Abstract
The tanning of hides is a practice deeply rooted in ancient times, but in the early 20th century, transitioning to an industrial model of leather and hide production, chrome tanning expanded globally due to its practicality, quality, and versatility. However, in recent decades, there has been a growing attention paid to the potential oxidation of the free chromium present in tanned leather, which could transform from an unharmful trivalent status into its carcinogenic hexavalent status. This phenomenon occurs in a very small fraction of hides, which is yet sufficient to exhibit significant activity. Hence, there is an evident need to explore further alternatives that allow avoiding oxidation. In this work, the performance of a sequence of selected antioxidants is evaluated in different oxidation conditions: simple stirring, UV–Vis–IR irradiation, and heating up to 75 °C. The official diphenylcarbazide–UV tool norm was used to quantify the hexavalent chromium amount. The results underline the effectiveness of 2,6-di-tert-butylphenol, and that its greatest preventative method of addition during the tanning process is together with the tanning agent. This approach will pave the way for researching alternative concepts and exploring perspectives to inhibit chromium issues.
1. Introduction
Leather has proven to be one of the fastest-growing segments within the global market for personal luxury goods and is expected to grow at rates above the market average [1]. The improvement stems from increasing consumer incomes and enhanced living standards, which have resulted in a greater demand for leather products [2]. Leather industries hold economic significance as they enhance the value of raw hides that, after the slaughterhouse phase, are considered almost totally a waste. Tanneries emphasize these useful animal resources, ensuring that the less-used parts of the animal do not go to waste [3].
From an industrial point of view, the transition process from raw hides to a refined product is called tanning and encompasses several steps. In its entirety, irreversible cross-links are established between polypeptide chains in this process, which constitute the smallest structural units of leather collagen fibers [4]. Historically, until the 19th century, vegetal tannins represented the prevalent method for stabilizing collagen, yielding a harder type of leather. However, in the late 19th century, chromium was introduced, offering a faster, cost-effective, and versatile product, suitable for many different industries [5]. The chemicals employed in tanning may vary according to the tanning mode, and chromium tanning has continued to be the widest employed method [6]. In particular, a Cr(III) salt is used, representing the most stable oxidation state of chromium [7] and characterized by the ability to make stable complexes that strongly stabilize collagen with coordinative bonds [8].
However, the unlikely but possible oxidation of trivalent chromium may lead to the observation of the hexavalent compound. Specific occupational risks are associated with the hexavalent metal, which is heavily problematic [9], not only causing direct tissue damage, but also containing carcinogenic and mutagenic potential [10]. Besides the lung and the nose mucous tissue, the kidney is the main target organ after uptake of Cr(VI). In more detail, after oral and/or dermal exposure, the different absorption rates of Cr(III) and Cr(VI) compounds account for specific respective profiles of toxicity [11], where Cr(VI) compounds easily penetrate a cell membrane in the state of chromate anions [12]. Additionally, in the case of the environment, chromium issues cannot be avoided. When a soil contains a large amount of soluble and biologically assimilable forms of chromium, the effects of soil pollution from the metal ion are observed [13]. The soluble ionic status of Cr(VI) formed in or added to soils or natural waters will persist indefinitely unless chromate is removed by leaching, adsorption, precipitation, uptake by living cells, or by reduction to the trivalent form [14]. All these reasons are behind the focus on the possible formation of Cr(VI) [15].
Fatliquoring is an additional key step of tanning for enhancing leather quality, specifically by improving softness, wear resistance, tear resistance, and porosity [16]. The fatliquoring agents’ role is to prevent the collapse of collagen fibers caused by the removal of water during the drying process by working as lubricants [17]. The chemicals used in the operation derive from four primary sources: fish, petroleum, vegetable, or animal origins. Some of the miscellaneous extracted products are rich in fatty acids, often with a significant proportion of unsaturated fatty acids [18]. The last mentioned ones, represented by fish oils, may, after photo-aging with UV light or thermal treatment, lead to the oxidation of Cr(III) [19]. This explains why, ever since testing began in tannery wastewaters, influenced by further external factors, it has been possible to find Cr(VI), even if in lower amounts thanks to the humidity effect. In fact, storage of fatliquored leather at a relative humidity above 35% may result in an increase of probable Cr(VI) formation [20]. These oxidative agents have physicochemical properties that can introduce hydroperoxides, free radicals, and other reactive species to double bonds, thereby inducing the oxidation of trivalent chromium to hexavalent chromium. This oxidation primarily affects the residual free metal within the tanned leather, which is more prone to oxidation compared to complexed metal.
In this context, there is an urgent need for solutions to mitigate or inhibit this oxidation process [21]. Other possibilities were studied in detail, with particular attention paid to tara tannin and ascorbic acid. Since no satisfying effects were presented in tanneries, the purpose of the present work is to find an optimum method that reasonably reduces chromium oxidation in tanning without any side effects on the final leather (typical of vegetal tannins). The performance of the antioxidant and the sequence of additions to be made to ensure the lowest amount of the oxidation product have been investigated. A selection of antioxidants has been made on the basis of a previous study [22], by choosing antioxidants among the most common of those already industrially used and a commercialized product, and their performance has been evaluated.
The study in question is a groundbreaking discovery that improves the tanning process, enabling the dual action of ensuring safety while simultaneously guaranteeing high product quality. The proposed comparison allows us to understand the effects of the chosen antioxidants on the formation of hexavalent chromium.
2. Materials and Methods
2.1. Materials
The compositions of the listed agents are based on those declared in the safety sheets.
The commercial fatliquoring agent used is Riveroil TIS, composed of sulphonate esters, polyglycol ethers, fish oil derivates, and alkanediols (supplied by River Chimica Industriale S.p.A., San Miniato (PI), Italy).
The tanning agent used is Cromo FD, composed of Cr(OH)SO4 + Na2SO4 > 75% (supplied by River Chimica Industriale S.p.A., San Miniato (PI), Italy).
The antioxidant screening involved the following chemicals: 2,6-di-tert-butylphenol (C14H22O, MW = 206.32 g/mol, CAS: 128-39-2, Fluka, Buchs, Switzerland); DL-α-tocopherol (C29H50O2, MW = 430.71 g/mol, CAS: 10191-41-0, Tokyo Chemical Industry Co., Tokyo, Japan); 2,2,6,6-tetramethylpiperidinoxy free radical, TEMPO (C9H18NO, MW = 156.25 g/mol, CAS: 2564-83-2, Fluorochem Ltd., Hadfield, UK); 2,5-di-tert-butylhydroquinone (C14H13O2, MW = 222.32 g/mol, CAS: 88-58-4, Sigma-Aldrich, St. Louis, MO, USA); and a declared antioxidant product available on the market and its active principle.
The solvents used for extractions are the following: ethyl acetate, EtOAc (C4H8O2, MW = 88.1 g/mol, CAS: 141-78-6, Sigma-Aldrich, St. Louis, MO, USA); dichloromethane, DCM (CH2Cl2, MW = 84.93 g/mol, CAS: 75-09-2, Sigma-Aldrich, St. Louis, MO, USA); and formic acid (CH2O2, MW = 46.03 g/mol, CAS: 64-18-6, Sigma-Aldrich, St. Louis, MO, USA).
Additionally, Freiberg hide powder (FILK, Freiberg Institute, Freiberg, Germany) was used.
To perform the Cr(VI) quantification in water and leather (EN ISO 17075-1), the following were used: potassium hydrogen phosphate (K2HPO4, MW = 174.18 g/mol, CAS: 7758-11-4, Sigma-Aldrich, St. Louis, MO, USA); 1,5-Diphenylcarbazide (DPC) (C13H14N4O, MW = 242.28 g/mol, CAS: 140-22-7, Sigma-Aldrich, St. Louis, MO, USA); sulfuric acid (H2SO4, MW = 98.08 g/mol, CAS: 7664-93-9, Sigma-Aldrich, St. Louis, MO, USA); phosphoric acid (H3PO4, MW = 97.99 g/mol, CAS: 7664-38-2, Sigma-Aldrich, St. Louis, MO, USA); and potassium dichromate (K2Cr2O7, MW = 294.19 g/mol, CAS: 7778-50-9, Sigma-Aldrich, St. Louis, MO, USA).
2.2. Methods
The study is divided into two steps, following the same principle: Cr(VI) becomes quantifiable because diphenylcarbazide (DPC) is oxidized by the hexavalent chromium and becomes diphenylcarbazone (DPCA), detectable on the UV spectrophotometer.
2.2.1. Antioxidant Efficacy in Tannery Wastewater
In a beaker, a primary solution of 60 mL reproduces the generic conditions of the substance combination in finished leather (percentages are based on the total volume). The detailed recipe is made up of 44.9% water, 24.9% Cromo FD, and 29.9% of Riveroil TIS [23]. The tests are conducted in a 6-well plate, and each has a volume of 6 mL. The antioxidant is added directly in the wells at 0.3% of the single well volume. The plates are kept in motion by magnetic stirrers at approximately 300 rpm. Three different oxidative conditions were tested: simple stirring for 5 days at room temperature, UV–Vis–IR irradiation for 3 days [24] at room temperature (25 °C), and heating at 75 °C for 2 days. After processing, the 6 mL are diluted with water in a 50 mL flask. To carry out prior isolation of interfering substances (solid powder/antioxidant/fattening agents), a Buchner filtration is performed for each, and 1 mL of filtrate is diluted with water in 25 mL volumetric flasks. Then, liquid–liquid extraction is done using a separating funnel, using EtOAc and DCM with a maximum of three washes per solvent. At this point, the solution is ready for the normative reference from the CNR-IRSA Italian national institute: a spectrophotometric determination of Cr(VI) using diphenylcarbazide (DPC) directly in a water solution [25].
2.2.2. Antioxidant Efficacy with Hide Powder
This research involves antioxidant experiments made on hide powder, with the normative reference following EN ISO 17075-1:2017 [26]. The weight of the experimental formulation is measured on the basis of the wet hide powder. The best-performing antioxidant selected in the first step is tested. The procedure consists of the following steps: 1 g of hide powder is wet with a few mL of water until it reaches the weight of 7.8 g; then, on the basis of the wet powder weight, 40% w/w of acidified water (water + formic acid) at pH 2.5 treats the hide [27]; after 24 h, 8% w/w of Cromo FD is added [28]; and after another 24 h, 9% w/w of Riveroil TIS is finally added to the solution. Each antioxidant is added at 1% w/w of the fatliquoring agent quantity, and for each one, three different solutions have been prepared, differentiated by the order in which the antioxidant is added. They are addressed, respectively, with the letters Q, W, and E. In solution Q, the antioxidant is added together with the fatliquoring; in solution W, it is added right after the acidic water (simulates pickling [29]); and in solution E, it is added right after the chromium addition. In this way, the extraction is followed by the specific normative steps.
2.3. Instruments
The hexavalent chromium quantification was conducted in both cases using UV spectrometry. The instrument used is a V-600 Series UV–Vis spectrophotometer from JASCO (Cremella (LC), Italy). A 540 nm wavelength was selected [30], with a range between 0.1 and 1 mg/L Cr(VI). The quantity of Cr(VI) in the liquid sample was calculated using two different calibration curves, according to the different normative references followed. In the first method (CNR-IRSA), cuvettes with a 1 cm optical path were used, while for the second procedure (ISO), the optical path is 5 cm, which is favorable for sensitive incrementation. The pH measurements were performed using a lab pH meter (HI98191, Hanna Instruments, Padova, Italy) with a standard error of 0.01.
3. Results and Discussion
The purpose of this work was to find the best antioxidant able to prevent the oxidation of trivalent chromium in tannery wastewaters, and then directly on leather. To this end, the quantities of each compound were chosen to reproduce the worst case in the industrial process: the excess of a sensible (but useful) recipe. To facilitate the reproducibility of the results, a recognized standard collagen matrix was used: Freiberg hide powder. Below are the steps that our research conducted to evaluate the performance of the antioxidants. The UV principle remains the same: diphenylcarbazide (DPC) is oxidized by the hexavalent chromium and becomes diphenylcarbazone (DPCA) [31], making a complex with the oxidized metal, which is detectable on the UV spectrophotometer.
3.1. Experimental Set-Up
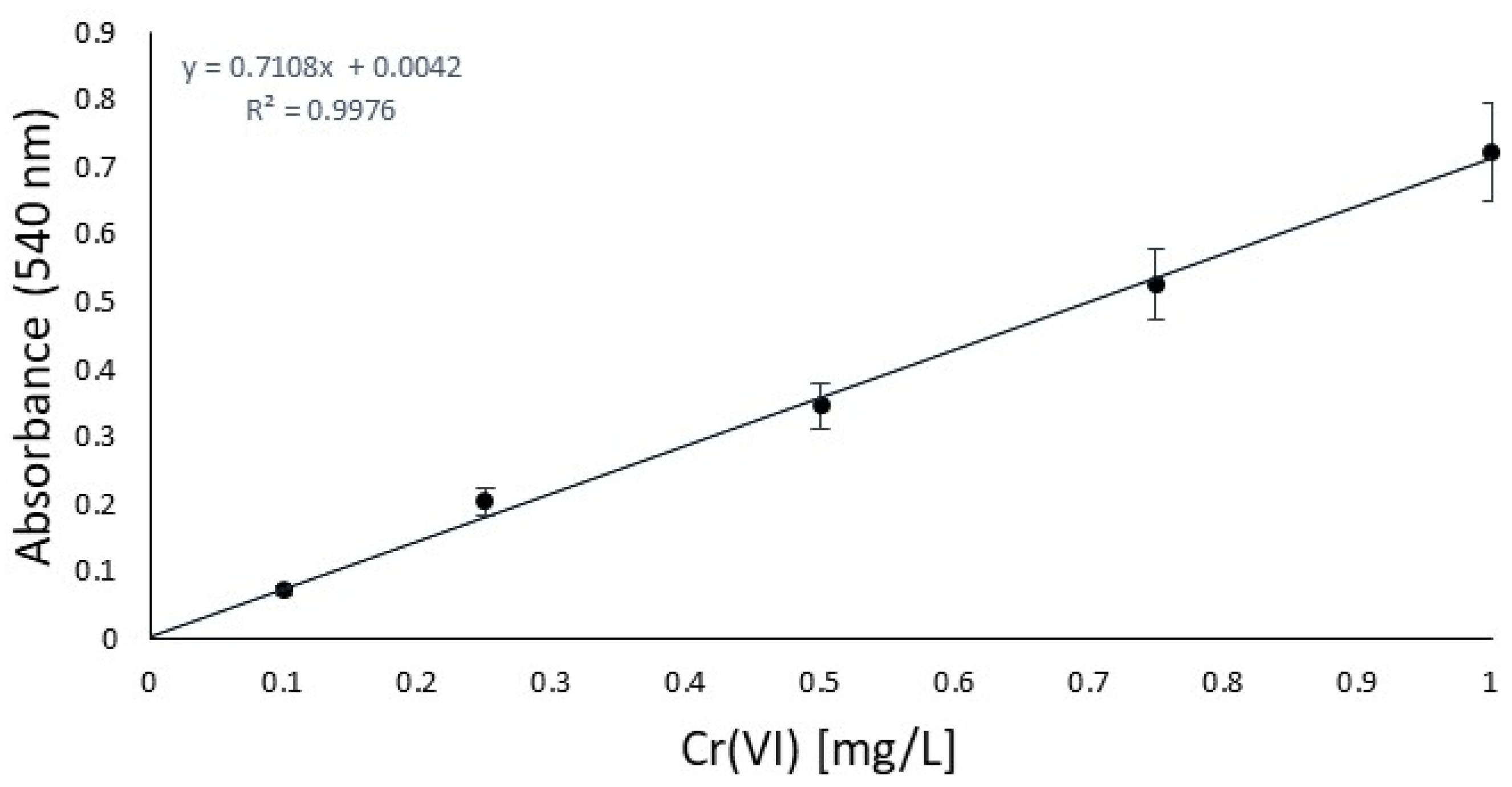
First of all, in order to focus on the antioxidant performance, a chromium salt as the tanning agent and a fatliquoring agent had to be chosen and kept unchanged for all the experiments to ensure that the results would be effectively compared. From a previous study [22], to represent the most sensible conditions for oxidation, Cromo FD and Riveroil TIS were selected, respectively. On the same basis, the series of antioxidants to be checked were decided, focusing our investigation on 2,6-di-tert-butylphenol, TEMPO, and tocopherol, which had already been tested in the previous work only under simple stirring conditions. To this list, a declared antioxidant product available on the market, its active principle, and 2,5-di-tert-butylhydroquinone were added. To control oxidation, the idea was to use the same sequence of steps as the industrial tanning process and quantities of chromium that simulate the real conditions in tanneries in the worst case, without the basification that enhances the stabilization of chromium complexes [32]. At this last point, the availability of free chromium is increased, directly related to the oxidation sensibility increasing. The decision to test three different operating conditions was due to the hexavalent chromium expected. The UV irradiation and temperature should facilitate the oxidation of trivalent chromium [33], and so represent the worst conditions for the analysis. After the oxidation time, to carry out prior isolation of interfering substances (antioxidant/fatliquoring agents), filtration was performed for each. Then, liquid–liquid extraction was performed to avoid the risk of running into an auxiliary reaction of DPC or measurement mistakes. In order to validate the procedure of Cr(VI) quantification, a blank study in the absence of an antioxidant was done [34]. Before the analysis, the calibration curve was obtained between 0.1 and 1 mg/L, as reported in Figure 1, and according to the normative reference [35]. This was essential for the quantification of Cr(VI) by UV absorption in the next step.
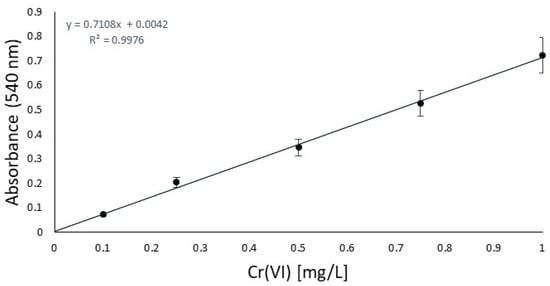
Figure 1.
Calibration curve according to the CNR-IRSA normative reference [35].
3.2. UV Analysis
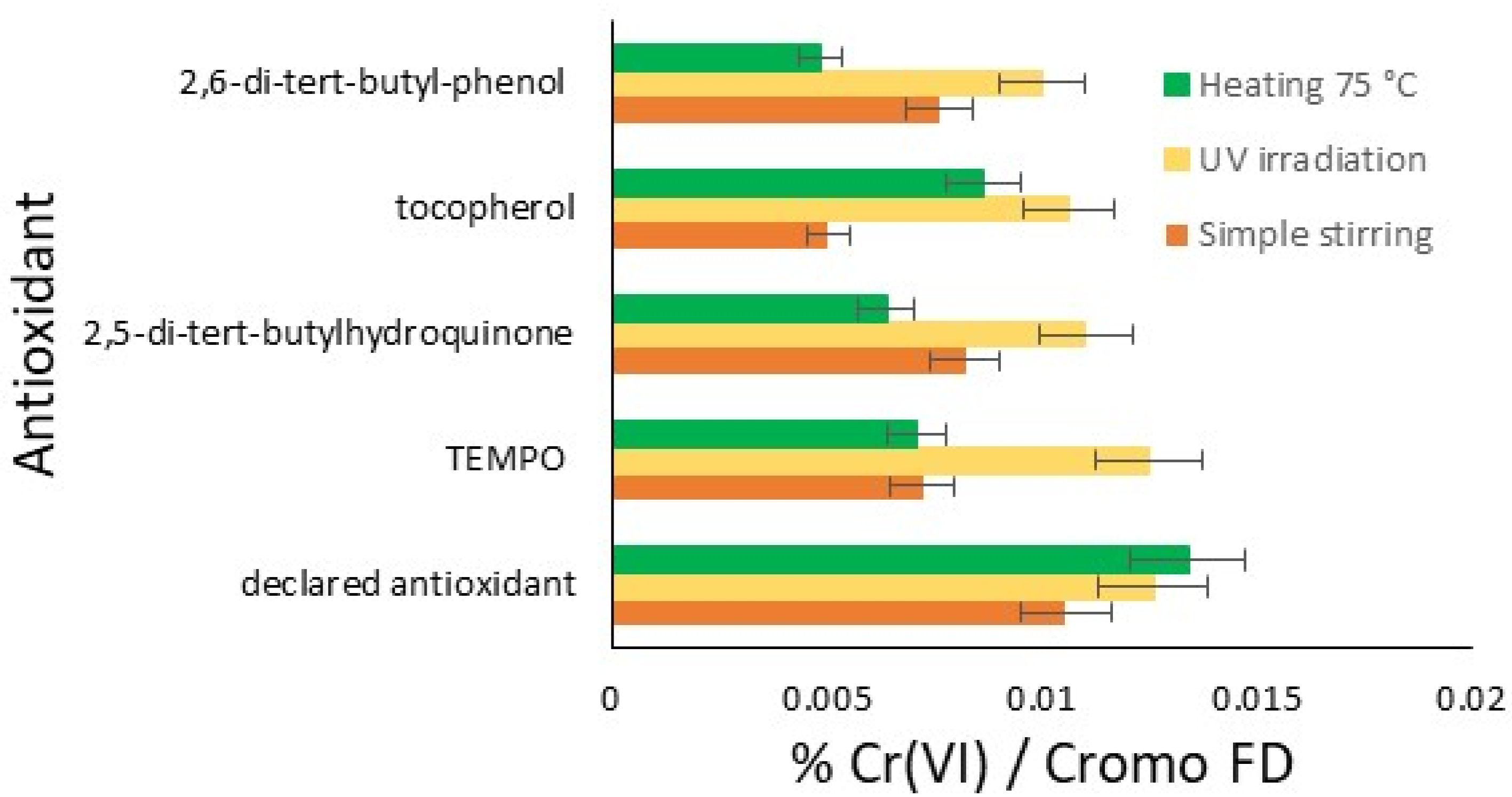
In the graph in Figure 2, the results of this initial comparative study are reported for the three conditions tested: stirring for five days, UV irradiation for three days, and the thermal effect at 75 °C for two days. Therefore, the amount of hexavalent chromium for each antioxidant under each oxidation condition is possible to see in the bar chart. Each trial resulted in an amount of chromium significantly lower than the blank, which was completely out of scale, resulting in 97.17% of hexavalent chromium with Cromo FD. The results are classified from the best to the lowest effect.
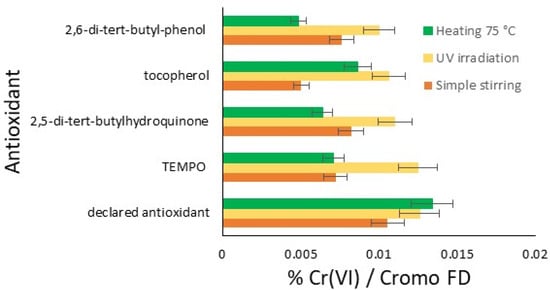
Figure 2.
Summarizing the results: comparison of the efficiency of the antioxidant on the amount of Cr(VI) formed from Cr(III) (%): simple stirring (orange); UV irradiation (yellow); and heating at 75 °C (green).
In general, UV irradiation tends to produce more Cr(VI) compared to heating and stirring, except in the case of the declared commercial antioxidant, where heating produces slightly more Cr(VI) than UV irradiation. This result represents a first important limitation of the studied antioxidants. With regard to heating, better antioxidant protective activity is observed with all the compounds, and some sensitiveness is only found in the cases of the declared antioxidant and tocopherol. Finally, the easiest stirring represents the best protective condition for the limitation of Cr(VI) development, with the exception of the declared antioxidant. The radical molecule TEMPO, now widely used in chemical environments and the plastics industry, which is of synthetic origin, exhibits interesting antioxidant activity, sensitive only to UV. Notably, an antioxidant effective under irradiation does not necessarily exhibit the same characteristics in tests conducted at elevated temperatures. In the tests at 75 °C, 2,5-di-tert-butylhydroquinone, TEMPO, and 2,6-di-tert-butylphenol show much greater efficacy. The declared antioxidant product available on the market, on the other hand, has relatively comparable activities across different conditions.
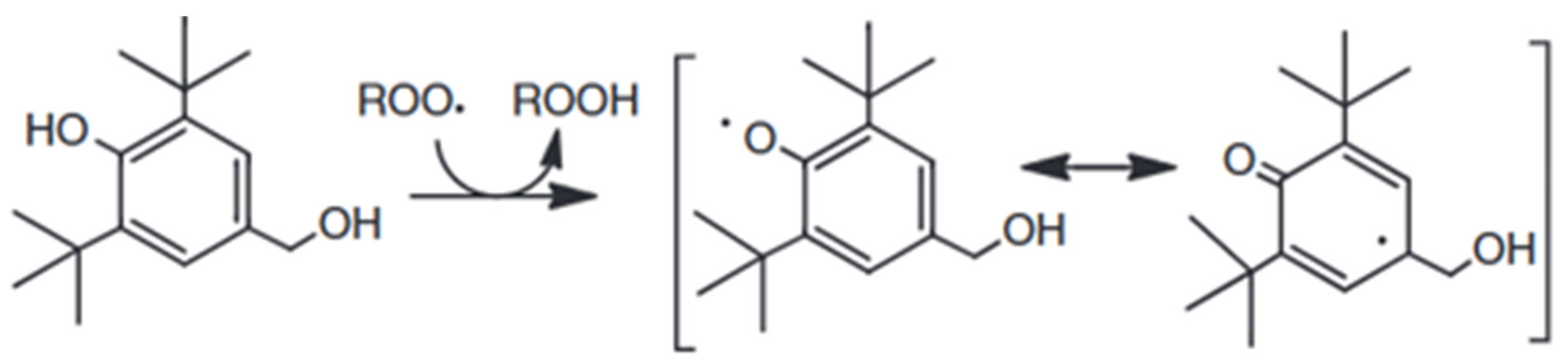
The indicated performance should be related to the chemical structure of these molecules. 2,6-di-tert-butylphenol and 2,5-di-tert-butylhydroquinone are two hindered phenols, characterized by a strong tendency to be oxidized and to remain stable for a long time [36]. In fact, their hindered structure permits the shear of the inducted charge along all the C-C bonds. The difference between them is the presence of one more hydroxide group and the lesser hindering of the tert-butyl group. This is why 2,6-di-tert-butylphenol shows better behavior [37]. A similar explanation is represented in Figure 3 [36].
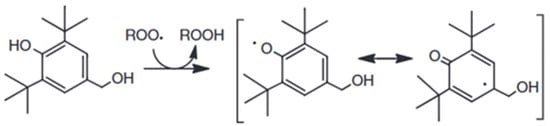
Figure 3.
Stabilization of hindered phenols by resonance.
In the case of tocopherol, the mechanism is different, and not comparable with the other chemicals. Its aromatic hydroxide is oxidized, becoming easily stabilized by the many aliphatic carbons in the chemical structure [38]. Additionally, in harder oxidative conditions (oxidating agent, heating, and irradiation), its behavior cannot be explained [39]. In the case of TEMPO, there is another method of preventing oxidation, and its power is related to its capacity to regenerate itself using a redox mechanism [40]. The possible problem that caused the strong oxidation under UV rays is related to its reduction. In fact, in order to regenerate itself, TEMPO must oxidize other compounds and oxidize fatty acids, eventually leading to the same oxidative behavior that it initially lacks; if chromium is oxidized by itself, it is even more problematic.
3.3. UV Analysis on Hide Powder
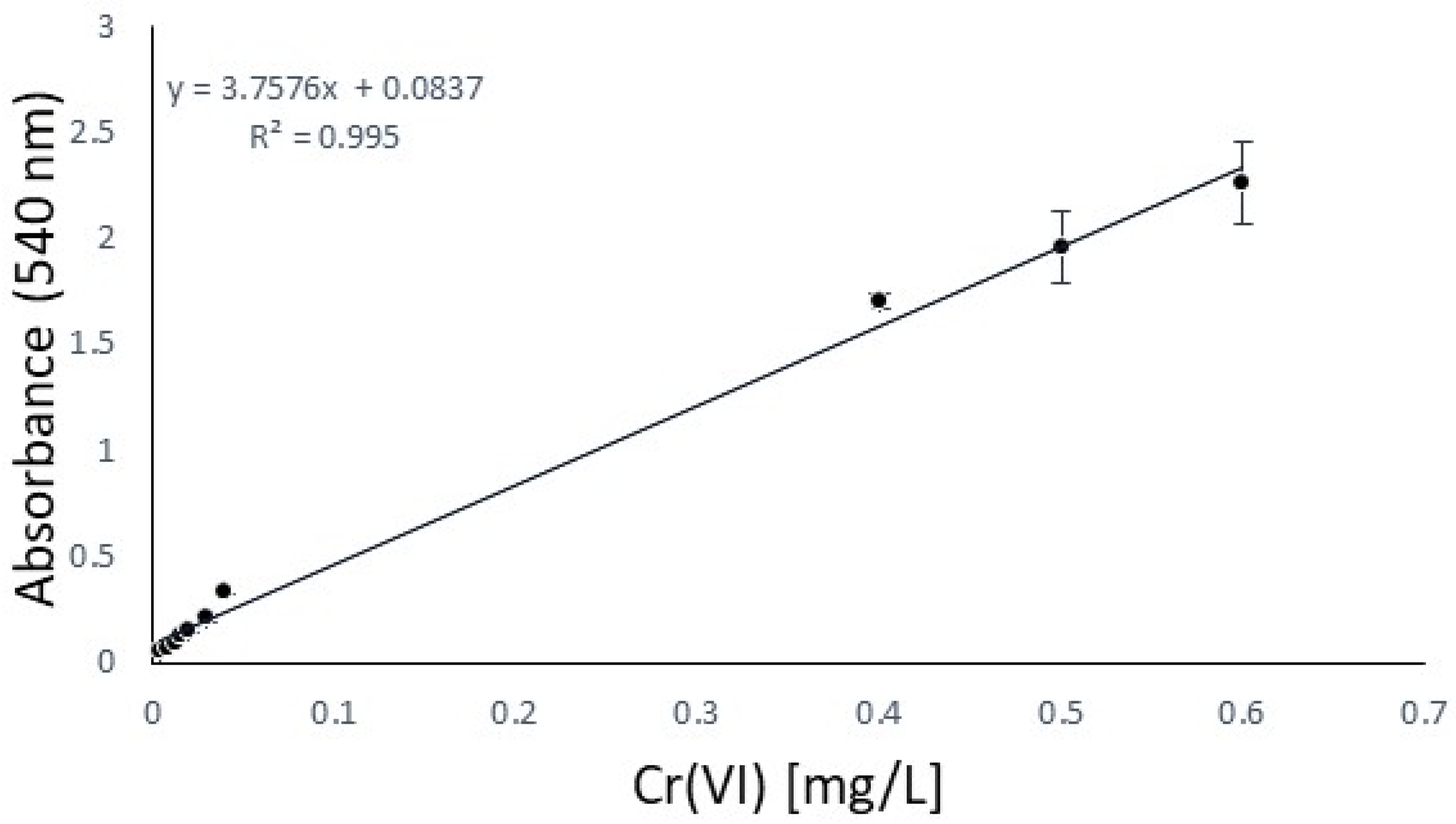
The hide powder is involved in this phase, where two different aspects are to be evaluated. First of all, this phase aims to find the most effective antioxidant, and secondly, it aims to assess whether adding it in the phase of acidification, tanning, or fatliquoring will produce the maximum effect on the inhibition of hexavalent chromium formation. From the previous step, further screening was conducted, selecting four antioxidants to be tested directly on the hide powder: 2,5-di-tert-butylhydroquinone, tocopherol, 2,6-di-tert-butyl-phenol, and the active principle of the declared antioxidant product available on the market. The calibration curve, using potassium dichromate according to the normative reference EN ISO 17075-1, is reported in Figure 4 [26], and permits the analytical quantification of Cr(VI) directly by UV absorption. In particular, the method is described in the relative ISO to be suitable to quantify the Cr(VI) content in leathers down to 3 mg/kg. Due to the low concentration of Cr(VI) extracted from leather after the standard procedure detectable with a UV spectrophotometer, cuvettes with a 5 cm cell optical path were chosen, which have a well-known application in this type of analysis to increase absorbance proportional to the optical path length [41]. Furthermore, for an improvement of UV quality, a filtration was done after the oxidation procedure to avoid the risk of running into an auxiliary reaction of DPC or measurement mistakes (as scattering in UV analysis) [42]. Blank trials were also carried out to evaluate the presence of Cr(VI) in the absence of an antioxidant.
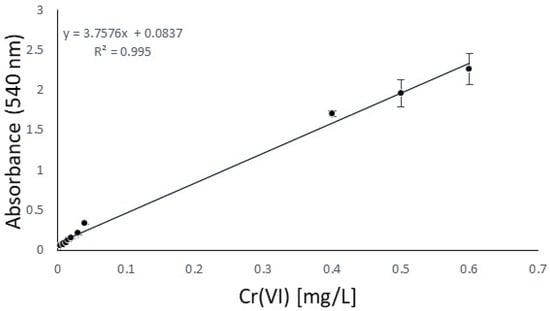
Figure 4.
Calibration curve: Cr(VI) in the extraction solution based on ISO 17075-1.
Three different conditions were also tested here, as in the previous step: simple stirring for 5 days, UV–Vis–IR irradiation for 3 days, and heating at 75 °C for 2 days.
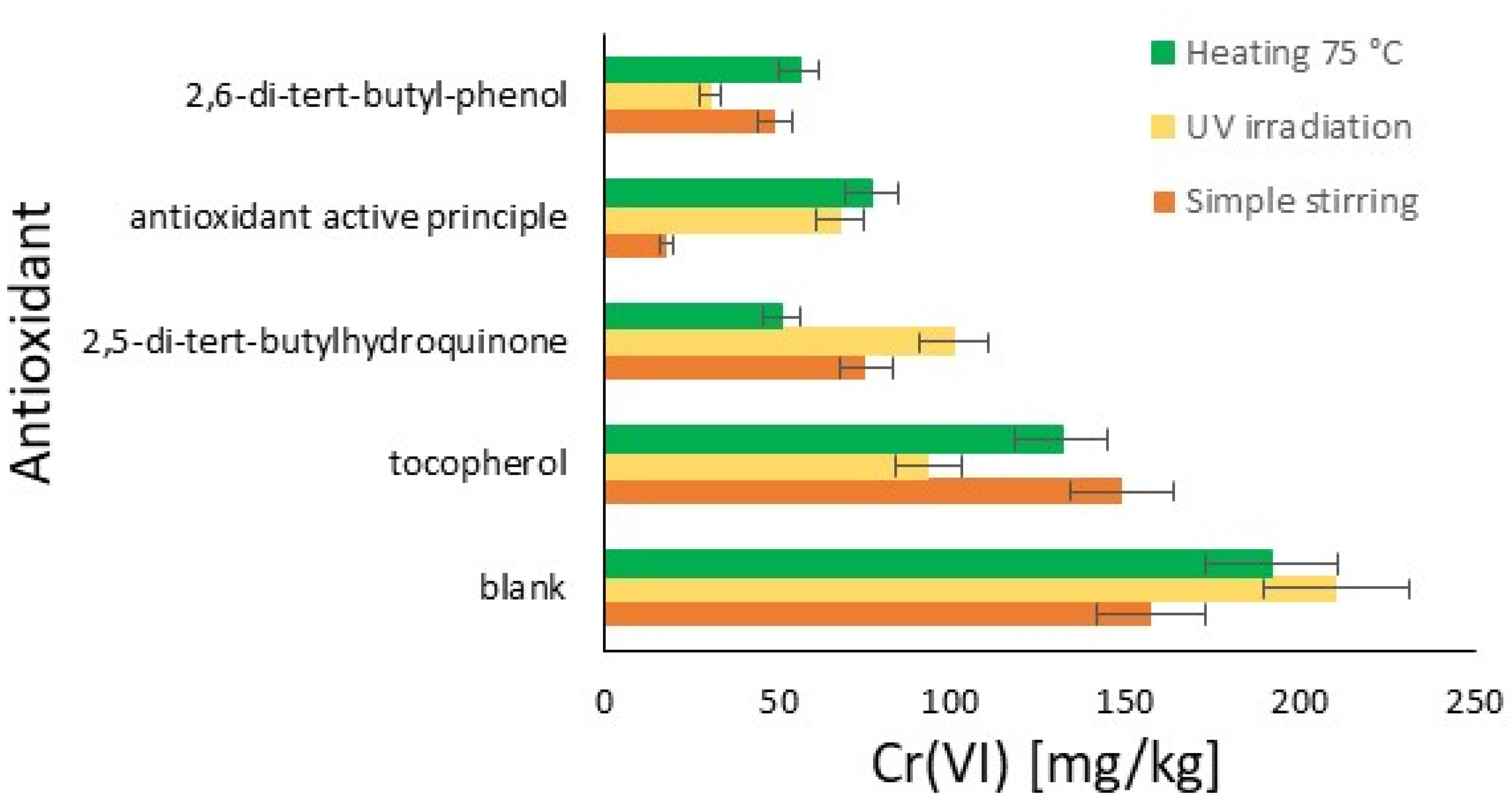
The graph in Figure 5 shows the quantity of Cr(VI) in the hide powder under various conditions, with and without the use of different antioxidants. The quantity of Cr(VI) is expressed in mg/kg on the x-axis, while the tested antioxidants are listed on the y-axis. The absence of antioxidants leads to the highest quantities of Cr(VI), confirming that exposure to UV light, agitation, and heating facilitates the formation of Cr(VI) in the absence of antioxidant protection. 2,5-di-tert-butylhydroquinone significantly reduces the formation of Cr(VI) compared to the blank, with the highest efficacy observed during heating at 75 °C. 2,6-di-tert-butylphenol proves to be the most effective antioxidant, reducing the formation of Cr(VI) to very low levels under all tested conditions, with success under UV light. The commercial antioxidant proves to be very effective during agitation but shows reduced efficacy under UV light and heating compared to 2,6-di-tert-butylphenol. Tocopherol is the least effective among the tested antioxidants, especially under conditions of agitation and heating, where it shows high quantities of Cr(VI).
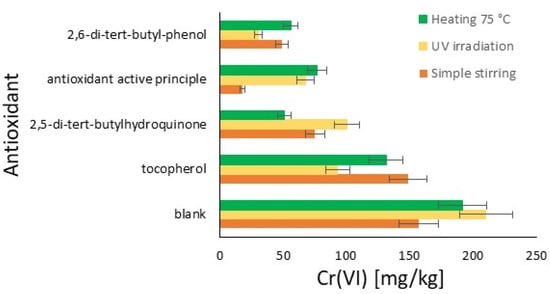
Figure 5.
Comparison of antioxidants’ effect on Cr(III) oxidation to Cr(VI) in hide powder with respect to a blank in different conditions: simple stirring (orange); UV irradiation (yellow); and heating at 75 °C (green).
The aim here is to evaluate how the characteristics change between Figure 2 and Figure 5 (with and without hide) in terms of their efficiency classification. 2,6-di-tert-butylphenol remains the substance with the most effectful characteristics, demonstrating that its structure provides important prevention for all the oxidizing phenomena [43]. Similar behavior between the experiments with and without hide powder is demonstrated also in the case of 2,5-di-tert-butylhydroquinone. The active principle of the commercial antioxidant’s behavior differs a lot from the formulated one, and demonstrates strong potential in application. The presence of additives in the formulation limits this effect, but otherwise may increase penetration into the raw skin and/or limit the interaction with other chemicals or effects on the organoleptic properties. A completely different behavior in powder hide was found in the case of tocopherol. In the case of the collagen presence, the antioxidant cannot provide the same effectiveness, representing a limit that could be due to some possibilities such as the different concentration or direct differences in the extraction. In fact, in the first case, the possibility should be due to the lower efficiency in leather at this concentration, as declared in the literature [44]. In the case of the second method, the hexavalent chromium is extracted by a solution of K2HPO4⋅3H2O and H3PO4 [45], so the high pH in this passage and the presence of a transition metal could limit the effect of tocopherol according to its hydrogen-donating ability (HAT) [46].
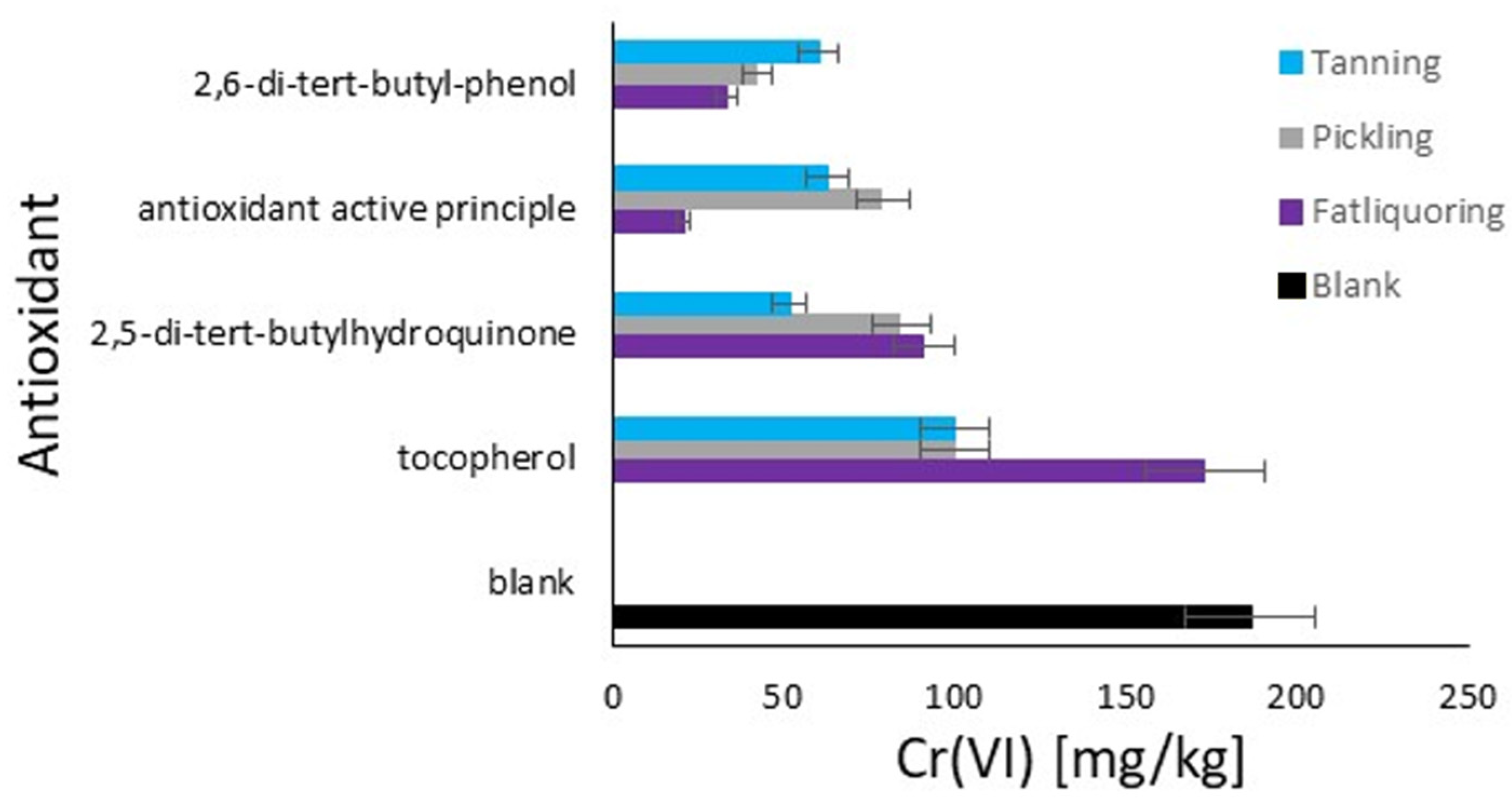
The graph in Figure 6 displays the quantity of hexavalent chromium in the hide powder based on the order of antioxidant additions. The quantities of Cr(VI) are expressed in mg/kg on the x-axis, while the tested antioxidants are listed on the y-axis. The different modes of antioxidant addition are represented by four colors, as described. In the blank, the reference value is 186 mg/kg of Cr(VI), representing the highest quantity, which resulted from the average value of the blank study of the data previously shown. This highlights, once again, the necessity of the antioxidant. 2,5-di-tert-butylhydroquinone shows the highest efficacy when added together with chromium (E), with a quantity of approximately 51 mg/kg of Cr(VI). When added before tanning (W) or together with fatliquor (Q), it is less effective. For 2,6-di-tert-butylphenol, when added together with fatliquor (Q), it is more effective, by a quantity of approximately 33 mg/kg of Cr(VI). Its efficacy decreases when added before tanning (W) and together with chromium (E). The active principle of the formulated product demonstrates a maximum efficacy when added together with fatliquor (Q), with only 21 mg/kg of Cr(VI). It is less effective when added together with chromium (E) and before tanning (W). Tocopherol appears to be the least effective among the tested antioxidants, with elevated quantities of Cr(VI) in all addition modes, especially when added together with fatliquor (Q), with approximately 173 mg/kg of Cr(VI).
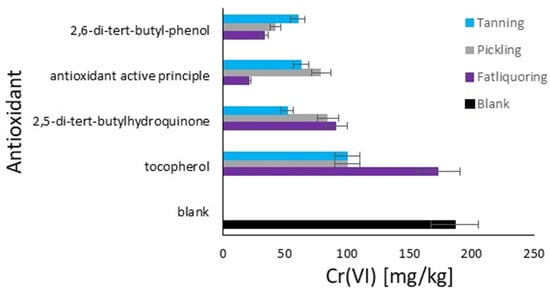
Figure 6.
Comparison of the antioxidants’ effect on Cr(III) oxidation to Cr(VI) in hide powder with different moments of addition: light blue (E)—tanning; grey (W)—pickling; and purple (Q)—fat liquoring. Black (B)—blank, no antioxidant.
The trend remains the same as in Figure 5 in terms of antioxidant activity, and an interest point arises about the addition with fatliquor for tocopherol, which demonstrates the highest problem with its use. The reason for this behavior should be related to its possible immediate deactivation by the direct mixing of sensible oxidative substances with tocopherol [47]. The rapid antioxidant reaction caused by its important affinity with fats represents a limit respective to the other agents [48], as it results in a short activity period. The study of the effects of additions during the pickling phase was decided to stress the system with an acidic pH, which improved or limited the antioxidant effects for the same reasons explained in the discussion of Figure 5, as the classifications remain the same. Finally, in the case of the addition with chromium, 2,5-di-tert-butylhydroquinone is the best for its affinity with chromium and lower affinity with the fatliquoring phase, due to its hydroxy groups that ensure a fundamental polarity respective to the other analyzed molecules [49].
The addition of antioxidants with tanning or fatliquoring agents is a practice already known and used in tanneries [50]; however, this study suggests which agents could explain the best effect. From another point of view, these results could indicate possible strategies to also obtain an additive effect, for example, by using the commercial antioxidant active principle with the fatliquoring agents, and 2,5-di-tert-butylhydroquinone with the tanning agent.
4. Conclusions
In terms of overall efficacy, 2,6-di-tert-butylphenol emerges as the most effective antioxidant in all tested conditions, showing the lowest levels of Cr(VI) in the hide powder and important safe activity in tannery wastewater. From the perspective of oxidation under specific conditions, the active principle is very effective under UV light, but less crucial during agitation and heating. The antioxidant with variable performance is 2,5-di-tert-butylhydroquinone, which shows good efficacy, particularly under heating. The least effective antioxidant is tocopherol, which, while reducing the Cr(VI) formation compared to the blank, is not as effective as the other antioxidants. The data suggest that the optimal choice of antioxidant depends on the specific conditions to which the leather material is exposed, according to a chemical configuration that improves stability. Furthermore, the best method of addition appears to be together with the chromium (E procedure). Regarding the use of antioxidants in combination with fatliquors, the least effective combination exhibits high quantities of Cr(VI), possibly due to a direct and rapid antioxidant deactivation effect. These results suggest that the optimal choice of antioxidant and the addition method can significantly reduce the formation of Cr(VI) in hide powder, thus improving the safety and quality of the final product.
Author Contributions
Conceptualization, M.M.; methodology, O.S.; software, O.S.; validation, F.R.; formal analysis, O.S. and G.L.; investigation, G.L.; resources, F.R. and M.M.; data curation, O.S. and G.L.; writing—original draft preparation, O.S. and G.L.; writing—review and editing, O.S.; visualization, F.R.; supervision, M.M.; project administration, M.M.; funding acquisition, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Lineapelle s.r.l., the PON “Ricerca e Innovazione” 2014–2020, Asse IV “Istruzione e Ricerca per il recupero” Azione IV.5 “Dottorati su tematiche green”, and MICS (Made in Italy Circolare e Sostenibile), and financed by the European Union—NextGenerationEU (National Recovery and Resilience Plan (PNRR)—mission 4 component 2, investment 1.3—D.D. 1551.11-10-2022, PE00000004) fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank Elisabetta Scaglia (Lineapelle) and Simone Gelosa (CFA lab) for their support and interest in this topic.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| TEMPO | Tetramethylpiperidinoxy free radical |
| EtOAc | Ethyl acetate |
| DPC | 1,5-Diphenylcarbazide |
| DPCA | Diphenylcarbazone |
References
- Razzaque, M.A.; Eusuf, A.; Uddin, M.; Rahman, J. Exports of Leather and Leather Goods: Performance, Prospects, and Policy Priorities. In Navigating New Waters: Unleashing Bangladesh’s Export Potential for Smooth LDC Graduation, Manchester Reasearch Explorer; Bangladesh Enterprise Institute: Dhaka, Bangladesh, 2020; pp. 229–266. [Google Scholar]
- Hailemichael, M.; Satya Raju, R. The Determinants of Growth of Leather and Leather Products Manufacturing Micro and Small Scale Enterprises. Eur. J. Bus. Manag. 2015, 7, 191–204. [Google Scholar]
- Limeneh, D.Y.; Tesfaye, T.; Ayele, M.; Husien, N.M.; Ferede, E.; Haile, A.; Mengie, W.; Abuhay, A.; Gelebo, G.G.; Gibril, M.; et al. A Comprehensive Review on Utilization of Slaughterhouse By-Product: Current Status and Prospect. Sustainability 2022, 14, 6469. [Google Scholar] [CrossRef]
- Fathima, N.N.; Baias, M.; Blumich, B.; Ramasami, T. Structure and dynamics of water in native and tanned collagen fibers: Effect of crosslinking. Int. J. Biol. Macromol. 2010, 47, 590–596. [Google Scholar] [CrossRef]
- Falcão, L.; Araújo, M. Vegetable Tannins Used in the Manufacture of Historic Leathers. Molecules 2018, 23, 1081. [Google Scholar] [CrossRef]
- Sreeram, K.J.; Ramasami, T. Sustaining tanning process through conservation, recovery and better utilization of chromium. Resour. Conserv. Recycl. 2003, 38, 185–212. [Google Scholar] [CrossRef]
- Imai, A.; Gloyna, E.F. Effects of pH and oxidation state of chromium on the behavior of chromium in the activated sludge process. Water Res. 1990, 24, 1143–1150. [Google Scholar] [CrossRef]
- Wu, L.-C.; Thomsen, M.K.; Madsen, S.R.; Schmoekel, M.; Jørgensen, M.R.V.; Cheng, M.-C.; Peng, S.-M.; Chen, Y.-S.; Overgaard, J.; Iversen, B.B. Chemical Bonding in a Linear Chromium Metal String Complex. Inorg. Chem. 2014, 53, 12489–12498. [Google Scholar] [CrossRef] [PubMed]
- Costa, M. Toxicity and Carcinogenicity of Cr(VI) in Animal Models and Humans. Crit. Rev. Toxicol. 1997, 27, 431–442. [Google Scholar] [CrossRef]
- McCarroll, N.; Keshava, N.; Chen, J.; Akerman, G.; Kligerman, A.; Rinde, E. An evaluation of the mode of action framework for mutagenic carcinogens case study II: Chromium (VI). Environ. Mol. Mutagen. 2010, 51, 89–111. [Google Scholar] [CrossRef]
- Zhao, N.; Wei, N.; Li, J.; Qiao, Z.; Cui, J.; He, F. Surface properties of chemically modified activated carbons for adsorption rate of Cr (VI). Chem. Eng. J. 2005, 115, 133–138. [Google Scholar] [CrossRef]
- De Flora, S. Threshold mechanisms and site specificity in chromium(VI) carcinogenesis. Carcinogenesis 2000, 21, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Song, Z.; Jeyakumar, P.; Shaheen, S.M.; Rinklebe, J.; Ok, Y.S.; Bolan, N.; Wang, H. A critical review on bioremediation technologies for Cr(VI)-contaminated soils and wastewater. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1027–1078. [Google Scholar] [CrossRef]
- Bartlett, R.J. Chromium cycling in soils and water: Links, gaps, and methods. Environ. Health Perspect. 1991, 92, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.B.; Johansen, J.D.; Menné, T. Chromium allergy: Significance of both Cr(III) and Cr(VI). Contact Dermat. 2003, 49, 206–212. [Google Scholar] [CrossRef]
- Newbury, E.W. Oils, fats and waxes in the leather industry. Oil Soap 1940, 17, 43–45. [Google Scholar] [CrossRef]
- Sizeland, K.H.; Wells, H.C.; Norris, G.E.; Edmonds, R.L.; Kirby, N.; Hawley, A. Collagen D-Spacing and the effect of fat liquor addition. J. Am. Leather Chem. Assoc. 2015, 110, 66–71. [Google Scholar]
- Saranya, R.; Tamil Selvi, A.; Jayapriya, J.; Aravindhan, R. Synthesis of Fat Liquor Through Fish Waste Valorization, Characterization and Applications in Tannery Industry. Waste Biomass Valorization 2020, 11, 6637–6647. [Google Scholar] [CrossRef]
- Fuck, W.F.; Gutterres, M.; Marcílio, N.R.; Bordingnon, S. The influence of Chromium supplied by tanning and wet finishing processes on the formation of cr(vi) in leather. Braz. J. Chem. Eng. 2011, 28, 221–228. [Google Scholar] [CrossRef]
- Kolomaznik, K.; Adamek, M.; Andel, I.; Uhlirova, M. Leather waste—Potential threat to human health, and a new technology of its treatment. J. Hazard. Mater. 2008, 160, 514–520. [Google Scholar] [CrossRef]
- Hedberg, Y.S. Chromium and leather: A review on the ch, emistry of relevance for allergic contact dermatitis to chromium. J. Leather Sci. Eng. 2020, 2, 20. [Google Scholar] [CrossRef]
- Salmi, O.; Molinelli, A.; Gelosa, S.; Sacchetti, A.; Rossi, F.; Masi, M. Use of Antioxidants to Reduce Chromium (VI) Formation during the Leather Tanning Process. Sustain. Chem. 2024, 5, 244–257. [Google Scholar] [CrossRef]
- Bajza, Z.; Vinkovic Vrcek, I. Fatliquoring agent and drying temperature effects on leather properties. J. Mater. Sci. 2001, 36, 5265–5270. [Google Scholar] [CrossRef]
- Hedberg, Y.S.; Lidén, C. Chromium (III) and chromium (VI) release from leather during 8 months of simulated use. Contact Dermat. 2016, 75, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.K.; De Maio, M. Assessment of risk to human health due to intake of chromium in the groundwater of the Aosta Valley region, Italy. Hum. Ecol. Risk Assess. Int. J. 2017, 23, 1153–1163. [Google Scholar] [CrossRef]
- ISO 17075-1:2017 IULTCS/IUC 18-1; Leather—Chemical Determination of Chromium (VI) Content in Leather Part 1: Colorimetric Method (n.d.). ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/67096.html (accessed on 17 November 2024).
- Biškauskaitė, R.; Valeikienė, V.; Valeika, V. Enzymes for leather processing: Effect on pickling and chroming. Materials 2021, 14, 1480. [Google Scholar] [CrossRef]
- Madhan, B.; Fathima, N.N.; Rao, J.R.; Nair, B.U. A new chromium-zinc tanning agent: A viable option for less chrome technology. J. Am. Leather Chem. Assoc. 2002, 97, 189–196. [Google Scholar]
- Yang, T.; Zeng, Y.; Sun, Q.; Lei, C.; Shi, B. Effect of pickling materials on leather quality from a hide surface charge perspective. J. Am. Leather Chem. Assoc. 2022, 117, 7. [Google Scholar] [CrossRef]
- Bregnbak, D.; Johansen, J.D.; Jellesen, M.S.; Zachariae, C.; Thyssen, J.P. Chromium (VI) release from leather and metals can be detected with a diphenylcarbazide spot test. Contact Dermat. 2015, 73, 281–288. [Google Scholar] [CrossRef]
- Dou, X.; Wang, Q.; Zhu, T.; Ding, Z.; Xie, J. Construction of effective nanosensor by combining semiconducting polymer dots with diphenylcarbazide for specific recognition of trace Cr (VI) ion in water and vitro. Nanomaterials 2022, 12, 2663. [Google Scholar] [CrossRef]
- Prokein, M.; Renner, M.; Weidner, E. Fast high-pressure tanning of animal skins by accelerated chromium sulphate complexation. Clean Technol. Environ. Policy 2020, 22, 1133–1143. [Google Scholar] [CrossRef]
- Xu, T.; Jiang, X.; Tang, Y.; Zeng, Y.; Zhang, W.; Shi, B. Oxidation of trivalent chromium induced by unsaturated oils: A pathway for hexavalent chromium formation in soil. J. Hazard. Mater. 2021, 405, 124699. [Google Scholar] [CrossRef]
- Scancar, J.; Osterman, T.; Bukovec, N.; Milacic, R. Critical Appraisal of Analytical Procedures for the Determination of CR (VI) in Dyed Leathrs by 1, 5 Diphenylcarbazide Spectrophotometry after Sample Dilutin or Color Removal. J. Am. Leather Chem. Assoc. 2007, 102, 85–92. [Google Scholar]
- Tiwari, A.K.; Orioli, S.; De Maio, M. Assessment of groundwater geochemistry and diffusion of hexavalent chromium contamination in an industrial town of Italy. J. Contam. Hydrol. 2019, 225, 103503. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.C.; Huang, Y. Relationship structure-antioxidant activity of hindered phenolic compounds. Grasas Aceites 2014, 65, e051. [Google Scholar] [CrossRef]
- Kolyada, M.N.; Osipova, V.P.; Berberova, N.T.; Shpakovsky, D.B.; Milaeva, E.R. Antioxidant activity of 2, 6-Di-tert-butylphenol derivatives in lipid peroxidation and hydrogen peroxide decomposition by human erythrocytes in vitro. Russ. J. Gen. Chem. 2018, 88, 2513–2517. [Google Scholar] [CrossRef]
- Beya, M.; Netzel, M.; Sultanbawa, Y.; Smyth, H.; Hoffman, L. Plant-Based Phenolic Molecules as Natural Preservatives in Comminuted Meats: A Review. Antioxidants 2021, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Kmiecik, D.; Fedko, M.; Siger, A.; Kulczyński, B. Degradation of tocopherol molecules and its impact on the polymerization of triacylglycerols during heat treatment of oil. Molecules 2019, 24, 4555. [Google Scholar] [CrossRef]
- Fuchs, J.; Groth, N.; Herrling, T.; Zimmer, G. Electron paramagnetic resonance studies on nitroxide radical 2, 2, 5, 5-tetramethyl-4-piperidin-1-oxyl (TEMPO) redox reactions in human skin. Free Radic. Biol. Med. 1997, 22, 967–976. [Google Scholar] [CrossRef]
- Mayerhöfer, T.G.; Mutschke, H.; Popp, J. Employing Theories Far beyond Their Limits—The Case of the (Boguer-) Beer–Lambert Law. ChemPhysChem 2016, 17, 1948–1955. [Google Scholar] [CrossRef]
- Davis, S.J.; Wise, W.R.; Recchia, S.; Spinazzè, A.; Masi, M. The Evaluation of the Detection of Cr (VI) in Leather. Analytica 2021, 3, 1–13. [Google Scholar] [CrossRef]
- Polovinkina, M.A.; Osipova, V.P.; Kolyada, M.N.; Osipova, A.D.; Berberova, N.T.; Pimenov, Y.T.; Milaeva, E.R. In silico, in vitro, in vivo Evaluation of Antioxidant Activity and Toxic Effects of Phosphorus-Containing Derivatives of 2, 6-Di-tert-Butylphenol. Environ. Res. Eng. Manag. 2019, 75, 13–23. [Google Scholar] [CrossRef]
- Jing, C.; Nan, H.; Wuyong, C.; Shiyu, S. Controlling Cr (VI) in leather: A review from passive prevention to stabilization of chromium complexes. J. Am. Leather Chem. Assoc. 2017, 112, 250–257. [Google Scholar]
- Arellano-Sánchez, M.G.; Vievard, J.; Moufarrej, L.; Devouge-Boyer, C.; Hubert-Roux, M.; Afonso, C.; Mignot, M. Separation, speciation and quantification of both chromium (VI) and chromium (III) in tanned leather samples: A comparative study and validation of analytical methods. Collagen Leather 2023, 5, 18. [Google Scholar] [CrossRef]
- Tasan, M.; Demirci, M. Total and individual tocopherol contents of sunflower oil at different steps of refining. Eur. Food Res. Technol. 2005, 220, 251–254. [Google Scholar] [CrossRef]
- Barouh, N.; Bourlieu-Lacanal, C.; Figueroa-Espinoza, M.C.; Durand, E.; Villeneuve, P. Tocopherols as antioxidants in lipid-based systems: The combination of chemical and physicochemical interactions determines their efficiency. Compr. Rev. Food Sci. Food Saf. 2022, 21, 642–688. [Google Scholar] [CrossRef] [PubMed]
- Kamal-Eldin, A.; Appelqvist, L.-Å. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.J.; Zhang, L.; Wang, X.Z. Solubility of 2,5-Di-tert-butylhydroquinone and Process Design for Its Purification Using Crystallization. J. Chem. Eng. Data 2015, 60, 1968–1974. [Google Scholar] [CrossRef]
- Çolak, S.M.; Dandar, U.; Kılıç, E. Antioxidant effect of tannic acid on formation of formaldehyde and hexavalent chromium compounds in leather. Text. Appar. 2014, 24, 105–110. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).