Effect of NaAlO2 Co-Electrical Parameters on the Wear Resistance of MAO/MoS2 Self-Lubricating Composite Coatings
Abstract
1. Introduction
2. Experimental
2.1. Fabrication of MAO Coatings
2.2. Fabrication of MAO/MoS2 Self-Lubricating Composite Coatings
2.3. Characterization of Coating Properties
3. Results
3.1. Orthogonal Experiment Results
3.2. Comprehensive Wear Performance
3.3. Optimized Wear Performance
3.4. Optimized Coating Properties
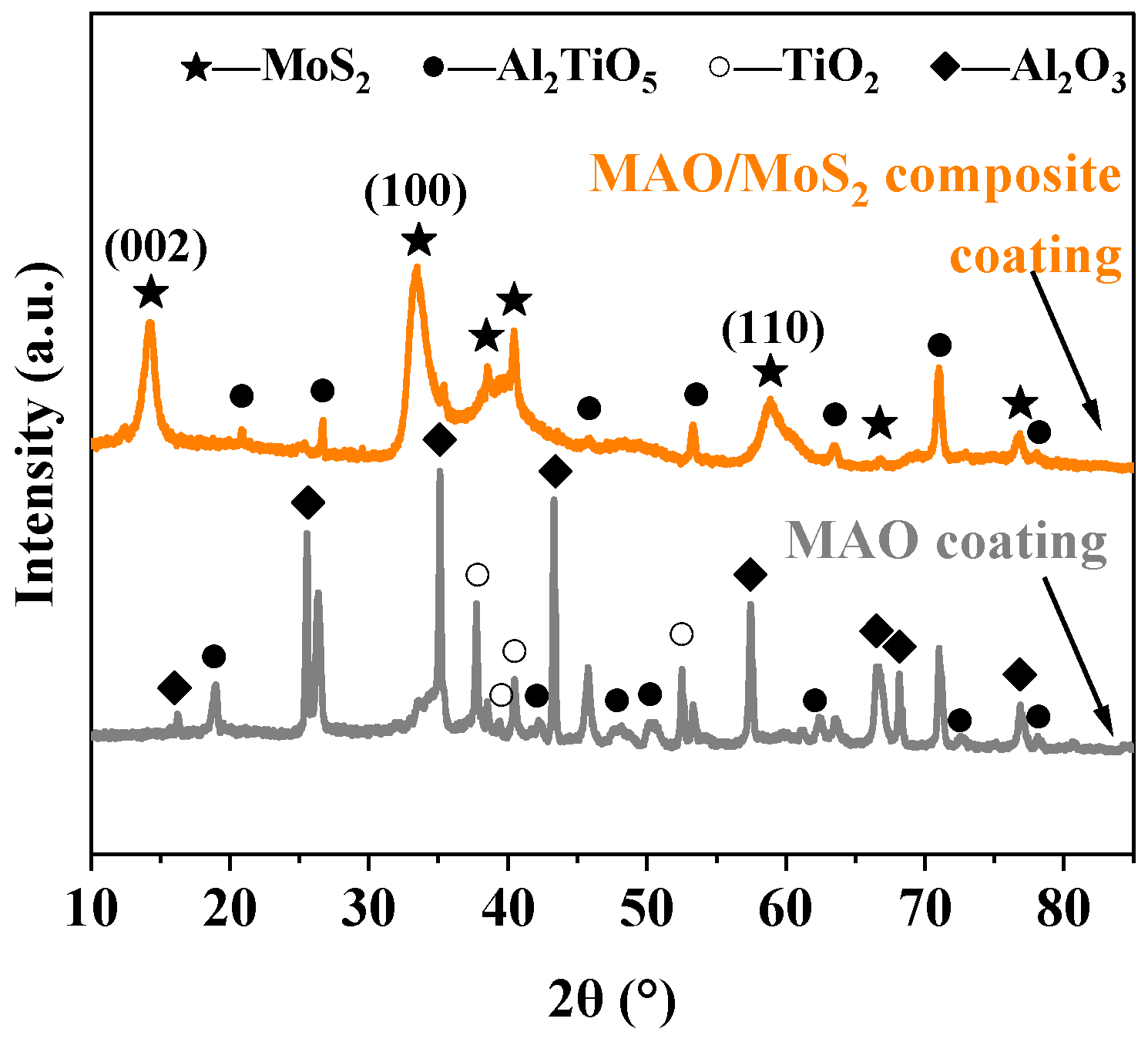
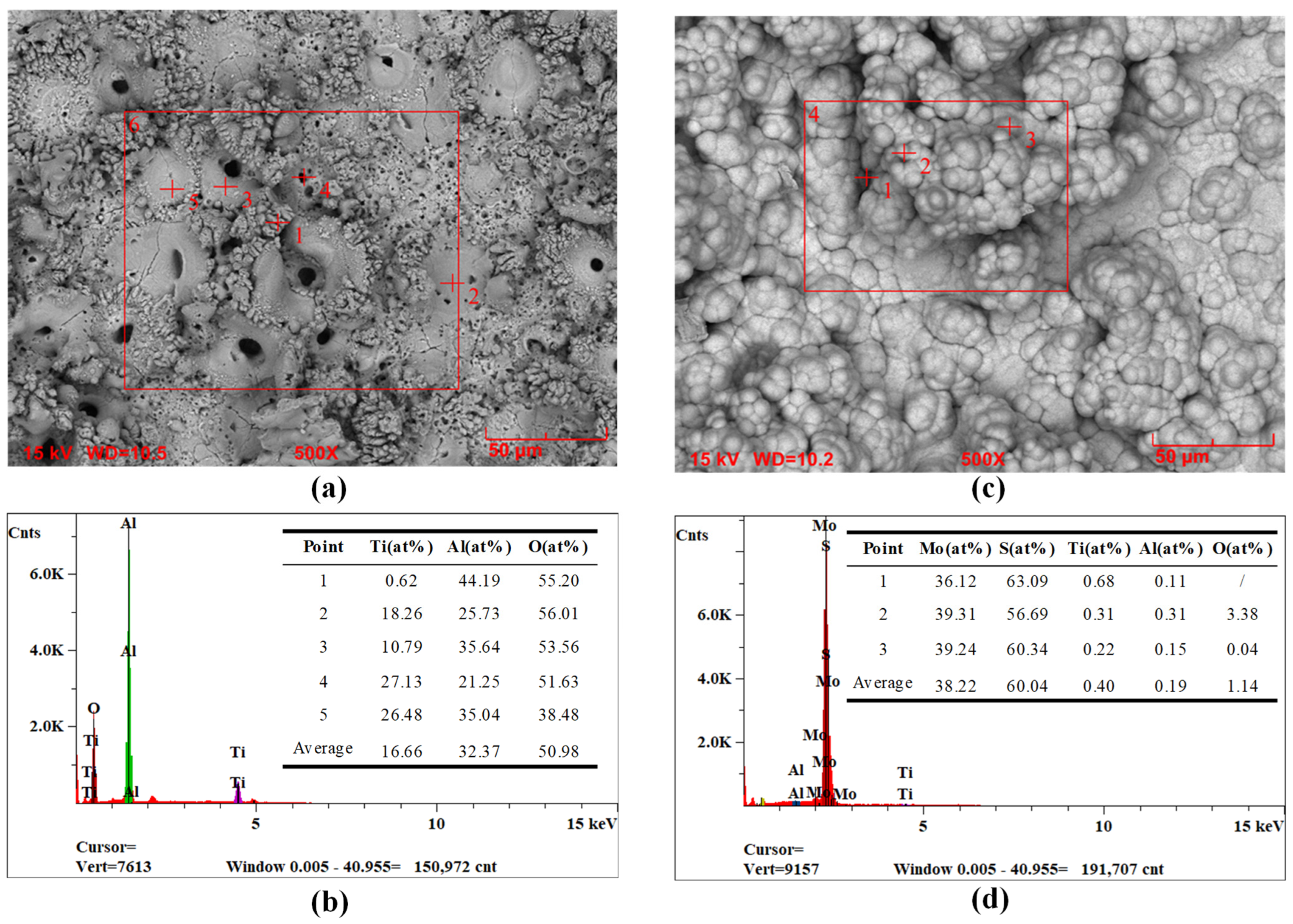
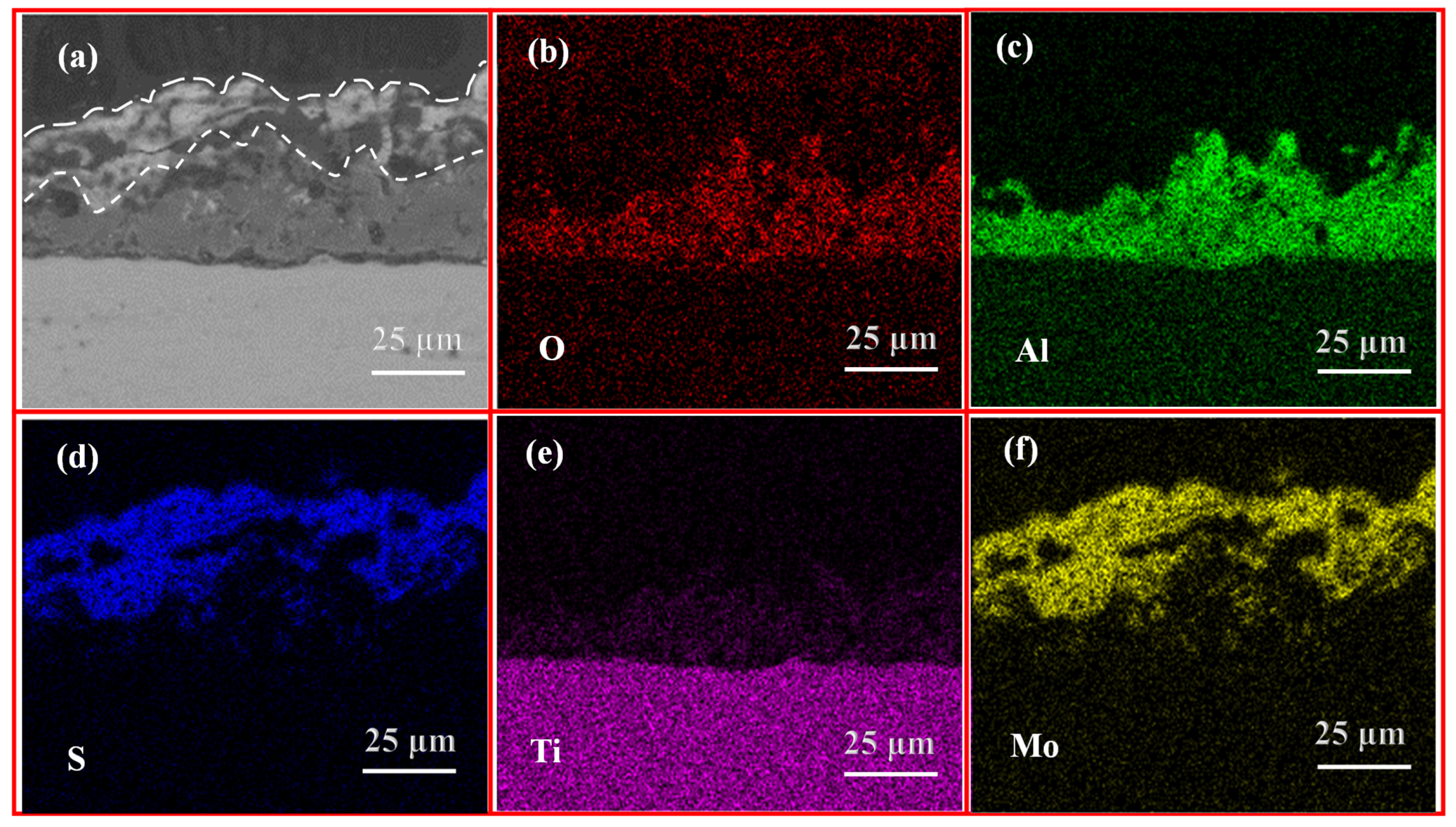
3.5. Wear Resistance Analysis
4. Conclusions
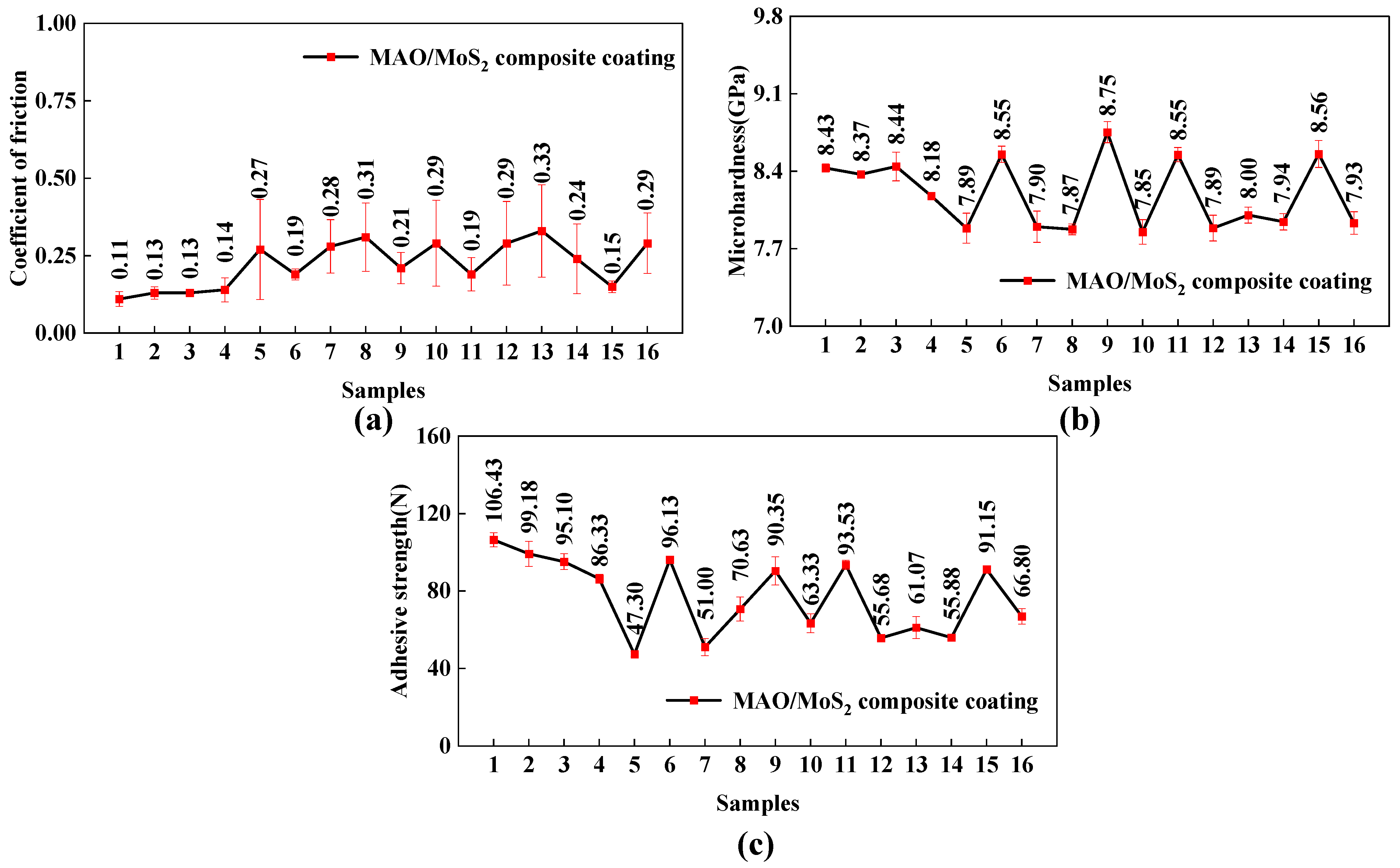
- The MAO/MoS2 composite coatings prepared under the eight groups of MAO process parameters (1#, 2#, 3#, 4#, 6#, 9#, 11#, and 15#) in orthogonal experiments exhibit a lower average CoF ranging from 0.10 to 0.22 over a 90-min wear period. Furthermore, the coatings display high microhardness and adhesive strength, with values ranging from 8.18 to 8.75 GPa and 82 to 107 N, respectively. These findings indicate that the composite coatings prepared using the aforementioned eight sets of parameters exhibit excellent comprehensive wear resistance.
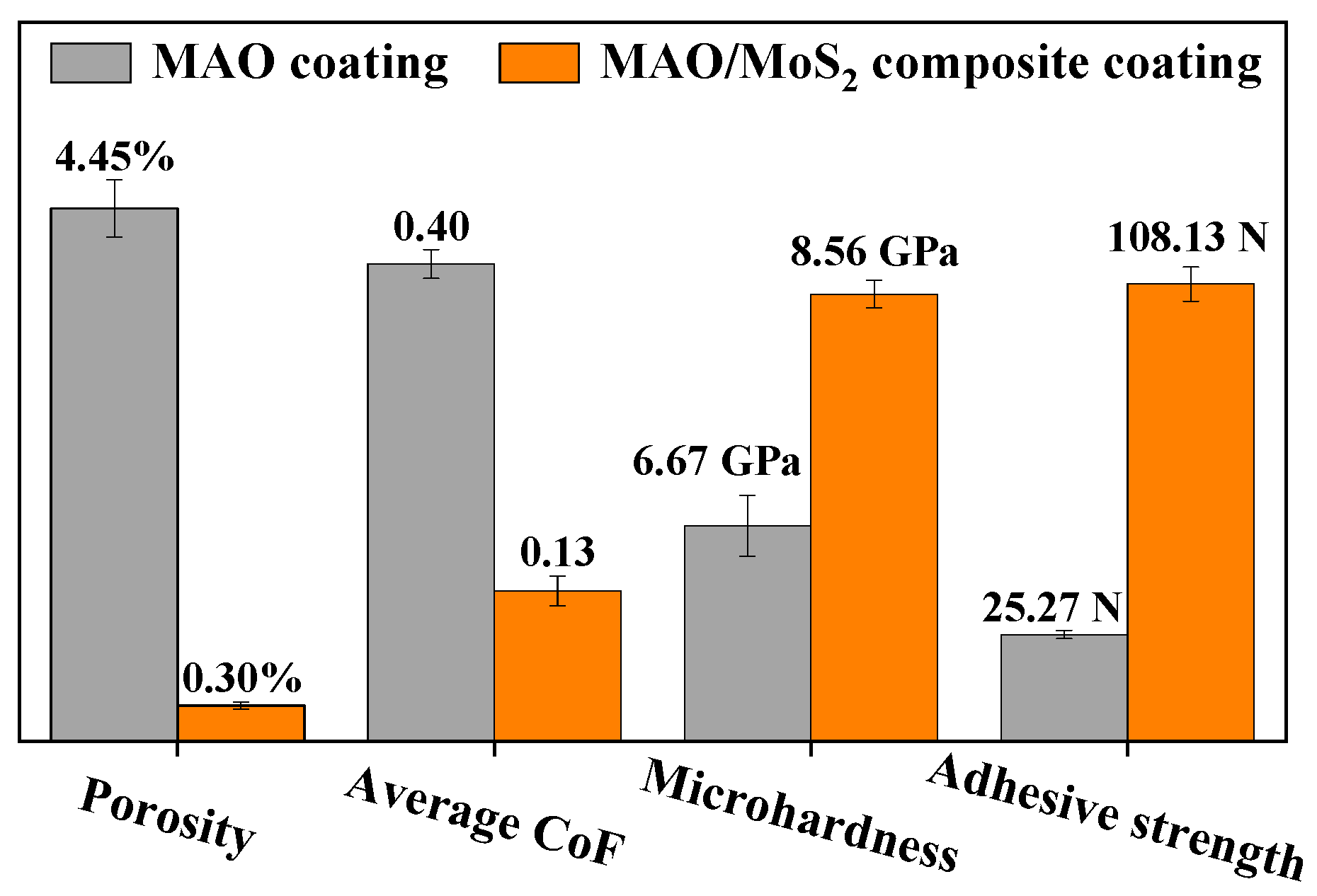
- The optimal process for MAO treatment on TC4 titanium alloy substrates is determined by an orthogonal experimental design. The process involves a NaAlO2 concentration of 10 g/L, a control voltage of 500 V, a frequency of 200 Hz, a duty cycle of 20%, and a treating time of 30 min. Following the optimization of the parameters, it is observed that the average CoF of the composite coating in the 20 N high-load dry friction experiments was ~68.5% lower than that of the MAO coating during the 20-min wear period. Following a 90-min wear period, a value of 0.13 is recorded. Additionally, the porosity of the composite coating was found to be ~93.3% lower, while the microhardness and adhesive strength exhibited increases of ~43.2% and 327.9%, respectively.
- The optimized composite coating displays superior wear resistance in comparison to the MAO coating. This is attributed to the fact that the MAO coating possesses a porosity of 4.45%, which is characterized by the presence of numerous or large-size pores, as well as a multitude of particle clusters. Consequently, the hydrothermally synthesized MoS2 is capable of growing and filling the pores in situ, thereby forming a tightly attached lubrication film. The porosity of the composite coating is reduced to 0.30%, and its flat surface exhibits high microhardness (8.56 GPa) and adhesive strength (108.13 N). Additionally, the CoF value is as low as 0.13 with a gradual increase in 90-min wear time, indicating a notable enhancement in the wear resistance of the titanium alloy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MAO | Micro-arc oxidation |
| CoF | Coefficient of friction |
| PTFE | Polytetrafluoroethylene |
| MAIP | Multi-arc ion plating |
| MoS2 | Molybdenum disulfide |
| PPL | Para-polyphenylene |
References
- Peters, M.; Kumpfert, J.; Ward, C.H.; Leyens, C. Titanium Alloys for Aerospace Applications. Adv. Eng. Mater. 2003, 5, 419–427. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Niinomi, M.; Boehlert, C.J. Titanium Alloys for Biomedical Applications. In Advances in Metallic Biomaterials: Tissues, Materials and Biological Reactions; Niinomi, M., Narushima, T., Nakai, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 179–213. [Google Scholar]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef]
- Rack, H.J.; Qazi, J.I. Titanium alloys for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1269–1277. [Google Scholar] [CrossRef]
- He, Z.; Wang, Z.; Wang, W.; Fan, A.; Xu, Z. Surface modification of titanium alloy Ti6Al4V by plasma niobium alloying process. Surf. Coat. Technol. 2007, 201, 5705–5709. [Google Scholar] [CrossRef]
- Li, Q.; Yang, W.; Wang, D.; Liang, J. Recent Advances of Wear Resistant and Lubricating Micro-arc Oxidation Coatings on Titanium Alloys. Mater. Prot. 2017, 50, 63–67. [Google Scholar]
- Walsh, F.C.; Low, C.T.J.; Wood, R.J.K.; Stevens, K.T.; Archer, J.; Poeton, A.R.; Ryder, A. Plasma electrolytic oxidation (PEO) for production of anodised coatings on lightweight metal (Al, Mg, Ti) alloys. Trans. IMF 2009, 87, 122–135. [Google Scholar] [CrossRef]
- Zhang, W. Titanium alloy surface wear-resisting processing engineering research present situation. Total Corros. Control 2017, 31, 25–29. [Google Scholar]
- Pogrebnyak, A.; Kaverina, A.S.; Kylyshkanov, M. Electrolytic plasma processing for plating coatings and treating metals and alloys. Prot. Met. Phys. Chem. Surf. 2014, 50, 72–87. [Google Scholar] [CrossRef]
- Qi, X.; Jiang, B.; Song, R. Effects of ageing treatment on corrosion behavior of 7075 aluminum alloy coated by micro arc oxidation (MAO). Corros. Sci. 2022, 199, 110164. [Google Scholar] [CrossRef]
- He, R.; Wang, B.; Xiang, J.; Pan, T. Effect of copper additive on microstructure and anti-corrosion performance of black MAO films grown on AZ91 alloy and coloration mechanism. J. Alloys Compd. 2021, 889, 161501. [Google Scholar] [CrossRef]
- Tu, W.; Cheng, Y.; Wang, X.; Zhan, T.; Han, J.; Cheng, Y. Plasma electrolytic oxidation of AZ31 magnesium alloy in aluminate-tungstate electrolytes and the coating formation mechanism. J. Alloys Compd. 2017, 725, 199–216. [Google Scholar] [CrossRef]
- Cheng, J.; Li, F.; Zhu, S.; Yu, Y.; Qiao, Z.; Yang, J. Electrochemical corrosion and tribological evaluation of TiAl alloy for marine application. Tribol. Int. 2017, 115, 483–492. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Q.; Liu, D.; Du, N. Microstructure and elevated temperature tribological behavior of TiO2/Al2O3 composite ceramic coating formed by microarc oxidation of Ti6Al4V alloy. Surf. Coat. Technol. 2015, 272, 343–349. [Google Scholar] [CrossRef]
- Zhang, R.; Han, L.; Tang, M.; Ren, Y.; Li, L. Research progress of micro-arc oxidation ceramic coatings and composite coatings on titanium alloy. J. Synth. Cryst. 2018, 40, 219–230. [Google Scholar]
- Li, H.; Sun, Y.; Zhang, J. Effect of ZrO2 particle on the performance of micro-arc oxidation coatings on Ti6Al4V. Appl. Surf. Sci. 2015, 342, 183–190. [Google Scholar] [CrossRef]
- Qi, Y.; Peng, Z.; Liu, B.; Liang, J.; Wang, P. Fabrication and wear resistance of hard micro arc oxidation coatings on Ti alloys. Surf. Technol. 2019, 48, 81–88. [Google Scholar]
- Li, W.; Yan, Z.; Shen, D.; Zhang, Z.; Yang, R. Microstructures and tribological properties of MoS2 overlayers on MAO Al alloy. Tribol. Int. 2023, 181, 108348. [Google Scholar] [CrossRef]
- Lu, X.; Mohedano, M.; Blawert, C.; Matykina, E.; Arrabal, R.; Kainer, K.U.; Zheludkevich, M.L. Plasma electrolytic oxidation coatings with particle additions—A review. Surf. Coat. Technol. 2016, 307, 1165–1182. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, X. Research status of influence of micro-nano particles in preparation of micro-arc oxidation composite layer. Hot Work. Technol. 2020, 49, 12–19. [Google Scholar]
- Ma, C.; Cheng, D.; Zhu, X.; Yan, Z.; Fu, J.; Yu, J.; Liu, Z.; Yu, G.; Zheng, S. Investigation of a self-lubricating coating for diesel engine pistons, as produced by combined microarc oxidation and electrophoresis. Wear 2018, 394, 109–112. [Google Scholar] [CrossRef]
- Mu, M.; Liang, J.; Zhou, X.; Xiao, Q. One-step preparation of TiO2/MoS2 composite coating on Ti6Al4V alloy by plasma electrolytic oxidation and its tribological properties. Surf. Coat. Technol. 2013, 214, 124–130. [Google Scholar] [CrossRef]
- Aliasghari, S.; Skeldon, P.; Thompson, G. Plasma electrolytic oxidation of titanium in a phosphate/silicate electrolyte and tribological performance of the coatings. Appl. Surf. Sci. 2014, 316, 463–476. [Google Scholar] [CrossRef]
- Martini, C.; Ceschini, L.; Tarterini, F.; Paillard, J.; Curran, J. PEO layers obtained from mixed aluminate–phosphate baths on Ti–6Al–4V: Dry sliding behaviour and influence of a PTFE topcoat. Wear 2010, 269, 747–756. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Wu, X.Q.; Xue, Z.G.; Matykina, E.; Skeldon, P.; Thompson, G. Microstructure, corrosion and wear performance of plasma electrolytic oxidation coatings formed on Ti–6Al–4V alloy in silicate-hexametaphosphate electrolyte. Surf. Coat. Technol. 2013, 217, 129–139. [Google Scholar] [CrossRef]
- Li, Y.; Ren, X.; Chen, Z. Influence of DLC film deposition on the tribological property of micro-arc oxidation films with different surface morphologies on titanium. Surf. Technol. 2019, 48, 112–121. [Google Scholar]
- Tuo, Y.; Yang, Z.; Guo, Z.; Chen, Y.; Hao, J.; Zhao, Q.; Kang, Y.; Zhang, Y.; Zhao, Y. Pore structure optimization of MoS2/Al2O3 self-lubricating ceramic coating for improving corrosion resistance. Vacuum 2023, 207, 111687. [Google Scholar] [CrossRef]
- Li, S.; Zhao, X.; An, Y.; Liu, D.; Zhou, H.; Chen, J. YSZ/MoS2 self-lubricating coating fabricated by thermal spraying and hydrothermal reaction. Ceram. Int. 2018, 44, 17864–17872. [Google Scholar] [CrossRef]
- Lv, X.; Zou, G.; Ling, K.; Yang, W.; Mo, Q.; Li, W. Tribological properties of MAO/MoS2 self-lubricating composite coating by microarc oxidation and hydrothermal reaction. Surf. Coat. Technol. 2021, 406, 126630. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, N.; Luo, H.; Han, T.; Jiao, J.; Luo, C. Alternating current poling conditions determination by orthogonal experimental design. Ceram. Int. 2024, 50, 51738–51745. [Google Scholar] [CrossRef]
- Liu, F.; Xu, J.L.; Yu, D.Z.; Wang, F.P.; Zhao, L.C. Effects of cathodic voltages on the structure and properties of ceramic coatings formed on NiTi alloy by micro-arc oxidation. Mater. Chem. Phys. 2010, 121, 172–177. [Google Scholar] [CrossRef]
- Tu, W.; Cheng, Y.; Zhan, T.; Han, J.; Cheng, Y. Influence of Sodium Tungstate and Sealing Treatment on Corrosion Resistance of Coatings Formed on AZ31 Magnesium Alloy by Plasma Electrolytic Oxidation. Int. J. Electrochem. Sci. 2017, 12, 10863–10881. [Google Scholar] [CrossRef]
- Chen, X.; Luo, C.; Liu, J. Microstructure distribution of the micro-arc oxidizing coating on magnesium alloys. J. Funct. Mater. 2010, 41, 2075–2079. [Google Scholar]
- Wang, H.Y.; Li, Y.Y.; Chen, Z.X.; Chen, C.; Shan, G. Optimization of preparation process and wear resistance of Ti micro-arc oxidation coatings. J. Yanshan Univ. 2021, 45, 496–504. [Google Scholar]
- Liu, Y.; Ou, H.; Xia, Y.J.; Chen, L.P.; Mo, S.Q.; Pan, J.R. Hydrothermal Synthesis of MoS2 Microspheres for New Electrodes. Jiangxi Sci. 2019, 37, 735–738. [Google Scholar]
- Hu, H.J.; Zhou, H.; Zheng, Y.G.; Zhang, K.F.; Wan, Z.H. The improved friction properties of bonded MoS2 films by MAO treating of Al substrate. Appl. Mech. Mater. 2013, 275, 1911–1914. [Google Scholar] [CrossRef]
- Tang, L.; Gao, C.; Huang, J.; Zhang, H.; Chang, W. Dry sliding friction and wear behaviour of hardened AISI D2 tool steel with different hardness levels. Tribol. Int. 2013, 66, 165–173. [Google Scholar] [CrossRef]
- ISO 6507-1:2018; Metallic Materials—Vickers Hardness Test. International Organization for Standardization: Geneva, Switzerland, 2018.
- ISO 2409:2013; Paints and Varnishes—Cross-Cut Test. International Organization for Standardization: Geneva, Switzerland, 2013.
- ASTM D3359-21; Standard Test Method for Determining the In Situ Rock Deformation Modulus and Other Associated Rock Properties Using a Flexible Volumetric Dilatometer. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM G99-23; Standard Test Method for Wear and Friction Testing with a Pin-on-Disk or Ball-on-Disk Apparatus. ASTM International: West Conshohocken, PA, USA, 2023.
- Liu, X.; Wu, Y.; Qiu, Z.; Lu, Z.; Yao, S.; Zhuo, S.; Zeng, D. Simultaneously enhancing wear and corrosion resistance of HVAF-sprayed Fe-based amorphous coating from Mo clad feedstock. J. Mater. Process. Technol. 2022, 302, 117465. [Google Scholar] [CrossRef]
- Zou, Y.; Qiu, Z.; Zheng, Z.; Wang, G.; Yan, X.; Yin, S.; Liu, M.; Zeng, D. Ex-situ additively manufactured FeCrMoCB/Cu bulk metallic glass composite with well wear resistance. Tribol. Int. 2021, 162, 107112. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, T.; Guo, L.; Jiang, B. Fretting wear behaviour of microarc oxidation coatings formed on titanium alloy against steel in unlubrication and oil lubrication. Appl. Surf. Sci. 2006, 252, 8113–8120. [Google Scholar] [CrossRef]
- Wheeler, J.; Collier, C.; Paillard, J.; Curran, J. Evaluation of micromechanical behaviour of plasma electrolytic oxidation (PEO) coatings on Ti–6Al–4V. Surf. Coat. Technol. 2010, 204, 3399–3409. [Google Scholar] [CrossRef]
- Peng, Z.; Cheng, Y.; Li, Y.; Cao, J.; Wu, Y.; Cai, C. Plasma electrolytic oxidation of Ti-6Al-4V alloy in aluminate electrolyte and properties of coatings. Chin. J. Nonferr. Met. 2015, 25, 133–142. [Google Scholar]
- Qin, Y.; Xiong, D.; Li, J.; Tyagi, R. Compositions and tribological properties of PEO coatings on Ti6Al4V alloy. Surf. Eng. 2017, 33, 895–902. [Google Scholar] [CrossRef]
- Deng, W.; Li, S.; Liu, X.; Zhao, X.; An, Y.; Zhou, H.; Chen, J. A novel approach to fabricate hybrid materials with excellent tribological properties from spray-formed ceramic. Mater. Lett. 2017, 193, 199–202. [Google Scholar] [CrossRef]
- Lv, J.; Yuan, M.; Sun, P.; Wei, C.; Zhang, S.; Wang, Y.; Liu, J.; Tan, F. Effect of Microarc Oxidation Treatment with Different Concentrations of MgSiF6 on Titanium-Porcelain Bonding Strength. Adv. Mater. Sci. Eng. 2020, 2020, 9542471. [Google Scholar] [CrossRef]
- Ma, X.; Shi, Q.; Huang, Y.; Liu, Y.; Yue, X. Microstructure and properties of TC4 titanium alloy micro-arc oxide composite coating based on laser surface texturing. Sci. Rep. 2025, 15, 9621. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, Q.; Chen, C.; Lian, G.; Huang, X. Effects of WC addition on the morphology, microstructure and mechanical properties of Fe50/TiC/WC laser claddings on AISI 1045 steel. Surf. Coat. Technol. 2021, 427, 127781. [Google Scholar] [CrossRef]
- Guo, J.; Feng, K.; Wang, X.; Yang, W.; Cheng, Z.; Sun, B.; Cao, W.; Meng, J. High-temperature tribological behavior of hydrothermal MoS2 coating on TC6 surface treated by MAO method. Int. J. Appl. Ceram. Technol. 2024, 22, e15014. [Google Scholar] [CrossRef]


| Factors | A NaAlO2 Concentration (g/L) | B Voltage (V) | C Frequency (Hz) | D Duty Ratio (%) | E Time (min) | |
|---|---|---|---|---|---|---|
| Levels | ||||||
| 1 | 10 | 450 | 200 | 5 | 15 | |
| 2 | 15 | 475 | 300 | 10 | 20 | |
| 3 | 20 | 500 | 400 | 15 | 25 | |
| 4 | 25 | 525 | 500 | 20 | 30 | |
| A | B | C | D | E | Composite Coating Samples | |
|---|---|---|---|---|---|---|
| 1# | 10 (1) | 450 (1) | 200 (1) | 5 (1) | 15 (1) | 1-1 |
| 2# | 10 (1) | 475 (2) | 300 (2) | 10 (2) | 20 (2) | 2-1 |
| 3# | 10 (1) | 500 (3) | 400 (3) | 15 (3) | 25 (3) | 3-1 |
| 4# | 10 (1) | 525 (4) | 500 (4) | 20 (4) | 30 (4) | 4-1 |
| 5# | 15 (2) | 450 (1) | 300 (2) | 15 (3) | 30 (4) | 5-1 |
| 6# | 15 (2) | 475 (2) | 200 (1) | 20 (4) | 25 (3) | 6-1 |
| 7# | 15 (2) | 500 (3) | 500 (4) | 5 (1) | 20 (2) | 7-1 |
| 8# | 15 (2) | 525 (4) | 400 (3) | 10 (2) | 15 (1) | 8-1 |
| 9# | 20 (3) | 450 (1) | 400 (3) | 20 (4) | 20 (2) | 9-1 |
| 10# | 20 (3) | 475 (2) | 500 (4) | 15 (3) | 15 (1) | 10-1 |
| 11# | 20 (3) | 500 (3) | 200 (1) | 10 (2) | 30 (4) | 11-1 |
| 12# | 20 (3) | 525 (4) | 300 (2) | 5 (1) | 25 (3) | 12-1 |
| 13# | 25 (4) | 450 (1) | 500 (4) | 10 (2) | 25 (3) | 13-1 |
| 14# | 25 (4) | 475 (2) | 400 (3) | 5 (1) | 30 (4) | 14-1 |
| 15# | 25 (4) | 500 (3) | 300 (2) | 20 (4) | 15 (1) | 15-1 |
| 16# | 25 (4) | 525 (4) | 200 (1) | 15 (3) | 20 (2) | 16-1 |
| A | B | C | D | E | Average CoF | Microhardness (GPa) | Adhesive Strength (N) | |
|---|---|---|---|---|---|---|---|---|
| 1# | 10 (1) | 450 (1) | 200 (1) | 5 (1) | 15 (1) | 0.11 | 8.43 | 106.43 |
| 2# | 10 (1) | 475 (2) | 300 (2) | 10 (2) | 20 (2) | 0.13 | 8.37 | 99.18 |
| 3# | 10 (1) | 500 (3) | 400 (3) | 15 (3) | 25 (3) | 0.13 | 8.44 | 95.10 |
| 4# | 10 (1) | 525 (4) | 500 (4) | 20 (4) | 30 (4) | 0.14 | 8.18 | 86.33 |
| 5# | 15 (2) | 450 (1) | 300 (2) | 15 (3) | 30 (4) | 0.27 | 7.89 | 47.30 |
| 6# | 15 (2) | 475 (2) | 200 (1) | 20 (4) | 25 (3) | 0.19 | 8.55 | 96.13 |
| 7# | 15 (2) | 500 (3) | 500 (4) | 5 (1) | 20 (2) | 0.28 | 7.90 | 51.00 |
| 8# | 15 (2) | 525 (4) | 400 (3) | 10 (2) | 15 (1) | 0.31 | 7.87 | 70.63 |
| 9# | 20 (3) | 450 (1) | 400 (3) | 20 (4) | 20 (2) | 0.21 | 8.75 | 90.35 |
| 10# | 20 (3) | 475 (2) | 500 (4) | 15 (3) | 15 (1) | 0.29 | 7.85 | 63.33 |
| 11# | 20 (3) | 500 (3) | 200 (1) | 10 (2) | 30 (4) | 0.19 | 8.55 | 93.53 |
| 12# | 20 (3) | 525 (4) | 300 (2) | 5 (1) | 25 (3) | 0.29 | 7.89 | 55.68 |
| 13# | 25 (4) | 450 (1) | 500 (4) | 10 (2) | 25 (3) | 0.33 | 8.00 | 61.07 |
| 14# | 25 (4) | 475 (2) | 400 (3) | 5 (1) | 30 (4) | 0.24 | 7.94 | 55.88 |
| 15# | 25 (4) | 500 (3) | 300 (2) | 20 (4) | 15 (1) | 0.15 | 8.56 | 91.15 |
| 16# | 25 (4) | 525 (4) | 200 (1) | 15 (3) | 20 (2) | 0.29 | 7.93 | 66.80 |
| k 1(CoF) | 0.13 | 0.23 | 0.19 | 0.23 | 0.21 | |||
| k 1(GPa) | 8.36 | 8.27 | 8.37 | 8.04 | 8.18 | |||
| k 1(N) | 96.76 | 76.29 | 90.72 | 67.24 | 82.88 | |||
| k 2(CoF) | 0.26 | 0.21 | 0.21 | 0.24 | 0.23 | |||
| k 2(GPa) | 8.05 | 8.18 | 8.17 | 8.20 | 8.24 | |||
| k 2(N) | 66.26 | 78.63 | 73.33 | 81.10 | 76.83 | |||
| k 3(CoF) | 0.24 | 0.19 | 0.22 | 0.24 | 0.23 | |||
| k 3(GPa) | 8.26 | 8.36 | 8.25 | 8.03 | 8.22 | |||
| k 3(N) | 75.72 | 82.70 | 77.99 | 68.13 | 76.99 | |||
| k 4(CoF) | 0.25 | 0.26 | 0.26 | 0.17 | 0.21 | |||
| k 4(GPa) | 8.11 | 7.97 | 7.98 | 8.51 | 8.14 | |||
| k 4(N) | 68.72 | 69.86 | 65.43 | 90.99 | 70.76 | |||
| R(CoF) | 0.14 | 0.07 | 0.06 | 0.07 | 0.02 | |||
| R(GPa) | 0.30 | 0.39 | 0.38 | 0.48 | 0.10 | |||
| R(N) | 30.49 | 12.84 | 25.29 | 22.86 | 6.07 |
| NaAlO2 Concentration | Voltage | Frequency | Duty Ratio | Time |
|---|---|---|---|---|
| 10 g/L | 500 V | 200 Hz | 20% | 30 min |
| Orthogonal Experimental Optimum | Experimental A1B3C1D4E4 | |
|---|---|---|
| Average CoF | 0.1067 | 0.1289 |
| Microhardness | 8.75 GPa | 8.56 GPa |
| Adhesive strength | 106.43 N | 108.13 N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, F.; Yang, E.; Jia, N.; Li, W.; Zhao, X.; Yang, R. Effect of NaAlO2 Co-Electrical Parameters on the Wear Resistance of MAO/MoS2 Self-Lubricating Composite Coatings. Materials 2025, 18, 1825. https://doi.org/10.3390/ma18081825
Liang F, Yang E, Jia N, Li W, Zhao X, Yang R. Effect of NaAlO2 Co-Electrical Parameters on the Wear Resistance of MAO/MoS2 Self-Lubricating Composite Coatings. Materials. 2025; 18(8):1825. https://doi.org/10.3390/ma18081825
Chicago/Turabian StyleLiang, Feiyan, Erhui Yang, Na Jia, Weizhou Li, Xiaolian Zhao, and Ruixia Yang. 2025. "Effect of NaAlO2 Co-Electrical Parameters on the Wear Resistance of MAO/MoS2 Self-Lubricating Composite Coatings" Materials 18, no. 8: 1825. https://doi.org/10.3390/ma18081825
APA StyleLiang, F., Yang, E., Jia, N., Li, W., Zhao, X., & Yang, R. (2025). Effect of NaAlO2 Co-Electrical Parameters on the Wear Resistance of MAO/MoS2 Self-Lubricating Composite Coatings. Materials, 18(8), 1825. https://doi.org/10.3390/ma18081825







