Valorization of Soybean Peel-Derived Humins for Carbon Dot (CD) Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Humin Production from Soybean Peels
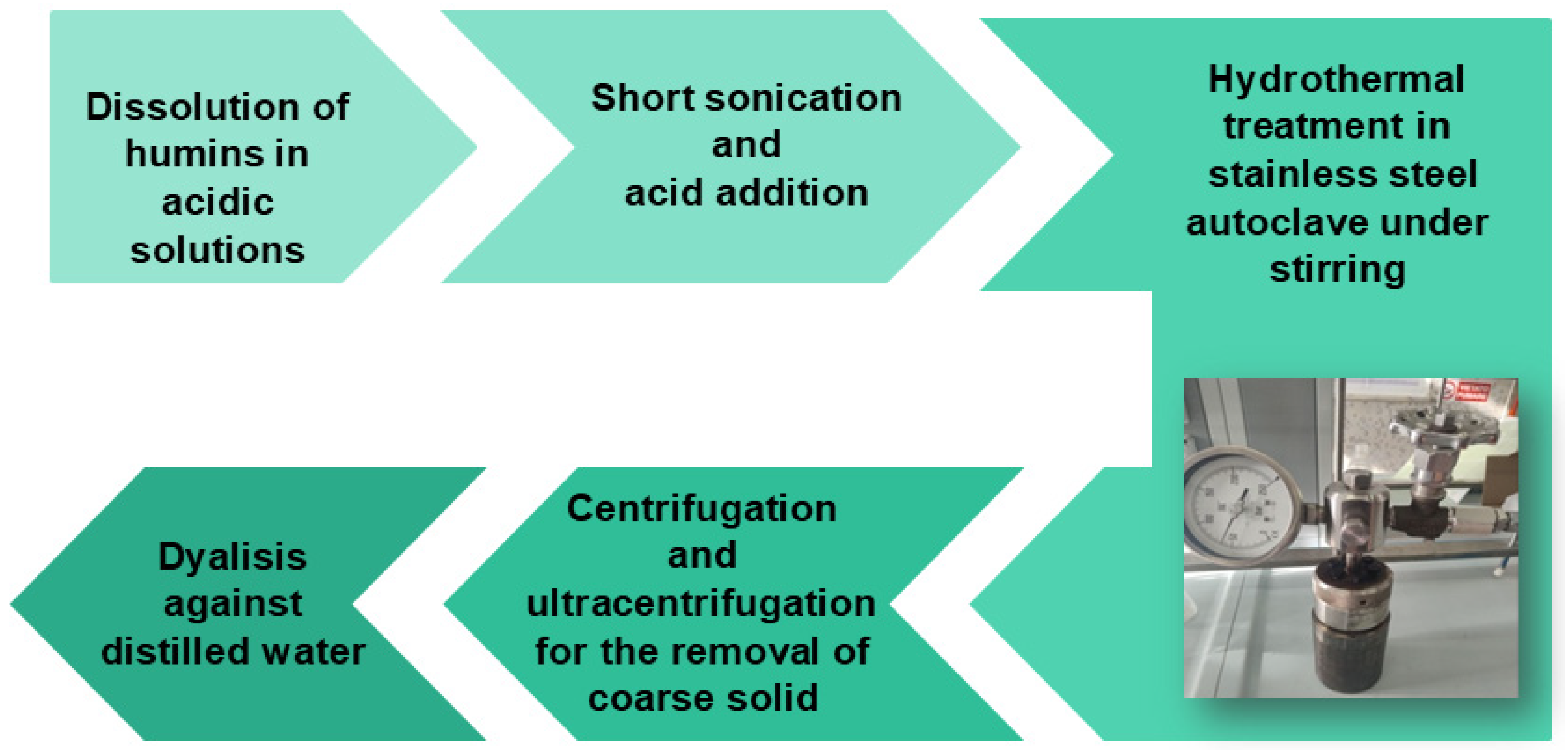
2.2.2. Synthesis of CDs by Hydrothermal Method
Hydrothermal Synthesis of CDs from Humins H1
Hydrothermal Synthesis of CDs from Humins H2
Hydrothermal Synthesis of CDs from Humins H3
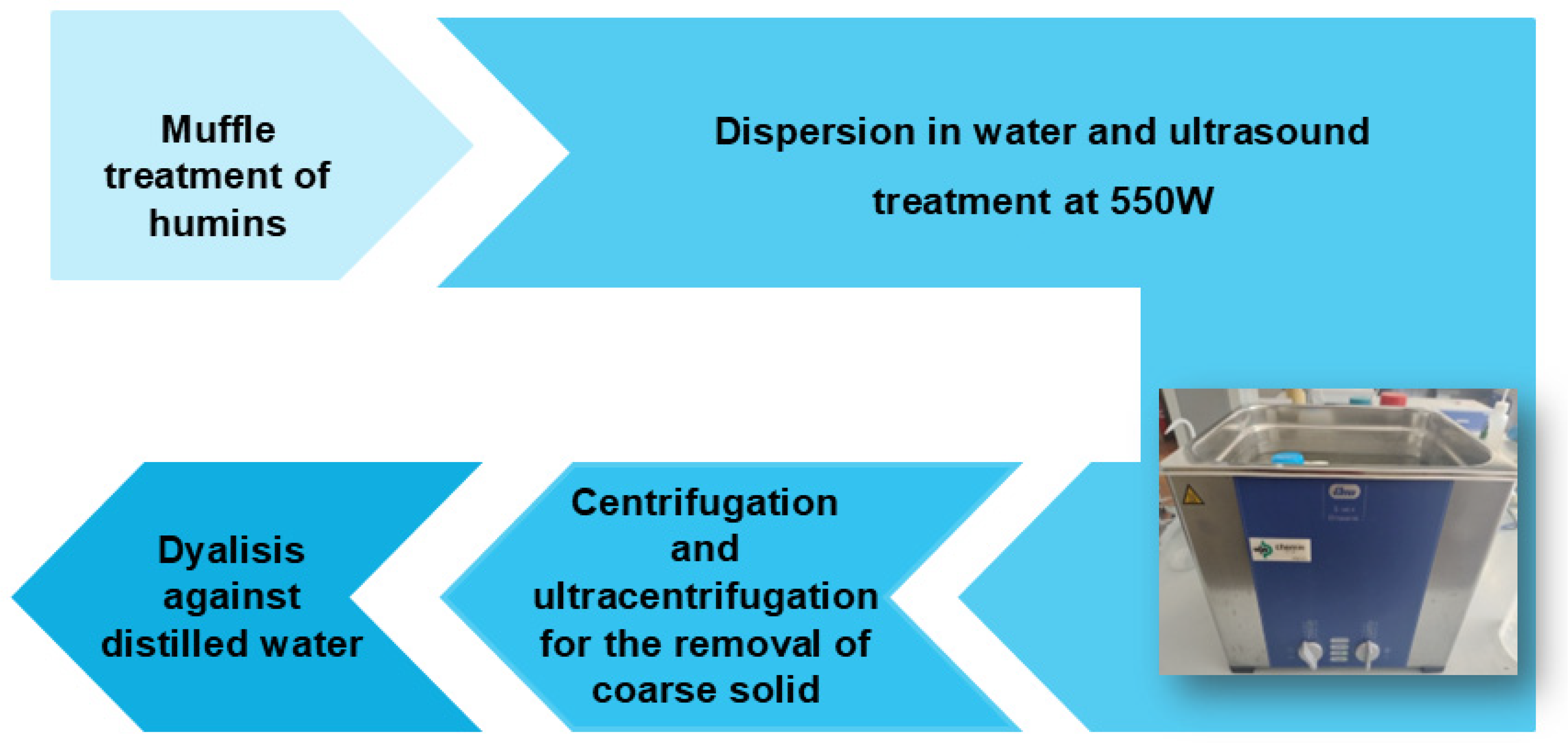
2.2.3. Synthesis of CDs by Low-Temperature Carbonization and Ultrasonication
Sonochemical Synthesis of CDs from Humins H1
Sonochemical Synthesis of CDs from Humins H2
Sonochemical Synthesis of CDs from Humins H3
2.3. Characterization of Humins and CDs
3. Results and Discussion
3.1. Humin Characterization
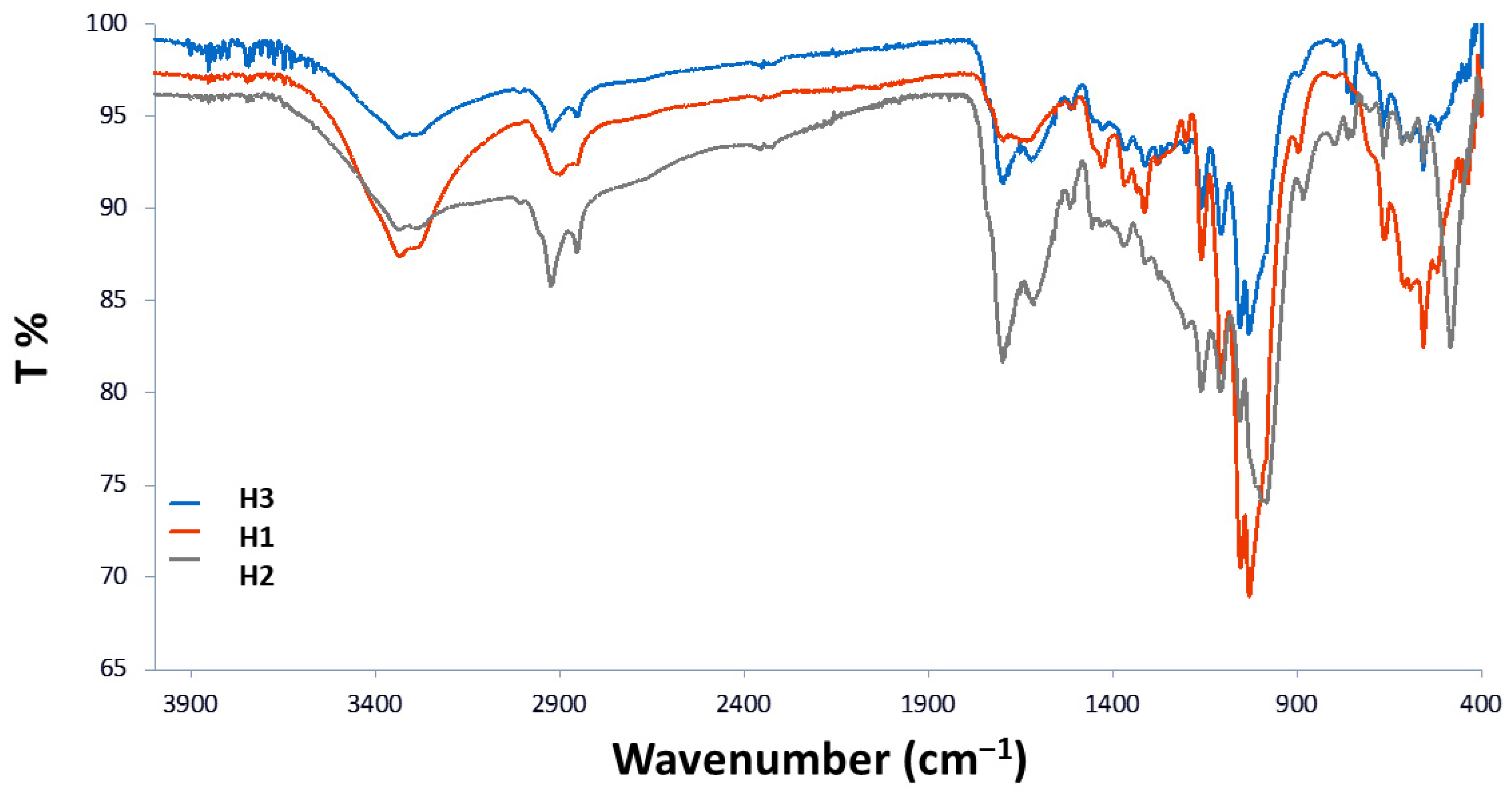
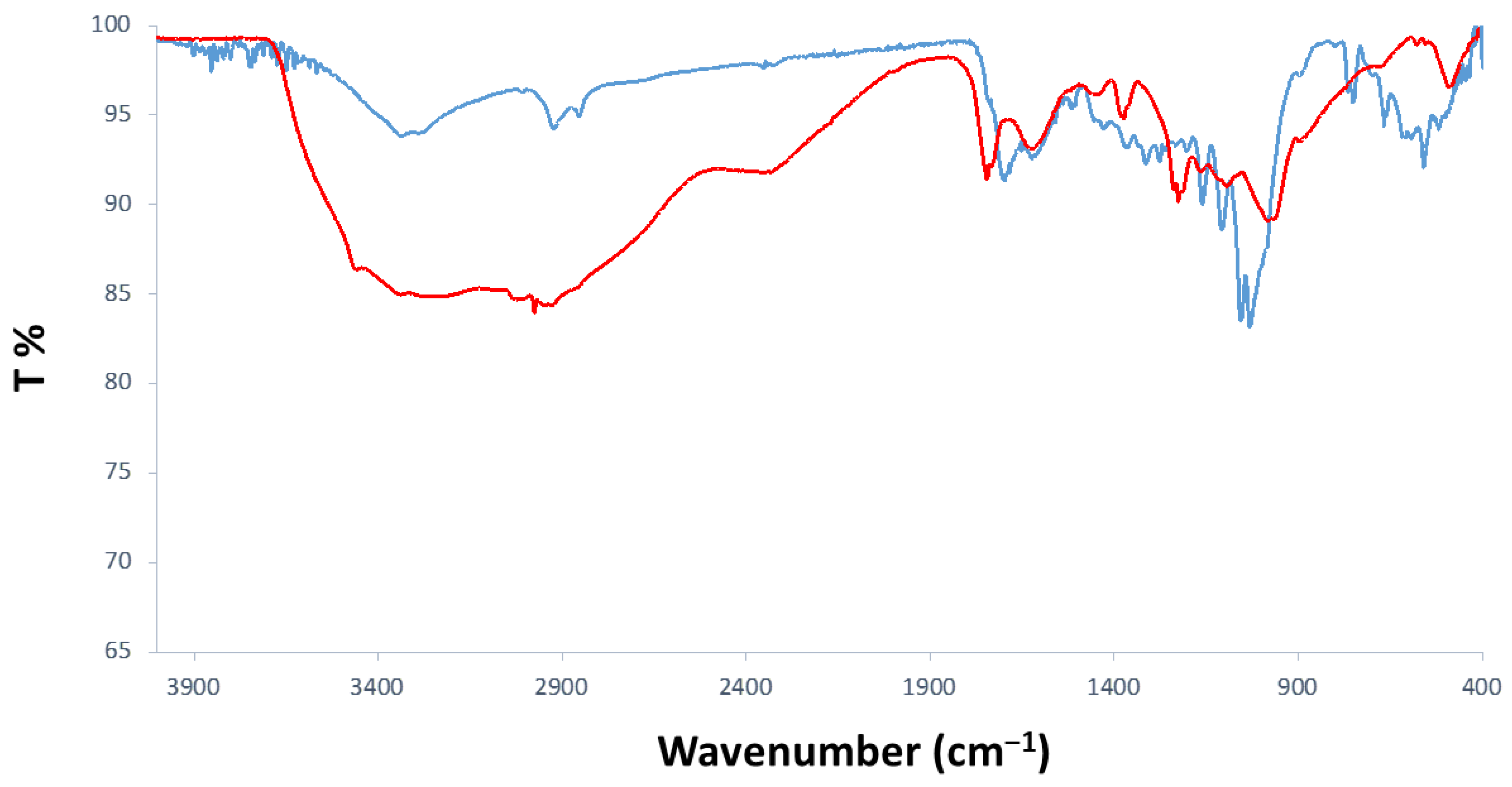
3.1.1. FTIR-ATR Analyses
3.1.2. TGA Analyses
3.2. Synthesis of CDs by Hydrothermal Method
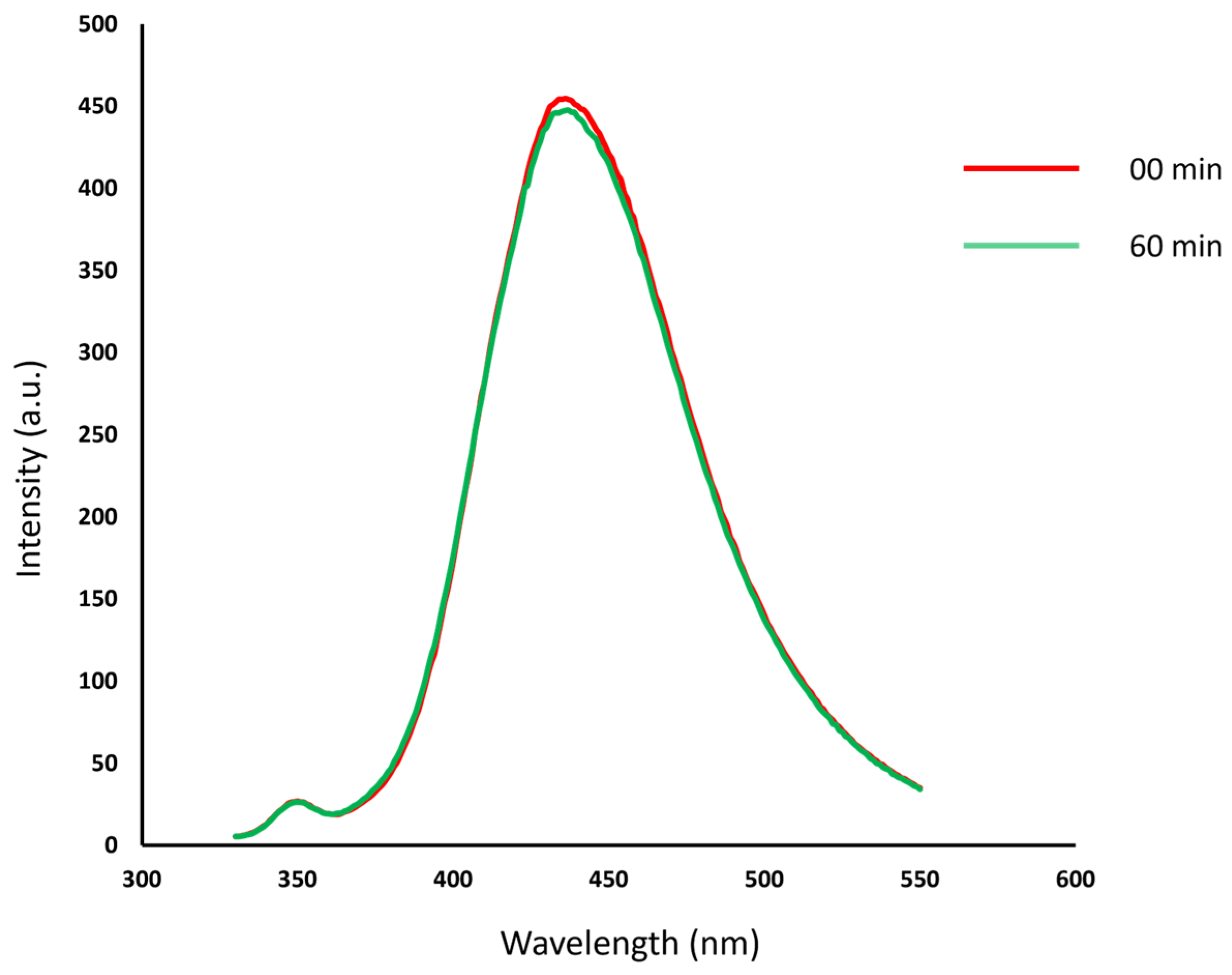
3.3. Synthesis of CDs by Ultrasonic Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keijer, T.; Bakker, V.; Slootweg, J.C. Circular Chemistry to Enable a Circular Economy. Nat. Chem. 2019, 11, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, R.; Markandan, K.; Khoo, K.S.; Cheng, C.K.; Ashokkumar, V.; Deepanraj, B.; Show, P.L. The Expansion of Lignocellulose Biomass Conversion into Bioenergy via Nanobiotechnology. Front. Nanotechnol. 2021, 3, 793528. [Google Scholar] [CrossRef]
- Laurenza, A.G.; Losito, O.; Casiello, M.; Fusco, C.; Nacci, A.; Pantone, V.; D’Accolti, L. Valorization of Cigarette Butts for Synthesis of Levulinic Acid as Top Value-Added Chemicals. Sci. Rep. 2021, 11, 15775. [Google Scholar] [CrossRef]
- Losito, O.; Casiello, M.; Fusco, C.; Mateos Cuadrado, H.; Monopoli, A.; Nacci, A.; D’Accolti, L. Eco-Friendly Catalytic Synthesis of Top Value Chemicals from Valorization of Cellulose Waste. Polymers 2023, 15, 1501. [Google Scholar] [CrossRef]
- De Bhowmick, G.; Sarmah, A.K.; Sen, R. Lignocellulosic Biorefinery as a Model for Sustainable Development of Biofuels and Value Added Products. Bioresour. Technol. 2018, 247, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Mika, L.T.; Cséfalvay, E.; Németh, Á. Catalytic Conversion of Carbohydrates to Initial Platform Chemicals: Chemistry and Sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Duarte, L.C.; Gírio, F.M. Hemicellulose Biorefineries: A Review on Biomass Pretreatments. J. Sci. Ind. Res. 2008, 67, 849–864. [Google Scholar]
- Liu, S.; Zhu, Y.; Liao, Y.; Wang, H.; Liu, Q.; Ma, L.; Wang, C. Advances in Understanding the Humins: Formation, Prevention and Application. Appl. Energy Combust. Sci. 2022, 10, 100062. [Google Scholar] [CrossRef]
- Sumerskii, I.V.; Krutov, S.M.; Zarubin, M.Y. Humin-like Substances Formed under the Conditions of Industrial Hydrolysis of Wood. Russ. J. Appl. Chem. 2010, 83, 320–327. [Google Scholar] [CrossRef]
- Patil, S.K.R.; Heltzel, J.; Lund, C.R.F. Comparison of Structural Features of Humins Formed Catalytically from Glucose, Fructose, and 5-Hydroxymethylfurfuraldehyde. Energy Fuels 2012, 26, 5281–5293. [Google Scholar] [CrossRef]
- Van Zandvoort, I.; Koers, E.J.; Weingarth, M.; Bruijnincx, P.C.A.; Baldus, M.; Weckhuysen, B.M. Structural Characterization of 13C-Enriched Humins and Alkali-Treated 13C Humins by 2D Solid-State NMR. Green Chem. 2015, 17, 4383–4392. [Google Scholar] [CrossRef]
- Liu, L. Biomass-Derived Humins: Formation, Chemistry and Structure, 1st ed.; Springer: Singapore, 2023; ISBN 978-981-99-1990-1. [Google Scholar]
- Kang, S.; Zhang, G.; Yang, Q.; Tu, J.; Guo, X.; Qin, F.G.F.; Xu, Y. A New Technology for Utilization of Biomass Hydrolysis Residual Humins for Acetic Acid Production. BioResources 2016, 11, 9496–9505. [Google Scholar] [CrossRef]
- Hoang, T.M.C.; Lefferts, L.; Seshan, K. Valorization of Humin-Based Byproducts from Biomass Processing—A Route to Sustainable Hydrogen. ChemSusChem 2013, 6, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Fu, J.; Zhang, G. From Lignocellulosic Biomass to Levulinic Acid: A Review on Acid-Catalyzed Hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Tosi, P.; van Klink, G.P.M.; Celzard, A.; Fierro, V.; Vincent, L.; de Jong, E.; Mija, A. Auto-Crosslinked Rigid Foams Derived from Biorefinery Byproducts. ChemSusChem 2018, 11, 2797–2809. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, N.; Wang, B.; Peng, F.; Fan, Y. Synthesis of Humin-Based Carbon Quantum Dots and Luminescent Properties. Pap. Biomater. 2018, 3, 8–15. [Google Scholar] [CrossRef]
- Dong, Y.; Wan, L.; Cai, J.; Fang, Q.; Chi, Y.; Chen, G. Natural Carbon-Based Dots from Humic Substances. Sci. Rep. 2015, 5, 10037. [Google Scholar] [CrossRef]
- Kang, C.; Huang, Y.; Yang, H.; Yan, X.F.; Chen, Z.P. A Review of Carbon Dots Produced from Biomass Wastes. Nanomaterials 2020, 10, 2316. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The Photoluminescence Mechanism in Carbon Dots (Graphene Quantum Dots, Carbon Nanodots, and Polymer Dots): Current State and Future Perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, V.B.; Gedanken, A. Sonochemical Synthesis of Carbon Dots, Mechanism, Effect of Parameters, and Catalytic, Energy, Biomedical and Tissue Engineering Applications. Ultrason. Sonochem. 2020, 64, 105009. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Bhunia, S.K.; Saha, A.; Maity, A.R.; Ray, S.C.; Jana, N.R. Carbon Nanoparticle-Based Fluorescent Bioimaging Probes. Sci. Rep. 2013, 3, 1473. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.amis-outlook.org/statistics/crops (accessed on 27 March 2025).
- Cerdan, K.; Brancart, J.; Roels, E.; Vanderborght, B.; Van Puyvelde, P. Humins Blending in Thermoreversible Diels–Alder Networks for Stiffness Tuning and Enhanced Healing Performance for Soft Robotics. Polymers 2022, 14, 1657. [Google Scholar] [CrossRef]
- Sokołowska, M.; Nowak-Grzebyta, J.; Stachowska, E.; Miądlicki, P.; Zdanowicz, M.; Michalkiewicz, B.; El Fray, M. Enzymatically Catalyzed Furan-Based Copolyesters Containing Dilinoleic Diol as a Building Block. RSC Adv. 2023, 13, 22234–22249. [Google Scholar] [CrossRef]
- Soni, H.; Pamidimukkala, P.S. Green Synthesis of N, S Co-Doped Carbon Quantum Dots from Triflic Acid Treated Palm Shell Waste and Their Application in Nitrophenol Sensing. Mater. Res. Bull. 2018, 108, 250–254. [Google Scholar] [CrossRef]
- Lu, W.; Qin, X.; Liu, S.; Chang, G.; Zhang, Y.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Economical, Green Synthesis of Fluorescent Carbon Nanoparticles and Their Use as Probes for Sensitive and Selective Detection of Mercury(II) Ions. Anal. Chem. 2012, 84, 5351–5357. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Zhang, Y. One-Step Green Synthesized Fluorescent Carbon Nanodots from Bamboo Leaves for Copper(II) Ion Detection. Sens. Actuators B Chem. 2014, 196, 647–652. [Google Scholar] [CrossRef]
- Dong, Z.; Qi, J.; Yue, L.; Zhou, H.; Chen, L.; Gu, J.; He, Y.; Wu, H. Biomass-Based Carbon Quantum Dots and Their Agricultural Applications. Plant Stress 2024, 11, 100411. [Google Scholar] [CrossRef]
- Li, J.; Xu, O.; Zhu, X. A Facile Green and One-Pot Synthesis of Grape Seed-Derived Carbon Quantum Dots as a Fluorescence Probe for Cu(ii) and Ascorbic Acid. RSC Adv. 2021, 11, 34107–34116. [Google Scholar] [CrossRef]
- Yu, C.; Qin, D.; Jiang, X.; Zheng, X.; Deng, B. Facile Synthesis of Bright Yellow Fluorescent Nitrogen-Doped Carbon Quantum Dots and Their Applications to an off–on Probe for Highly Sensitive Detection of Methimazole. Microchem. J. 2021, 168, 106480. [Google Scholar] [CrossRef]
- Kasinathan, K.; Samayanan, S.; Marimuthu, K.; Yim, J.-H. Green Synthesis of Multicolour Fluorescence Carbon Quantum Dots from Sugarcane Waste: Investigation of Mercury (II) Ion Sensing, and Bio-Imaging Applications. Appl. Surf. Sci. 2022, 601, 154266. [Google Scholar] [CrossRef]
- Zhao, W.-B.; Liu, K.-K.; Song, S.-Y.; Zhou, R.; Shan, C.-X. Fluorescent Nano-Biomass Dots: Ultrasonic-Assisted Extraction and Their Application as Nanoprobe for Fe3+ Detection. Nanoscale Res. Lett. 2019, 14, 130. [Google Scholar] [CrossRef]
- Dwandaru, W.S.B.; Hidayatullah, A.R.; Listari, E.S.A.; Sl, D.G. Utilization of Luminescent Carbon Nanodots from Soybean Husk Wastes for Fingerprint Identification Using Tracker Software Spectrum Analysis. Positron 2023, 13, 41. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Q.; Long, Y.; Zhang, H.; Huang, X.; Zhu, R. Enhancing the Luminescence of Carbon Dots with a Reduction Pathway. Chem. Commun. 2011, 47, 10650. [Google Scholar] [CrossRef] [PubMed]
- Atchudan, R.; Jebakumar Immanuel Edison, T.N.; Shanmugam, M.; Perumal, S.; Somanathan, T.; Lee, Y.R. Sustainable Synthesis of Carbon Quantum Dots from Banana Peel Waste Using Hydrothermal Process for in Vivo Bioimaging. Phys. E Low-Dimens. Syst. Nanostruct. 2021, 126, 114417. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Wang, J.; Wan, H.; Zhang, Y.; Ning, Y.; Yang, B. Photoluminescence Mechanism in Graphene Quantum Dots: Quantum Confinement Effect and Surface/Edge State. Nano Today 2017, 13, 10–14. [Google Scholar] [CrossRef]
- Manioudakis, J.; Victoria, F.; Thompson, C.A.; Brown, L.; Movsum, M.; Lucifero, R.; Naccache, R. Effects of Nitrogen-Doping on the Photophysical Properties of Carbon Dots. J. Mater. Chem. C 2019, 7, 853–862. [Google Scholar] [CrossRef]
- De Leo, V.; Catucci, L.; Falqui, A.; Marotta, R.; Striccoli, M.; Agostiano, A.; Comparelli, R.; Milano, F. Hybrid Assemblies of Fluorescent Nanocrystals and Membrane Proteins in Liposomes. Langmuir 2014, 30, 1599–1608. [Google Scholar] [CrossRef]
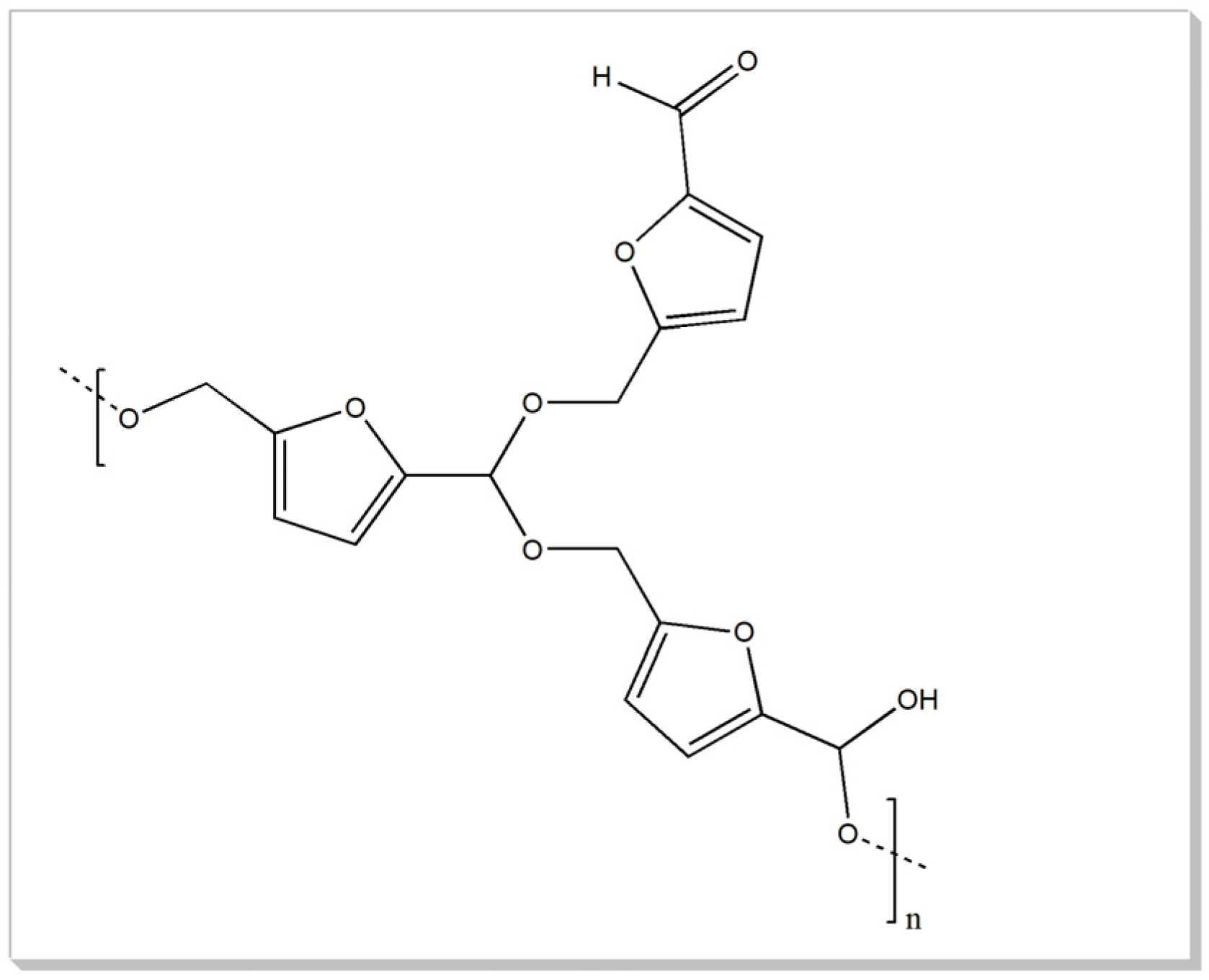
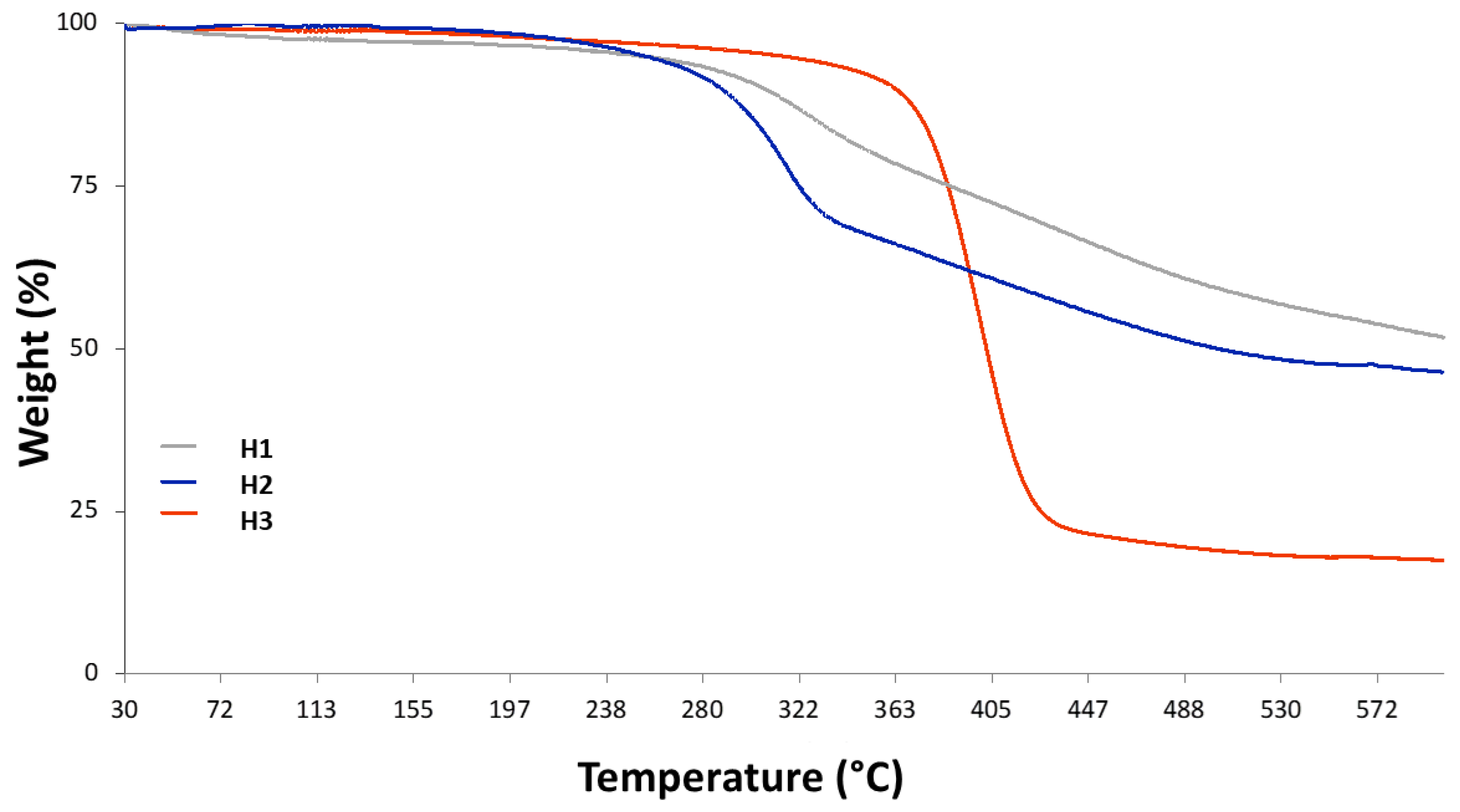


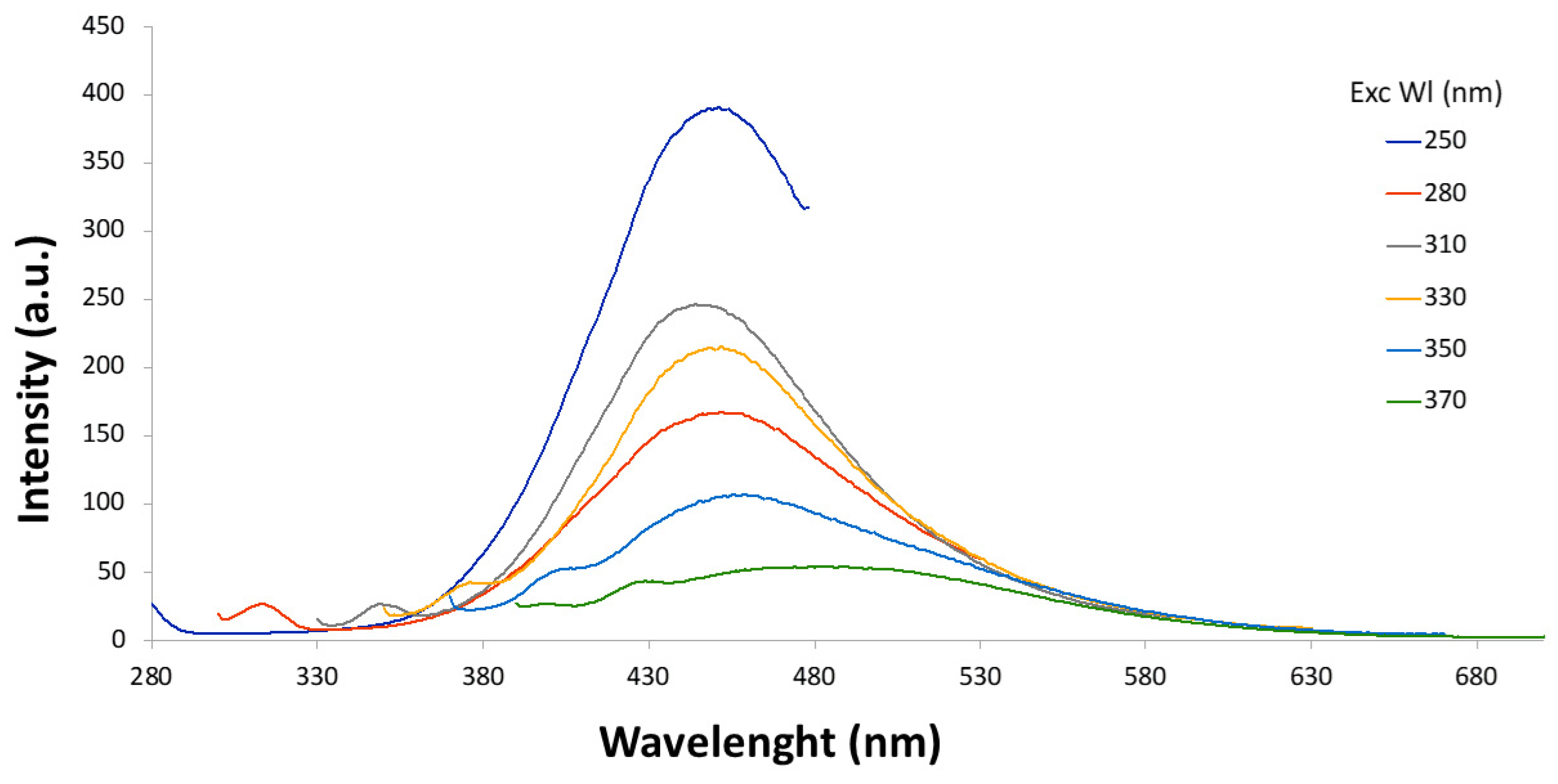

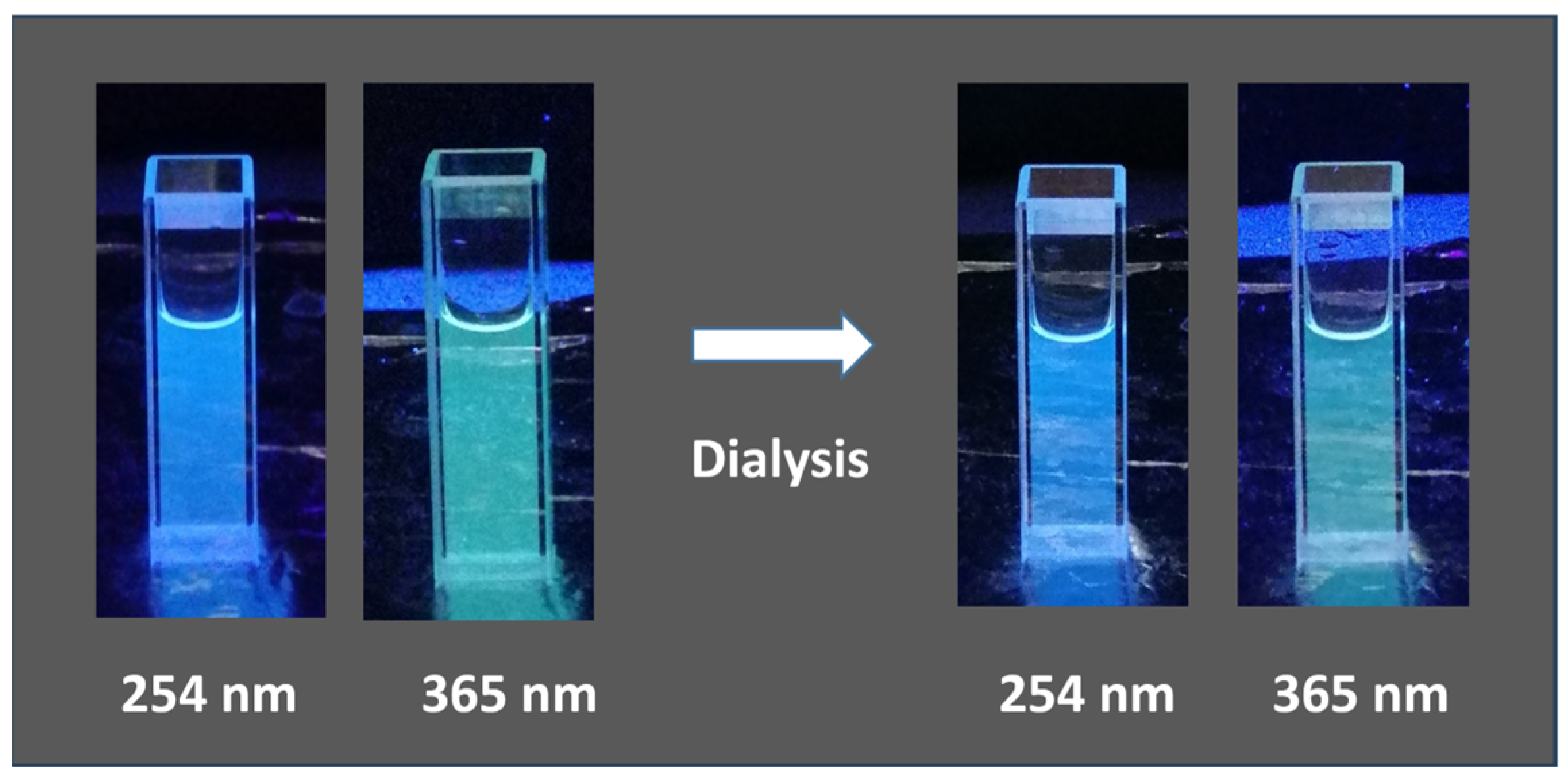
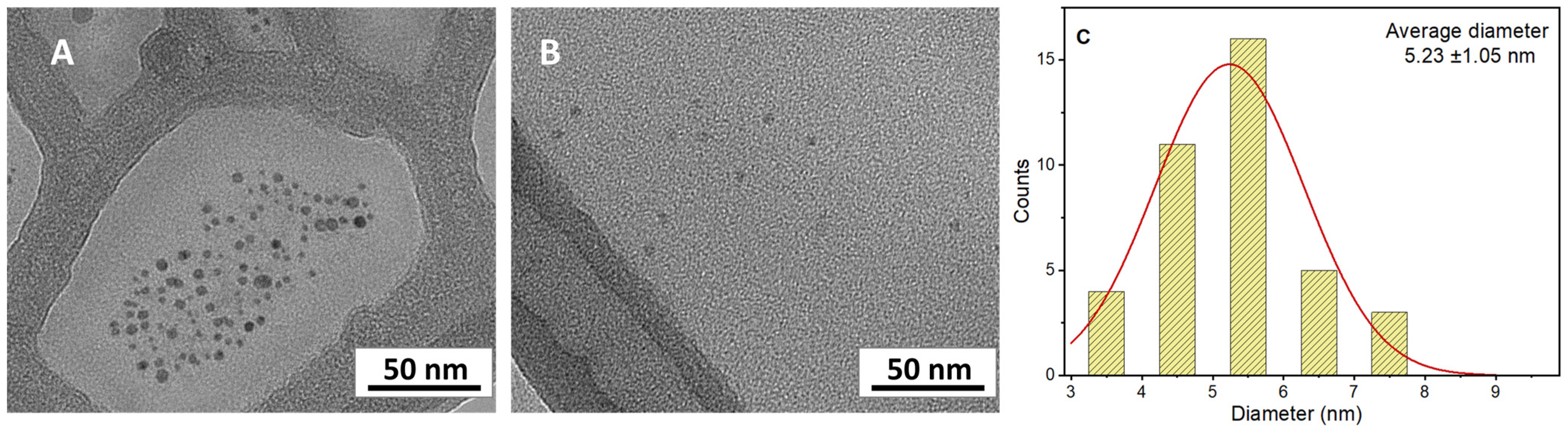
| Humin Sample | Starting Material | Methodology | Pretreatment | Condition | Temp. and Time |
| H1 | Soybean peels | none | CH3COOH | 200 °C 2 h | |
| H2 | Soybean peels | none | H3PO4 | 200 °C 2 h | |
| H3 | Soybean peels | H3PO4 (80 °C 2 h) | H3PO4 | 200 °C 2 h | |
| CD Sample | Starting Material | Methodology | Pretreatment | Condition | Temp. and Time |
| T1A | H1 | Hydrothermal | H3PO4 | 200 °C 3 h | |
| T1B | H1 | Hydrothermal | H3PO4 | 200 °C 6 h | |
| T2A | H2 | Hydrothermal | H3PO4 | 200 °C 3 h | |
| T2B | H2 | Hydrothermal | H3PO4 | 200 °C 6 h | |
| T3A | H3 | Hydrothermal | H3PO4 | 200 °C 3 h | |
| T3B | H3 | Hydrothermal | H3PO4 | 200 °C 6 h | |
| T4 | H1 | Sonochemical | air 250 °C 2 h | 550 W | RT 30′ min |
| T5 | H2 | Sonochemical | air 250 °C 2 h | 550 W | RT 30′ min |
| T6 | H3 | Sonochemical | air 250 °C 2 h | 550 W | RT 30′ min |
| Signal (cm−1) | Description |
|---|---|
| 3275 | Stretching O-H |
| 2854/2924 | Symm. and asymm. CH2 stretching |
| 1699 | C=O stretching of conjugated aldehyde |
| 750–800 | C-H (furanic ring) out-of-plane bending |
| Substrate | Catalyst | Sample Name | UV | Fluorescence | QY (%) |
|---|---|---|---|---|---|
| Soybean peel | Acetic acid | T1 | Yes | Very low | // |
| Soybean peel | Phosphoric acid | T2 | Yes | Very low | // |
| Soybean peel | Phosphoric acid and pretreatment | T3 | Yes | Very low | // |
| Substrate | Catalyst | Sample Name | UV | Fluorescence | QY (%) |
|---|---|---|---|---|---|
| Soybean peel | Acetic acid | T4 | Yes | Very low | // |
| Soybean peel | Phosphoric acid | T5 | Yes | Very low | // |
| Soybean peel | Phosphoric acid and pretreatment | T6 | Yes | Yes |
|
| Entry | Precursors | Method | Size (nm) | Quantum Yield (%) | Reference |
|---|---|---|---|---|---|
| 1 | Lettuce leaves | 180 °C 6 h (hydrothermal) | 2.8 | 0.38 | [33] |
| 2 | Grape seeds | 200 °C 8 h (hydrothermal) | 8.9 | 0.275 | [32] |
| 3 | Sugarcane bagasse | 200 °C 12 h (hydrothermal) | From 2 to 8 | 0.091 | [34] |
| 4 | Soybeans | Sonochemical 2 h | 2.4 | 16.7 | [35] |
| 5 | Soybean husks | Pretreatment (250 °C for 2 h) Microwave (450 W for 50 min) | Not reported a | Not reported a | [36] |
| 6 | Humins | (T1)200 °C 3 h (hydrothermal) | // | // | This work |
| 7 | Humins | Pretreatment (air 250 °C 2 h) Sonochemical method (550 W) | 7.3 ± 1.2 | 2.6 ± 0.1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Losito, O.; Netti, T.; Kost, V.; Annese, C.; Catucci, L.; Da Ros, T.; De Leo, V.; D’Accolti, L. Valorization of Soybean Peel-Derived Humins for Carbon Dot (CD) Production. Materials 2025, 18, 1865. https://doi.org/10.3390/ma18081865
Losito O, Netti T, Kost V, Annese C, Catucci L, Da Ros T, De Leo V, D’Accolti L. Valorization of Soybean Peel-Derived Humins for Carbon Dot (CD) Production. Materials. 2025; 18(8):1865. https://doi.org/10.3390/ma18081865
Chicago/Turabian StyleLosito, Onofrio, Thomas Netti, Veronika Kost, Cosimo Annese, Lucia Catucci, Tatiana Da Ros, Vincenzo De Leo, and Lucia D’Accolti. 2025. "Valorization of Soybean Peel-Derived Humins for Carbon Dot (CD) Production" Materials 18, no. 8: 1865. https://doi.org/10.3390/ma18081865
APA StyleLosito, O., Netti, T., Kost, V., Annese, C., Catucci, L., Da Ros, T., De Leo, V., & D’Accolti, L. (2025). Valorization of Soybean Peel-Derived Humins for Carbon Dot (CD) Production. Materials, 18(8), 1865. https://doi.org/10.3390/ma18081865










