Evolution of the Phase Composition in a Nickel-Predominant NiTi Shape Memory Alloy During High-Energy Ball Milling
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
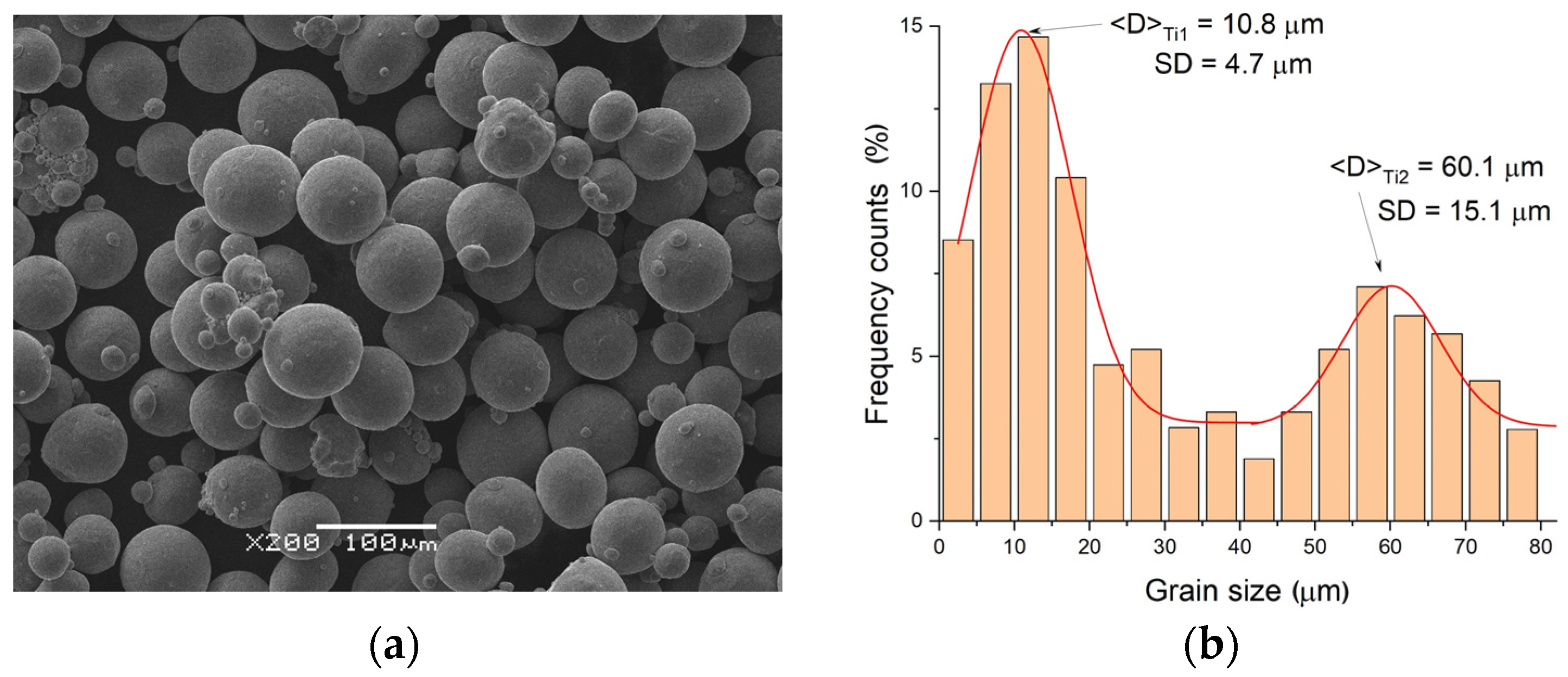
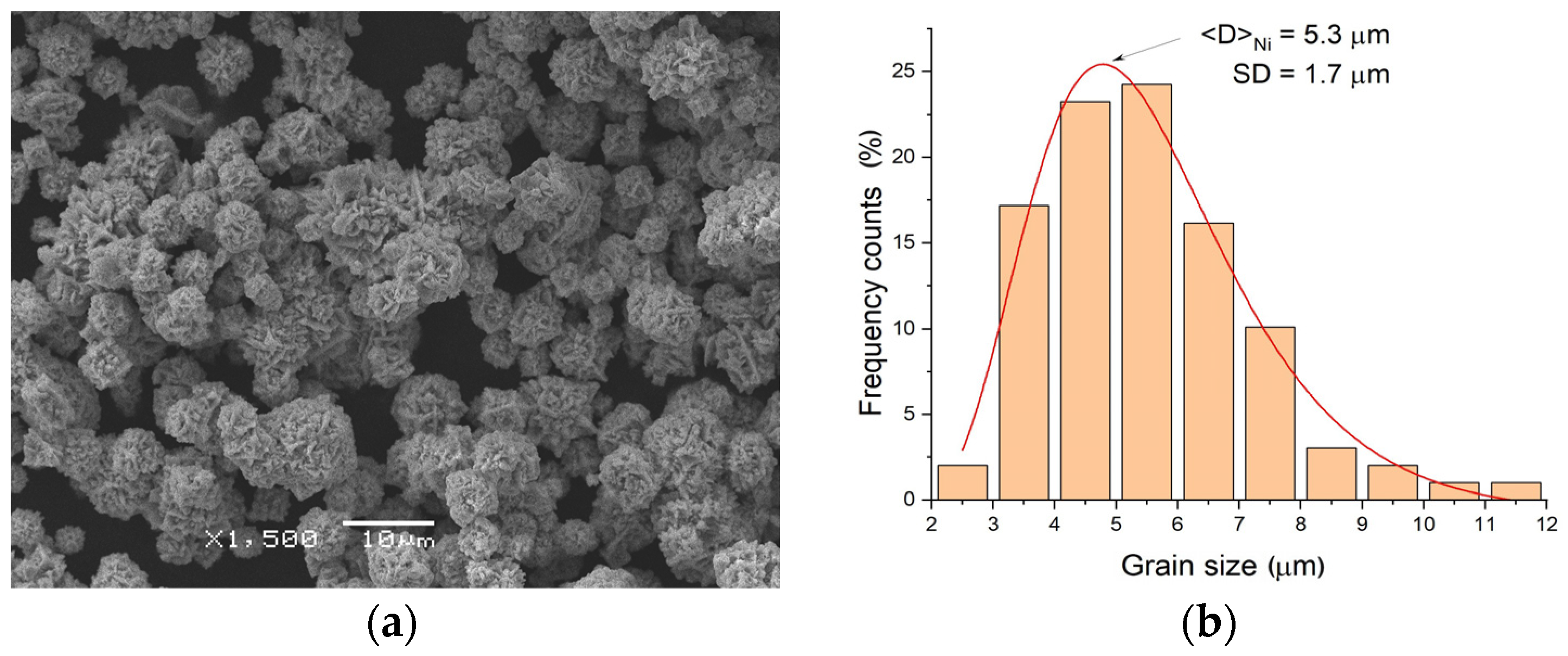
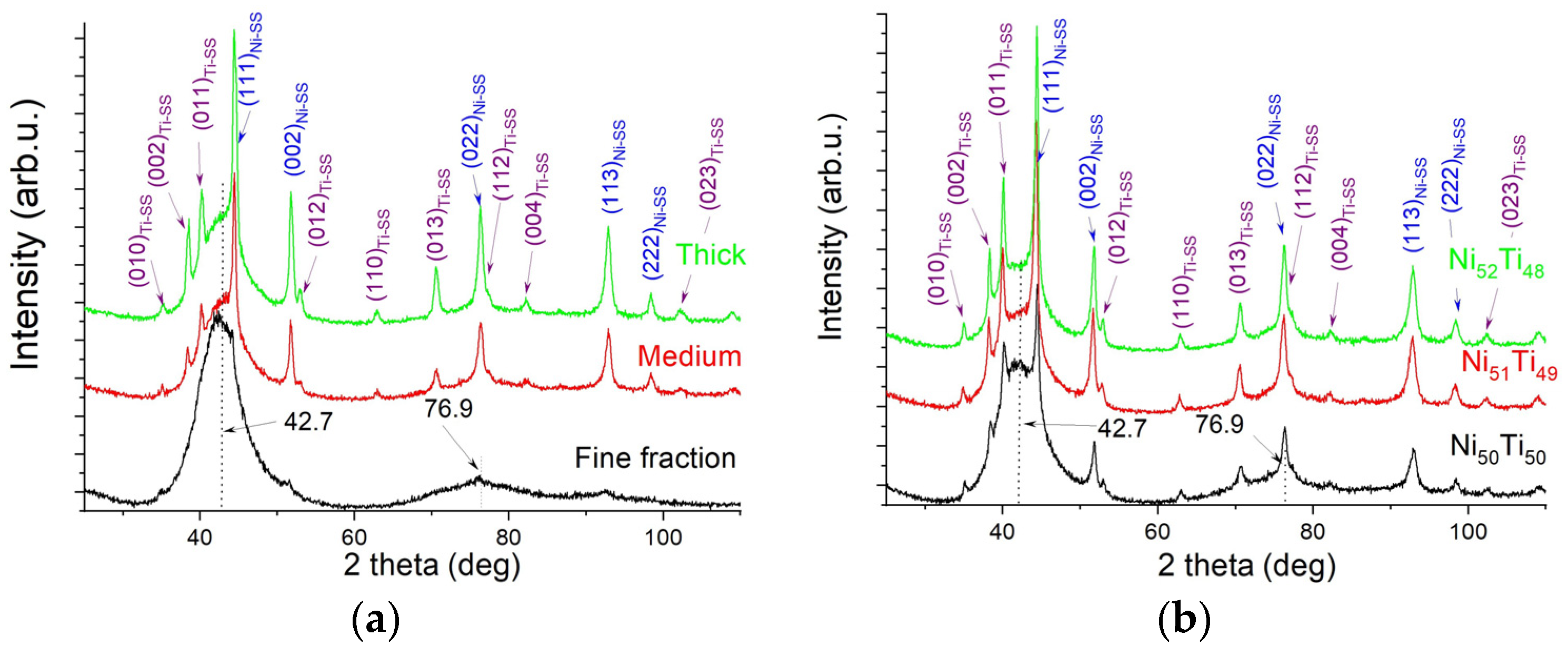
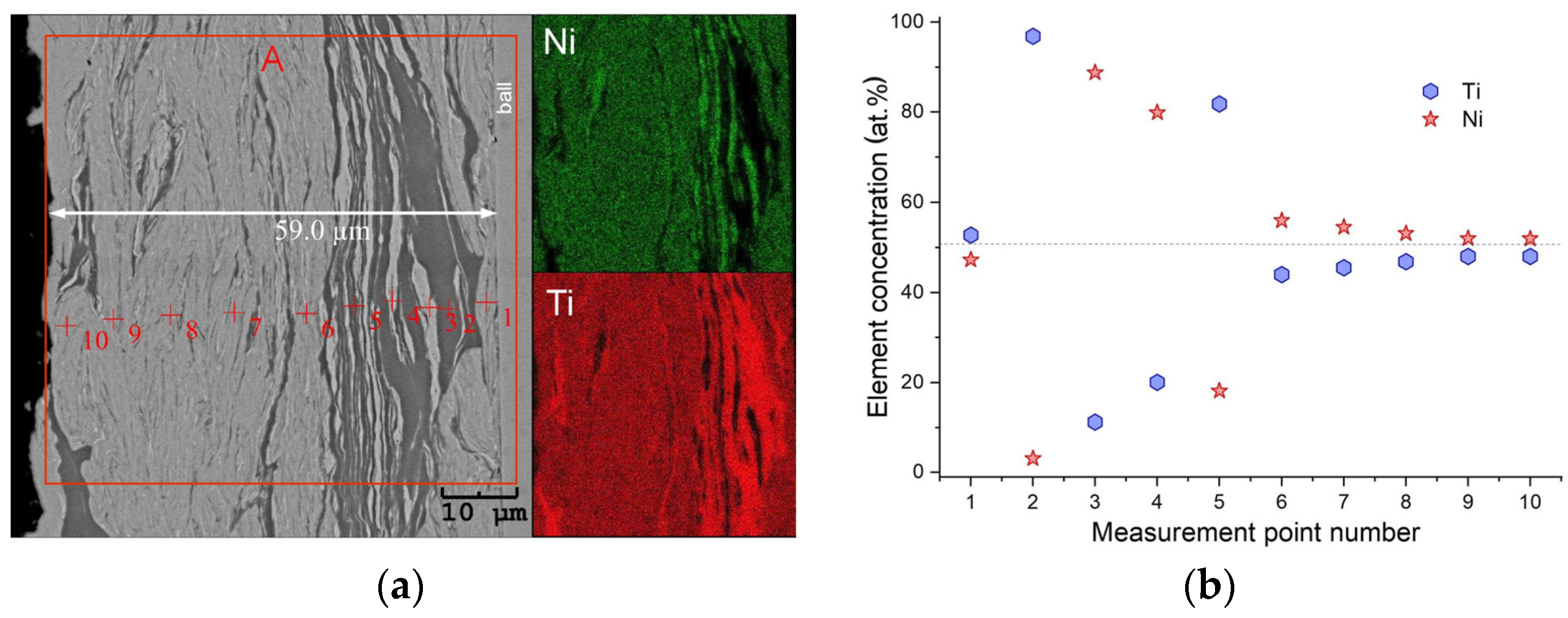
3.1. Characterization of Elemental Powders
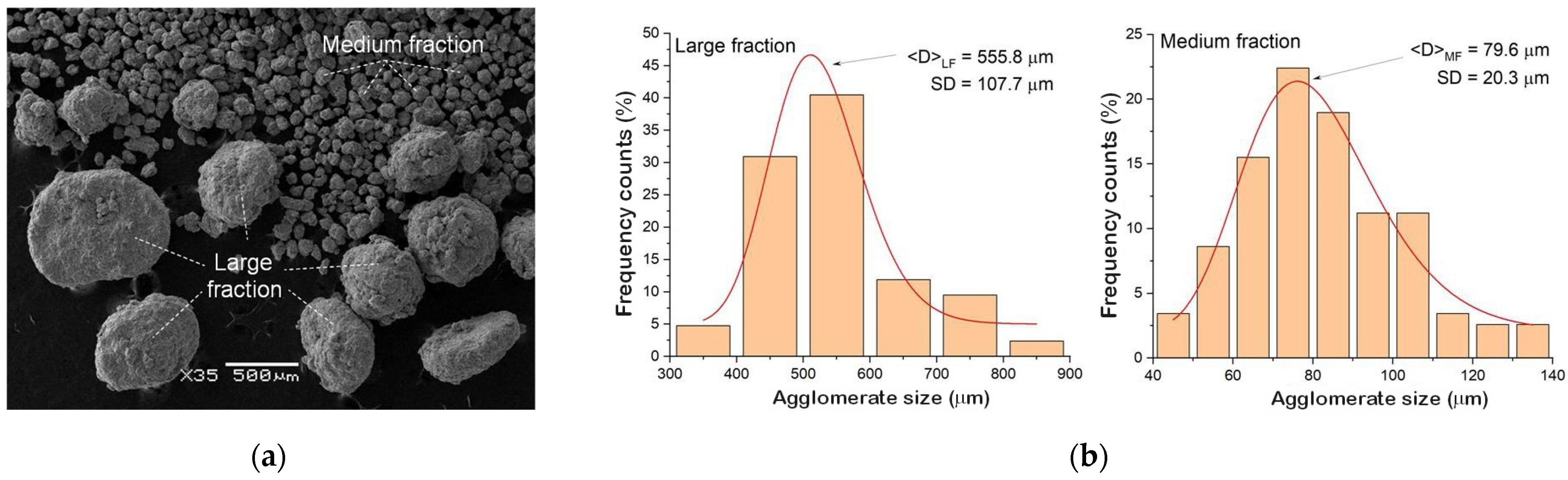
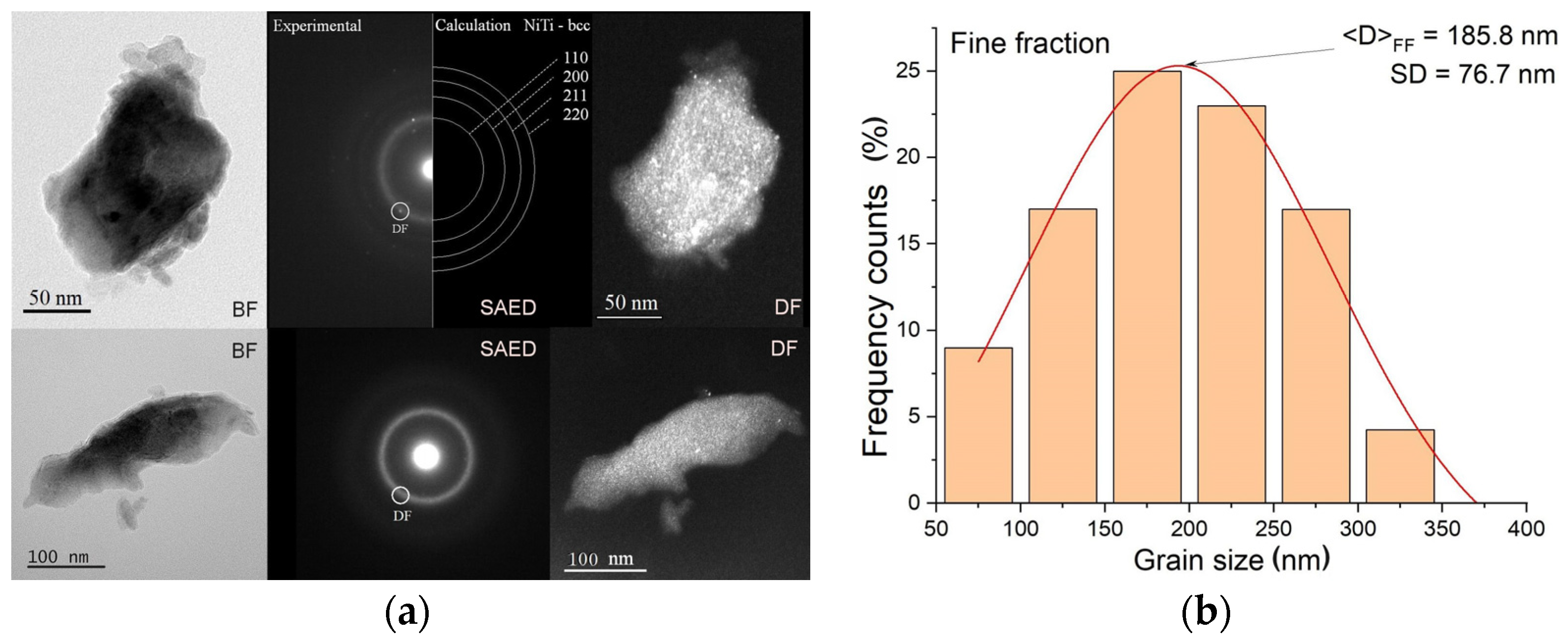
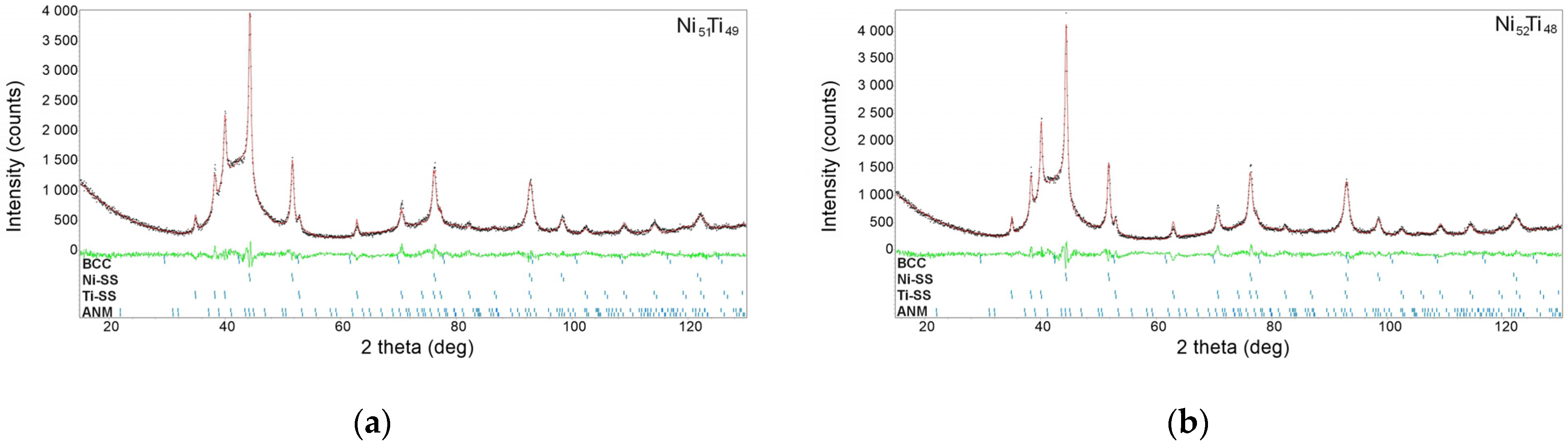
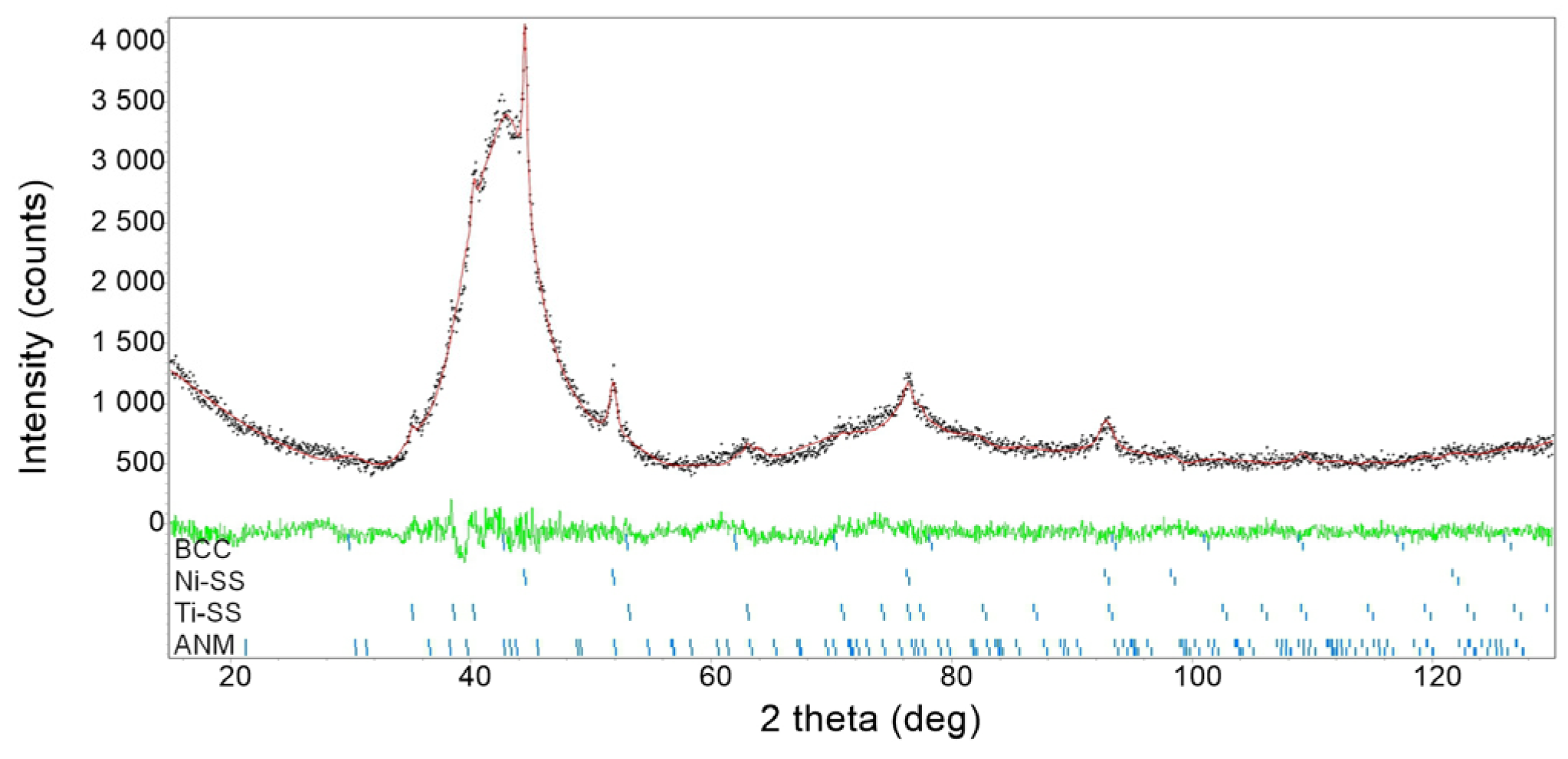
3.2. Characterization of As-Milled Alloys
4. Conclusions
- High-energy milling, carried out for 140 h, produced NiTi alloys with a chemical composition close to the nominal one, in the form of powders consisting of three fractions differing in the average grain/agglomerate size: 200 nm; 80 μm; and 556 μm.
- The alloys consisted of four basic phases: amorphous–nanocrystalline mixtures, solid solutions based on nickel and titanium, and a phase with a bcc structure, a precursor of the parent phase undergoing a reverse martensitic transformation.
- Regardless of the chemical composition of the produced alloy, the unit cell of the titanium-based solid solution underwent contraction; the lattice parameter decreased by approximately 0.15%, indicating a limitation in the content of dissolved nickel. In turn, the nickel unit cell parameters expanded, revealing the possibility of dissolving approximately 6% of titanium.
- The increase in the nickel content in the NiTi alloys contributed to an increase in the weight content of the amorphous–nanocrystalline mixture. The content of this mixture in the produced alloys was over 80 wt.%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Massalski, T.B.; Okamoto, H.; Subramanian, P.R.; Kacprzac, L. Binary Alloy Phase Diagrams, 2nd ed.; ASM International: Materials Park, OH, USA, 1990; Volume 3, p. 2875. [Google Scholar]
- Zhang, J.; Cai, W.; Ren, X.; Otsuka, K.; Asai, M. Reversible change in transformation temperatures of a Ti-51at%Ni alloy associated with alternating aging. Mat. Trans. 1999, 40, 1367–1375. [Google Scholar] [CrossRef]
- Khalil Allafi, J.; Dlouhy, A.; Eggeler, G. Ni4Ti3-precipitation during aging of NiTi shape memory alloys and its influence on martensitic phase transformations. Acta Mat. 2002, 50, 4255–4274. [Google Scholar] [CrossRef]
- Khalil Allafi, J.; Ren, X.; Eggeler, G. The mechanism of multistage martensitic transformations in aged Ni-rich NiTi shape memory alloys. Acta Mat. 2002, 50, 793–803. [Google Scholar] [CrossRef]
- Miyazaki, S. Thermal and stress cycling effects and fatigue properties of Ni-Ti alloys. In Engineering Aspects of Shape Memory Alloys, 1st ed.; Duerig, T.W., Stöckel, D., Melton, K.N., Wayman, C.M., Eds.; Butterworth-Heinemann: London, UK; Boston, MA, USA, 1990; pp. 394–413. [Google Scholar]
- Khalil-Allafi, J.; Amin-Ahmadi, B. The effect of chemical composition on enthalpy and entropy changes of martensitic transformations in binary NiTi shape memory alloys. J. Alloys Comp. 2009, 487, 363–366. [Google Scholar] [CrossRef]
- Morawiec, H.; Stróż, D.; Goryczka, T.; Chrobak, D. Two-stage martensitic transformation in a deformed and annealed NiTi alloy. Scr. Mater. 1996, 35, 485–490. [Google Scholar] [CrossRef]
- Otsuka, K.; Ren, X. Physical metallurgy of Ti–Ni-based shape memory alloys. Progress Mat. Sci. 2005, 50, 511–678. [Google Scholar] [CrossRef]
- Dunkley, J.J. Metal powder atomisation methods for modern manufacturing. Johns. Matthey Technol. Rev. 2019, 63, 226–232. [Google Scholar] [CrossRef]
- Hsu, T.I.; Wei, C.M.; Wu, L.D.; Li, Y.P.; Chiba, A.; Tsai, M.H. Nitinol powders generate from Plasma Rotation Electrode Process provide clean powder for biomedical devices used with suitable size, spheroid surface and pure composition. Sci. Rep. 2018, 8, 13776. [Google Scholar] [CrossRef]
- Tingskog, T. An introduction to metal powders for AM: Manufacturing processes and properties. Met. Addit. Manuf. 2018, 4, 111–119. [Google Scholar]
- Wang, J.-W.; He, D.-Y.; Wu, X.; Guo, X.-Y.; Tan, Z.; Zhou, Z.; Shao, W. Characterization of pre-alloyed NiTi powders produced by electrode induction-melting inert gas atomization for additive manufacturing. J. Min. Metall. Sect. B-Metall. 2022, 58, 219–228. [Google Scholar] [CrossRef]
- Tomochika, H.; Kikuchi, H.; Araki, T.; Nishida, M. Fabrication of NiTi intermetallic compound by a reactive gas laser atomization process. Mat. Sci. Eng. A 2003, 356, 122–129. [Google Scholar] [CrossRef]
- Özbilen, S. Satellite formation mechanism in gas atomised powders. Powder Metall. 1999, 42, 70–78. [Google Scholar] [CrossRef]
- Goryczka, T.; Dercz, G. Effect of milling time on crystallization sequence and microstructure of NiTi alloys produced via high-energy ball milling. Arch. Metall. Mater. 2023, 68, 1127–1135. [Google Scholar] [CrossRef]
- Goryczka, T.; Salwa, P.; Zubko, M. High-energy ball milling conditions in formation of NiTiCu shape memory alloys. Microsc. Microanal. 2021, 28, 939–945. [Google Scholar] [CrossRef]
- Kurtz, S.K.; Carpay, F.M.A. Microstructure and normal grain growth in metals and ceramics. Part II. Experiment. J. App. Phys. 1980, 51, 5725–5744. [Google Scholar] [CrossRef]
- Scardi, P.; Leoni, M. Diffraction line profiles from polydisperse crystalline systems. Acta Crystallogr. A 2001, 57, 604–613. [Google Scholar] [CrossRef]
- Hunter, B.A. Rietica—A visual Rietveld program. Comm. Powder Diffr. 1998, 20, 21. [Google Scholar]
- SRM 660a; Lanthanum Hexaboride Powder. National Institute of Standards & Technology: Gaithersburg, MD, USA, 2000.
- McCusker, L.B.; Von Dreele, R.B.; Cox, D.E.; Louer, D.; Scardi, P. Rietveld refinement guidelines. J. Appl. Cryst. 1999, 32, 36–50. [Google Scholar] [CrossRef]
- Toby, B.H. R factors in Rietveld analysis: How good is good enough. Powder Diff. 2006, 21, 67–70. [Google Scholar] [CrossRef]
- Salwa, P.; Goryczka, T. Influence of milling time on formation of NiTi alloy produced by high-energy ball milling. Arch. Metall. Mater. 2019, 64, 1017–1022. [Google Scholar] [CrossRef]
- Slater, J.C. Atomic radii in crystals. J. Chem. Phys. 1964, 41, 3199–3203. [Google Scholar] [CrossRef]


| Lattice Parameter [Å] | ||||
|---|---|---|---|---|
| a0 | c0 | |||
| Calculated | ICDD | Calculated | ICDD | |
| Ti | 2.951(4) | 2.9505 1 | 4.689(9) | 4.6826 1 |
| Ni | 3.324(8) | 3.5238 2 | - | - |
| Sample | Lattice Parameter [Å] | ||||
|---|---|---|---|---|---|
| Ti-SS | Ni-SS | bcc | |||
| Calculated | Calculated | Calculated | ICDD 1 | ||
| a0 | c0 | a0 | a0 | a0 | |
| Ni50Ti50 | 2.948(5) | 4.683(3) | 3.527(6) | 3.008(5) | 3.015 |
| Ni51Ti49 | 2.949(6) | 4.682(3) | 3.526(1) | 3.002(1) | |
| Ni52Ti48 | 2.949(3) | 4.683(1) | 3.525(9) | 3.004(9) | |
| Alloy | Weight Percentage of Phases [%] | Reliability Factors [%] | ||||||
|---|---|---|---|---|---|---|---|---|
| ANM | Solid Solutions | bcc | Total | Rp | Rwp | Rexp | ||
| Ni-SS | Ti-SS | |||||||
| Ni50Ti50 | 80.3 | 3.6 | 0.3 | 15.8 | 100 | 4.41 | 5.62 | 4.10 |
| Ni51Ti49 | 80.6 | 0.9 | 5.9 | 12.6 | 100 | 4.76 | 6.20 | 4.36 |
| Ni52Ti48 | 83.0 | 0.8 | 6.6 | 9.6 | 100 | 5.06 | 6.49 | 4.47 |
| Alloy | Content of Alloying Elements [at.%] | |||
|---|---|---|---|---|
| Nominal | Experimental | |||
| Ni | Ti | Ni | Ti | |
| Ni50Ti50 | 50 | 50 | 49.8 ± 0.3 | 50.2 ± 0.3 |
| Ni51Ti49 | 51 | 49 | 50.9 ± 0.3 | 49.1 ± 0.3 |
| Ni52Ti48 | 52 | 48 | 51.7 ± 0.3 | 48.3 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goryczka, T.; Dercz, G.; Zubko, M. Evolution of the Phase Composition in a Nickel-Predominant NiTi Shape Memory Alloy During High-Energy Ball Milling. Materials 2025, 18, 1882. https://doi.org/10.3390/ma18081882
Goryczka T, Dercz G, Zubko M. Evolution of the Phase Composition in a Nickel-Predominant NiTi Shape Memory Alloy During High-Energy Ball Milling. Materials. 2025; 18(8):1882. https://doi.org/10.3390/ma18081882
Chicago/Turabian StyleGoryczka, Tomasz, Grzegorz Dercz, and Maciej Zubko. 2025. "Evolution of the Phase Composition in a Nickel-Predominant NiTi Shape Memory Alloy During High-Energy Ball Milling" Materials 18, no. 8: 1882. https://doi.org/10.3390/ma18081882
APA StyleGoryczka, T., Dercz, G., & Zubko, M. (2025). Evolution of the Phase Composition in a Nickel-Predominant NiTi Shape Memory Alloy During High-Energy Ball Milling. Materials, 18(8), 1882. https://doi.org/10.3390/ma18081882







