Optothermal Properties of Donor–Acceptor Layers, Including PTB7, PTB7th, Y5, and Y6, for Organic Photovoltaic Cell Applications
Abstract
:1. Introduction
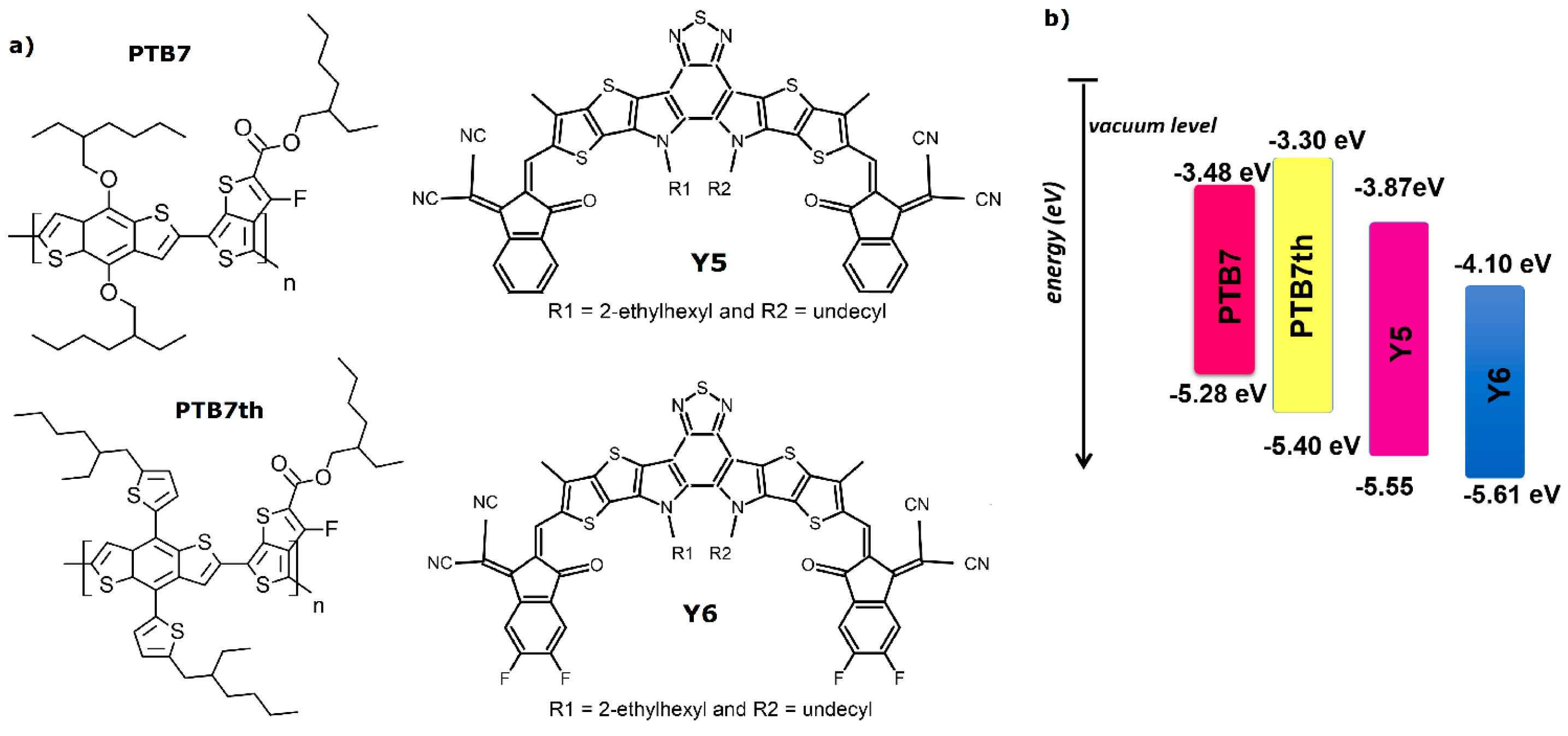
2. Materials and Methods
3. Results and Discussion
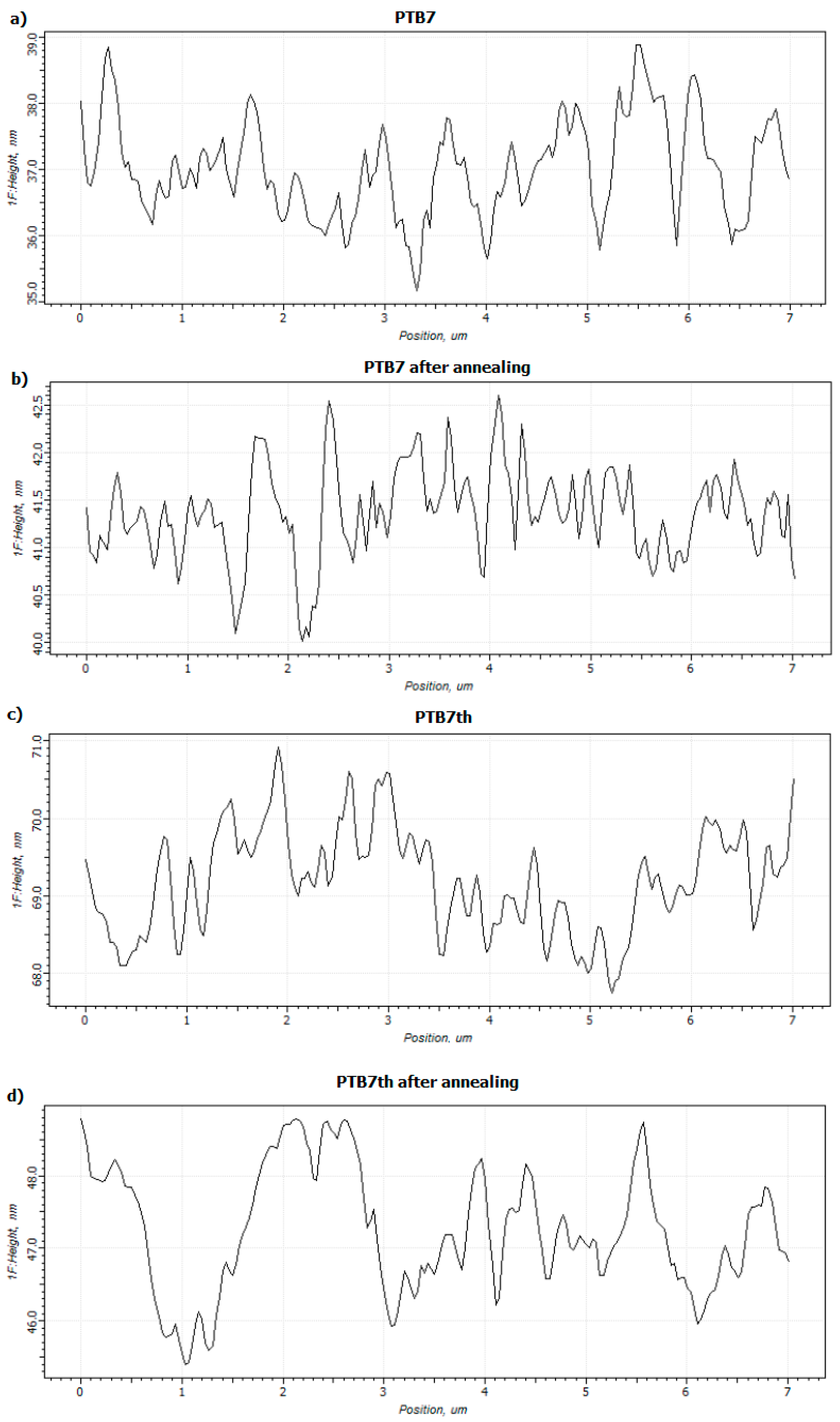
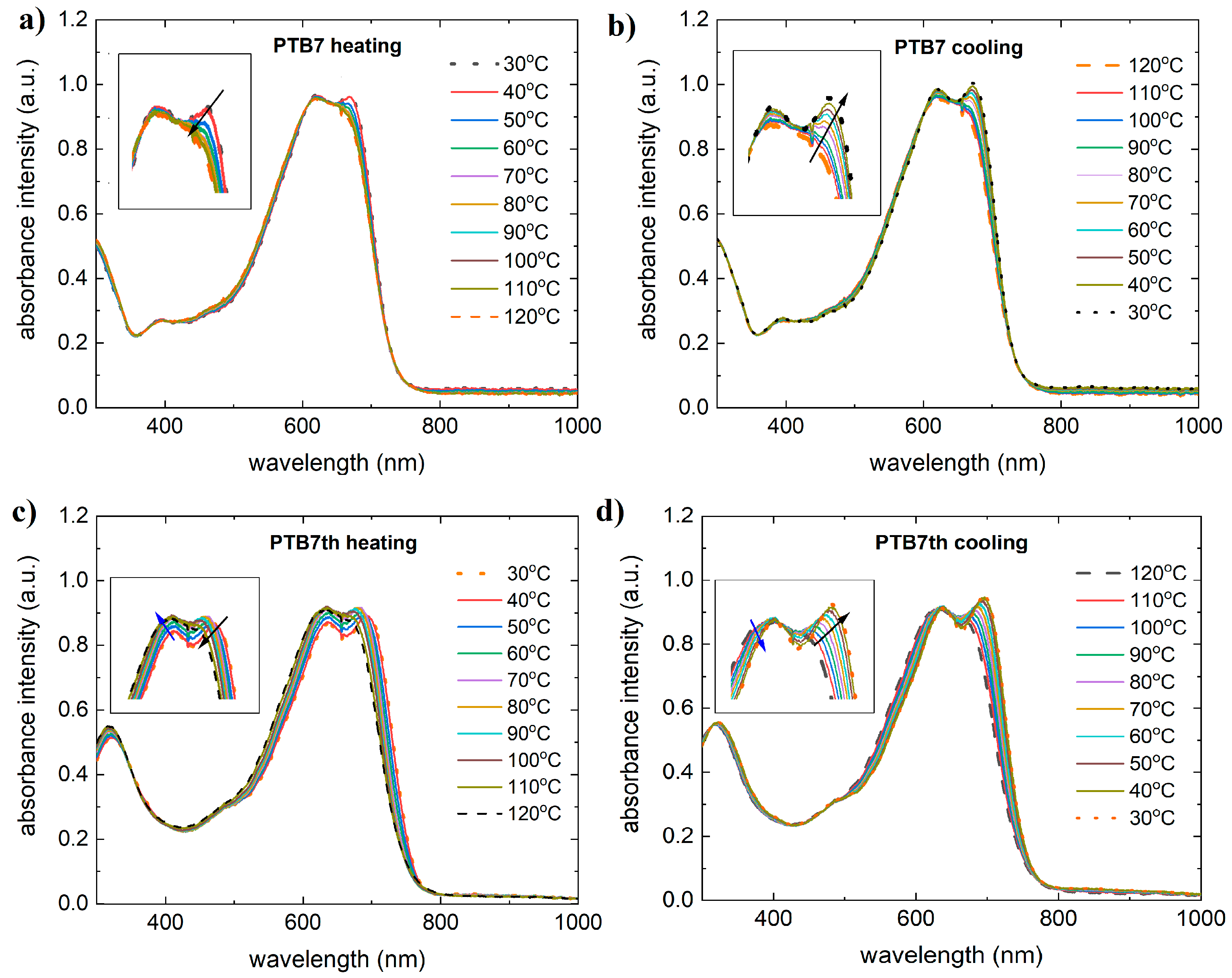
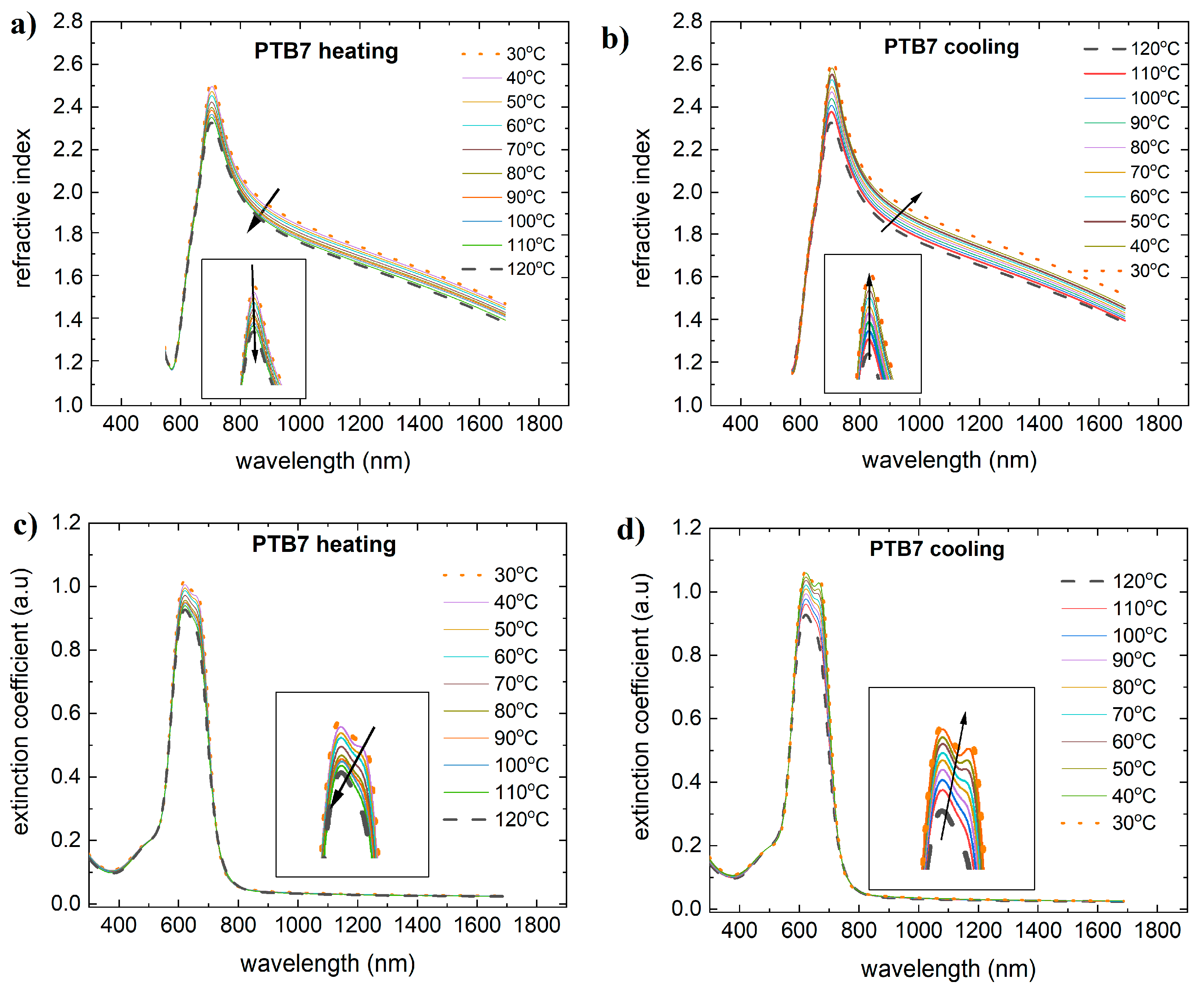
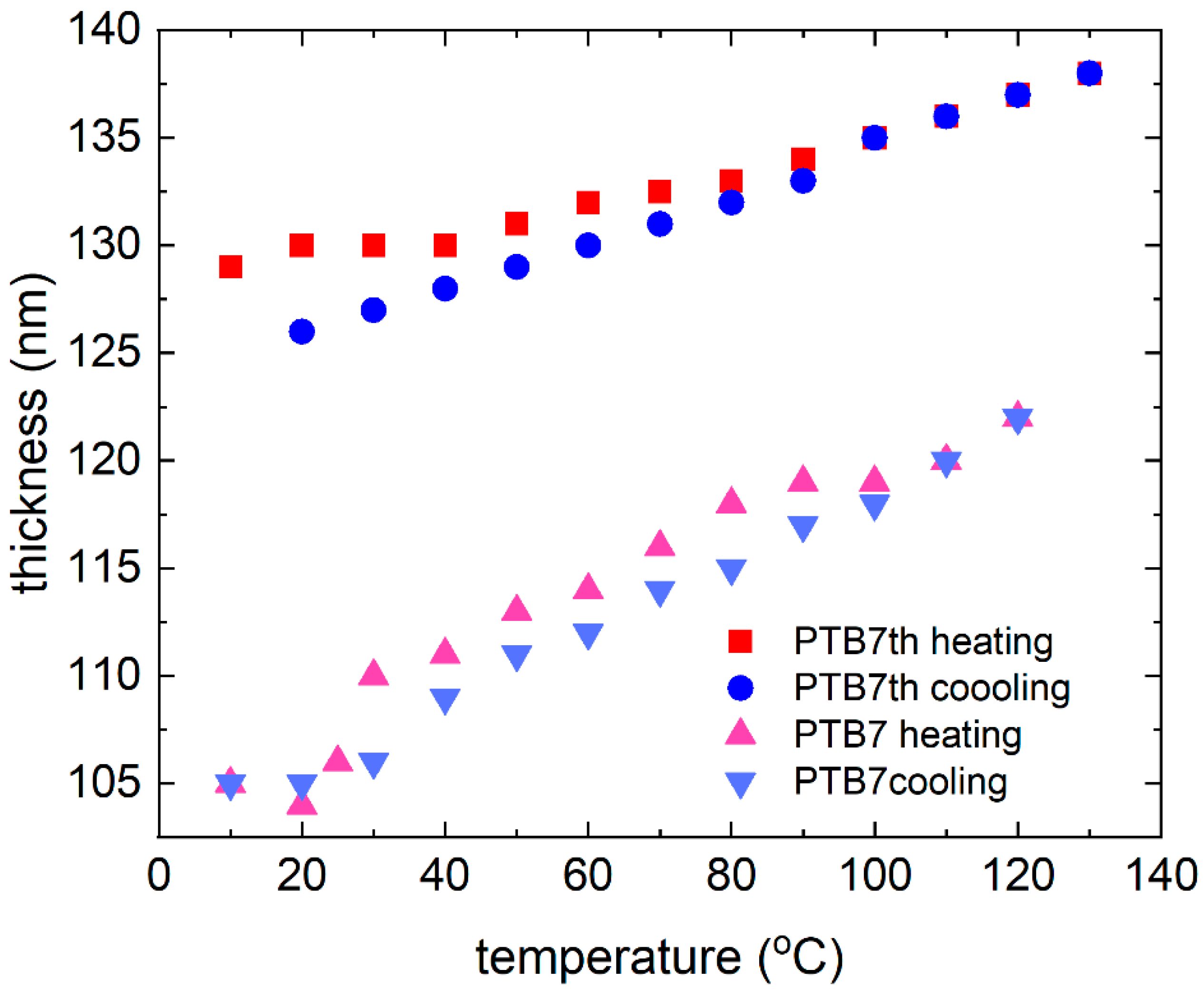
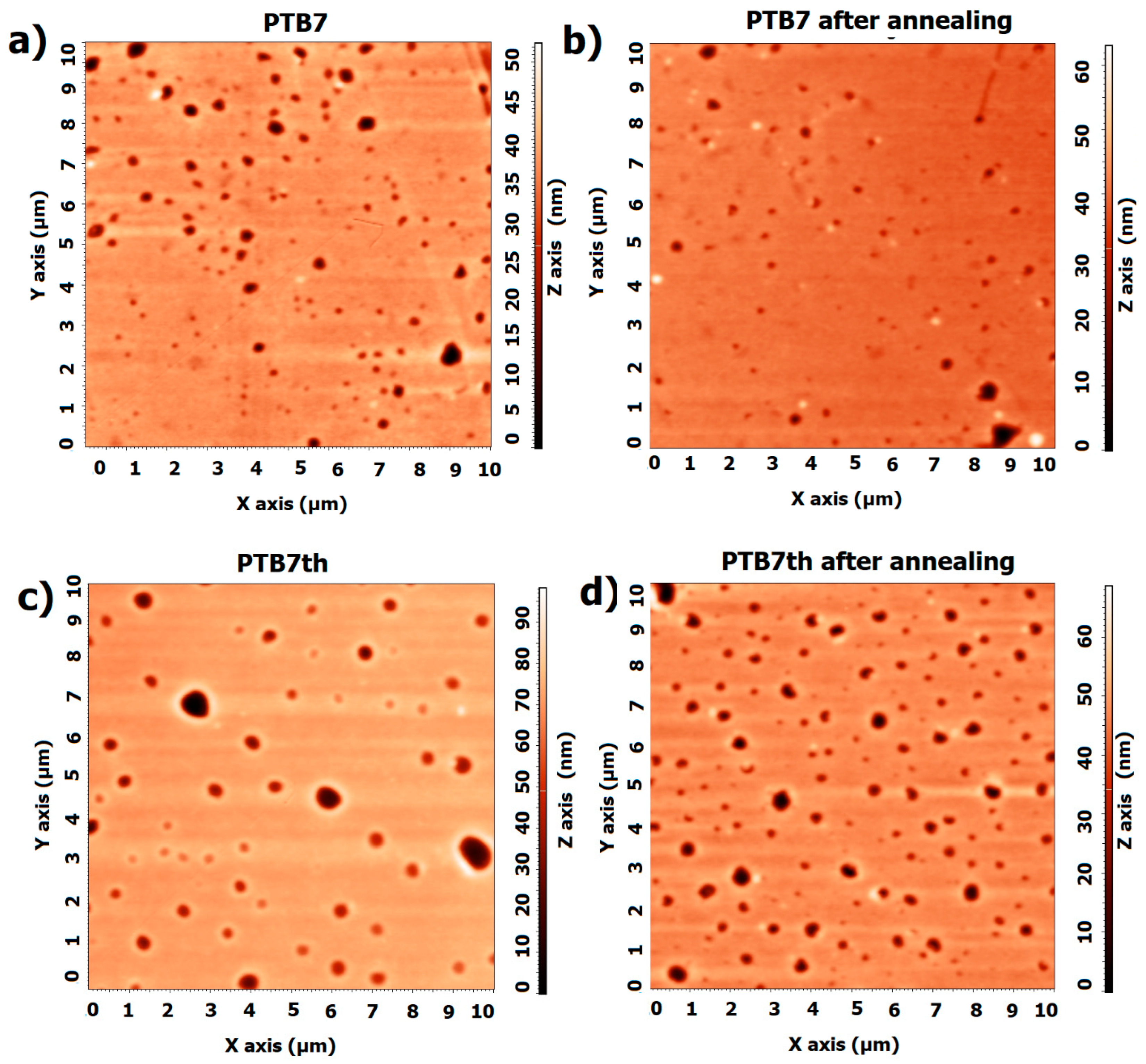
3.1. Single-Compound Thermal Stability of Donors: PTB7 and PTB7th
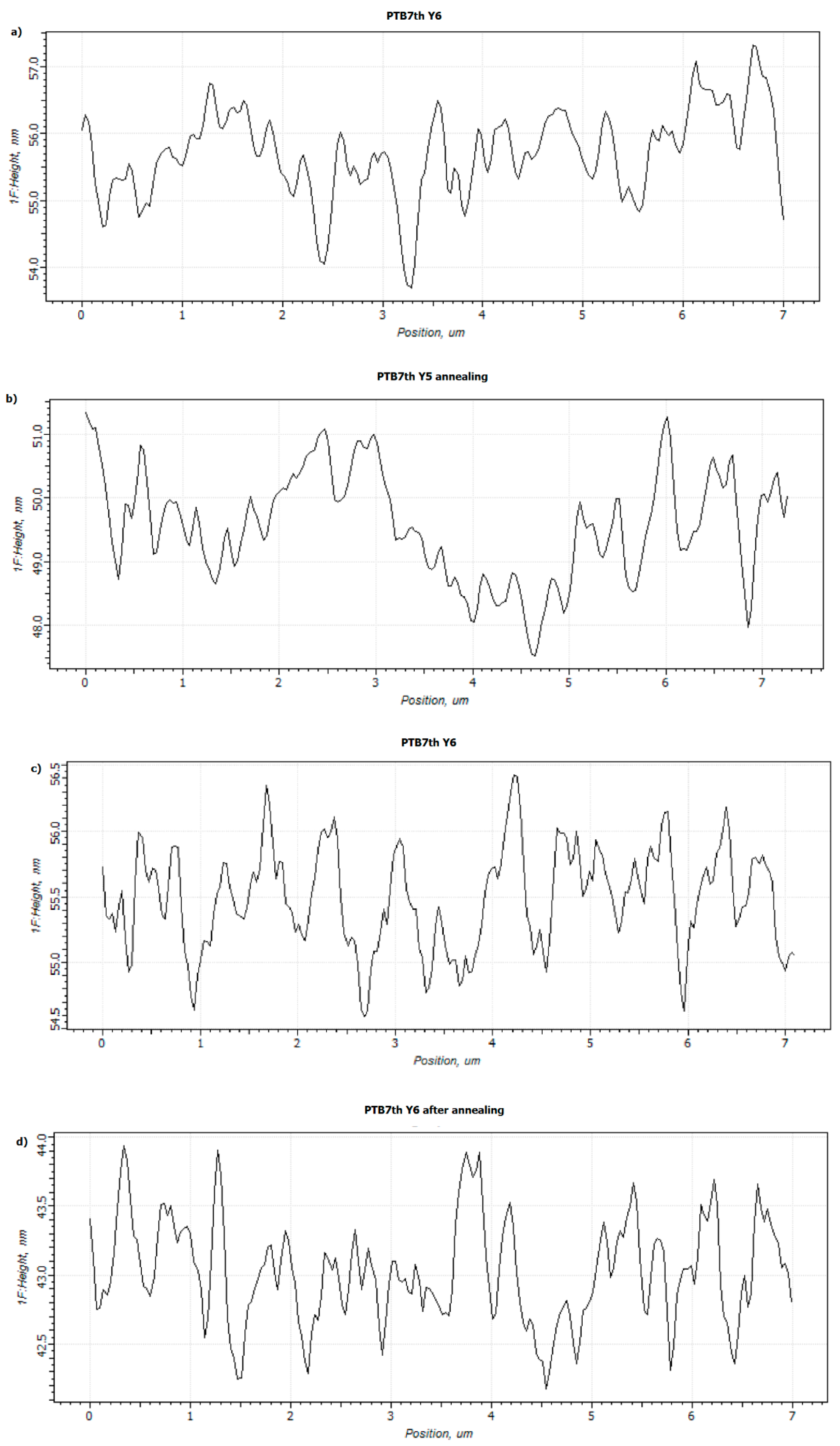
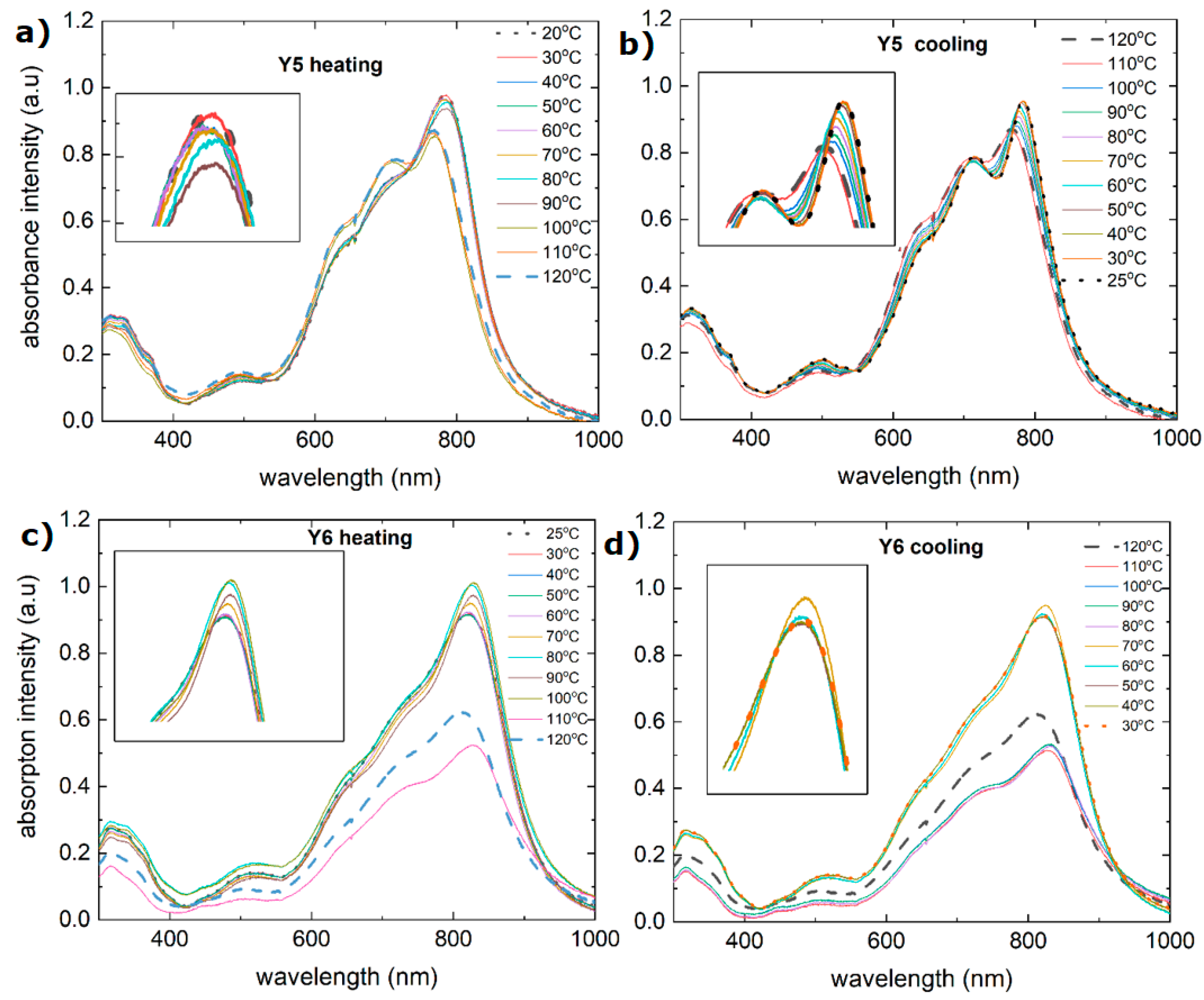
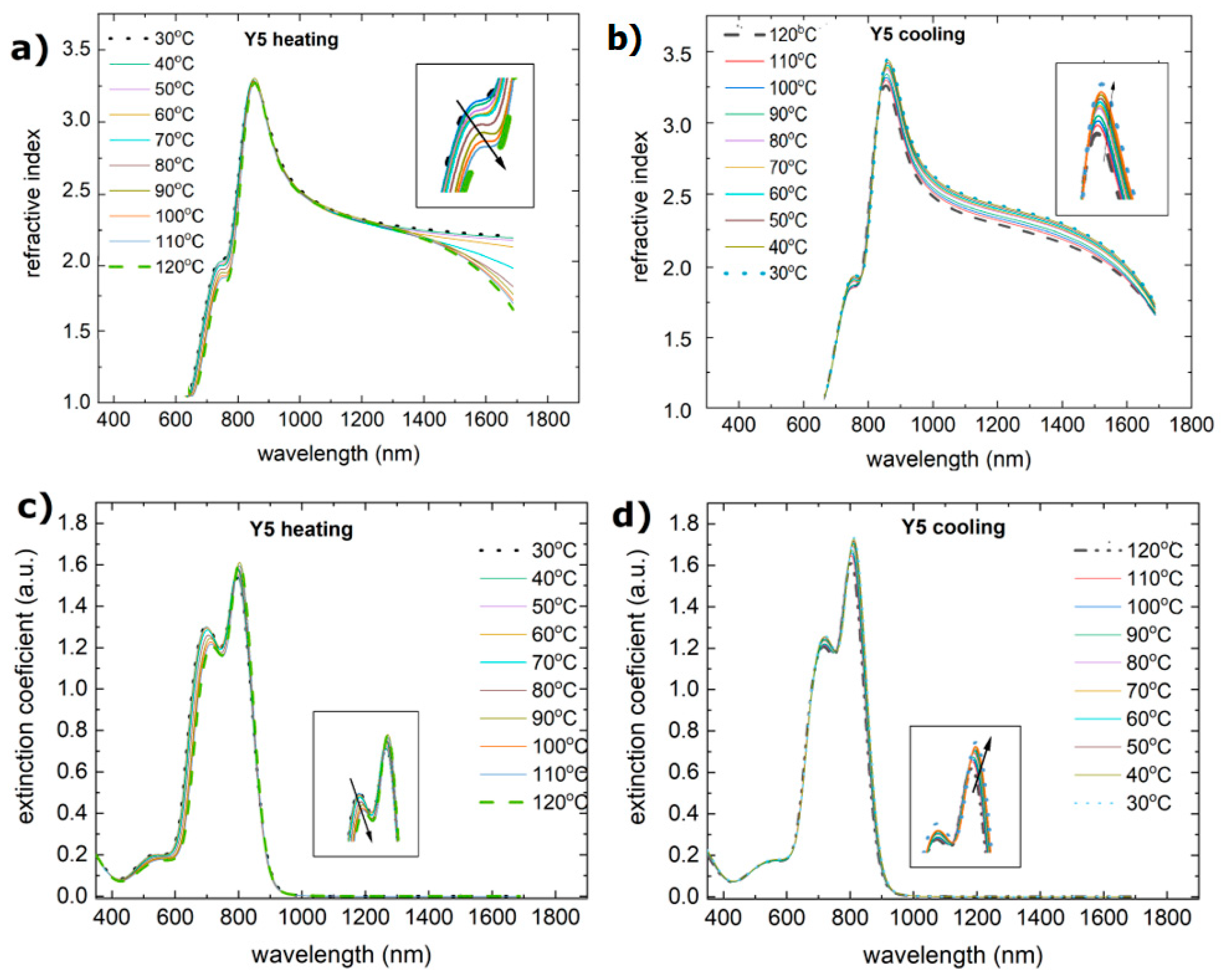
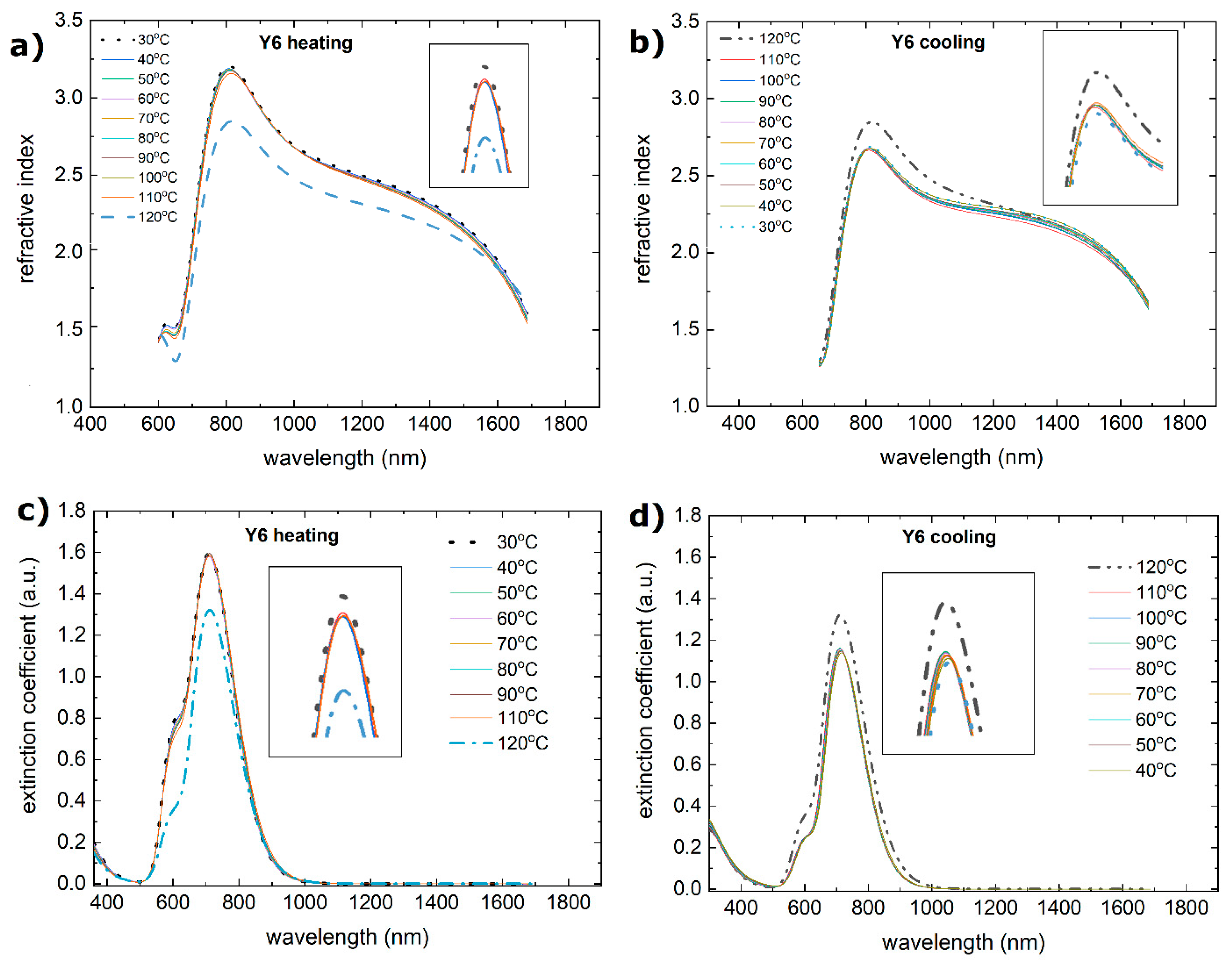
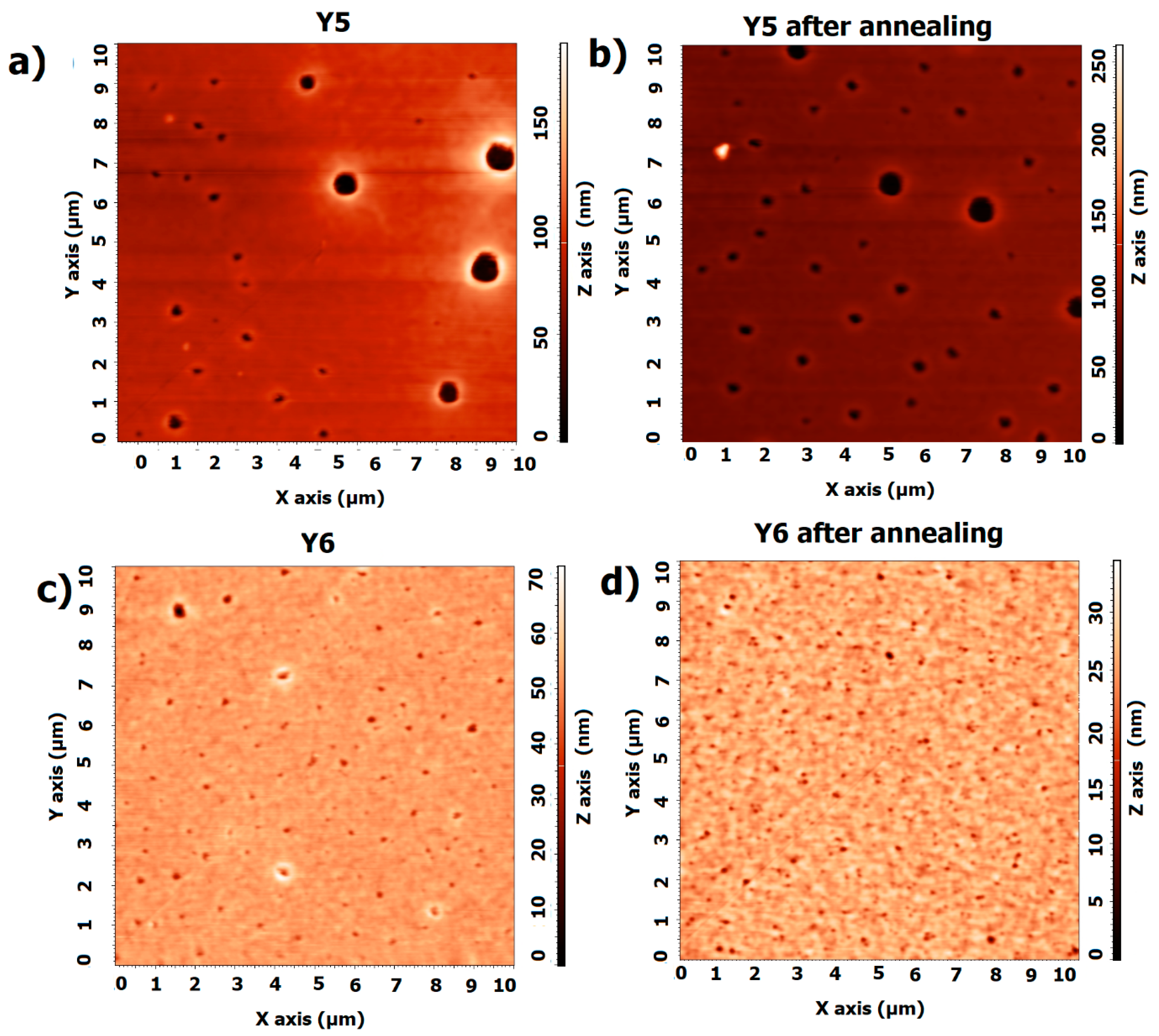
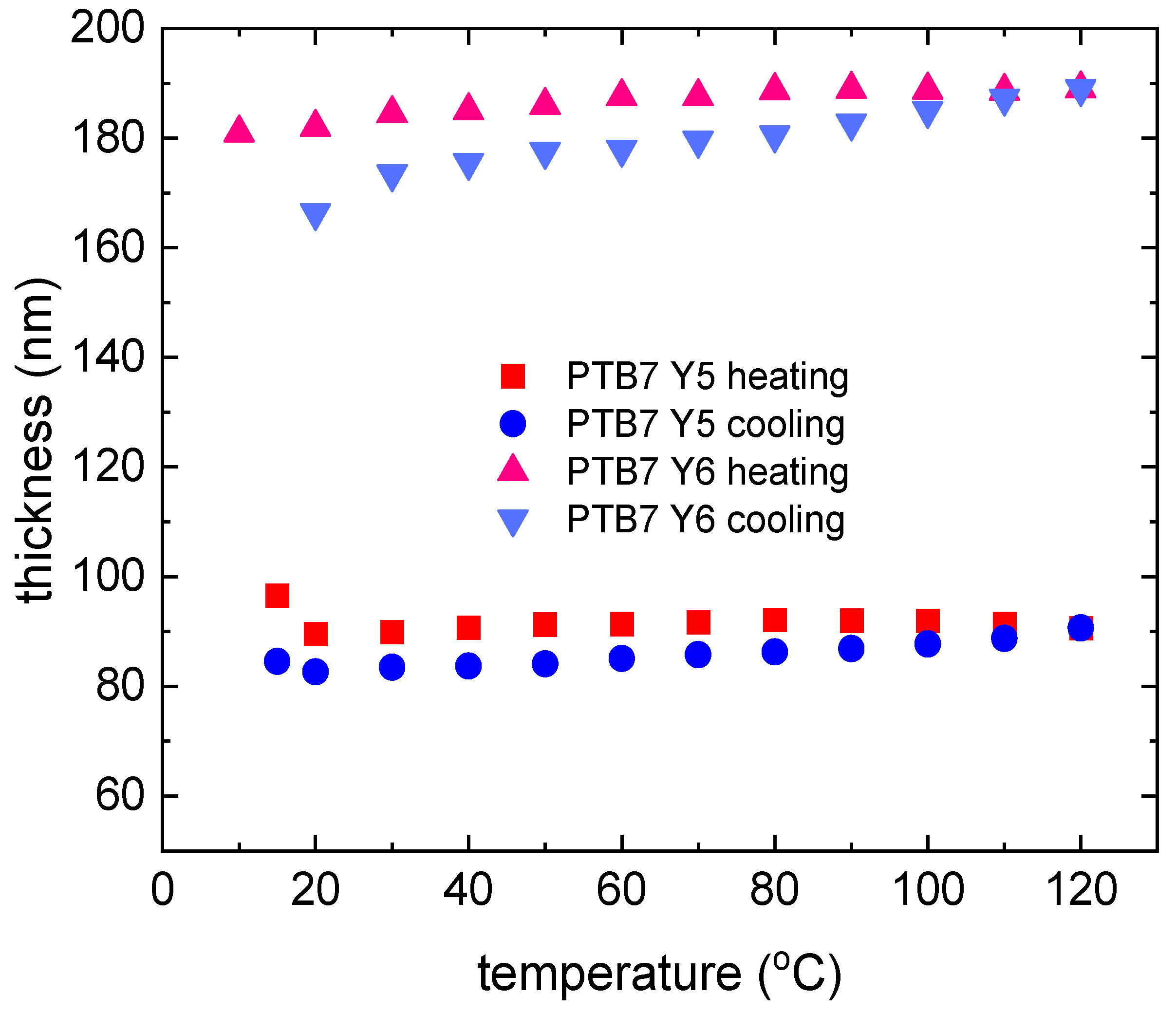
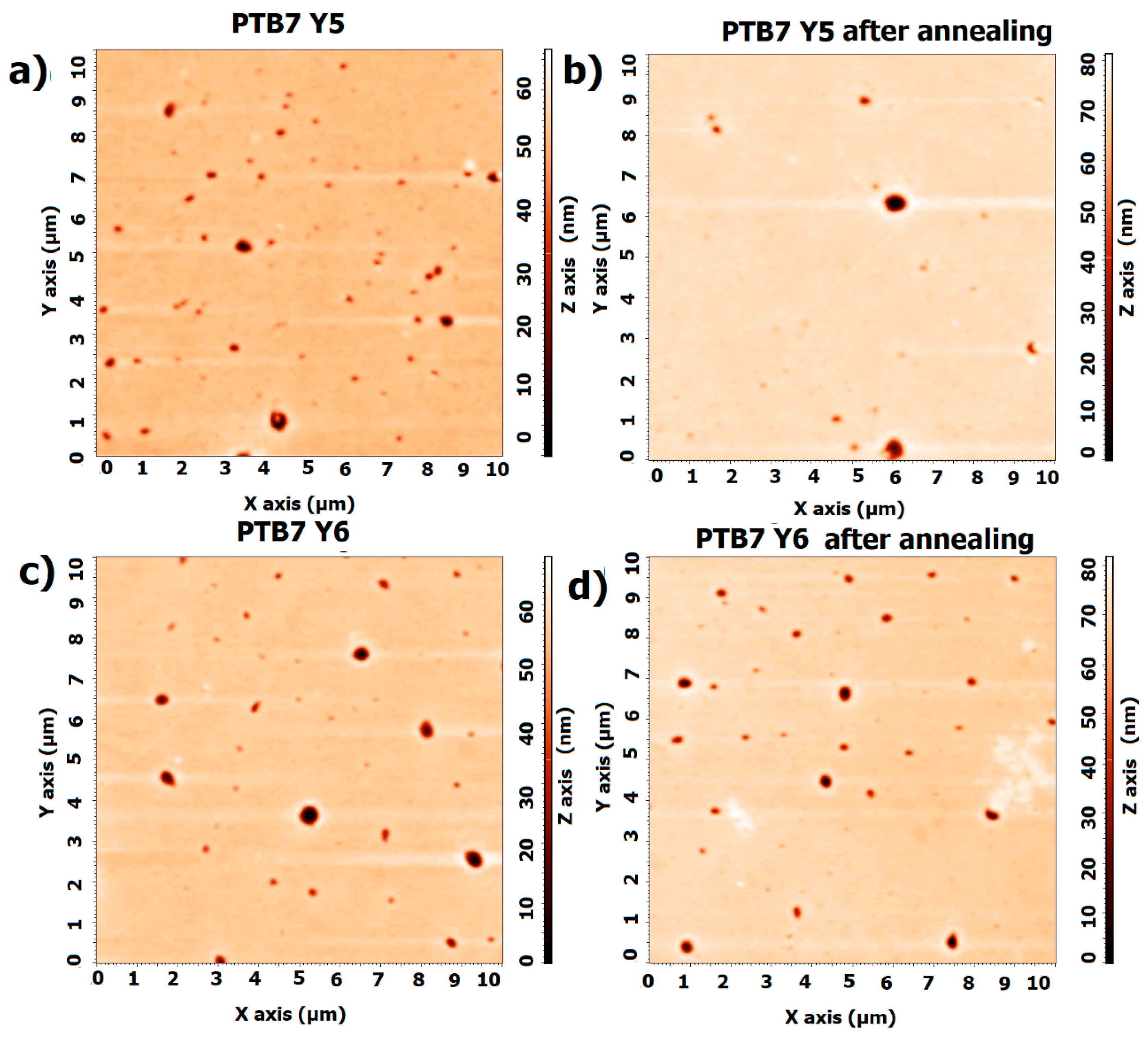
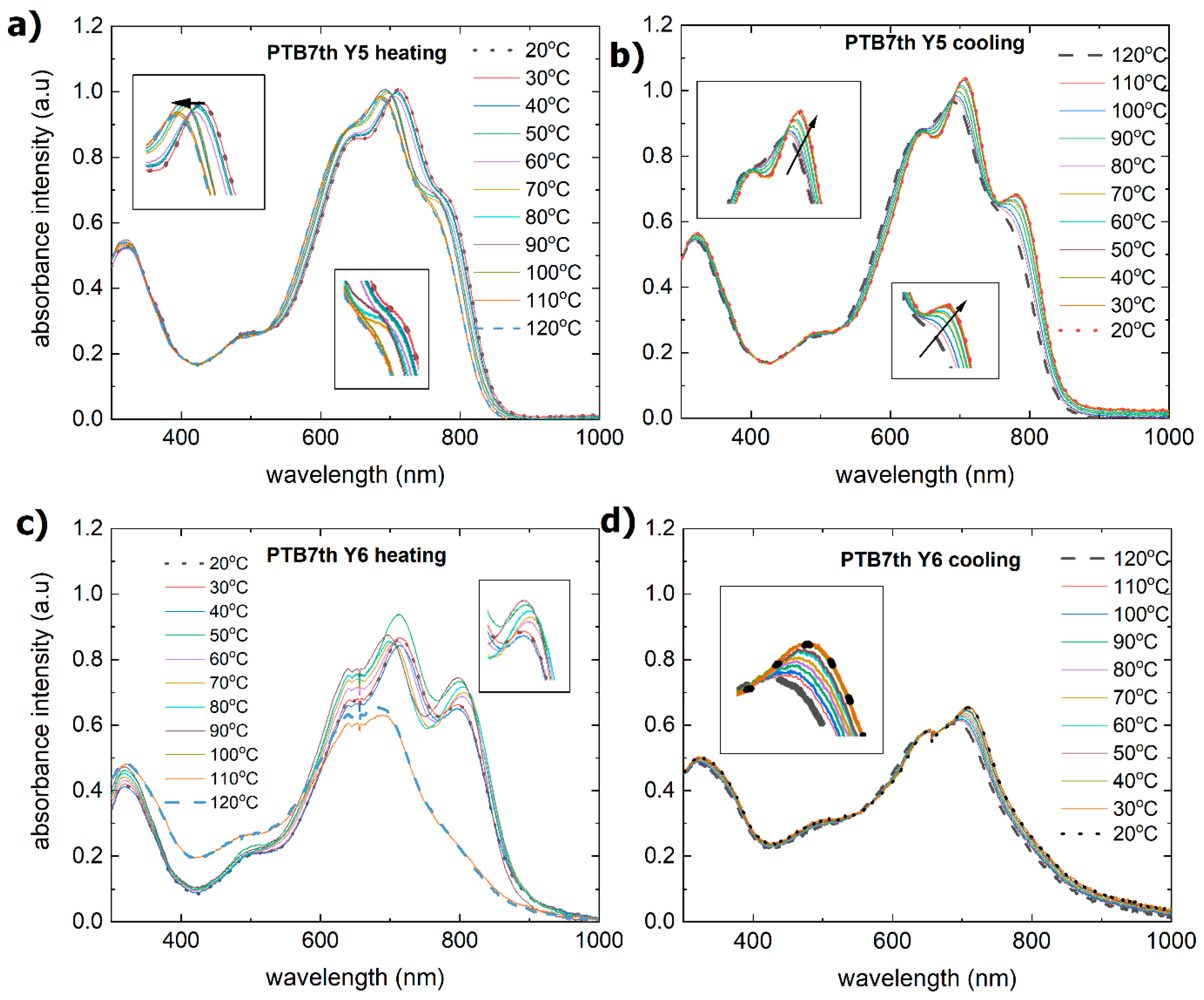
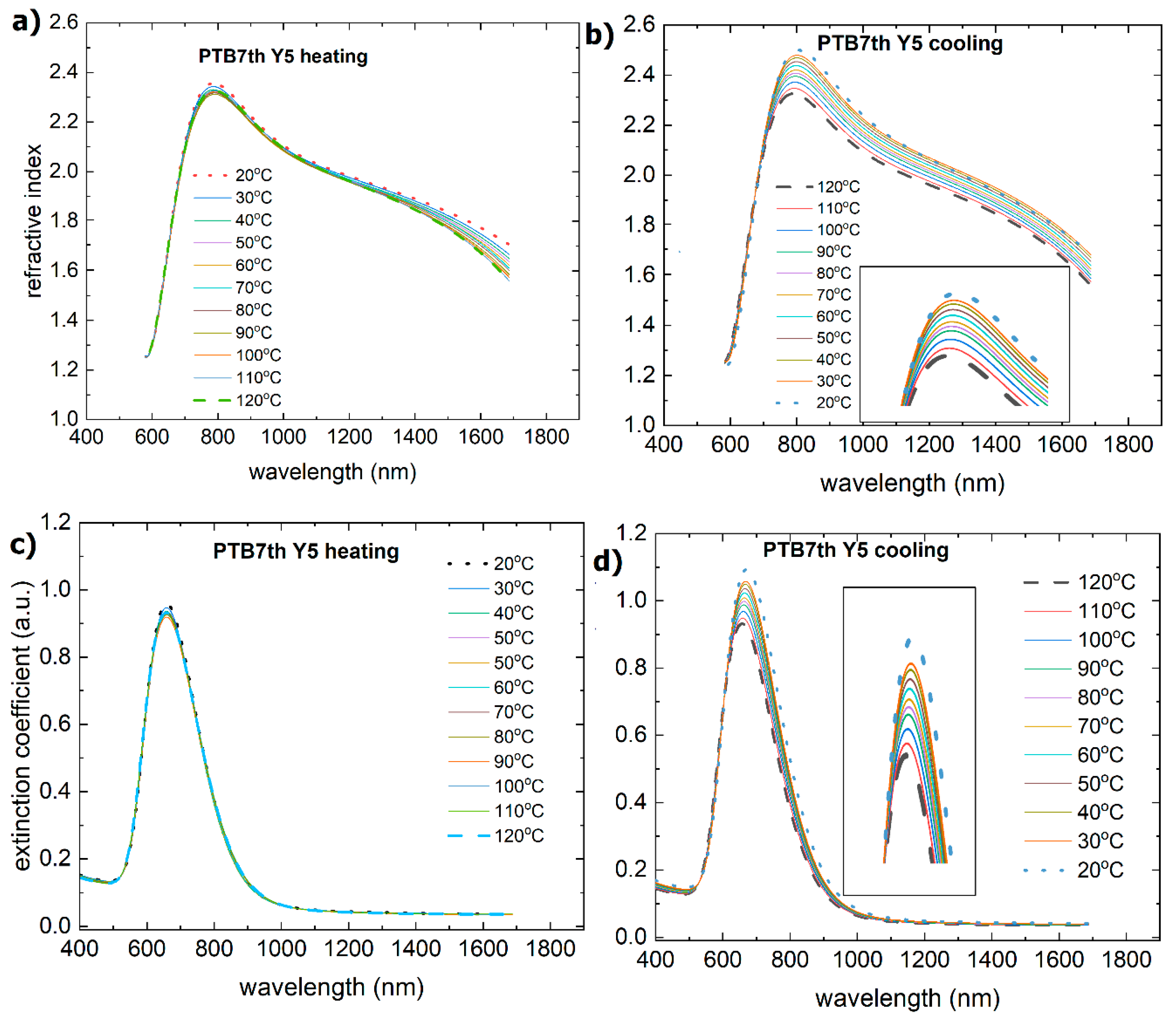
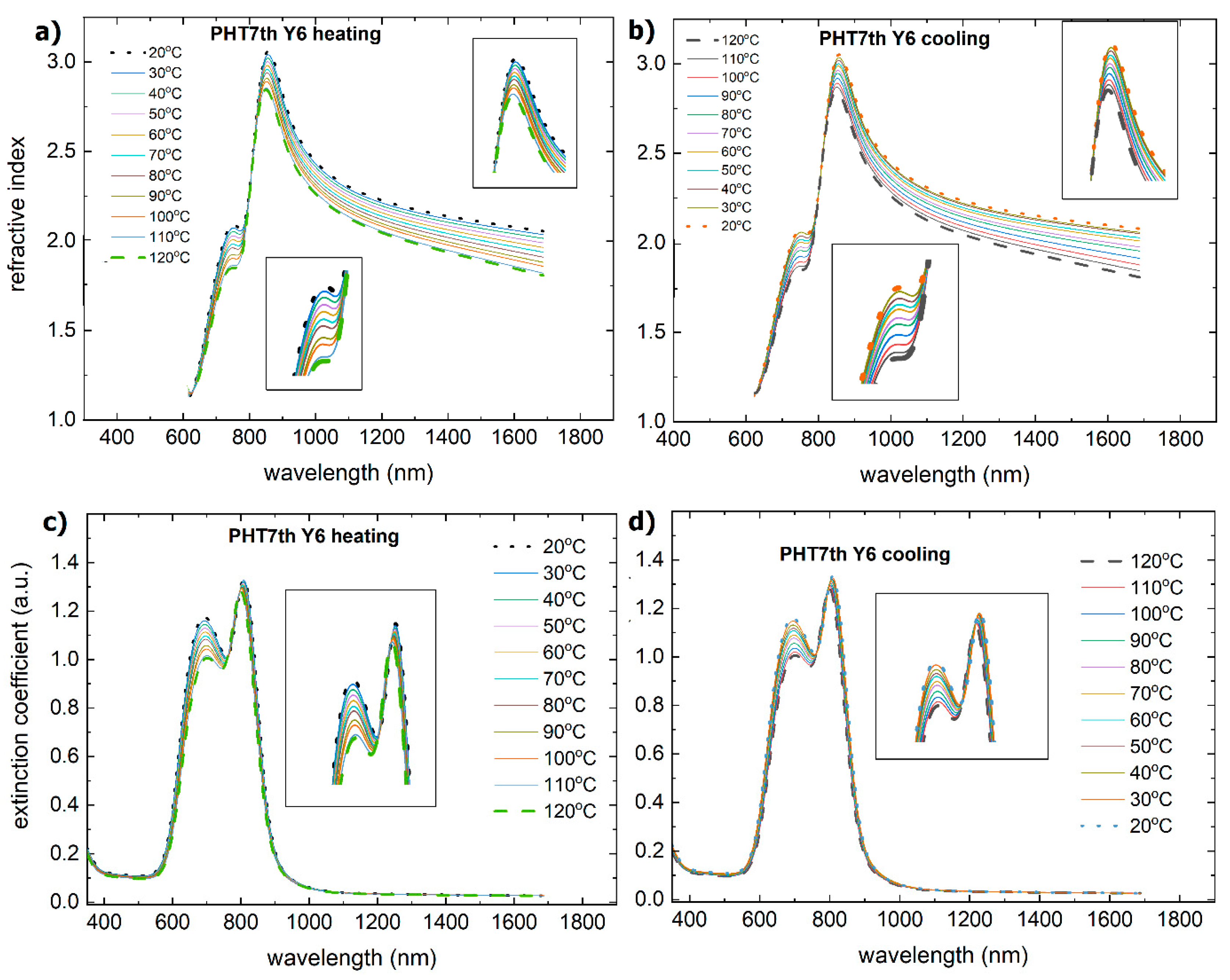
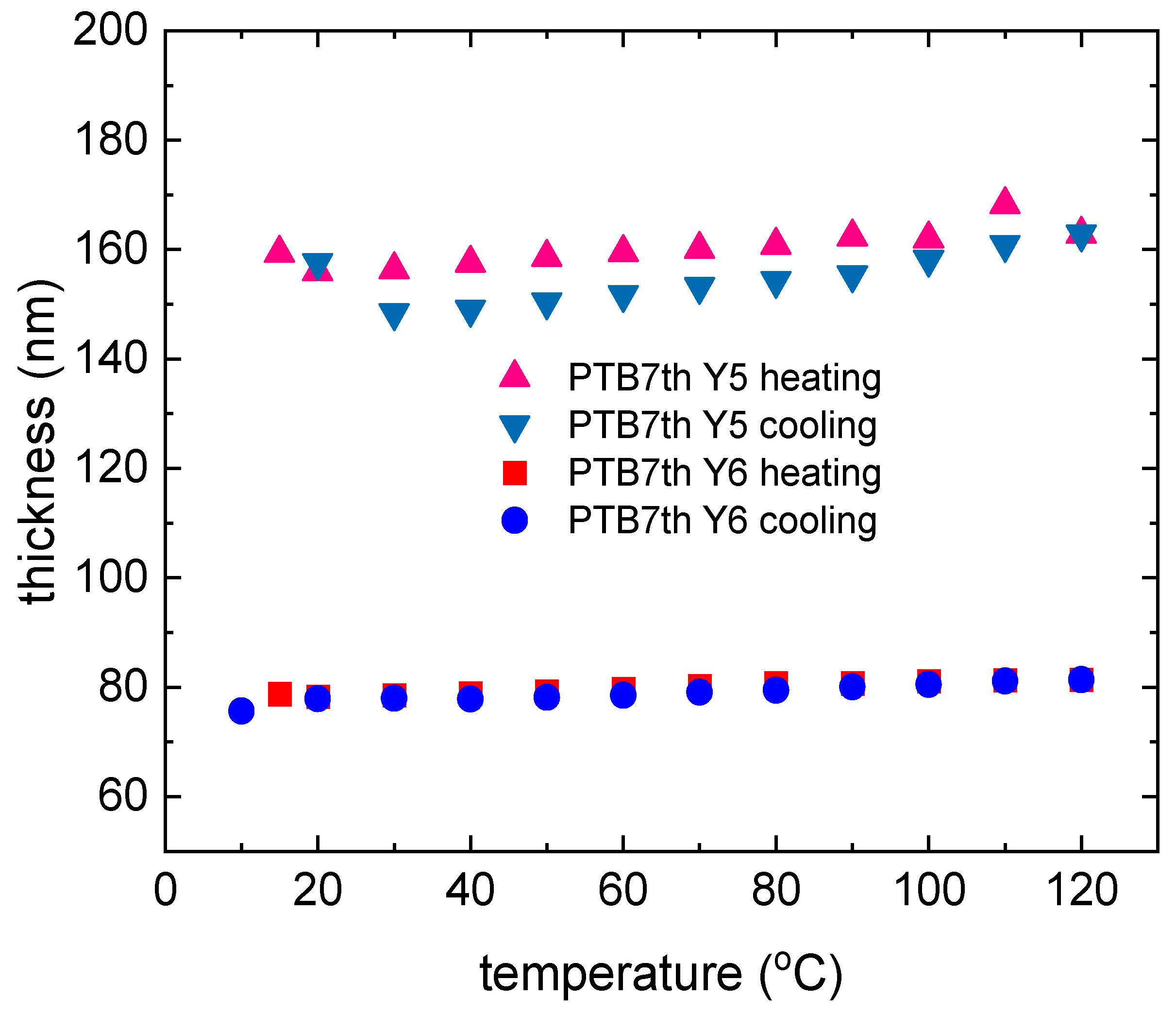
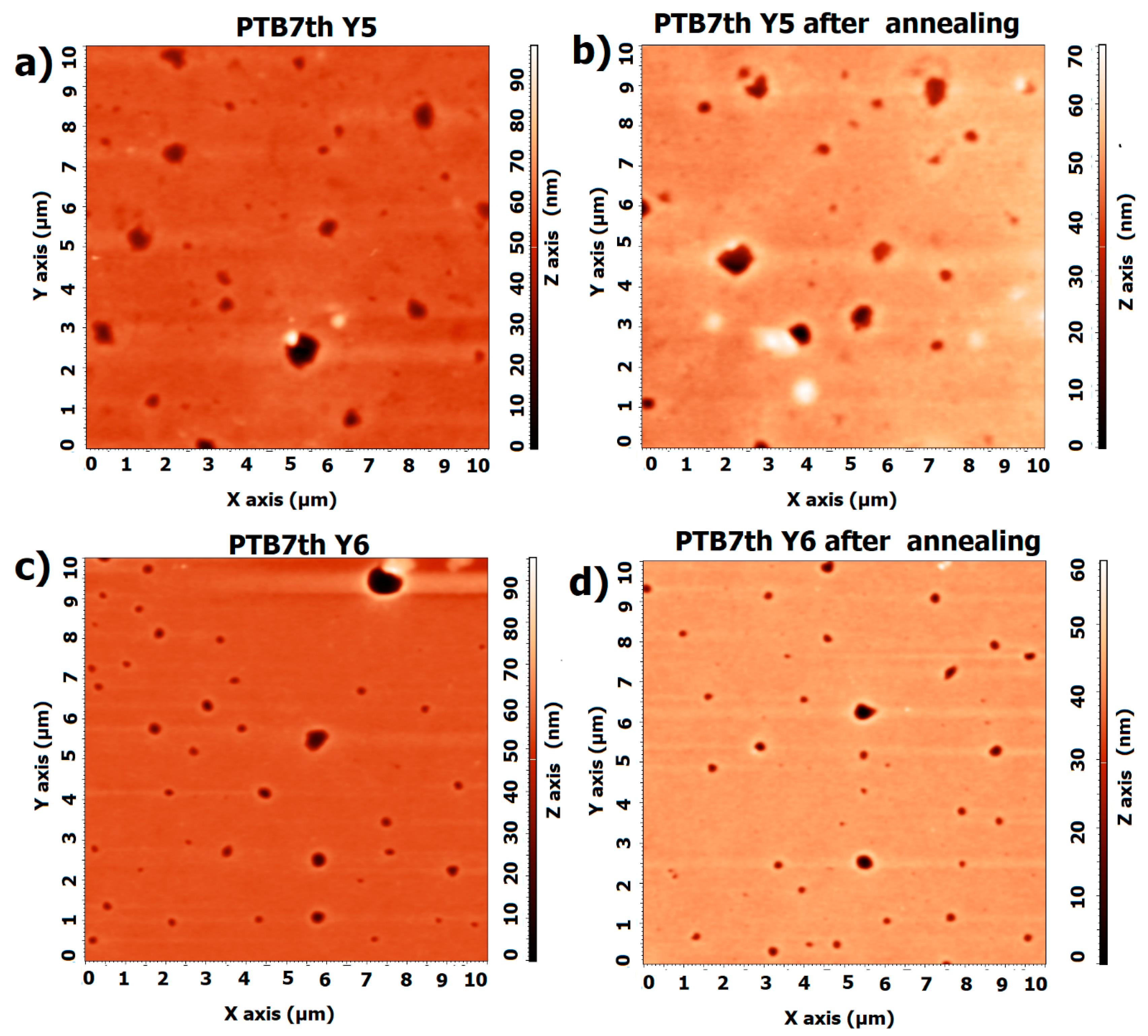
3.2. Single-Compound Thermal Stability of Non-Fullerene Acceptors Y5 and Y6
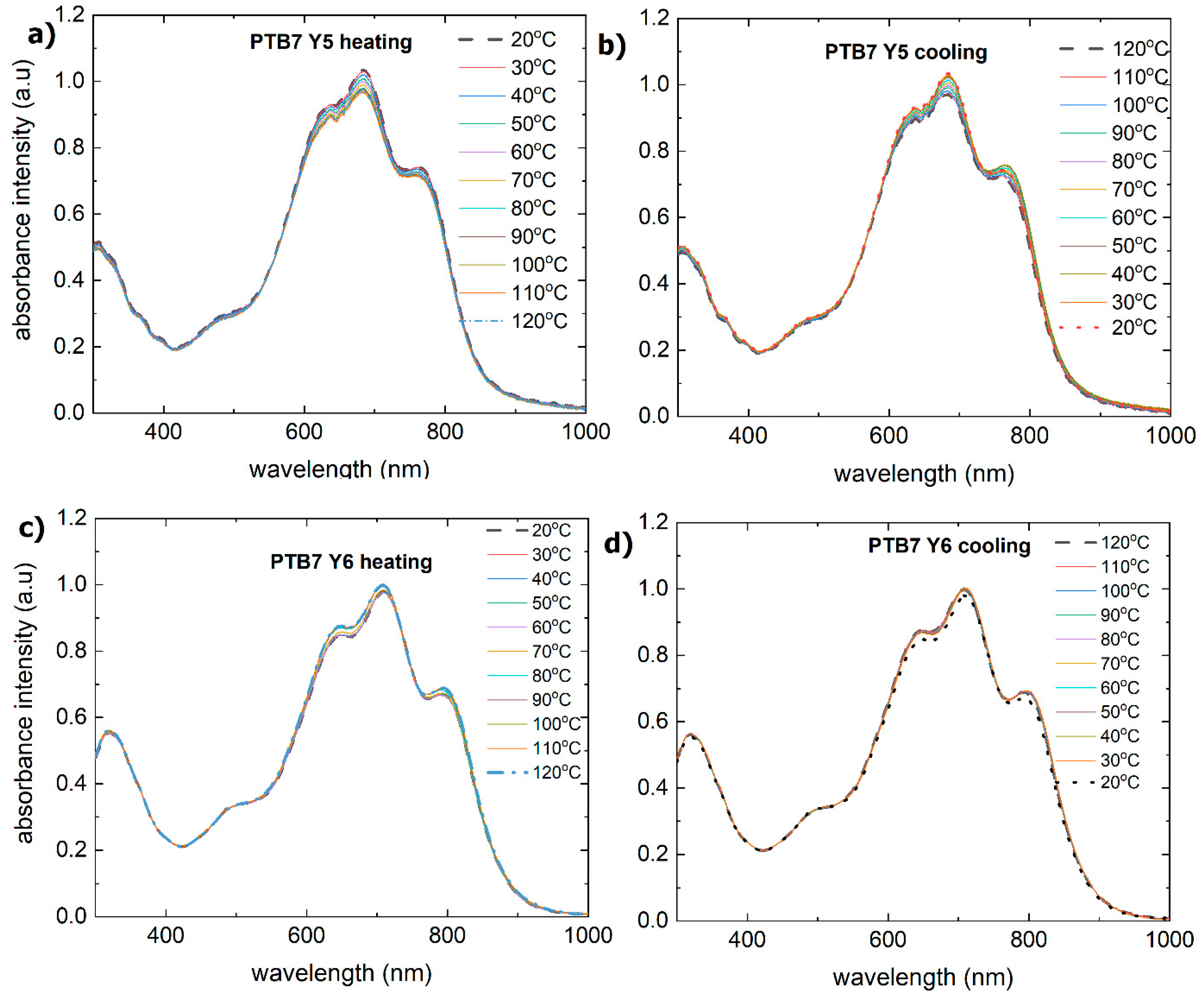
3.3. Binary Mixtures Based on PTB7 Donor Thermal Stability
3.4. Binary Mixtures Based on PTB7th Donor Thermal Stability
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Li, S.; Ma, T.; Wang, D. Photovoltaic pavement and solar road: A review and perspectives. Sustain. Energy Technol. Assess. 2023, 55, 102933. [Google Scholar] [CrossRef]
- Akram Cheema, H.; Ilyas, S.; Kang, H.; Kim, H. Comprehensive review of the global trends and future perspectives for recycling of decommissioned photovoltaic panels. Waste Manag. 2024, 174, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Abo-Zahhad, E.M.; Hachicha, A.A.; Radwan, A.; Said, Z.; Rahman, S.M.A.; Haridy, S. Comprehensive Performance Evaluation of New Photovoltaic/Serpentine Collectors using Response Surface Methodology: Energy, Exergy, and Environmental Perspectives. Sol. Energy 2024, 268, 112258. [Google Scholar] [CrossRef]
- Yan, L.L.; Han, C.; Shi, B.; Zhao, Y.; Zhang, X.D. A review on the crystalline silicon bottom cell for monolithic perovskite/silicon tandem solar cells. Mater. Today Nano 2019, 7, 100045. [Google Scholar] [CrossRef]
- Warden, G.K.; Juel, M.; Gaweł, B.A.; Di Sabatino, M. Recent developments on manufacturing and characterization of fused quartz crucibles for monocrystalline silicon for photovoltaic applications. Open Ceram. 2023, 13, 100321. [Google Scholar] [CrossRef]
- Ma, J.; Yuan, Y.; Sun, P. Approaching 23% silicon heterojunction solar cells with dual-functional SiOx/MoS2 quantum dots interface layers. Sol. Energy Mater. Sol. Cells 2021, 227, 111110. [Google Scholar] [CrossRef]
- Aghgonbad, M.M.; Sedghi, H. Influence of annealing temperature on optical properties of zinc oxide thin films analyzed by spectroscopic ellipsometry method. Chin. J. Phys. 2018, 56, 2129–2138. [Google Scholar] [CrossRef]
- Lee, C.W.; Chou, C.H.; Huang, J.H.; Hsu, C.S.; Nguyen, T.P. Investigations of organic light emitting diodes with CdSe(ZnS) quantum dots. Mater. Sci. Eng. B 2008, 147, 307–311. [Google Scholar] [CrossRef]
- Heisler, C.; Schnohr, C.S.; Hädrich, M.; Oertel, M.; Kraft, C.; Reislöhner, U.; Metzner, H.; Wesch, W. Transparent CdTe solar cells with a ZnO:Al back contact. Thin Solid Film. 2013, 548, 627–631. [Google Scholar] [CrossRef]
- Prakash, P.; Janarthanan, B. Review on the progress of light harvesting natural pigments as DSSC sensitizers with high potency. Inorg. Chem. Commun. 2023, 152, 110638. [Google Scholar] [CrossRef]
- Chauhan, R. Scanning prevalent technologies to promote scalable devising of DSSCs: An emphasis on dye component precisely with a shift to ambient algal dyes. Inorg. Chem. Commun. 2022, 139, 109368. [Google Scholar] [CrossRef]
- Arslan, B.S.; Ülüş, S.N.; Gezgin, M.; Arkan, B.; Güzel, E.; Avcı, D.; Nebioğlu, M.; Şişman, İ. Insight into the effects of the donors and pi-spacers on the photovoltaic performance of quinoline and pyridocarbazole based DSSCs. Opt. Mater. 2020, 106, 109974. [Google Scholar] [CrossRef]
- Cevher, D.; Cevher, S.C.; Cirpan, A. Recently developed benzodithiophene based organic solar cells: A review on materials and strategies. Mater. Today Commun. 2023, 37, 107524. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, D.; Xu, Z.; Pu, Y.; Lan, S.; Wang, F.; Wu, F.; Hu, B.; Tong, Y.; Lv, R.; et al. Recent progress of hybrid cathode interface layer for organic solar cells. J. Energy Chem. 2024, 91, 383–406. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, X.; Liu, W.; Liu, Y.; Zhan, X. Organic interlayer materials for non-fullerene solar cells. Trends Chem. 2024, 6, 37–50. [Google Scholar] [CrossRef]
- Lewińska, G.; Danel, K.S.; Sanetra, J. The bulk heterojunction cells based on new helicenes—Preparation, implementation and surface examination. Sol. Energy 2016, 135, 848–853. [Google Scholar] [CrossRef]
- Xu, Z.; Chin, S.-H.; Park, B.-I.; Meng, Y.; Kim, S.; Han, S.; Li, Y.; Kim, D.-H.; Kim, B.-S.; Lee, J.-W.; et al. Advancing perovskite solar cell commercialization: Bridging materials, vacuum deposition, and AI-assisted automation. Next Mater. 2024, 3, 100103. [Google Scholar] [CrossRef]
- Kumar, P.; Thokala, S.; Singh, S.P.; Singh, R. Research progress and challenges in extending the infra-red absorption of perovskite tandem solar cells. Nano Energy 2024, 121, 109175. [Google Scholar] [CrossRef]
- Brabec, C.J. Organic photovoltaics: Technology and market. Sol. Energy Mater. Sol. Cells 2004, 83, 273–292. [Google Scholar] [CrossRef]
- Sharma, A.; Masoumi, S.; Gedefaw, D.; O’Shaughnessy, S.; Baran, D.; Pakdel, A. Flexible solar and thermal energy conversion devices: Organic photovoltaics (OPVs), organic thermoelectric generators (OTEGs) and hybrid PV-TEG systems. Appl. Mater. Today 2022, 29, 101614. [Google Scholar] [CrossRef]
- Jain, A.; Kothari, R.; Tyagi, V.V.; Kumar Rajamony, R.; Shakeel Ahmad, M.; Mohan Singh, H.; Raina, S.; Pandey, A.K. Advances in organic solar cells: Materials, progress, challenges and amelioration for sustainable future. Sustain. Energy Technol. Assessments 2024, 63, 103632. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, R.; Li, Y.; Li, Y. Applications of organic solar cells in wearable electronics. Wearable Electron. 2024, 1, 26–40. [Google Scholar] [CrossRef]
- Mohamed El Amine, B.; Zhou, Y.; Li, H.; Wang, Q.; Xi, J.; Zhao, C. Latest Updates of Single-Junction Organic Solar Cells up to 20% Efficiency. Energies 2023, 16, 3895. [Google Scholar] [CrossRef]
- Li, Y.; Huang, W.; Zhao, D.; Wang, L.; Jiao, Z.; Huang, Q.; Wang, P.; Sun, M.; Yuan, G. Recent Progress in Organic Solar Cells: A Review on Materials from Acceptor to Donor. Molecules 2022, 27, 1800. [Google Scholar] [CrossRef]
- Uddin, A. Organic solar cells. In Comprehensive Guide on Organic and Inorganic Solar Cells: Fundamental Concepts to Fabrication Methods; Academic Press: Cambridge, MA, USA, 2022; pp. 25–55. [Google Scholar]
- Chander, S.; Purohit, A.; Nehra, A.; Nehra, S.P.; Dhaka, M.S. Influence of temperature on photovoltaic parameters of mono-crystalline silicon solar cell. AIP Conf. Proc. 2015, 1661, 050003. [Google Scholar]
- Savadogo, M.; Soro, B.; Konate, R.; Sourabié, I.; Zoungrana, M.; Zerbo, I.; Bathiebo, D.J.; Savadogo, M.; Soro, B.; Konate, R.; et al. Temperature Effect on Light Concentration Silicon Solar Cell’s Operating Point and Conversion Efficiency. Smart Grid Renew. Energy 2020, 11, 61–72. [Google Scholar] [CrossRef]
- Wang, S.; Qu, Y.; Li, S.; Ye, F.; Chen, Z.; Yang, X. Improved Thermal Stability of Polymer Solar Cells by Incorporating Porphyrins. Adv. Funct. Mater. 2015, 25, 748–757. [Google Scholar] [CrossRef]
- Wang, X.; Sangtarash, S.; Lamantia, A.; Dekkiche, H.; Forcieri, L.; Kolosov, O.V.; Jarvis, S.P.; Bryce, M.R.; Lambert, C.J.; Sadeghi, H.; et al. Thermoelectric properties of organic thin films enhanced by π–π stacking. J. Phys. Energy 2022, 4, 024002. [Google Scholar] [CrossRef]
- Yang, H.N.; He, S.J.; Zhang, T.; Man, J.X.; Zhao, Y.; Jiang, N.; Wang, D.K.; Lu, Z.H. Molecular orientation and thermal stability of thin-film organic semiconductors. Org. Electron. 2021, 88, 106014. [Google Scholar] [CrossRef]
- Lewinska, G.; Kanak, J.; Danel, K.S.; Sanetra, J.; Marszalek, K.W. Effect of benzene-based dyes on optothermal properties of active layers for ternary organic solar cells. Appl. Surf. Sci. 2023, 641, 158535. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, M.; Li, Z.; Wang, X.; Kang, X.; Ying, L. Efficient and stable organic solar cells enabled by incorporation of titanium dioxide doped PEDOT:PSS as hole transport layer. Prog. Org. Coat. 2023, 183, 107819. [Google Scholar] [CrossRef]
- Kumar, H.; Kumar, P.; Chaudhary, N.; Bhardwaj, R.; Chand, S.; Jain, S.C.; Kumar, V. Effect of temperature on the performance of CuPc/C60 photovoltaic device. J. Phys. D. Appl. Phys. 2008, 42, 015102. [Google Scholar] [CrossRef]
- Bristow, N.; Kettle, J. Outdoor performance of organic photovoltaics: Diurnal analysis, dependence on temperature, irradiance, and degradation. J. Renew. Sustain. Energy 2015, 7, 13111. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, Q.; Zhao, H.; Wang, H.; Li, N.; Song, W. 17.13% Efficiency and Superior Thermal Stability of Organic Solar Cells Based on a Comb-Shape Active Blend. Energy Environ. Mater. 2023, 6, e12443. [Google Scholar] [CrossRef]
- Zhang, Z.; Miao, J.; Ding, Z.; Kan, B.; Lin, B.; Wan, X.; Ma, W.; Chen, Y.; Long, X.; Dou, C.; et al. Efficient and thermally stable organic solar cells based on small molecule donor and polymer acceptor. Nat. Commun. 2019, 10, 3271. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Luo, Z.; Sun, R.; Guo, J.; Wang, T.; Wu, Y.; Wang, W.; Guo, J.; Wu, Q.; Shi, M.; et al. Simultaneous enhanced efficiency and thermal stability in organic solar cells from a polymer acceptor additive. Nat. Commun. 2020, 11, 1218. [Google Scholar] [CrossRef]
- Di Giacomo, F.; Razza, S.; Matteocci, F.; D’Epifanio, A.; Licoccia, S.; Brown, T.M.; Di Carlo, A. High efficiency CH3NH3PbI(3-x)Cl x perovskite solar cells with poly(3-hexylthiophene) hole transport layer. J. Power Sources 2014, 251, 152–156. [Google Scholar] [CrossRef]
- Züfle, S.; Christ, N.; Kettlitz, S.W.; Valouch, S.; Lemmer, U. Influence of temperature-dependent mobilities on the nanosecond response of organic solar cells and photodetectors. Appl. Phys. Lett. 2010, 97, 63306. [Google Scholar] [CrossRef]
- Tetreault, A.R.; Dang, M.T.; Bender, T.P. PTB7 and PTB7-Th as universal polymers to evaluate materials development aspects of organic solar cells including interfacial layers, new fullerenes, and non-fullerene electron acceptors. Synth. Met. 2022, 287, 117088. [Google Scholar] [CrossRef]
- Lu, B.; Wang, J.; Zhang, Z.; Wang, J.; Yuan, X.; Ding, Y.; Wang, Y.; Yao, Y. Recent progress of Y-series electron acceptors for organic solar cells. Nano Sel. 2021, 2, 2029–2039. [Google Scholar] [CrossRef]
- Yuan, J.; Zou, Y. The history and development of Y6. Org. Electron. 2022, 102, 106436. [Google Scholar] [CrossRef]
- Fernandes, L.; Gaspar, H.; Tomé, J.P.C.; Figueira, F.; Bernardo, G. Thermal stability of low-bandgap copolymers PTB7 and PTB7-Th and their bulk heterojunction composites. Polym. Bull. 2018, 75, 515–532. [Google Scholar] [CrossRef]
- Yin, Z.; Mei, S.; Chen, L.; Gu, P.; Huang, J.; Li, X.; Wang, H.Q.; Song, W. Efficient PTB7-Th:Y6:PC71BM ternary organic solar cell with superior stability processed by chloroform. Org. Electron. 2021, 99, 106308. [Google Scholar] [CrossRef]
- Lu, L.; Yu, L.; Lu, L.Y.; Yu, L.P. Understanding Low Bandgap Polymer PTB7 and Optimizing Polymer Solar Cells Based on It. Adv. Mater. 2014, 26, 4413–4430. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Yuan, J.; Yi, Y.; Zhang, C.; Zou, Y. Y6 and its derivatives: Molecular design and physical mechanism. Natl. Sci. Rev. 2021, 8, 2021. [Google Scholar] [CrossRef]
- Sharma, V.V.; Landep, A.; Lee, S.; Park, S.; Kim, Y.; Kim, G.-H. Recent advances in polymeric and small molecule donor materials for Y6 based organic solar cells. Next Energy 2023, 2, 100086. [Google Scholar] [CrossRef]
- Doumon, N.Y.; Houard, F.V.; Dong, J.; Christodoulis, P.; Dryzhov, M.V.; Portale, G.; Koster, L.J.A. Improved photostability in ternary blend organic solar cells: The role of [70] PCBM. J. Mater. Chem. C 2019, 7, 5104–5111. [Google Scholar] [CrossRef]
- Khoshman, J.M.; Hilfiker, J.N.; Tabet, N.; Kordesch, M.E. Multiple oscillator models for the optical constants of polycrystalline zinc oxide thin films over a wide wavelength range. Appl. Surf. Sci. 2014, 307, 558–565. [Google Scholar] [CrossRef]
- Jellison, G.E.; Modine, F.A. Parameterization of the optical functions of amorphous materials in the interband region. Appl. Phys. Lett. 1996, 69, 371–373. [Google Scholar] [CrossRef]
- Ferlauto, A.S.; Ferreira, G.M.; Pearce, J.M.; Wronski, C.R.; Collins, R.W.; Deng, X.; Ganguly, G. Analytical model for the optical functions of amorphous semiconductors from the near-infrared to ultraviolet: Applications in thin film photovoltaics. J. Appl. Phys. 2002, 92, 2424–2436. [Google Scholar] [CrossRef]
- Di, M.; Bersch, E.; Diebold, A.C.; Consiglio, S.; Clark, R.D.; Leusink, G.J.; Kaack, T. Comparison of methods to determine bandgaps of ultrathin HfO2 films using spectroscopic ellipsometry. J. Vac. Sci. Technol. 2011, 29, 41001. [Google Scholar] [CrossRef]
- Schwartz, B.J. Conjugated Polymers as Molecular Materials: How Chain Conformation and Film Morphology Influence Energy Transfer and Interchain Interactions. Annu. Rev. Phys. Chem. 2003, 54, 141–172. [Google Scholar] [CrossRef] [PubMed]
- McQuade, D.T.; Kim, J.; Swager, T.M. Two-dimensional conjugated polymer assemblies: Interchain spacing for control of photophysics. J. Am. Chem. Soc. 2000, 122, 5885–5886. [Google Scholar] [CrossRef]
- Chang, H.; Zhang, J.; Wu, X.; Lin, H.; Mou, Y.; Wu, Z.; Zhou, J.; Qian, H.; Yao, J.; Yang, K.; et al. Thermal-induced bandgap broadening phenomenon in copolymer organic semiconductors. Appl. Phys. Lett. 2024, 124, 252101. [Google Scholar] [CrossRef]
- Sun, B.; Hong, W.; Aziz, H.; Li, Y. Diketopyrrolopyrrole-based semiconducting polymer bearing thermocleavable side chains. J. Mater. Chem. 2012, 22, 18950–18955. [Google Scholar] [CrossRef]
- Savikhin, V.; Jagadamma, L.K.; Purvis, L.J.; Robertson, I.; Oosterhout, S.D.; Douglas, C.J.; Samuel, I.D.W.; Toney, M.F. Morphological, Chemical, and Electronic Changes of the Conjugated Polymer PTB7 with Thermal Annealing. iScience 2018, 2, 182–192. [Google Scholar] [CrossRef]
- Gao, M.; Liu, Y.; Xian, K.; Peng, Z.; Zhou, K.; Liu, J.; Li, S.; Xie, F.; Zhao, W.; Zhang, J.; et al. Thermally stable poly(3-hexylthiophene): Nonfullerene solar cells with efficiency breaking 10%. Aggregate 2022, 3, 1–12. [Google Scholar] [CrossRef]
- Kerremans, R.; Kaiser, C.; Li, W.; Zarrabi, N.; Meredith, P.; Armin, A. The Optical Constants of Solution-Processed Semiconductors-New Challenges with Perovskites and Non-Fullerene Acceptors. Adv. Opt. Mater. 2020, 8, 2000319. [Google Scholar] [CrossRef]
- Ibrahim, B.A.; Kadum, K.M. Influence of Polymer Blending on Mechanical and Thermal Properties. Mod. Appl. Sci. 2010, 4, 157. [Google Scholar] [CrossRef]
- Tomić, N.Z. Thermal studies of compatibilized polymer blends. In Compatibilization of Polymer Blends: Micro and Nano Scale Phase Morphologies, Interphase Characterization and Properties; Elsevier: Amsterdam, The Netherlands, 2020; pp. 489–510. [Google Scholar]
- Hinderhofer, A.; Hosokai, T.; Yonezawa, K.; Gerlach, A.; Kato, K.; Broch, K.; Frank, C.; Novák, J.; Kera, S.; Ueno, N.; et al. Post-growth surface smoothing of thin films of diindenoperylene. Appl. Phys. Lett. 2012, 101, 33307. [Google Scholar] [CrossRef]
- Ye, R.; Baba, M.; Suzuki, K.; Ohishi, Y.; Mori, K. Effect of thermal annealing on morphology of pentacene thin films. Jpn. J. Appl. Phys. Part 1 Regul. Pap. Short Notes Rev. Pap. 2003, 42, 4473–4475. [Google Scholar] [CrossRef]
- Park, S.K.; Kim, Y.H.; Han, J.I. Effect of annealing treatment and surface morphology on power conversion in organic photovoltaics. Jpn. J. Appl. Phys. 2009, 48, 0815051–0815053. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, H.; Cao, J.; Zhang, S.; Li, W.; Yang, B. Unusual temperature-sensitive excimer fluorescence from discrete π–π dimer stacking of anthracene in a crystal. Phys. Chem. Chem. Phys. 2019, 21, 14511–14515. [Google Scholar] [CrossRef] [PubMed]
- Keshri, S.K.; Nakanishi, W.; Takai, A.; Ishizuka, T.; Kojima, T.; Takeuchi, M. Discrete π Stack of a Tweezer-Shaped Naphthalenediimide–Anthracene Conjugate. Chem. A Eur. J. 2020, 26, 13288–13294. [Google Scholar] [CrossRef] [PubMed]
- Paleti, S.H.K.; Hultmark, S.; Han, J.; Wen, Y.; Xu, H.; Chen, S.; Järsvall, E.; Jalan, I.; Villalva, D.R.; Sharma, A.; et al. Hexanary blends: A strategy towards thermally stable organic photovoltaics. Nat. Commun. 2023, 14, 4608. [Google Scholar] [CrossRef]
- Tian, H.; Wucher, A.; Winograd, N. Reducing the Matrix Effect in Organic Cluster SIMS Using Dynamic Reactive Ionization. J. Am. Soc. Mass Spectrom. 2016, 27, 2014–2024. [Google Scholar] [CrossRef]
- Shard, A.G.; Miisho, A.; Vorng, J.L.; Havelund, R.; Gilmore, I.S.; Aoyagi, S. A two-point calibration method for quantifying organic binary mixtures using secondary ion mass spectrometry in the presence of matrix effects. Surf. Interface Anal. 2022, 54, 363–373. [Google Scholar] [CrossRef]
- Oh-E, M.; Ogata, H.; Araoka, F. Randomization and Constraint of Molecular Alignment and Orientation: Temperature-Dependent Anisotropy and Phase Transition in Vapor-Deposited Thin Films of an Organic Cross-Shaped Molecule. ACS Omega 2019, 4, 39–47. [Google Scholar] [CrossRef]
- Guskova, O.; Schünemann, C.; Eichhorn, K.J.; Walzer, K.; Levichkova, M.; Grundmann, S.; Sommer, J.U. Light Absorption in Organic Thin Films: The Importance of Oriented Molecules. J. Phys. Chem. C 2013, 117, 17285–17293. [Google Scholar] [CrossRef]
- Baldacchini, G.; Chiacchiaretta, P.; Pode, R.B.; Vincenti, M.A.; Wang, Q.M. Phase transitions in thermally annealed films of Alq3. Low Temp. Phys. 2012, 38, 786–791. [Google Scholar] [CrossRef]
- Ebenhoch, B.; Thomson, S.A.J.; Genevičius, K.; Juška, G.; Samuel, I.D.W. Charge carrier mobility of the organic photovoltaic materials PTB7 and PC71BM and its influence on device performance. Org. Electron. 2015, 22, 62–68. [Google Scholar] [CrossRef]
- Tang, Y.; Lakhwani, G.; McNeill, C.R. The critical role of temperature-dependent mobilities in determining the open-circuit voltage of bulk-heterojunction organic solar cells. Appl. Phys. Lett. 2023, 123, 213501. [Google Scholar] [CrossRef]
- Lee, D.; Kim, J.; Park, G.; Bae, H.W.; An, M.; Kim, J.Y. Enhanced Operating Temperature Stability of Organic Solar Cells with Metal Oxide Hole Extraction Layer. Polymers 2020, 12, 992. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, H.; Hu, M.; Wang, X.; Hu, L.; Mao, H.; Yuan, Z.; Ma, W.; Chen, Y.; Zhang, L.; et al. Enhanced Efficiency and Excellent Thermostability in Organic Photovoltaics via Ternary Strategy with Twisted Conjugated Compound. Small 2021, 17, 2103537. [Google Scholar] [CrossRef]
- Dong, Y.; Zou, Y.; Yuan, J.; Yang, H.; Wu, Y.; Cui, C.; Li, Y. Ternary Polymer Solar Cells Facilitating Improved Efficiency and Stability. Adv. Mater. 2019, 31, 1904601. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewinska, G.; Kanak, J.; Sanetra, J.; Marszalek, K.W. Optothermal Properties of Donor–Acceptor Layers, Including PTB7, PTB7th, Y5, and Y6, for Organic Photovoltaic Cell Applications. Materials 2025, 18, 1841. https://doi.org/10.3390/ma18081841
Lewinska G, Kanak J, Sanetra J, Marszalek KW. Optothermal Properties of Donor–Acceptor Layers, Including PTB7, PTB7th, Y5, and Y6, for Organic Photovoltaic Cell Applications. Materials. 2025; 18(8):1841. https://doi.org/10.3390/ma18081841
Chicago/Turabian StyleLewinska, Gabriela, Jarosław Kanak, Jerzy Sanetra, and Konstanty W. Marszalek. 2025. "Optothermal Properties of Donor–Acceptor Layers, Including PTB7, PTB7th, Y5, and Y6, for Organic Photovoltaic Cell Applications" Materials 18, no. 8: 1841. https://doi.org/10.3390/ma18081841
APA StyleLewinska, G., Kanak, J., Sanetra, J., & Marszalek, K. W. (2025). Optothermal Properties of Donor–Acceptor Layers, Including PTB7, PTB7th, Y5, and Y6, for Organic Photovoltaic Cell Applications. Materials, 18(8), 1841. https://doi.org/10.3390/ma18081841








