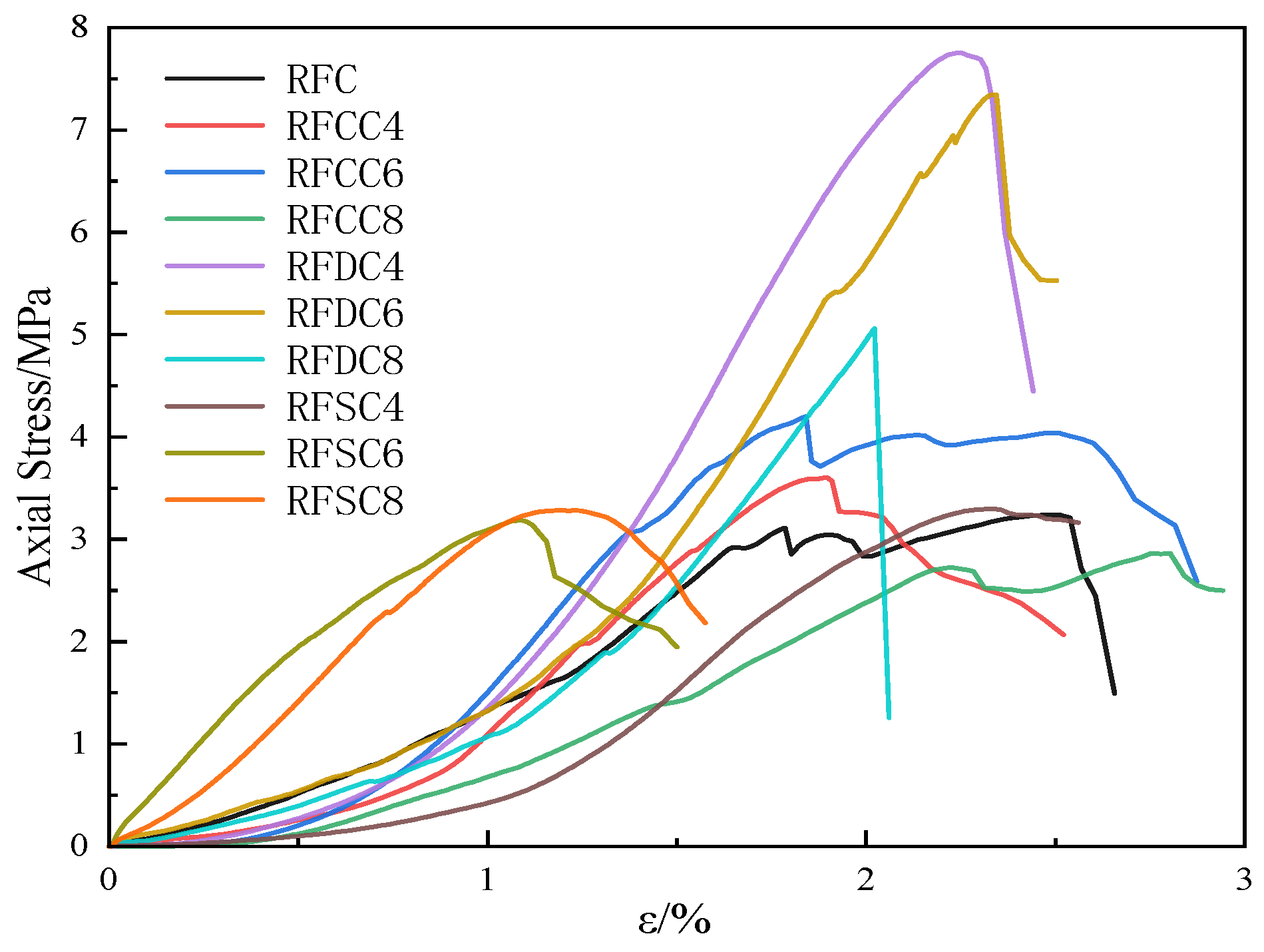
3.1.1. Compressive Strength Analysis
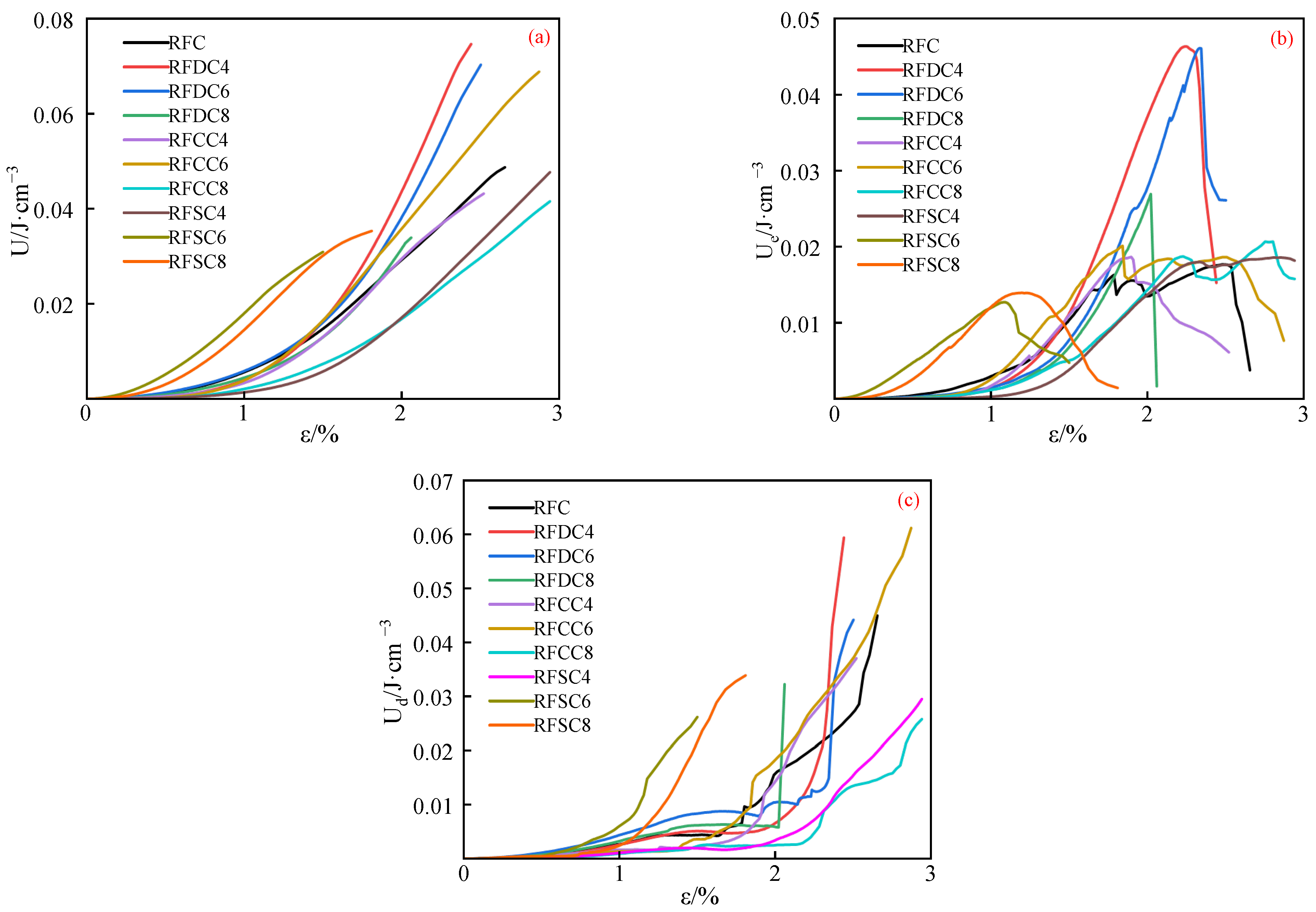
3.1.2. Energy Evolution Patterns
- (1)
- Initial damage stage (OA). The dissipated energy increases nonlinearly with deformation, primarily due to the closure of micropores and internal friction within the specimen. Both the input energy and elastic energy also increase with deformation, but the elastic energy remains lower than the dissipated energy during this stage. As the micropores close, the growth rate of the elastic strain energy begins to accelerate. The specimen transitions into the stable damage stage when the elastic strain energy equals the dissipated energy (after the intersection point of the curves). Therefore, the point where the dissipated energy equals the elastic energy can be regarded as the boundary between the initial damage stage and the stable damage stage.
- (2)
- Stable damage stage (AB). After the intersection point of the dissipated energy and elastic strain energy curves, the specimen enters the stable damage stage. During this stage, as the micropores are further compacted and internal friction continues, the dissipated energy curve increases approximately linearly. Both the total energy and elastic strain energy increase at the same rate, with their growth rates exceeding that of the dissipated energy. This stage is primarily characterized by the accumulation of elastic strain energy.
- (3)
- Stationary damage stage (BC). As the micropores become fully compacted, the specimen enters the stationary damage stage. During this stage, the dissipated energy remains nearly constant, and its evolution curve appears approximately horizontal. At this point, the micropores in the rock sample are completely closed, and almost no new dissipated energy is generated. As a result, the slope of the dissipated energy evolution curve is minimal (close to zero). Meanwhile, both the total energy and elastic energy continue to increase at an accelerated rate as the rock sample is further compacted, with their growth trends remaining consistent. In this stage, the energy input into the rock sample is primarily converted into elastic energy.
- (4)
- Accelerated damage stage (CD). As the deformation of the fractured rock sample increases, internal cracks begin to propagate and new cracks form, leading to the dissipation of the elastic strain energy stored in the rock sample. During this stage, the growth rate of the dissipated energy gradually increases (indicated by the rising slope of the dissipated energy evolution curve), while the growth rate of the elastic strain energy gradually decreases (indicated by the declining slope of the elastic strain energy evolution curve). As the specimen reaches its maximum compressive strength, the total input energy essentially peaks, and the elastic strain energy stored in the rock sample reaches its limit. At this point, the damage evolution of the fractured rock mass enters the failure stage.
- (5)
- Damage failure stage (DE). After the rock sample reaches its peak strength, the damage evolution of the specimen enters the damage failure stage. During this stage, the dissipated energy evolution curve exhibits a sharp and sudden increase, while the elastic strain energy evolution curve shows a sharp and sudden decrease, and the total energy remains essentially constant. The further propagation and interconnection of cracks in the fractured rock mass cause the specimen to ultimately lose its load-bearing capacity. The elastic strain energy accumulated in the rock sample is rapidly released in the form of dissipated energy, resulting in a sharp increase in the dissipated energy curve and a sharp decrease in the elastic strain energy curve [48].
3.2. Microstructure Analysis
3.2.1. Analysis of Hydration Product Phases
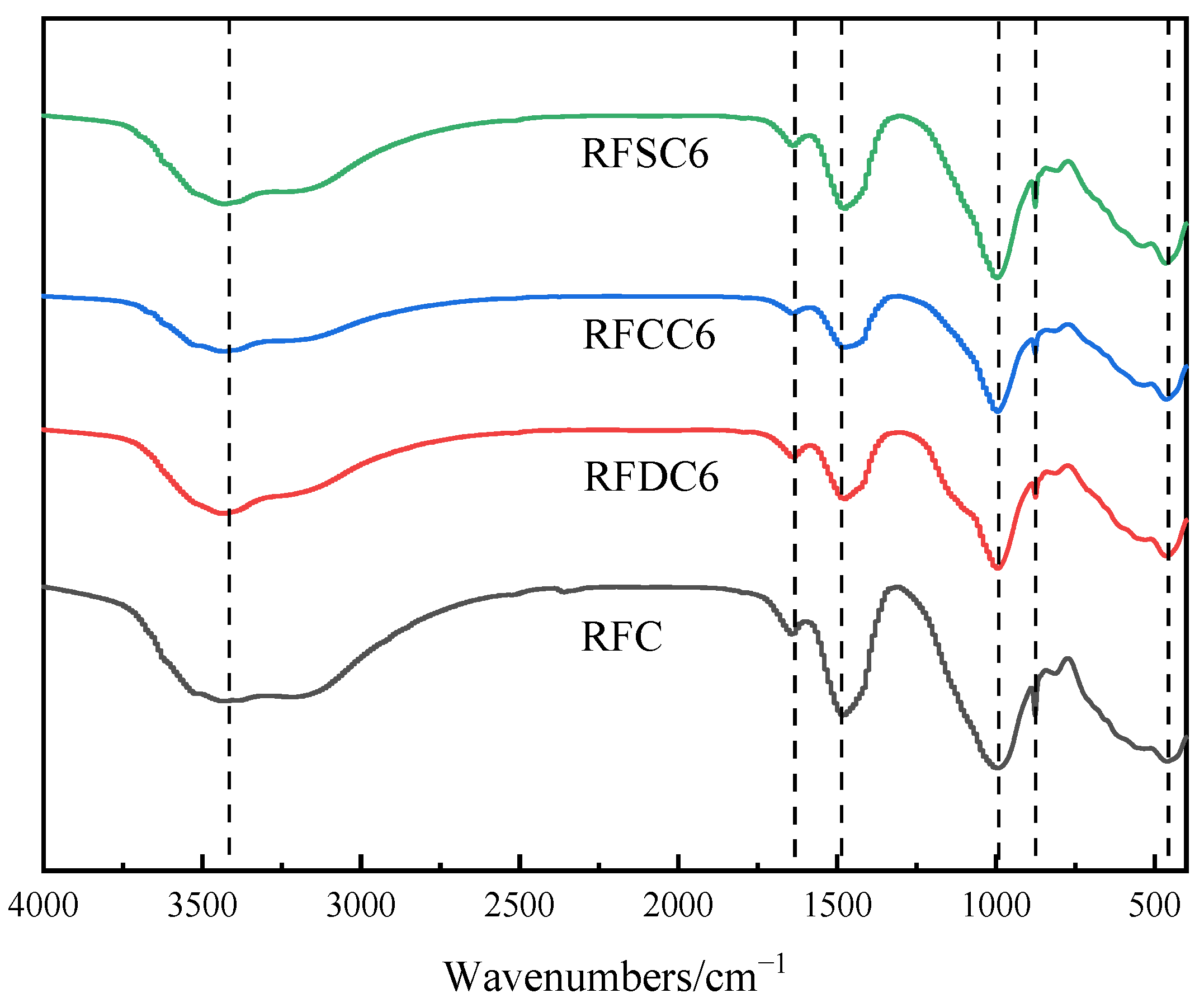
3.2.2. Chemical Bond Analysis of Hydration Products
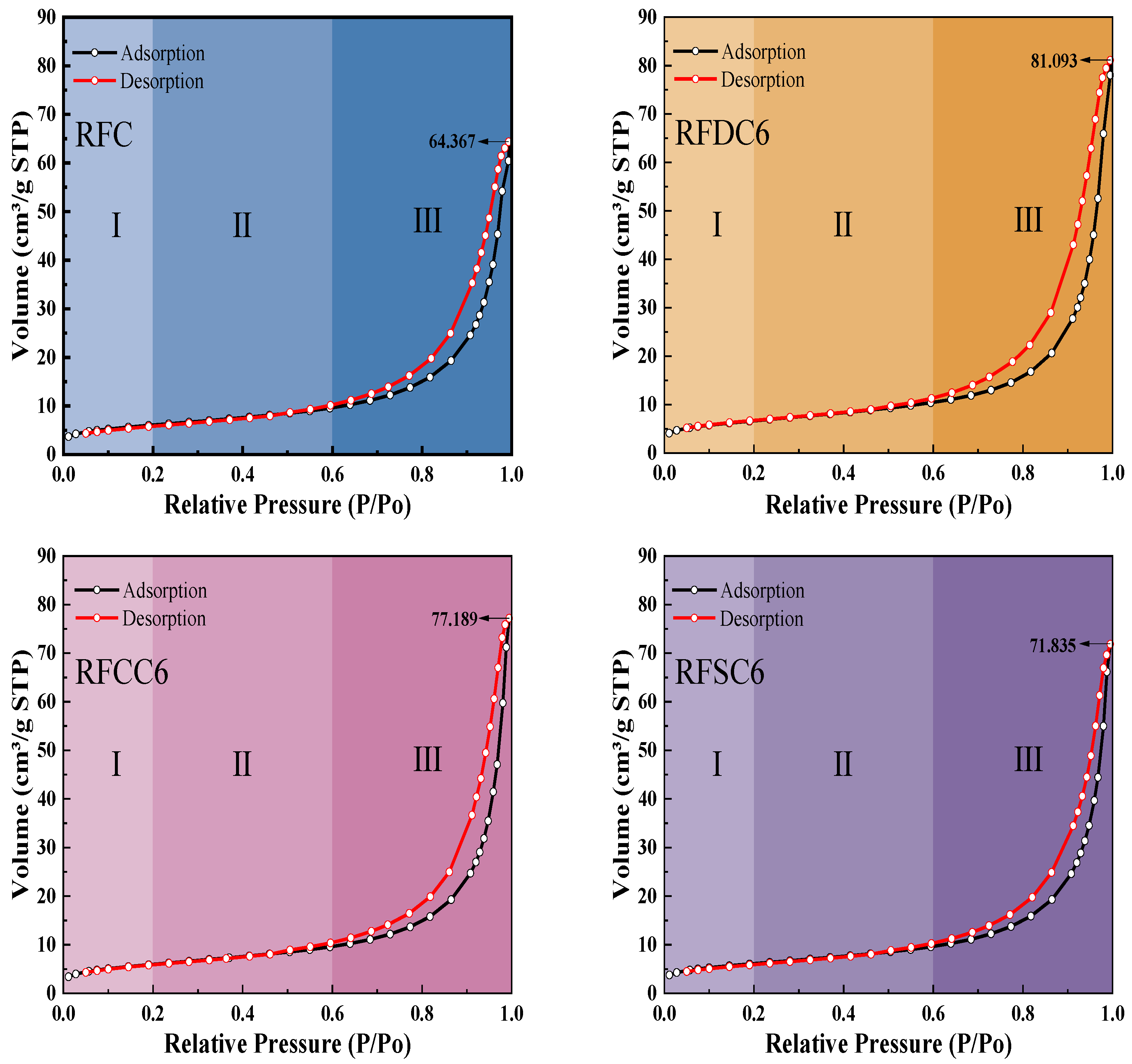
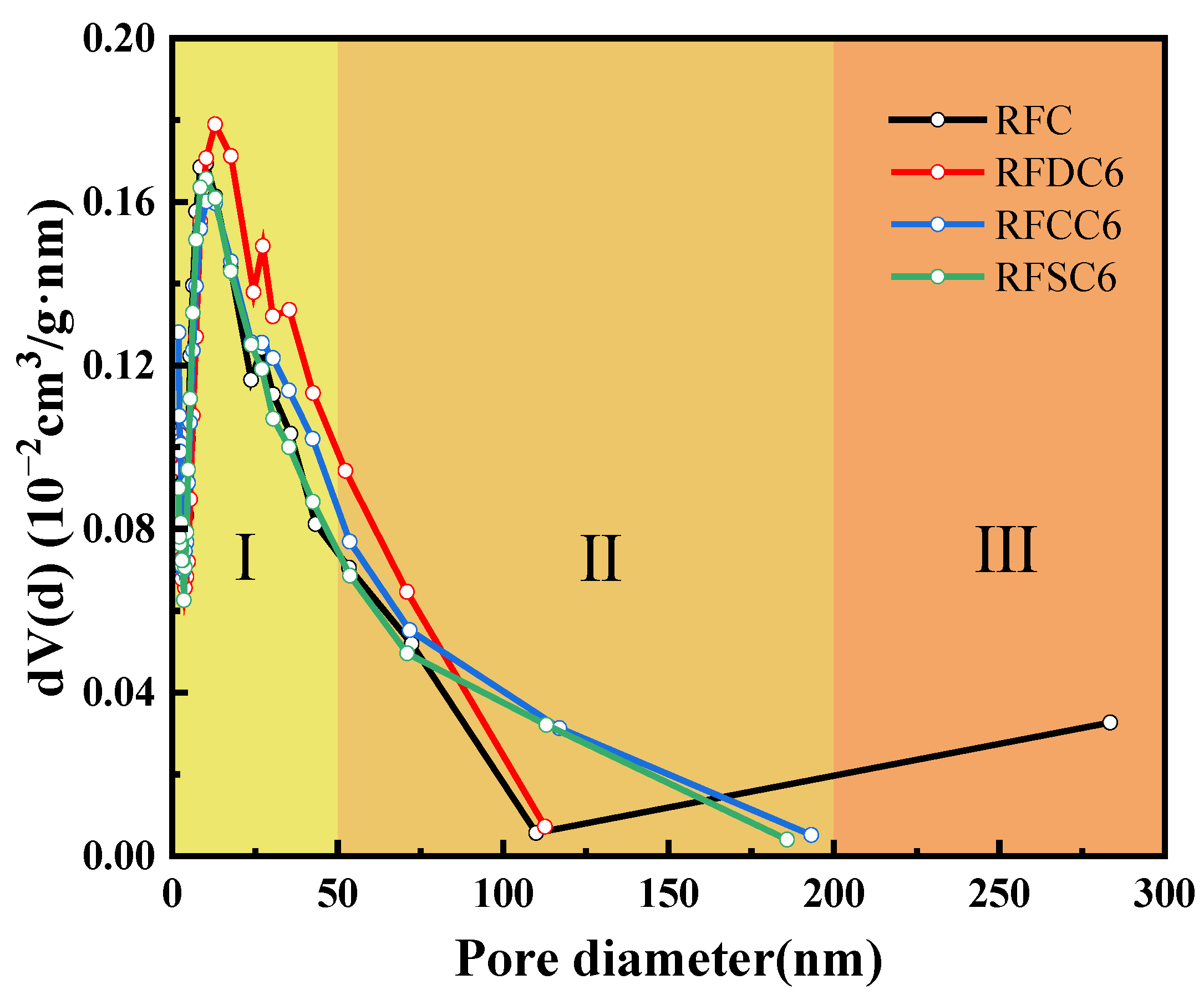
3.2.3. Pore Structure Analysis
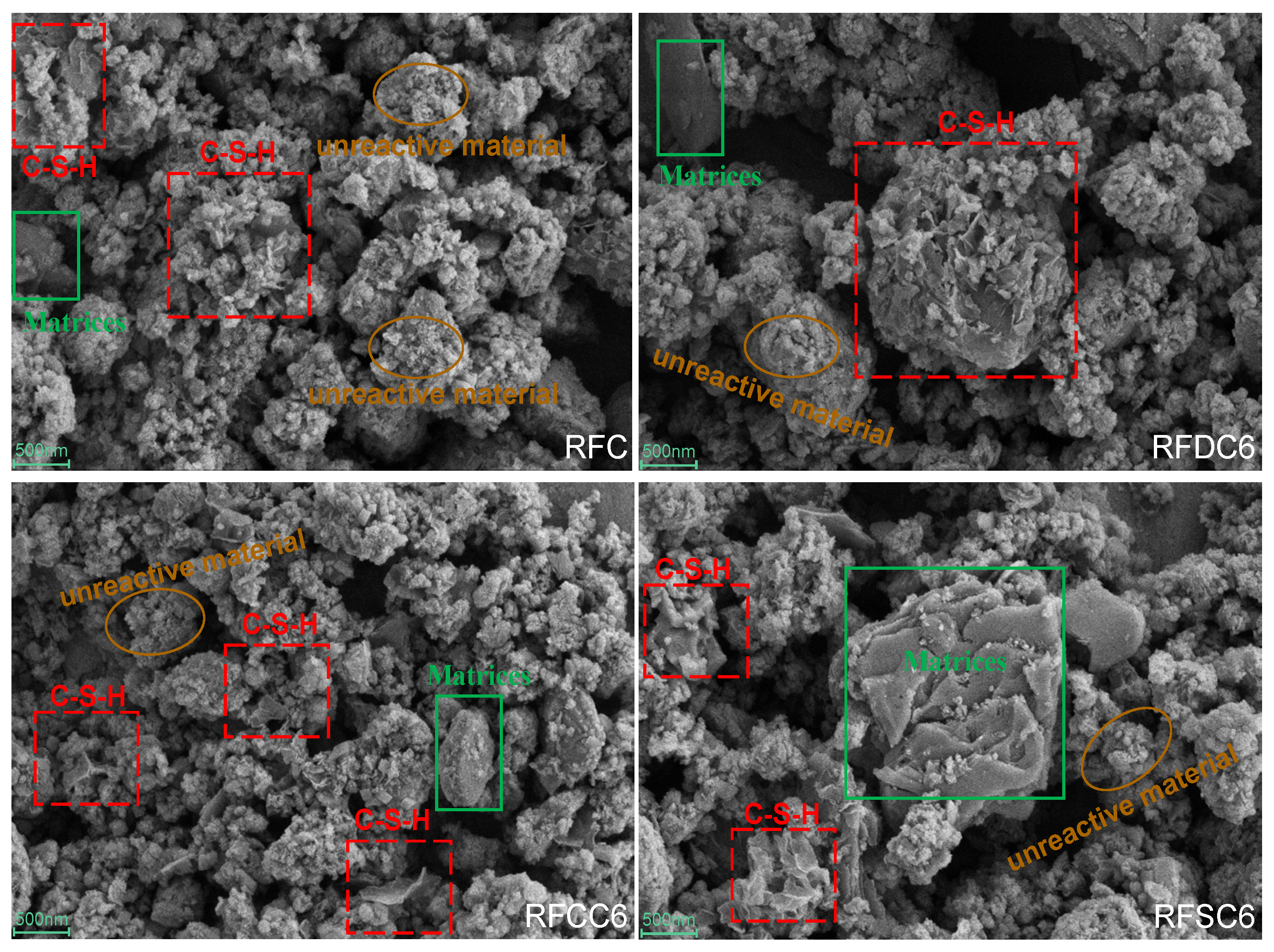
3.2.4. Micro Morphology Analysis
3.3. Economic and Environmental Impacts
3.4. Mechanism Analysis
4. Conclusions
- (1)
- RFDC6 exhibits excellent mechanical properties at different curing ages. The compressive strength of the material increases by 80.0% from 3 days to 28 days. Particularly at the 28-day curing age, due to the high integrity of the specimen and fewer microcracks, it demonstrates higher rigidity and can more effectively absorb and dissipate energy during the damage process. Its compressive strength is significantly higher than that of other systems. RFCC6 performs well in early strength improvement, but the compressive strength of the material only increases by 18.0% from 3 days to 28 days. At the 28-day curing age, its compressive strength improvement is limited, primarily enhancing the elastic properties of the material, which helps maintain the integrity of the filling structure and prevent structural collapse due to local overload. RFSC6 shows lower mechanical properties than the RFC reference system at different curing ages. Although the compressive strength of the material increases by 36.7% from 3 days to 28 days, at the 28-day curing age, its input energy, elastic energy, and dissipated energy are generally low. The insufficient energy absorption and load-bearing capacity, along with the lower overall structural integrity, result in less ideal mechanical performance.
- (2)
- Through microscopic analysis, it can be observed that the main hydration products of red mud-based filling materials include C(-A)-S-H gel and ettringite. These products interweave and adsorb to form a dense structural network, which is the primary factor contributing to the improvement in the material’s mechanical properties. In the RFDC6 system, the synergistic effect of SO42− promotes the generation of more C(-A)-S-H gel and ettringite, filling the internal pore structure of the material and enhancing its density. This transforms larger pores into smaller gel pores, making the pore structure more complete and more conducive to improving compressive strength. In the RFCC6 system, the increase in OH− concentration promotes the early formation of C(-A)-S-H gel. This gel fills the pores, converting larger pores into larger capillary pores. However, due to the limited solid phase content, the material’s density does not significantly improve, but the substantial gel content significantly enhances the material’s elastic properties. In the RFSC6 system, there is a considerable amount of unreacted raw materials. Carbonate precipitates are formed due to the carbon capture capability of steel slag, and there are gaps between the unreacted raw materials and the hydration products. The internal structure of the material is relatively fragmented, lacking a complete load-bearing structure, which results in a decline in various properties.
- (3)
- By testing the leachate of materials from each system, it can be observed that the heavy metal ion concentrations in the leachate of red mud-based filling materials all comply with the limits specified in the “GB 8978-1996 Integrated Wastewater Discharge Standard”. The synergistic effects of desulfurized gypsum, carbide slag, and steel slag with red mud effectively immobilize heavy metal ions in red mud-based filling materials, demonstrating excellent environmental performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, X.; Zeng, M.; Yu, H.; Lin, F.; Li, J.; Wang, W.; Chen, G. Critical review for the potential analysis of material utilization from inorganic industrial solid waste. J. Clean. Prod. 2024, 459, 142457. [Google Scholar] [CrossRef]
- Wu, F.; Ren, Y.; Qu, G.; Liu, S.; Chen, B.; Liu, X.; Zhao, C.; Li, J. Utilization path of bulk industrial solid waste: A review on the multi-directional resource utilization path of phosphogypsum. J. Environ. Manag. 2022, 313, 114957. [Google Scholar] [CrossRef] [PubMed]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilisation of Bayer red mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, M.; Zhang, G.; Mann, D.; Lumsden, K.; Tao, M. Durability of red mud-fly ash based geopolymer and leaching behavior of heavy metals in sulfuric acid solutions and deionized water. Constr. Build. Mater. 2016, 124, 373–382. [Google Scholar] [CrossRef]
- Liu, W.; Chen, X.; Li, W.; Yu, Y.; Yan, K. Environmental assessment, management and utilization of red mud in China. J. Clean. Prod. 2014, 84, 606–610. [Google Scholar] [CrossRef]
- Wang, F.; Xu, J.; Yin, H.; Zhang, Y.; Pan, H.; Wang, L. Sustainable stabilization/solidification of the Pb, Zn, and Cd contaminated soil by red mud-derived binders. Environ. Pollut. 2021, 284, 117178. [Google Scholar] [CrossRef] [PubMed]
- Ram Kumar, B.A.V.; Ramakrishna, G. Performance evaluation of red mud as a construction material—A review. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Reddy, P.S.; Reddy, N.G.; Serjun, V.Z.; Mohanty, B.; Das, S.K.; Reddy, K.R.; Rao, B.H. Properties and Assessment of Applications of Red Mud (Bauxite Residue): Current Status and Research Needs. Waste Biomass Valorization 2021, 12, 1185–1217. [Google Scholar] [CrossRef]
- Pei, J.; Pan, X.; Wang, Y.; Lv, Z.; Yu, H.; Tu, G. Effects of alkali and alkaline-earth oxides on preparation of red mud based ultra-lightweight ceramsite. Ceram. Int. 2023, 49, 18379–18387. [Google Scholar] [CrossRef]
- Wang, S.; Jin, H.; Deng, Y.; Xiao, Y. Comprehensive utilization status of red mud in China: A critical review. J. Clean. Prod. 2021, 289, 125136. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, Z.; Wang, K.; Wang, F.; Jiang, H.; Liang, M.; Wei, J.; Airey, G. Sustainable utilization of bauxite residue (Red Mud) as a road material in pavements: A critical review. Constr. Build. Mater. 2021, 270, 121419. [Google Scholar] [CrossRef]
- Krivenko, P.; Kovalchuk, O.; Pasko, A.; Croymans, T.; Hult, M.; Lutter, G.; Vandevenne, N.; Schreurs, S.; Schroeyers, W. Development of alkali activated cements and concrete mixture design with high volumes of red mud. Constr. Build. Mater. 2017, 151, 819–826. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Wang, K.-T.; Tang, Q.; Cui, X.-m. Synthesis and characterization of low temperature (<800 °C) ceramics from red mud geopolymer precursor. Constr. Build. Mater. 2017, 131, 564–573. [Google Scholar] [CrossRef]
- Wang, L.; Yu, I.K.M.; Tsang, D.C.W.; Li, S.; Li, J.-S.; Poon, C.S.; Wang, Y.-S.; Dai, J.-G. Transforming wood waste into water-resistant magnesia-phosphate cement particleboard modified by alumina and red mud. J. Clean. Prod. 2017, 168, 452–462. [Google Scholar] [CrossRef]
- Guru, S.; Amritphale, S.S.; Mishra, J.; Joshi, S. Multicomponent red mud-polyester composites for neutron shielding application. Mater. Chem. Phys. 2019, 224, 369–375. [Google Scholar] [CrossRef]
- Tang, W.C.; Wang, Z.; Donne, S.W.; Forghani, M.; Liu, Y. Influence of red mud on mechanical and durability performance of self-compacting concrete. J. Hazard. Mater. 2019, 379, 120802. [Google Scholar] [CrossRef]
- Gautam, M.; Agrawal, M. Phytoremediation of metals using vetiver (Chrysopogon zizanioides (L.) Roberty) grown under different levels of red mud in sludge amended soil. J. Geochem. Explor. 2017, 182, 218–227. [Google Scholar] [CrossRef]
- Egerić, M.; Smičiklas, I.; Dojčinović, B.; Sikirić, B.; Jović, M.; Šljivić-Ivanović, M.; Čakmak, D. Interactions of acidic soil near copper mining and smelting complex and waste-derived alkaline additives. Geoderma 2019, 352, 241–250. [Google Scholar] [CrossRef]
- Ma, L.; Han, L.; Chen, S.; Hu, J.; Chang, L.; Bao, W.; Wang, J. Rapid synthesis of magnetic zeolite materials from fly ash and iron-containing wastes using supercritical water for elemental mercury removal from flue gas. Fuel Process. Technol. 2019, 189, 39–48. [Google Scholar] [CrossRef]
- Xiao, K.; Guan, R.; Yang, J.; Li, H.; Yu, Z.; Liang, S.; Yu, W.; Hu, J.; Hou, H.; Liu, B. Effects of red mud on emission control of NOx precursors during sludge pyrolysis: A protein model compound study. Waste Manag. 2019, 85, 452–463. [Google Scholar] [CrossRef]
- Oliveira, A.A.S.; Martins, A.R.; Ferreira, R.V.; Cunha, I.T.; Serp, P.; de Mesquita, J.P.; Moura, F.C.C. N-doped carbon nanotubes grown on red mud residue: Hybrid nanocomposites for technological applications. Catal. Today 2020, 344, 247–258. [Google Scholar] [CrossRef]
- Samouhos, M.; Taxiarchou, M.; Pilatos, G.; Tsakiridis, P.E.; Devlin, E.; Pissas, M. Controlled reduction of red mud by H2 followed by magnetic separation. Miner. Eng. 2017, 105, 36–43. [Google Scholar] [CrossRef]
- Lu, F.; Xiao, T.; Lin, J.; Li, A.; Long, Q.; Huang, F.; Xiao, L.; Li, X.; Wang, J.; Xiao, Q.; et al. Recovery of gallium from Bayer red mud through acidic-leaching-ion-exchange process under normal atmospheric pressure. Hydrometallurgy 2018, 175, 124–132. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Zhang, Q.; Zhang, C.; Chen, L. Separation characteristics of vanadium from leach liquor of red mud by ion exchange with different resins. Hydrometallurgy 2018, 176, 42–48. [Google Scholar] [CrossRef]
- Chen, T.; Gao, Y.; Li, Y.; Zhu, J.; Cheng, Z.; Xiong, H. The strength, reaction mechanism, sustainable potential of full solid waste alkali-activated cementitious materials using red mud and carbide slag. Constr. Build. Mater. 2024, 449, 138454. [Google Scholar] [CrossRef]
- Han, Z.; Ma, H.; Shi, G.; He, L.; Wei, L.; Shi, Q. A review of groundwater contamination near municipal solid waste landfill sites in China. Sci. Total Environ. 2016, 569, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, Z.; Li, Y.; Cao, W. Strength properties of Bayer red mud stabilized by lime-fly ash using orthogonal experiments. Constr. Build. Mater. 2018, 166, 554–563. [Google Scholar] [CrossRef]
- Zhou, K.; Gong, K.; Zhou, Q.; Zhao, S.; Guo, H.; Qian, X. Estimating the feasibility of using industrial solid wastes as raw material for polyurethane composites with low fire hazards. J. Clean. Prod. 2020, 257, 120606. [Google Scholar] [CrossRef]
- Bao, Z.; Ptacek, C.J.; Blowes, D.W. Extracting resources from abandoned mines. Science 2023, 381, 731–732. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Y.; Zhou, L.; Hou, H.; Zhang, Y.; Liang, J.; Zhang, S. Ecological restoration in mining areas in the context of the Belt and Road initiative: Capability and challenges. Environ. Impact Assess. Rev. 2022, 95, 106767. [Google Scholar] [CrossRef]
- Cooke, J.A.; Johnson, M.S. Ecological restoration of land with particular reference to the mining of metals and industrial minerals: A review of theory and practice. Environ. Rev. 2002, 10, 41–71. [Google Scholar] [CrossRef]
- Dong, Z.; Bian, Z.; Jin, W.; Guo, X.; Zhang, Y.; Liu, X.; Wang, C.; Guan, D. An integrated approach to prioritizing ecological restoration of abandoned mine lands based on cost-benefit analysis. Sci. Total Environ. 2024, 924, 171579. [Google Scholar] [CrossRef]
- Yao, Y.; Li, Y.; Liu, X.; Jiang, S.; Feng, C.; Rafanan, E. Characterization on a cementitious material composed of red mud and coal industry byproducts. Constr. Build. Mater. 2013, 47, 496–501. [Google Scholar] [CrossRef]
- Liu, R.-X.; Poon, C.-S. Utilization of red mud derived from bauxite in self-compacting concrete. J. Clean. Prod. 2016, 112, 384–391. [Google Scholar] [CrossRef]
- Ye, N.; Yang, J.; Liang, S.; Hu, Y.; Hu, J.; Xiao, B.; Huang, Q. Synthesis and strength optimization of one-part geopolymer based on red mud. Constr. Build. Mater. 2016, 111, 317–325. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Wu, L.; Wu, A.; Ruan, Z.; Zhang, M.; Zhao, R. Effective reuse of red mud as supplementary material in cemented paste backfill: Durability and environmental impact. Constr. Build. Mater. 2022, 328, 127002. [Google Scholar] [CrossRef]
- Li, Y.; Min, X.; Ke, Y.; Liu, D.; Tang, C. Preparation of red mud-based geopolymer materials from MSWI fly ash and red mud by mechanical activation. Waste Manag. 2019, 83, 202–208. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, G.; Bai, X.; Kong, S.; Li, J.; Li, G.; Ge, Z.; Huang, J. Resource Utilization Potential of Red Mud: A Study on the Micro-Mechanism of the Synergistic Effect of Multiple Solid Waste Filling Materials. Sustainability 2023, 15, 5532. [Google Scholar] [CrossRef]
- Singh, S.B.; Munjal, P.; Thammishetti, N. Role of water/cement ratio on strength development of cement mortar. J. Build. Eng. 2015, 4, 94–100. [Google Scholar] [CrossRef]
- Zhou, G.; Qiu, H.; Zhang, Q.; Xu, M.; Wang, J.; Wang, G. Experimental Investigation of Coal Dust Wettability Based on Surface Contact Angle. J. Chem. 2016, 2016, 9452303. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, Q.; Chen, Q.; Kero, J.; Andersson, A.; Ahmed, H.; Engström, F.; Samuelsson, C. Characterization and evaluation of the pozzolanic activity of granulated copper slag modified with CaO. J. Clean. Prod. 2019, 232, 1112–1120. [Google Scholar] [CrossRef]
- HJ 557-2010; Solid Waste Leaching Toxicity Leaching Method Horizontal Oscillation Method. Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 2010.
- Ke, G.; Li, Z.; Jiang, H. Study on long-term solidification of all-solid waste cementitious materials based on circulating fluidized bed fly ash, red mud, carbide slag, and fly ash. Constr. Build. Mater. 2024, 427, 136284. [Google Scholar] [CrossRef]
- Xu, J.; Chen, P. Synergistic effect of iron tailings, steel slag and red mud cementitious materials on mechanical and microstructure properties. J. Build. Eng. 2024, 95, 110131. [Google Scholar] [CrossRef]
- Peng, R.; Ju, Y.; Wang, J.G.; Xie, H.; Gao, F.; Mao, L. Energy Dissipation and Release During Coal Failure Under Conventional Triaxial Compression. Rock Mech. Rock Eng. 2015, 48, 509–526. [Google Scholar] [CrossRef]
- Zhang, M.; Meng, Q.; Liu, S. Energy Evolution Characteristics and Distribution Laws of Rock Materials under Triaxial Cyclic Loading and Unloading Compression. Adv. Mater. Sci. Eng. 2017, 2017, 5471571. [Google Scholar] [CrossRef]
- Gong, F.; Yan, J.; Luo, S.; Li, X. Investigation on the Linear Energy Storage and Dissipation Laws of Rock Materials Under Uniaxial Compression. Rock Mech. Rock Eng. 2019, 52, 4237–4255. [Google Scholar] [CrossRef]
- Li, D.; Sun, Z.; Xie, T.; Li, X.; Ranjith, P.G. Energy evolution characteristics of hard rock during triaxial failure with different loading and unloading paths. Eng. Geol. 2017, 228, 270–281. [Google Scholar] [CrossRef]
- Xu, L.; Gong, F.; Luo, S. Effects of pre-existing single crack angle on mechanical behaviors and energy storage characteristics of red sandstone under uniaxial compression. Theor. Appl. Fract. Mech. 2021, 113, 102933. [Google Scholar] [CrossRef]
- Yang, H.; Sinha, S.K.; Feng, Y.; McCallen, D.B.; Jeremić, B. Energy dissipation analysis of elastic–plastic materials. Comput. Methods Appl. Mech. Eng. 2018, 331, 309–326. [Google Scholar] [CrossRef]
- Zhang, N.; Li, H.; Zhao, Y.; Liu, X. Hydration characteristics and environmental friendly performance of a cementitious material composed of calcium silicate slag. J. Hazard. Mater. 2016, 306, 67–76. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Li, Z.; Ren, Y.; Wang, Y.; Zhang, W. Preparation, characterization and application of red mud, fly ash and desulfurized gypsum based eco-friendly road base materials. J. Clean. Prod. 2021, 284, 124777. [Google Scholar] [CrossRef]
- Pallav, V.; Teppand, T.; Leinpuu, A.; Shanskiy, M.; Mets, M.; Mändar, H.; Rikmann, E.; Liiv, J. Stabilization of Road Embankments on Peat Soils Using Oil Shale Ash and Pozzolanic Additives. Appl. Sci. 2023, 13, 8366. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, Q.; Xue, C.; Jia, Y.; Guo, W.; Zhang, Y.; Qiu, Y. Preparation and curing method of red mud-calcium carbide slag synergistically activated fly ash-ground granulated blast furnace slag based eco-friendly geopolymer. Cem. Concr. Compos. 2023, 139, 104999. [Google Scholar] [CrossRef]
- Jiang, H.; Ke, G.; Li, Z. Synergistic activation mechanism and long-term properties of a solid waste cementitious materials based on red mud and calcium carbide slag. Constr. Build. Mater. 2024, 438, 137313. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Xu, Y.; Tang, B.; Wang, Y.; Mukiza, E. Preparation and characterization of cement treated road base material utilizing electrolytic manganese residue. J. Clean. Prod. 2019, 232, 980–992. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Cheilas, A.; Katsioti, M.; Georgiades, A.; Malliou, O.; Teas, C.; Haniotakis, E. Impact of hardening conditions on to stabilized/solidified products of cement–sewage sludge–jarosite/alunite. Cem. Concr. Compos. 2007, 29, 263–269. [Google Scholar] [CrossRef]
- Ortega, J.M.; Esteban, M.D.; Rodríguez, R.R.; Pastor, J.L.; Ibanco, F.J.; Sánchez, I.; Climent, M.Á. Long-Term Behaviour of Fly Ash and Slag Cement Grouts for Micropiles Exposed to a Sulphate Aggressive Medium. Materials 2017, 10, 598. [Google Scholar] [CrossRef]
- Koshy, N.; Dondrob, K.; Hu, L.; Wen, Q.; Meegoda, J.N. Synthesis and characterization of geopolymers derived from coal gangue, fly ash and red mud. Constr. Build. Mater. 2019, 206, 287–296. [Google Scholar] [CrossRef]
- GB 8978-1996; Integrated Wastewater Discharge Standard. Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 1996.
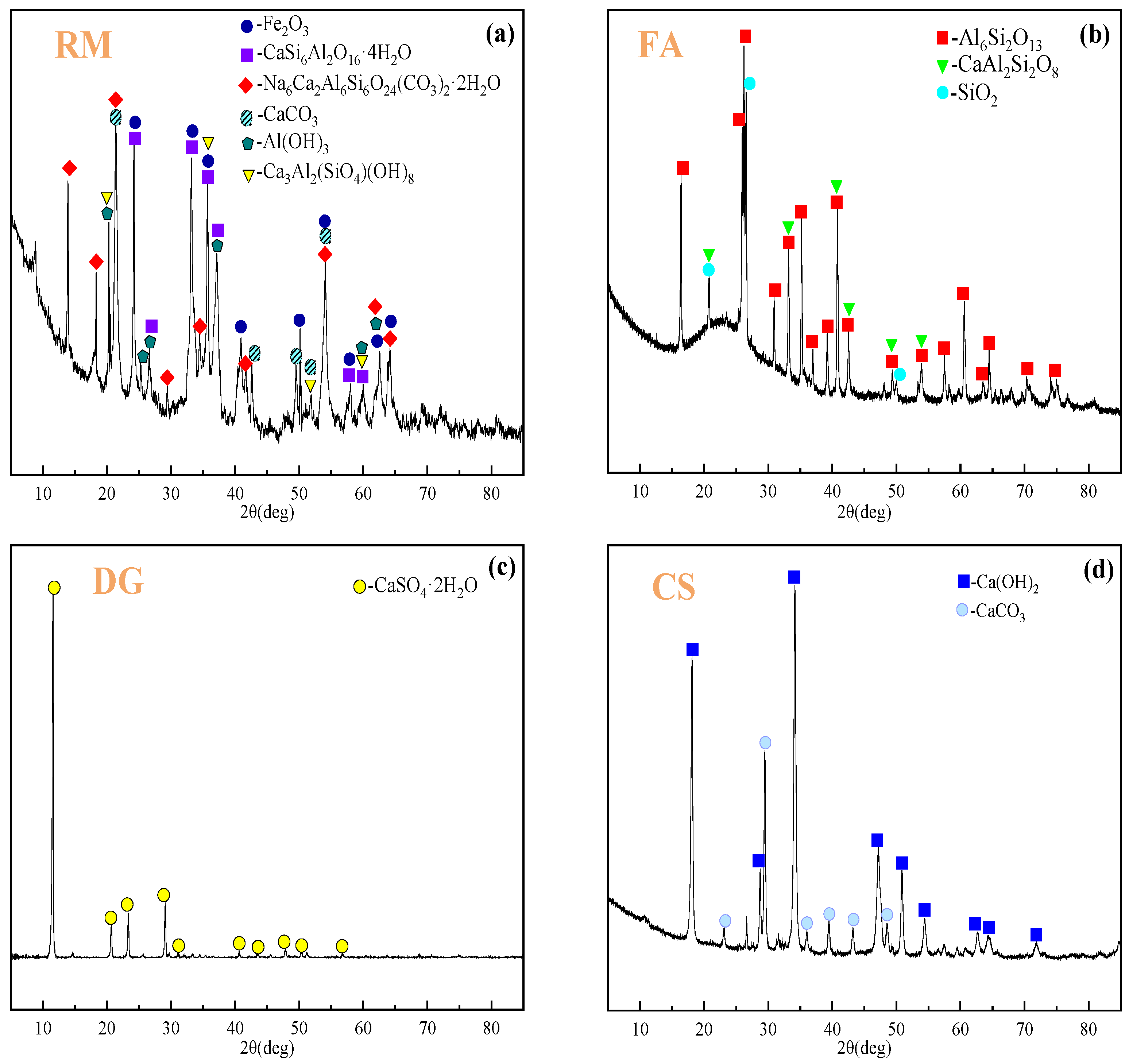
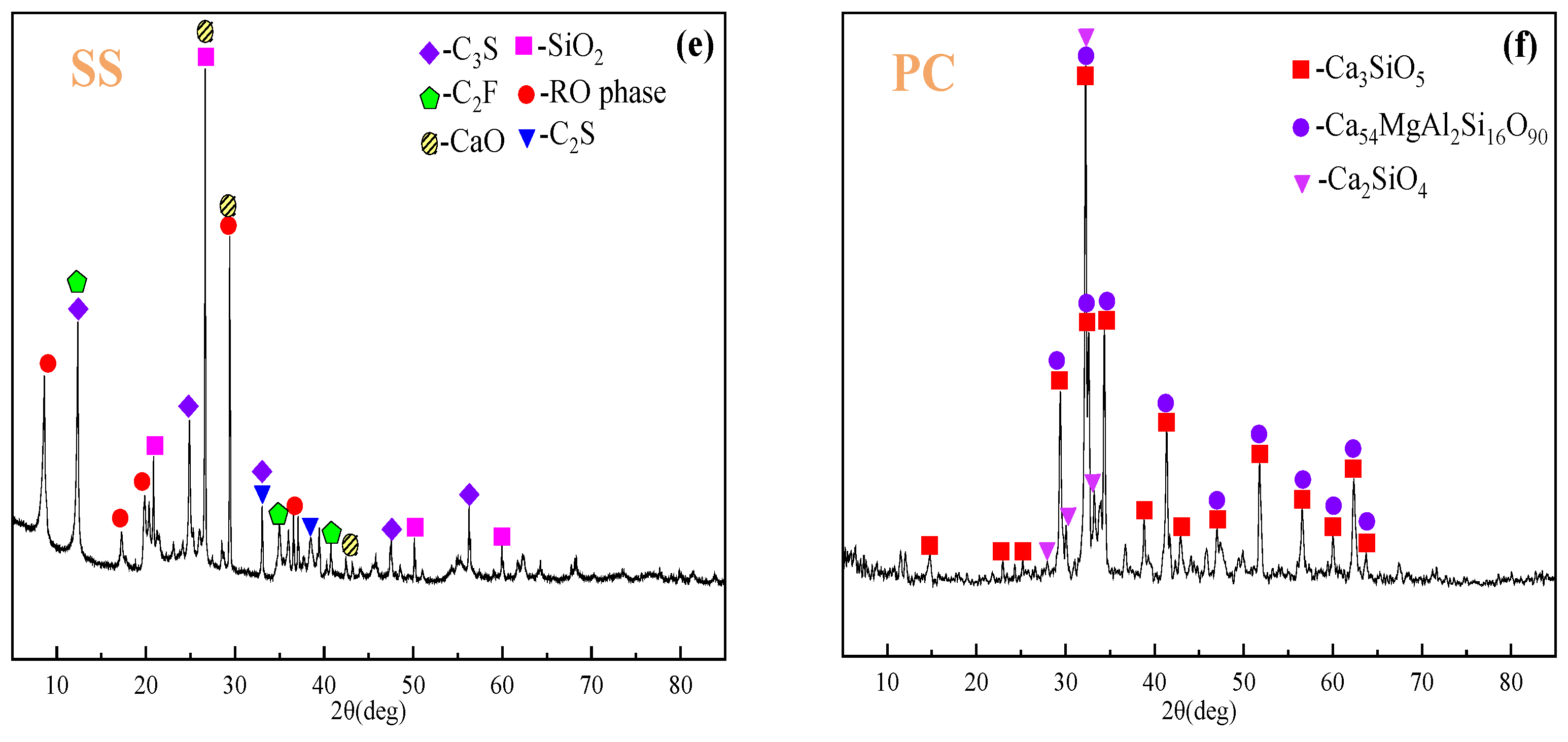
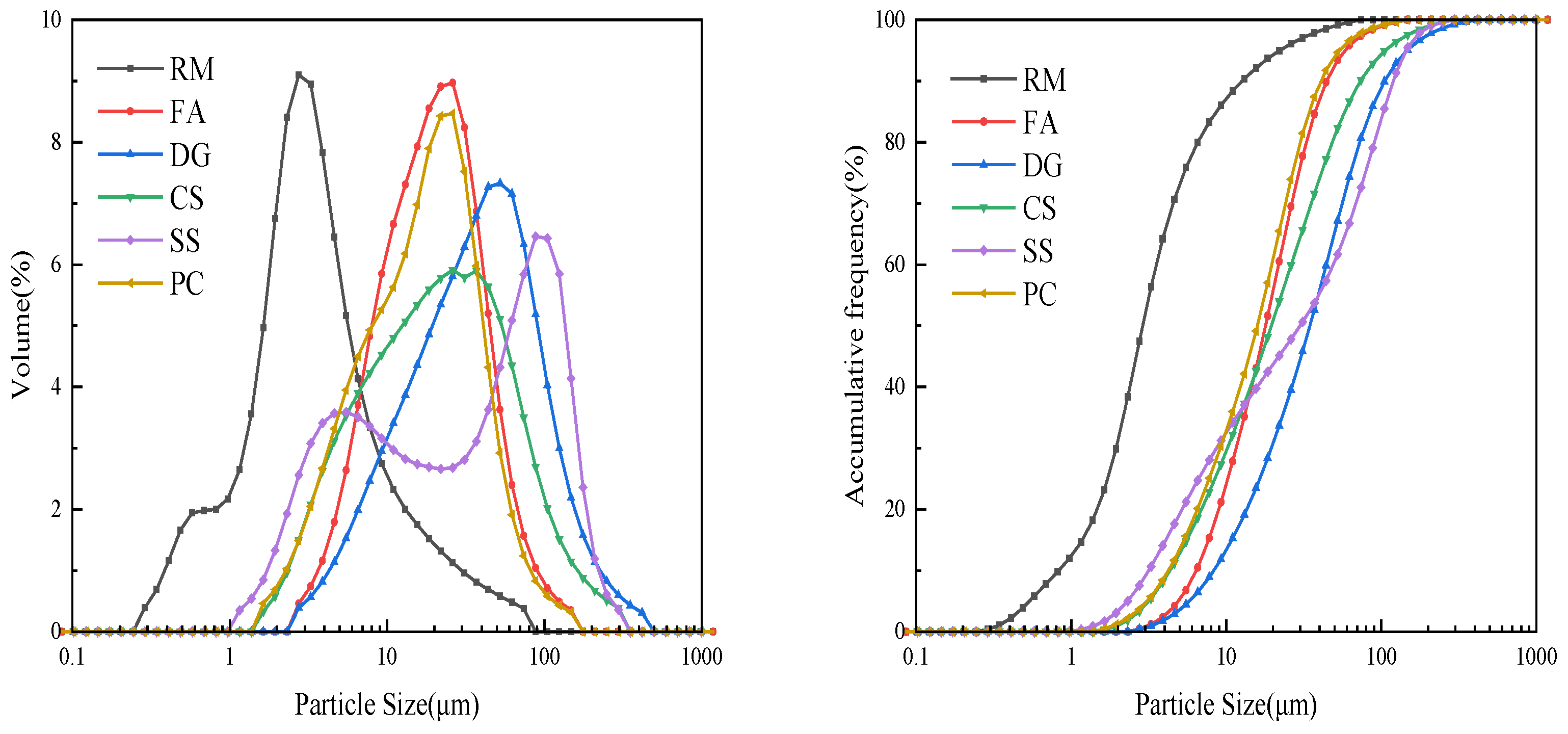



| Components (Wt%) | Fe2O3 | Al2O3 | SiO2 | Na2O | TiO2 | CaO | SO3 | P2O5 | K2O |
|---|---|---|---|---|---|---|---|---|---|
| RM | 49.84 | 19.33 | 12.20 | 8.53 | 7.93 | 0.87 | 0.55 | 0.19 | 0.16 |
| FA | 8.60 | 28.15 | 38.79 | 0.24 | 1.84 | 17.16 | 3.02 | 1.06 | 1.14 |
| DG | 0.00 | 0.03 | 0.04 | 0.06 | 0.00 | 49.19 | 50.64 | 0.00 | 0.00 |
| CS | 1.45 | 3.93 | 3.53 | 0.00 | 0.00 | 88.75 | 1.51 | 0.83 | 0.00 |
| SS | 31.83 | 6.23 | 21.20 | 3.26 | 1.79 | 25.66 | 3.68 | 2.13 | 4.22 |
| PC | 3.35 | 4.22 | 16.15 | 0.21 | 0.42 | 68.77 | 3.41 | 0.08 | 0.67 |
| Sample | RM | FA | DG | CS | SS | PC |
|---|---|---|---|---|---|---|
| RFC | 70 | 20 | - | - | - | 10 |
| RFDC4 | 67.2 | 19.2 | 4 | - | - | 9.6 |
| RFDC6 | 65.8 | 18.8 | 6 | - | - | 9.4 |
| RFDC8 | 64.4 | 18.4 | 8 | - | - | 9.2 |
| RFCC4 | 67.2 | 19.2 | - | 4 | - | 9.6 |
| RFCC6 | 65.8 | 18.8 | - | 6 | - | 9.4 |
| RFCC8 | 64.4 | 18.4 | - | 8 | - | 9.2 |
| RFSC4 | 67.2 | 19.2 | - | - | 4 | 9.6 |
| RFSC6 | 65.8 | 18.8 | - | - | 6 | 9.4 |
| RFSC8 | 64.4 | 18.4 | - | - | 8 | 9.2 |
| Sample | Compressive Strength/MPa | |||||
|---|---|---|---|---|---|---|
| 3 d | Rangeability/% | 7 d | Rangeability/% | 28 d | Rangeability/% | |
| RFC | 2.65 | - | 2.73 | - | 3.72 | - |
| RFDC4 | 3.76 | 41.9% | 4.66 | 70.7% | 6.82 | 83.3% |
| RFDC6 | 4.09 | 54.3% | 6.36 | 133.0% | 7.36 | 97.8% |
| RFDC8 | 3.48 | 31.3% | 4.38 | 60.4% | 5.62 | 51.1% |
| RFCC4 | 2.56 | −3.4% | 2.98 | 9.2% | 3.14 | −15.6% |
| RFCC6 | 3.66 | 38.11% | 4.00 | 46.5% | 4.32 | 16.1% |
| RFCC8 | 3.07 | 15.85% | 3.24 | 18.7% | 3.63 | −2.4% |
| RFSC4 | 2.33 | −12.1% | 2.52 | −7.7% | 3.26 | −12.4% |
| RFSC6 | 2.45 | −7.5% | 2.67 | −2.19% | 3.35 | −9.9% |
| RFSC8 | 1.81 | −31.7% | 2.36 | −13.6% | 3.10 | −16.7% |
| Sample | RFC | RFDC6 | RFCC6 | RFSC6 |
|---|---|---|---|---|
| Specific surface area (m2/g) | 21.8451 | 23.8856 | 21.6598 | 21.9245 |
| Total pore volume (cm3/g) | 0.099563 | 0.125435 | 0.119396 | 0.111114 |
| Average pore size (nm) | 17.5122 | 18.6526 | 19.6011 | 19.4901 |
| Sample | Leaching Ion (mg/L) | ||||||
|---|---|---|---|---|---|---|---|
| As | Pb | Cd | Cr | Cu | Mn | Hg | |
| RFC | 0.0173 | 0.0066 | <0.0012 | 0.0817 | 0.069.0 | <0.0036 | 0.0005 |
| RFDC6 | 0.0162 | <0.0042 | <0.0012 | 0.0691 | 0.022.6 | <0.0036 | 0.0002 |
| RFCC6 | 0.0157 | <0.0042 | <0.0012 | 0.0591 | 0.037.9 | <0.0036 | 0.0003 |
| RFSC6 | 0.0169 | 0.0057 | <0.0012 | 0.0535 | 0.010.2 | <0.0036 | 0.0001 |
| GB8978-1996 limits | 0.5 | 0.1 | 1.5 | 1.0 | 2.0 | 0.5 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Yan, G.; Kong, S.; Bai, X.; Zhang, J.; Ge, Z. Study on the Microscale Mechanisms of Multi-Source Solid Waste Synergy in Enhancing Physicochemical Properties of Red Mud-Based Backfill Materials. Materials 2025, 18, 1822. https://doi.org/10.3390/ma18081822
Huang J, Yan G, Kong S, Bai X, Zhang J, Ge Z. Study on the Microscale Mechanisms of Multi-Source Solid Waste Synergy in Enhancing Physicochemical Properties of Red Mud-Based Backfill Materials. Materials. 2025; 18(8):1822. https://doi.org/10.3390/ma18081822
Chicago/Turabian StyleHuang, Jinjing, Guochao Yan, Shaoqi Kong, Xuyang Bai, Jiawei Zhang, and Zhiguo Ge. 2025. "Study on the Microscale Mechanisms of Multi-Source Solid Waste Synergy in Enhancing Physicochemical Properties of Red Mud-Based Backfill Materials" Materials 18, no. 8: 1822. https://doi.org/10.3390/ma18081822
APA StyleHuang, J., Yan, G., Kong, S., Bai, X., Zhang, J., & Ge, Z. (2025). Study on the Microscale Mechanisms of Multi-Source Solid Waste Synergy in Enhancing Physicochemical Properties of Red Mud-Based Backfill Materials. Materials, 18(8), 1822. https://doi.org/10.3390/ma18081822







